Abstract
Background
Noma is a neglected tropical disease and a global health concern.
Objectives
To elucidate the epidemiology, management, prevention, and public health implications of Noma.
Methods
PubMed, Scopus, and Web of Science, supplemented by Google Scholar and World Health Organization databases, were searched using keywords to gather both published and grey literature from 1970 to 2023 in English.
Results
Approximately 30,000–40,000 cases occur annually, with varying incidences across various African countries, such as Nigeria, Niger, and Chad. Incidence in Nigerian and Ethiopian states range from 0.6 to 3300 and 1.64 to 13.4 per 100,000 population, respectively. Mortality is approximately 8.5% in Niger. Risk factors include malnutrition, immunocompromised status, poor dental hygiene, inadequate sanitation, gingival lesions, low socioeconomic status, chronic and infectious diseases, low birth weight, high parity, diarrhoea, and fever. Diagnosis is primarily made based on clinical signs/symptoms and accordingly staging of disease is done. Stage I, II and II presents with acute necrotizing gingivitis, facial edema with halitosis, and necrotizing stomatitis, respectively. If the patient survives acute stages, the progress to Stage IV and Stage V manifests as trismus, difficulty in deglutition and phonation, and facial disfigurement, with increased severity in last stage. Treatment encompasses antibiotic therapy (amoxicillin, metronidazole, chlorhexidine, ampicillin, gentamicin), surgical interventions, wound management (honey dressing, ketamine), and nutritional support. Prevention strategies include oral hygiene, vaccination, health education, and community-based interventions.
Conclusion
Noma’s recent inclusion in WHO list of neglected tropical diseases is a milestone in recognizing the importance of prevention and early intervention to globally enhance health outcomes.
Keywords: Noma, Neglected tropical disease, Poverty, Prevention, Public health
1. Introduction
Noma, or necrotizing ulcerative stomatitis, emerges as a devastating orofacial gangrene that predominantly affects malnourished children in subtropical and tropical regions (Baratti-Mayer et al., 2003). This condition, characterized by alarmingly high mortality and disability rates, not only claims the lives of many but also leaves its survivors with severe deformities. These physical scars subject them to a lifetime of stigma and isolation, further exacerbating their suffering. Noma is an immunopathological condition triggered by potent bacterial agents that incite an unchecked cytokine storm and produce other inflammatory mediators, leading to acute oral mucosal inflammation (Phillips et al., 2005). It is a polymicrobial infection, referred to as the “Face of Poverty,” Noma targets young children living in extreme poverty, especially in remote areas where healthcare facilities are severely limited (Uzochukwu et al., 2023.).
Originating from the Greek word “νομή” (nómi), meaning “to devour,” Noma carries a rich historical narrative. Once known in the British Commonwealth as “cancrum oris,” its history is well-documented, with mentions by prominent medical figures such as Hippocrates and Galen. Dutch physicians in the 16th and 17th centuries associated it with poverty and malnutrition (Marck, 2003). Despite its decline in the Western world, Noma remains prevalent in sub-Saharan Africa, central Latin America, and parts of Asia, posing a significant threat, especially to immunocompromised individuals, including those who are HIV-seropositive, in regions such as South Africa and Zimbabwe (Khammissa et al., 2022). While the disease's prevalence has declined in the Western world thanks to economic advancements and improved child nutrition, it continues to affect an estimated 140,000 individuals globally each year (Uzochukwu et al., 3 March 2023., Maguire et al., 2023, The World health report , 1998). It is important to acknowledge that the estimates of the epidemiology of Noma, largely based on the 1998 World Health Report, were derived from an expert Delphi consensus and limited available evidence (Unit WHOrganizationOH, 1998). The mortality rate hovers around 90 %, underscoring a major global health challenge (Reksodiputro and Noma, 2021). This reflects the disease's lethal nature and the profound deformities that survivors endure. Furthermore, the difficulties in preventing Noma through nutrition and measles vaccination and the challenges of treating those afflicted highlight the complexities of addressing this health crisis (Marck, 2003, Ashok et al., 2015, Marck, 2001). Hence, adopting a holistic approach is essential for treating Noma and preventing its occurrence, thus easing the burden on affected communities and contributing to global health equity (Feller et al., 2022).
The World Health Organization's (WHO) inclusion of Noma in its list of neglected tropical diseases (NTDs) signifies a critical moment (WHO, 2024). It highlights the WHO’s dedication to support the most vulnerable and emphasizing the need for global cooperation, increased research, funding, and awareness to combat this disease. A recent study underscores the potential for international collaboration in addressing the systemic issues underpinning Noma's prevalence (Ogbureke, 2022). This recognition could spur support for targeted interventions aimed at the root causes of Noma, such as poverty, malnutrition, and inadequate access to clean water and healthcare services. The WHO's decision is expected to catalyze an integrated approach to addressing Noma in affected regions, encompassing active case surveillance, joint training, social mobilization, and leveraging existing NTD wound management and support systems. Also, this integration seeks to foster advocacy, funding, research, and global visibility, thereby enhancing political commitment and facilitating efforts to eradicate extreme poverty and improve living conditions—critical factors in controlling Noma (Marck, 2003). This recognition by WHO followed collective action by 32 countries marking a significant milestone in the fight against Noma (WHO, 2024). This collaboration effort was spearheaded by Nigeria, and included 14 other African nations coming together for a common cause (WHO, 2024). These efforts have led to developing and implementing national action plans, training for primary care and community health workers, and enhanced intercountry collaboration for patient referrals. Despite these advances, challenges persist, including a lack of epidemiological data, substantial knowledge gaps, social stigma, and low awareness among healthcare workers, which impede prevention and control efforts (Srour and Baratti-Mayer, 2020). In this review, we aim to explore the epidemiology, pathogenesis, clinical features, management, and prevention of Noma and discuss the prospects of the disease.
2. Methodology
2.1. Literature search strategy
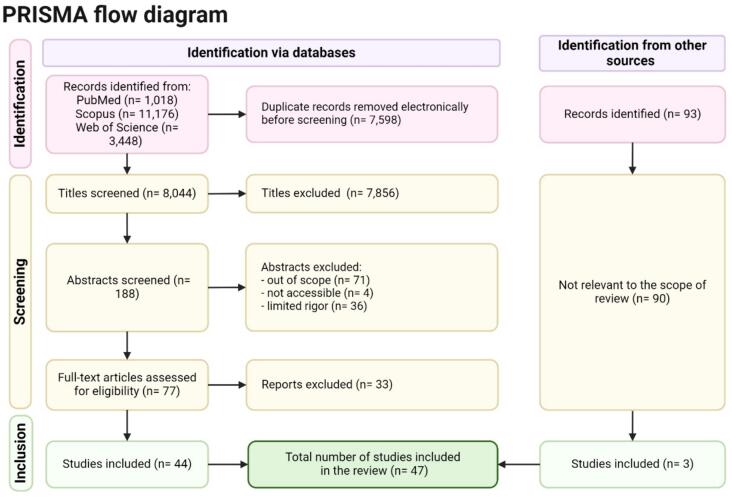
The search strategy encompassed identifying pertinent literature concerning Noma, spanning its epidemiology, etiology, clinical manifestations, diagnosis, treatment, prevention, and public health implications. Comprehensive searches were conducted across electronic databases including PubMed, Scopus, and Web of Science (Fig. 1). Additionally, grey literature sources such as Google Scholar and the organizational databases of the World Health Organization (WHO) were meticulously explored to access unpublished reports, theses, and other non-peer-reviewed documents. The search strategy was meticulously crafted utilizing a combination of keywords and terms related to Noma and its various dimensions, encompassing terms such as 'noma', 'NTD' (neglected tropical disease), 'necrotizing gingivitis ulcer', 'cancrum oris', 'facial gangrene', 'tropical ulcer', 'fusospirochetal gangrene', 'gangrenous stomatitis', 'oral microbiota', 'malnutrition', 'poverty-related diseases', 'oral health disparities', and 'community health interventions'. Articles published in English from 1970 to January 2024 were considered to ensure alignment with contemporary understanding and documentation of Noma. The need for Institutional Review Board (IRB) permission was waived due to the utilization of publicly available data for this narrative review.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for identification of studies included in the review.
2.2. Study selection
Upon retrieval of relevant articles, titles and abstracts were independently screened to identify potentially eligible studies. Full-text articles were obtained for further scrutiny if they met the predetermined inclusion criteria or if there was ambiguity based on the title and abstract. Inclusion criteria encompassed studies offering insights into the epidemiology, etiology, clinical features, diagnostic methods, treatment modalities, preventive strategies, and public health implications of Noma. Non-peer-reviewed documents, theses, and unpublished reports were included if they contributed valuable information pertinent to the objectives of the review.
2.3. Data extraction
Data extraction encompassed key findings pertaining to the epidemiology, etiology, clinical manifestations, diagnostic methods, treatment modalities, preventive strategies, and public health implications of Noma. Any discrepancies in data extraction were resolved through discussion and consensus between reviewers.
2.4. Data analysis and interpretation
Extracted data were subjected to thematic analysis to discern patterns, trends, and knowledge gaps within the literature. Synthesized findings were categorized according to thematic areas, including epidemiology, etiology, clinical features, diagnostic methods, treatment modalities, preventive strategies, and public health implications. Meta-analyses or subgroup analyses were not performed due to lack of homogenous quantitative data from included studies. In addition, a qualitative synthesis of the data was performed.
3. Epidemiology
Although epidemiological data are scarce, current estimates suggest a global incidence of 30,000–40,000 cases yearly (Maguire et al., 2023, Gebretsadik, 2023). From to 1950–2019, Noma has been reported in 88 countries across the globe. In the past decade, cases have been documented in 23 countries, particularly in Niger, Senegal, Mali, Togo and Zambia (Srour and Baratti-Mayer, 2020). Notably, the annual incidence of Noma is estimated at 140,000 cases, with a prevalence of 770,000 cases, indicating a significant public health concern (Unit WHOrganizationOH, 1998). Noma remains a critical issue in Africa, particularly in the “Noma belt” countries such as Nigeria, Niger, and Chad, with cases also reported in other African countries including Ethiopia, South Africa and Zimbabwe (Gebretsadik, 2023, Galli et al., 2022). The prevalence and incidence rates of Noma exhibit significant geographic variability, as evidenced by studies across different regions. For instance, while some areas like Kogi State, Nigeria, report lower prevalence rates of 0.6 per 100,000 people, others such as Sokoto and Kebbi states present much higher prevalence figures, reaching 3300 per 100,000 people, highlighting the disease's prevalence in developing countries near the Sahara Desert (Farley et al., 2023, Galli et al., 2022). Similarly, the incidence rates also vary greatly; eastern Ethiopia has an annual incidence between 1.64 and 13.4 per 100,000 children aged 0–9 years, whereas northwest Nigeria reports rates as high as 640 per 100,000 individuals, which is particularly high (Galli et al., 2022). Mortality data remain scarce, with disease severity highlighted by an 8.5 % mortality rate among treated cases in the Zinder region of Niger (Galli et al., 2022). The estimated incidence in the north-central zone is 8.3 per 100,000, varying from 4.1 to 17.9 per 100,000 in different states (Bello et al., 2019). The period prevalence, accounting for all cases observed during the study period, was 1.6 per 100,000 of the population at risk (Bello et al., 2019). These disparities underscore the complex interplay of factors influencing Noma's burden, necessitating targeted interventions and comprehensive data collection to address the impact of the disease effectively.
4. Risk factors
The development of Noma is influenced by a constellation of factors such as malnutrition, compromised immunity, inadequate oral hygiene, lesions on the gingival mucosal barrier, and an unidentified bacterial component serving as a trigger (Baratti-Mayer et al., 2003). Socio-economic conditions, such as substandard living conditions, extreme poverty, insufficient sanitation, and close proximity to livestock, play pivotal roles. Oral conditions like poor oral hygiene and certain forms of gingivitis, systemic conditions including severe malnutrition, debilitating diseases, malaria, tuberculosis, HIV infection, or other immunosuppressive conditions (e.g., leukemia and other blood dyscrasias), along with miscellaneous factors like low birth weight, inadequate weaning, the child's birth order, severe stunting, presence of respiratory disease, diarrhea, or fever in past three months, and the absence of the mother as the primary caregiver, are significant predisposing factors (Kagoné et al., 2022, Baratti-Mayer et al., 2013, Prado-Calleros et al., 2018).
Noma predominantly affects vulnerable children under ten years old (Maguire et al., 2023, Srour and Baratti-Mayer, 2020, Gebretsadik, 2023). Poverty escalates this risk due to restricted access to clean water, sanitation, and adequate nutrition. Overcrowding and inadequate healthcare services create an environment that is conducive to Noma transmission and progression. Insufficient healthcare infrastructure in isolated or rural areas leads to delayed diagnosis and treatment, hampering early intervention. Malnutrition weakens children's immune systems, making them more susceptible to infections and less capable of fighting diseases like Noma (Enwonwu, 1972).
5. Pathogenesis and clinical manifestations
Noma typically follows an intraoral ulcer or acute necrotizing gingivitis (ANG) rather than initiating as necrosis (Feller et al., 2022). ANG, prevalent among African children due to poverty and malnutrition, can progress to necrotizing stomatitis without proper treatment, destroying oral tissues and leading to Noma (Idigbe et al., 1999). A study revealed that among children under 15 years old, 3.1 % were diagnosed with simple gingivitis, 0.1 % with acute necrotizing gingivitis, and 0.05 % with edema, with an estimated total of 129,120 cases of Noma in this age group, predominantly consisting of simple gingivitis cases (93 %) (Fieger et al., 2003, Farley et al., 2020).
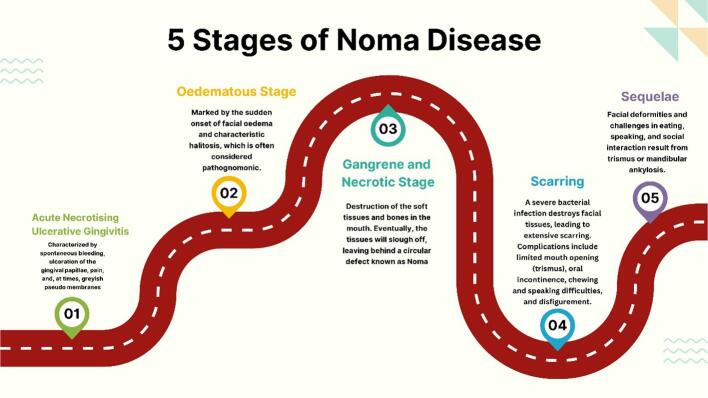
WHO classifies Noma into five stages: acute necrotizing gingivitis, edema, gangrene, scarring, and sequelae (Fig. 2) (Noma, 2024). This classification underscores the disease's rapid progression, necessitating immediate medical intervention.
Fig. 2.
Five Stages of Noma Disease. [Created with Biorender.com].
In a retrospective analysis spanning 8 years, 12 cases of acute Noma were observed, with 91.7 % displaying features indicative of the gangrenous stage and one individual showing symptoms consistent with the edema stage, whereas among the 66 participants with post-noma defects, 19.7 % exhibited nascent scarring suggestive of recent active disease, and 80.3 % displayed signs consistent with stage five (full-blown sequelae) of the Noma disease spectrum (Bello et al., 2019). In terms of Noma defects' locations on the face, Bello et al. report that the nose constituted half of the cases 50 % (n = 39), with lesions affecting the right and left cheeks accounting for 28.2 % (n = 22) and 32.1 % (n = 25) of the cases, respectively. Additionally, the majority of affected lips were the upper ones rather than the lower ones, with 13.6 % (n = 9) of patients with Noma sequelae experiencing trismus (Bello et al., 2019). In a rare case of a 32-year-old woman with no known pathological history exhibiting symptoms suggestive of acute Noma, the onset of symptoms, such as pain, swelling of the left cheek, hyperthermia, and lack of appetite, manifested 16 days prior to her admission to the hospital (Traore et al., 2021). These observations emphasizes the need for early diagnosis and timely intervention to mitigate the devastating impact of Noma.
5.1. Stage I (Acute Necrotizing Gingivitis)
Noma does not usually begin as a necrotizing process. Instead, it is preceded by a small intraoral ulcer, an aphthous lesion, or, commonly, by ANG (Idigbe et al., 1999). ANG is characterized by spontaneous bleeding, ulceration of the gingival papillae, pain, and, at times, greyish pseudo membranes. If untreated, ANG may progress into necrotizing stomatitis, destroying the attached gingival mucosa, the surrounding oral mucosa, and the underlying bone (Horning, 1996). The treatment of ANG requires a multifactorial approach. This approach involves superficial debridement, oral hygiene instruction, utilization of antimicrobial mouthwash and oral antibiotics, and initiation of a comprehensive prophylaxis plan involving root planning and predisposing factor management (Dufty et al., 2017).
5.2. Stage II (Edema Phase)
This stage of Noma is marked by the sudden onset of facial edema and characteristic halitosis, which is often considered pathognomonic. Facial swelling is usually severe, and can cause significant disfigurement. Halitosis is a distinctive feature of this stage and results from necrotic tissue in the oral cavity. This stage is usually brief, lasting only a few days, but it requires urgent medical attention to prevent the disease's progression (Srour et al., 2017).
5.3. Stage III (Acute/Gangrenous Stage)
Necrotizing stomatitis is a condition that destroys soft tissues and bones in the mouth. Without proper treatment, the necrotizing process can quickly spread to the mucosa of the cheek or lip, resulting in destruction of the mucosa, muscle, and skin. The affected tissues become swollen, macerated, and blue-gray in color on the skin side. Eventually, the tissues will slough off, leaving behind a circular defect known as Noma (Feller et al., 2000) A patient with necrosis requires hospitalization for antibiotic therapy, fluid and electrolyte replenishment, nutritional supplementation, and general supportive care (Feller et al., 2014).
5.4. Stage IV (Scarring Phase)
If someone survives Noma, a severe bacterial infection that destroys the tissues of the face, the margins of the defect caused by the disease heal with extensive fibrosis and scarring. This can lead to various complications such as limited mouth opening (trismus), oral incontinence, difficulty chewing and speaking, and disfigurement. It is common for scar tissue to form between the mandible, maxilla, and zygomatic arch, which can severely restrict mouth opening (Simon et al., 2015).
5.5. Stage V (Sequelae)
It is worth noting that only about 10–15 % of children who suffer from acute Noma can survive (Farley et al., 2023). Those who survive often have to deal with facial deformities and difficulties with eating, speaking, and socializing due to trismus or ankylosis of the mandible (Gebretsadik and de Kiev, 2022). This condition can also lead to further facial disfigurement and functional impairment due to growth disturbances (Srour et al., 2017, Wali and Regmi, 2017, Gebretsadik, 2024).
6. Diagnosis and treatment
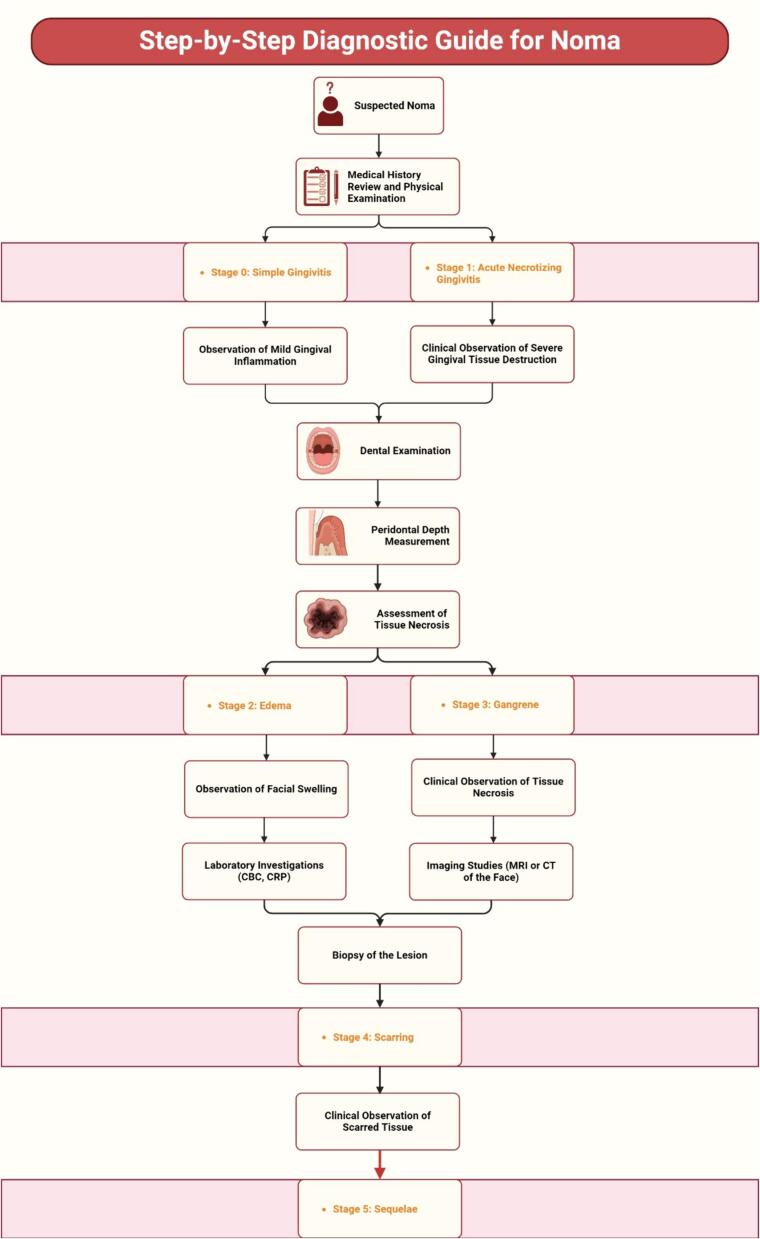
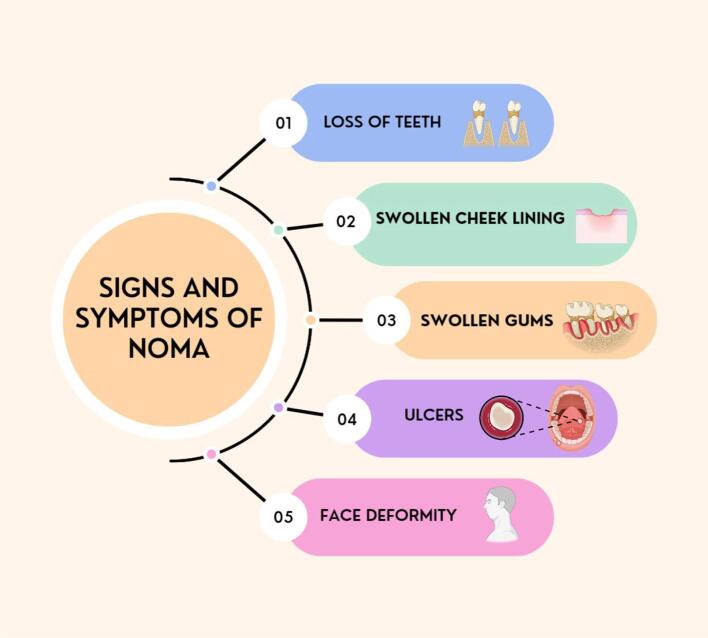
The diagnosis of Noma primarily relies on observing clinical signs and symptoms that manifest throughout its progression stages (Fig. 3). Noma progresses through several distinct stages, from gingival inflammation to severe necrosis affecting the face. Each stage presents specific clinical features, ranging from gingival ulcers to extensive facial tissue destruction (Fig. 4). The absence of point-of-care diagnostic tests means that healthcare professionals must rely on their clinical acumen to identify Noma. This necessitates a thorough understanding of the disease's progression and its ability to differentiate it from other conditions causing orofacial lesions, such as primary herpetic gingivostomatitis, acute necrotizing ulcerative gingivitis, and cancrum oris.
Fig. 3.
Stepwise Diagnostic Guide for Noma. CBC: Complete Blood Count, CRP: C-Reactive Protein, MRI: Magnetic Resonance Imaging, CT: Computed Tomography. [Created with Biorender.com].
Fig. 4.
Clinical Features of Noma Disease. [Created with Biorender.com].
The WHO recommends a multi-faceted treatment approach at different stages of Noma, emphasizing early detection, preventive measures, and comprehensive care for all stages (Srour et al., 2017, Wali and Regmi, 2017). Antibiotics, such as penicillin or metronidazole, are commonly used for prompt administration, which is crucial for controlling bacterial infection and preventing its spread (Marty et al., 2016). Also, sufficient nutrition is essential to bolster the body’s immune response against infections and to promote tissue recovery. Supplementing with high-protein and high-calorie diets may become necessary (Weledji and Njong, 2015). In severe Noma cases with tissue destruction, surgical intervention, including debridement to remove dead tissue, and reconstruction procedures like skin grafting or tissue flap surgery may be required (Rakhorst et al., 2023). Furthermore, ensuring adequate dental hygiene is essential to manage Noma as dental infections can lead to tooth loss and damage to the gums and jawbone. Dental treatment may include extraction of infected teeth, restoration of dental structures, and provision of dental hygiene education (Khammissa et al., 2022, Gebretsadik, 2023). However, data regarding post-operative follow-up is limited (Speiser et al., 2021). A summary of the treatment protocols for Noma at different stages is presented in Table 1 (WHO Regional Office for Africa, 2024).
Table 1.
Treatment protocols for Noma disease at different stages as recommended by WHO.
| Stage of Noma | Treatment |
|---|---|
| Early Detection/Preventive | Basic hygiene, antibiotics, improved nutrition, oral and maxillofacial surgery, or reconstructive plastic surgery for physical effects |
| Stage I (Acute Necrotizing Gingivitis) | Amoxicillin and metronidazole, chlorhexidine, and hydrogen peroxide for oral cleaning |
| Stage II (Edema Phase) | Either amoxicillin, clavulanic acid, gentamicin, and metronidazole OR ampicillin, gentamicin, and metronidazole; chlorhexidine mouthwash |
| Stage III (Acute/Gangrenous Stage) | Same antibiotic options as Stage II; chlorhexidine mouthwash, ketamine, and honey for dressing lesions |
| Stage IV (Scarring Phase) | Same antibiotic options as Stage II and III; chlorhexidine mouthwash, ketamine, and honey for dressing lesions |
| All Stages | Antibiotics, vitamin A or other nutritional supplements, high-protein diet, proper hydration |
7. Psychosocial aspects of Noma
About one in three individuals with Noma experience mental health conditions, with social disadvantage and beliefs in supernatural disease causation being common (Onu et al., 2023). A study involving Noma survivors and healthcare professionals in Burkina Faso highlighted the disease's economic and social impacts (Kagoné et al., 2022). Survivors face discrimination and stigma, with many turning to traditional and modern medicine for treatment (Kagoné et al., 2022). The stigma surrounding the disease often results in forced isolation, with many affected children living in seclusion rather than receiving medical care. Despite the evident need, there is a significant gap in psychosocial support for these patients. Hence, appropriate psychosocial interventions are required to address mental health issues.
8. Prevention
Preventing Noma focuses on addressing its underlying risk factors, such as malnutrition and inadequate healthcare access. Vaccination against measles and the early treatment of oral infections are critical preventive measures. Since WHO's Noma Control Initiative in 1994, efforts have been made to integrate Noma into healthcare systems, enhance data availability, and foster collaboration across sectors to reduce the incidence of Noma in at-risk populations. Table 2 delves deeper into each aspect of prevention mentioned, including additional strategies that can be employed to combat Noma's underlying causes and risk factors.
Table 2.
Comprehensive Noma Prevention Strategies.
| Strategy | Description | Implementation |
|---|---|---|
| Enhancing Nutritional Access | Malnutrition is a critical factor in the development of Noma, especially in children under five. Ensuring access to nutritious food and supplements is essential. | Expand access to high-quality food, promote breastfeeding, launch community nutrition and school feeding programs, and distribute vitamin supplements. |
| Broadening Healthcare Services | Limited healthcare access can exacerbate Noma's severity. Early detection and treatment of predisposing conditions are crucial. | Deploy mobile health clinics, embrace telemedicine, and train healthcare workers locally, focusing on early detection and management of oral infections and measles in remote areas. |
| Vaccination Initiatives | Vaccinations against measles and other diseases can prevent Noma by strengthening the immune system. | Conduct nationwide vaccination drives and integrate vaccinations into routine pediatric healthcare to mitigate Noma risk significantly. |
| Oral Hygiene Awareness | Educating on oral hygiene prevents initial infections leading to Noma. | Initiate school-based and community educational workshops focusing on proper toothbrushing, regular dental check-ups, and fluoride toothpaste usage. |
| Improving Water and Sanitation | Access to clean water and proper sanitation prevents infections that could lead to Noma. | Execute water purification and sanitation projects, including constructing latrines and community water initiatives. |
| Economic and Poverty Alleviation | Addressing poverty can indirectly combat Noma, as it's closely linked to economic conditions. | Enhance job creation, educational opportunities, and social welfare programs, including microfinance, job training, and policy reforms. |
| Global and Community Cooperation | Partnership among WHO, local governments, NGOs, and communities integrates Noma prevention into broader health initiatives. | Foster WHO-led initiatives, engage with NGOs for partnerships and encourage community involvement in prevention efforts. |
9. Future direction and challenges
The fight against Noma is beset with numerous challenges stemming from a complex interplay of factors, such as limited awareness, insufficient resources, and the intricate nature of its causes and progression. One of the primary hurdles in combating Noma is limited global awareness of the disease. This lack of understanding, even among healthcare professionals in areas where Noma is endemic, leads to delays in diagnosis and treatment, critically affecting chances of survival and recovery. Additionally, the scarcity of resources in the affected regions poses significant challenges to disease management and prevention. These areas often suffer from inadequate healthcare infrastructure, an absence of medical supplies, and a shortage of trained healthcare personnel, further complicating efforts to combat Noma.
Beyond immediate healthcare challenges, the socioeconomic conditions that underpin Noma prevalence cannot be overlooked. Poverty, malnutrition, and lack of access to clean water and sanitation are key factors contributing to the incidence of Noma. Thus, any effective strategy against Noma must address these underlying social determinants through broader public health and development initiatives. Future research should focus on several key areas to better combat Noma. The development of vaccines against bacterial pathogens associated with Noma represents a promising avenue for reducing the incidence of this disease. Similarly, creating point-of-care diagnostic tools would enable the early detection of Noma and significantly improve treatment outcomes. Additionally, a deeper understanding of disease mechanisms, including the roles of infection, malnutrition, and immune response, could lead to innovative therapeutic and preventive strategies.
Integrating Noma control strategies into global health policies is crucial for garnering the attention and resources necessary to fight the disease. Advocacy efforts should aim to raise awareness among policymakers and stakeholders regarding the impact of Noma and the need for targeted interventions. Strengthening international collaborations among governments, non-governmental organizations, research institutions, and WHO is essential for pooling resources and coordinating global efforts against Noma. Furthermore, engaging directly with communities affected by Noma through educational and empowerment initiatives is vital. It is possible to mitigate the primary risk factors associated with this disease by improving access to nutrition, sanitation, and clean water at the community level.
Overcoming the challenges Noma poses requires a concerted, multifaceted approach spanning research, public health policy, and international collaboration. By focusing on these areas, the global health community can make significant strides to improve the lives of those affected by Noma, and move closer to eradicating this devastating disease. The journey ahead involves medical and scientific efforts and a commitment to addressing the socioeconomic disparities that fuel Noma's persistence in vulnerable populations.
10. Conclusion
The recognition of Noma by the WHO as an NTD represents a pivotal advancement in the global health landscape. This inclusion not only underscores the critical need for collective action against this devastating condition, but also paves the way for enhanced research, funding, and international collaboration to mitigate its impact on the world's most vulnerable populations. Noma's epidemiology, pathogenesis, and challenges in its prevention and treatment reveal the complexity of addressing a disease deeply intertwined with socioeconomic factors, such as poverty, malnutrition, and inadequate access to healthcare. The WHO's decision to include Noma among NTDs is a clarion call to the global health community to allocate resources and expertise to eradicate this affliction. This highlights the necessity of adopting a multi-sectoral approach that goes beyond medical treatment to address the root causes of Noma, including poverty alleviation, nutritional support, and improvements in sanitation and hygiene. This recognition highlights the importance of bolstering healthcare systems in affected regions, enhancing the capabilities of healthcare workers to diagnose and treat Noma early, and implementing effective surveillance systems to monitor and prevent its spread. The path forward in the fight against Noma is laden with several challenges. Nevertheless, with concerted multi-sectoral efforts and a commitment to address the root causes of the disease, significant progress can be made.
CRediT authorship contribution statement
Amogh Verma: Writing – review & editing, Writing – original draft, Project administration, Methodology, Conceptualization, Validation, Visualization. Amna Zaheer: Writing – review & editing, Writing – original draft, Visualization. Areeba Ahsan: Writing – review & editing, Writing – original draft. Ayush Anand: Writing – review & editing, Writing – original draft, Visualization, Supervision, Methodology, Conceptualization. Hashem Abu Serhan: Writing – review & editing, Writing – original draft. Mahalaqua Nazli Khatib: Writing – review & editing, Writing – original draft. Quazi Syed Zahiruddin: Writing – review & editing, Writing – original draft. Abhay M Gaidhane: Writing – review & editing, Writing – original draft. Neelima Kukreti: Writing – review & editing, Writing – original draft. Sarvesh Rustagi: Writing – review & editing, Writing – original draft. Prakasini Satapathy: Writing – review & editing, Writing – original draft. Divya Sharma: Writing – original draft, Writing – review & editing. Mithhil Arora: Writing – original draft, Writing – review & editing. Rakesh Kumar Sharma: Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The open access publication of this article was funded by Qatar National Library.
Data availability
No data was used for the research described in the article.
References
- Ashok N., Tarakji B., Darwish S., et al. A Review on Noma: A Recent Update. Glob J Health Sci. 2015;8:53. doi: 10.5539/gjhs.v8n4p53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratti-Mayer D., Pittet B., Montandon D., et al. Noma: an “infectious” disease of unknown aetiology. Lancet Infect. Dis. 2003;3:419–431. doi: 10.1016/s1473-3099(03)00670-4. [DOI] [PubMed] [Google Scholar]
- Baratti-Mayer D., Gayet-Ageron A., Hugonnet S., et al. Risk factors for noma disease: a 6-year, prospective, matched case-control study in Niger. Lancet Glob. Health. 2013;1:e87–e96. doi: 10.1016/S2214-109X(13)70015-9. [DOI] [PubMed] [Google Scholar]
- Bello S.A., Adeoye J.A., Oketade I., et al. Estimated incidence and Prevalence of noma in north central Nigeria, 2010–2018: A retrospective study. PLoS Negl. Trop. Dis. 2019;13:e0007574. doi: 10.1371/journal.pntd.0007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufty J., Gkranias N., Donos N. Necrotising Ulcerative Gingivitis: A Literature Review. Oral Health Prev. Dent. 2017;15:321–327. doi: 10.3290/j.ohpd.a38766. [DOI] [PubMed] [Google Scholar]
- Enwonwu C.O. Epidemiological and biochemical studies of necrotizing ulcerative gingivitis and noma (cancrum oris) in Nigerian children. Arch. Oral Biol. 1972;17:1357–1371. doi: 10.1016/0003-9969(72)90169-0. [DOI] [PubMed] [Google Scholar]
- Farley E., Oyemakinde M.J., Schuurmans J., et al. The prevalence of noma in northwest Nigeria. BMJ Glob. Health. 2020;5:e002141. doi: 10.1136/bmjgh-2019-002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley E., Karinja M.N., Lawal A.M., et al. Proportion of paediatric admissions with any stage of noma at the Anka General Hospital, northwest Nigeria. PLoS Negl. Trop. Dis. 2023;17:e0011508. doi: 10.1371/journal.pntd.0011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller L., Khammissa R.A.G., Altini M., et al. Noma (cancrum oris): An unresolved global challenge. Periodontol. 2000;2019(80):189–199. doi: 10.1111/prd.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller L., Altini M., Chandran R., et al. Noma (cancrum oris) in the <scp>S</scp> outh <scp>A</scp> frican context. J. Oral Pathol. Med. 2014;43:1–6. doi: 10.1111/jop.12079. [DOI] [PubMed] [Google Scholar]
- Feller L., Lemmer J., Khammissa R.A.G. Is noma a neglected/overlooked tropical disease? Trans. R. Soc. Trop. Med. Hyg. 2022;116:884–888. doi: 10.1093/trstmh/trac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieger A., Marck K.W., Busch R., et al. An estimation of the incidence of noma in north-west Nigeria. Trop. Med. Int. Health. 2003;8:402–407. doi: 10.1046/j.1365-3156.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- Galli A., Brugger C., Fürst T., et al. Prevalence, incidence, and reported global distribution of noma: a systematic literature review. Lancet Infect. Dis. 2022;22:e221–e230. doi: 10.1016/S1473-3099(21)00698-8. [DOI] [PubMed] [Google Scholar]
- Gebretsadik H.G. Estimation of the Prevalence of Noma in Ethiopia, 2007–2019: A Retrospective Study. Am. J. Trop. Med. Hyg. 2023;109:1388–1392. doi: 10.4269/ajtmh.23-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebretsadik H.G. Surgical procedures, complications, and durations in patients with Noma disease: a cross-sectional study. Plast. Reconstr. Surg. Glob. Open. 2023;11:e5496. doi: 10.1097/GOX.0000000000005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebretsadik H.G. Noma is a facial disfiguring childhood disease: Insights from cases of Noma in Ethiopia. Int. J. Pediatr. Otorhinolaryngol. 2024;177 doi: 10.1016/j.ijporl.2023.111845. [DOI] [PubMed] [Google Scholar]
- Gebretsadik H.G., de Kiev L.C. A retrospective clinical, multi-center cross-sectional study to assess the severity and sequela of Noma/Cancrum oris in Ethiopia. PLoS Negl. Trop. Dis. 2022;16:e0010372. doi: 10.1371/journal.pntd.0010372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebretsadik HG. An Update on the Epidemiology of Noma (Facial Gangrene) in Ethiopia. Fortune Journal of Health Sciences; 06. Epub ahead of print 2023. DOI: 10.26502/fjhs.105.
- Horning GM. Necotizing gingivostomatitis: NUG to noma. Compend Contin Educ Dent 1996; 17: 951–4, 956, 957–8 passim; quiz 964. [PubMed]
- Idigbe E., Enwonwu C., Falkler W., et al. Living conditions of children at risk for noma: Nigerian experience. Oral Dis. 1999;5:156–162. doi: 10.1111/j.1601-0825.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Information brochure for early detection and management of noma | WHO | Regional Office for Africa, https://www.afro.who.int/publications/information-brochure-early-detection-and-management-noma (accessed 23 February 2024).
- Kagoné M., Mpinga E.K., Dupuis M., et al. Noma: experiences of survivors, opinion leaders and healthcare professionals in Burkina Faso. Trop. Med. Infect. Dis. 2022;7:142. doi: 10.3390/tropicalmed7070142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khammissa R.A.G., Lemmer J., Feller L. Noma staging: a review. Trop Med. Health. 2022;50:40. doi: 10.1186/s41182-022-00431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire B.J., Shrestha P., Rashan S., et al. Protocol for a systematic review of the evidence-based knowledge on the distribution, associated risk factors, the prevention and treatment modalities for noma. Wellcome Open Res. 2023;8:125. [Google Scholar]
- Marck K.W. Spijkervet FK [Noma: ’the face of poverty’] Ned. Tijdschr. Tandheelkd. 2001;108:496–499. [PubMed] [Google Scholar]
- Marck K.W. A History of Noma, the “Face of Poverty”. Plast. Reconstr. Surg. 2003;111:1702–1707. doi: 10.1097/01.PRS.0000055445.84307.3C. [DOI] [PubMed] [Google Scholar]
- Marty M., Palmieri J., Noirrit-Esclassan E., et al. Necrotizing Periodontal Diseases in Children: A Literature Review and Adjustment of Treatment. J. Trop. Pediatr. 2016;62:331–337. doi: 10.1093/tropej/fmw005. [DOI] [PubMed] [Google Scholar]
- Noma, https://www.who.int/news-room/fact-sheets/detail/noma (accessed 23 February 2024).
- Ogbureke K.U.E. Noma: A Neglected Area for Research. J. Dent. Res. 2022;101:1424–1429. doi: 10.1177/00220345221100399. [DOI] [PubMed] [Google Scholar]
- Onu J.U., Aluh D.O., Ononiwu C.N. Psychosocial aspects of Noma (Cancrum Oris) in sub-Saharan Africa: A scoping review. Trop. Doct. 2023;53:470–474. doi: 10.1177/00494755231175529. [DOI] [PubMed] [Google Scholar]
- Phillips R.S., Enwonwu C.O., Falkler W.A. Pro- versus anti-inflammatory cytokine profile in African children with acute oro-facial noma (cancrum oris, noma) Eur. Cytokine Netw. 2005;16:70–77. [PubMed] [Google Scholar]
- Prado-Calleros H.M., Castillo-Ventura B.B., Jiménez-Escobar I., et al. Noma and Noma-like disease in HIV/AIDS patients, a comorbid interaction: A systematic review. J. Infection Dev. Countries. 2018;12:89–96. doi: 10.3855/jidc.9716. [DOI] [PubMed] [Google Scholar]
- Rakhorst H.A., Gresnigt T.M., van Kooten O., et al. Reconstruction of Noma Sequelae: a surgical treatment algorithm developed from lessons from 210 Cases in Ethiopia. Plast. Reconstr. Surg. Glob. Open. 2023;11:e4844. doi: 10.1097/GOX.0000000000004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reksodiputro MH, Yosia M. Noma: Penyakit Tropis yang Terabaikan. Oto Rhino Laryngologica Indonesiana; 51. Epub ahead of print 2 July 2021. DOI: 10.32637/orli.v51i1.429.
- Simon F., Wolfe S.A., Guichard B., et al. Paul Tessier facial reconstruction in 1970 Iran, a series of post-noma defects. J. Cranio-Maxillofac. Surg. 2015;43:503–509. doi: 10.1016/j.jcms.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Speiser S., Langridge B., Birkl M.M., et al. Update on Noma: systematic review on classification, outcomes and follow-up of patients undergoing reconstructive surgery after Noma disease. BMJ Open. 2021;11:e046303. doi: 10.1136/bmjopen-2020-046303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M.L., Baratti-Mayer D. Why is noma a neglected-neglected tropical disease? PLoS Negl. Trop. Dis. 2020;14:e0008435. doi: 10.1371/journal.pntd.0008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M.L., Marck K., Baratti-Mayer D. Noma: Overview of a Neglected Disease and Human Rights Violation. Am. J. Trop. Med. Hyg. 2017;96:268–274. doi: 10.4269/ajtmh.16-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World health report : 1998 : Life in the 21st century : a vision for all : report of the Director-General, https://iris.who.int/handle/10665/42065 (accessed 23 February 2024).
- Traore H., Sogodogo E., Coulibaly A., et al. Case report: a rare case of NOMA (cancrum oris) in a Malian woman. New Microbes New Infect. 2021;42 doi: 10.1016/j.nmni.2021.100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unit WHOrganizationOH. Noma today: a public health problem? : report of an expert consultation organized by the Oral Health Unit of the World Health Organization using the Delphi method, https://iris.who.int/handle/10665/63908 (1998).
- Uzochukwu I., Moyes D., Proctor G., et al. The key players of dysbiosis in Noma disease; A systematic review of etiological studies. Front. Oral Health. 2023;4 doi: 10.3389/froh.2023.1095858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali I.M., Regmi K. People living with facial disfigurement after having had noma disease: A systematic review of the literature. J. Health Psychol. 2017;22:1243–1255. doi: 10.1177/1359105315624751. [DOI] [PubMed] [Google Scholar]
- Weledji E.P., Njong S. Cancrum Oris (Noma): the role of nutrition in management. J. Am. College Clinical Wound Specialists. 2015;7:50–52. doi: 10.1016/j.jccw.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO officially recognizes noma as a neglected tropical disease, https://www.who.int/news/item/15-12-2023-who-officially-recognizes-noma-as-a-neglected-tropical-disease (accessed 22 February 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.