ABSTRACT
Vaccination against COVID-19 is vital for achieving herd immunity, and the Government of India has adopted several strategies to achieve coverage. Vaccine hesitancy was identified as a potential obstacle in combating COVID-19. This study aimed to review the COVID-19 vaccine acceptance and hesitancy, and factors associated with vaccine hesitancy based on studies conducted in Indian populations. The data sources (PubMed, Scopus, and Google Scholar) were searched by following PRISMA guidelines, and the search was done in September 2022. We performed a meta-analysis through a random effect model to estimate pooled hesitancy rate with 95% confidence intervals (CI). A total of 3,339 records were searched, of which 46 studies were found to be eligible for inclusion in the review. The included studies covered 65,551 respondents, 55% were female. Studies reported COVID-19 vaccine acceptance rate of 65.7% in January-February 2021, which increased to 92.8% in May-August 2021. Likewise, the rate of vaccine hesitancy in December 2020 was 37%, dropping to 12.1% through November 2021. The estimated pooled COVID-19 vaccine hesitancy was 31% [95% CI: 27% − 36%, I2 = 99.3%]. Most studies highlighted that fear of the vaccine’s side effects, efficacy, and safety were major barriers to vaccine acceptance. However, as the review indicates, it is important to consider and address all factors contributing to vaccine hesitancy.
KEYWORDS: COVID-19, vaccine acceptance, vaccine hesitancy, associated factors, review, India
Introduction
The Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Distress Syndrome Coronavirus-2 (SARS-CoV-2), a positive-stranded RNA virus, classified into alpha, beta, delta, and gamma [1], was declared ‘a global public health emergency of international concern (PHEIC)’ by the World Health Organization (WHO) on 30 January 2020. The disease has posed significant challenges across nations’ healthcare systems [2,3]. SARS-CoV-2, with an incubation period of 2–14 days, is rapidly transmitted through close contact, droplet, and aerosol transmission. The common signs and symptoms were breathing difficulties, myalgia, anosmia and ageusia, dyspnea, chest pain, and hypoxia. The first case of SARS-CoV-2 was reported in December 2019 [4]. India reported its first case of COVID-19 in January 2020 [5], and since then, it rapidly progressed to a pandemic [6]. In India, the first wave commenced in March 2020 and continued till November 2020, while the second wave began in March 2021 and lasted till May 2021 [7]. The first case of COVID-19 caused by delta-variant was detected in India in October 2020 [8]. With increased transmissibility, the second wave of COVID-19 hit India harder with an exponential increase in new and severe cases, approximately double the first peak [9]. Later, SARS-CoV-2 Omicron, a new variant of COVID-19 detected in November 2021, was designated as a Variant of Concern (VOC) by the World Health Organization (WHO) [10]. The spread of COVID-19 has caused massive disruption worldwide, and the pandemic adversely affected mortality, mental health, and social and economic stability across the globe [11,12]. India, a densely populated country with a population of over 1.4 billion by January 2023 [13], recorded 441 million discharged/cured/migrated COVID-19 cases and 530,707 deaths attributed to the disease [14]. Various measures, such as quarantine, lockdown, social distancing, the mandatory wearing of face masks, frequent hand washing and use of sanitizers, travel restrictions and closure of schools and offices, were enacted by governments to combat COVID-19 [15]. However, the virus is still evolving into variants affecting populations worldwide.
Vaccination is the most efficacious solution to combat COVID-19 [16]. Mass vaccination against infectious viruses remains the most powerful, cost-efficient and effective strategy for widespread immune protection and to prevent disease spread, as evident from previous epidemics including Influenza H1N1, Pneumococcal, Ebola, Nipah, Zika, HPV, MERS, etc. The current evidence suggests that a coverage rate of 70% for a vaccine that is more than 90% effective would be required to achieve herd immunity [17,18]. It has been a key challenge for scientists to develop new vaccines in a shorter period, which usually takes 10–15 years for development and regulatory approval [19].
The WHO has approved several vaccines for emergency usage [20]. Presently, India is using the following COVID-19 vaccines – COVAXIN (Bharat Biotech, Hyderabad, India), COVISHIELD (developed by the University of Oxford, Cambridge, UK and manufactured by Serum Institute of India, Pune) and SPUTNIK V (Gamaleya Research Institute of Epidemiology and Microbiology, Moscow, Russia), CORBEVAX (Biological E. Limited, Telangana, India), and Covovax (manufactured by M/s Serum Institute of India) [21,22]. The first COVID-19 vaccination campaign was launched for healthcare and frontline workers in India on 16 January 2021. From March 2021 onwards, the second stage of the campaign started, and it was expanded to the general population, elderly (>60 years old), and co-morbid individuals aged (>45 years old). The third stage of vaccination was started in April 2021 for all >45 year old individuals; then, from May 2021, it was expanded to all >18 year olds. From January 2022, the fifth stage started for 15–18 year olds, and then extended to 12–14 year old children on 16 March 2022. Vaccination was provided free of cost to all the eligible population. COVID-19 vaccination was carried out through COVID-19 vaccination centers, special COVID-19 vaccination drives, and even domiciliary visits in villages. Registration for vaccination was done through the Indian Government Web Portal ‘CoWIN’ [23]. However, success of the vaccination program depends on public acceptance and vaccine uptake.
The World Health Organization (WHO) listed ‘Vaccine Hesitancy’ as one of the ten significant threats to global health [24]. The SAGE Working Group on Vaccine Hesitancy defined vaccine hesitancy as ‘delay in acceptance or refusal of vaccination despite the availability of services and influenced by factors such as complacency, convenience and confidence’ [25]. Vaccine hesitancy affects not only the hesitant individual but the whole community. As of 4 January 2023, the cumulative number of COVID-19 vaccine doses administered in India was more than 2 billion [26] for all the doses − 1st dose, 2nd dose and precautionary dose. It included all eligible age groups. The data highlights that population groups are still hesitant about vaccination, thus making it difficult to reach the threshold to confer herd immunity [27]. Considering that COVID-19 is unlikely to be eliminated, acquiring herd immunity against the virus is vital to limit its mortality risk. Individual perceptions about vaccines and their effectiveness, fear of side effects, etc., influence vaccine acceptance. It is important to identify the factors contributing to COVID-19 vaccine hesitancy. Therefore, to understand COVID-19 vaccine hesitancy and factors associated with it, we conducted a systematic review and meta-analysis of published studies reporting COVID-19 vaccine hesitancy, acceptance, and various factors associated with vaccine hesitancy among the Indian population. Based on the PICOT framework, the review attempted to address the following questions: (1) What is the proportion of COVID-19 vaccine hesitancy among eligible populations in India? and (2) What are the factors associated with COVID-19 vaccination hesitancy among the Indian population?
Methods
We employed a systematic review following the Center for Reviews and Dissemination standards [28]. We reported as per the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) [29,30].
Search strategy and key search terms
The literature search was done in PubMed, Scopus, and Google Scholar in September 2022. The search was restricted to research conducted in India and published in English. Medical Subject Headings (MeSH) and keywords were used in the search approach to identify the studies. The MeSH/keywords included for the current review were: COVID-19 OR SARS-CoV-2 OR severe acute respiratory syndrome OR Coronavirus; vaccine OR vaccination OR COVID-19 vaccines; vaccine hesitancy; vaccine acceptance; vaccine uptake; vaccine willingness; vaccine confidence; vaccine rejection; vaccine resistance; vaccine motivation; vaccine readiness; vaccine intention; vaccine refusal; vaccine perception; vaccine literacy; vaccine concerns; vaccine attitude; AND India. These key terms were combined with the Boolean operators ‘AND’. For, e.g. ’COVID-19 Vaccine Acceptance AND India’. Also, the references in the selected articles were manually screened for identifying further relevant articles.
Study selection criteria
The examination of the complete records for duplicate studies followed an electronic search in the database. After removing duplicates, the lists were reviewed to select relevant articles based on inclusion and exclusion criteria per PICOT criteria (Table 1). The inclusion criteria are limited to studies based on data from India. Original articles reporting COVID-19 vaccine-related beliefs and behaviors were considered for inclusion. Those studies unrelated to COVID-19 vaccine behavior, those articles solely focusing on healthcare providers, various non-data-driven publications, studies assessing the impact of COVID-19 infection and post-vaccination effects, and COVID-19 vaccine trials were excluded.
Table 1.
Eligibility criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
|
We have adopted a three-step process for selecting the studies for the current review. In the first step, the titles of the retrieved studies from all the databases were screened. Then, the selected studies based on titles were checked for duplication. Duplicates were removed. In the second step, the abstracts were read, and in the third step, the full texts of the studies were independently read by SD and YSK for inclusion. Any disagreements were resolved through consulting and discussion among the authors.
Data extraction
The following information was retrieved from the selected studies [31–76]: author name with the year of publication; study state; study community; study design; survey duration; age group, sample size with male and female segregation; acceptance/hesitancy/willingness rate; and factors associated with hesitancy or unwillingness (Table 2).
Table 2.
Details of the included studies.
| S. No. | Authors | Coverage | Mode | Study Design | Study Community | Survey Duration | Age/years | Sample Size, n | Female % | Vaccine Acceptance/Hesitancy (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Sharun et al. 2020 [31] | National | Online | Cross-Sectional | General | Oct − 2020 | Majority: 18–29 (73.5%) | 351 | 41.9 | Hesitancy rate = 13.7% |
| 2. | Gautam et al. 2020 [32] | West Bengal | Online | Cross-Sectional | General | Oct – Nov 2020 | NA | 1078 | NA | Acceptance rate = 77.27% |
| 3. | Leelawathy et al. 2021 [33] | Kerala | Online | Cross-Sectional | General | January 16 – February 22 2021 | Majority:18–29 (59.1%) | 1345 | 54.4 | Acceptance rate = 88.2% |
| 4. | Ain et al. 2021 [34] | Kashmir | Online | Cross-Sectional | General | 17–27 Dec 2020 | Majority: ≤30 (66.7%) | 487 | 55.2 | Acceptance rate = 46% |
| 5. | Kumar et al. 2021 [35] | Uttarakhand | Off-line | Cross-Sectional | General | 2 months | 34.03 ± 12.68 years | 841 | 48.5 | Acceptance rate = 53.4% |
| 6. | Anandraj et al. 2021 [36] | Puducherry | Off-line | Longitudinal | General | March 2021 – round-1 May 2021 – round-2 | Majority: Round 1: <45 (51.6%); Round 2: <45 (51.9%) | 861 | 62.6 | Hesitancy rate = 27.8% in round-1 to 32.7% in round-2 |
| 7. | Ekstrand et al. 2021 [37] | Karnataka | Online | Longitudinal | Patients | 18 Jan − 19 Feb 2021 | Median = 39.5 years | 438 | 51.6 | Hesitancy rate = 38.4% |
| 8. | Uvais, 2021 [38] | Kerala | Off-line | Cross-Sectional | Patients | March 29–21 April 2021 | Majority:26–60 (80%) | 90 | 58.9 | Hesitancy rate = 37.8% |
| 9. | Umakanthan et al. 2021 [39] | Multi-state | Online | Longitudinal | General | Feb – May 2021 | 46.64 ± 14.3 years | 3000 | 100 | Hesitancy rate = 59% |
| 10. | Chandani et al. 2021 [40] | National | Online | Cross-Sectional | General | Dec − 2020 | Majority:18–24 (46%) | 1638 | 45 | Hesitancy rate = 37% |
| 11. | Kishore et al. 2021 [41] | National | Online | Cross-Sectional | General | October 26 – November 10 2020 | Majority:21–40 (52.5%) | 270 | NA | Willingness rate = 68.8% |
| 12. | Danabal et al. 2021 [42] | Tamil Nadu | Off-line | Cross-Sectional | General | NA | Majority:18–45 (68.6%) | 564 | 62.9 | Hesitancy rate = 40.7% |
| 13. | Gaur et al. 2021 [43] | Gujarat | Off-line | Cross-Sectional | Patients | March 22 t – April 22 2021 | 47 ± 13 years | 382 | NA | Acceptance rate = 54% (Patients) and 67% (Controls) |
| 14. | Jacob et al. 2021 [44] | National | Online | Cross-Sectional | General | 2–14 Jan 2021 | Majority:18–24 (49.65%) | 2032 | 62.5 | Acceptance rate = 78.6% |
| 15. | Kumari et al. 2021 [45] | National | Online | Cross-Sectional | General | 13–25 March 2021 | 38.02 ± 13.34 years | 1294 | 41.6 | Acceptance rate = 83.6 % |
| 16. | Islam et al. 2021 [46] | Delhi | Dual Mode | Cross-Sectional | General | July – Oct 2020 | Majority: Middle-aged 41.7% | 513 | 48.3 | Acceptance rate = 79.5% |
| 17. | Bharadwaj et al. 2021 [47] | National | Online | Cross-Sectional | General | NA | NA | 754 | NA | Hesitancy rate = 53.9% |
| 18. | Shah et al. 2021 [48] | Gujarat | Off-line | Cross-Sectional | General | Jan − 2021 | 18 years and above | 25 | NA | Willingness rate = 72% |
| 19. | Bhondve et al. 2021 [49] | National | Online | Cross-Sectional | General | NA | Median = 43 (26) | 363 | 43.3 | Hesitancy rate = 51.2% |
| 20. | Bhangale et al. 2021 [50] | Maharashtra | Online | Cross-Sectional | General | February 20– March 15 2021 | 18 years and above | 772 | 40.7 | Hesitancy rate = 22.5% |
| 21. | Goruntla et al. 2021 [51] | National | Online | Cross-Sectional | General | 5–20 Oct 2020 | Majority: 20–29 (56.06%) | 2451 | 39.9 | Acceptance rate = 89.3% |
| 22. | Suresh et al. 2021 [52] | National | Online | Cross-Sectional | General | 1–15 Feb 2021 | Majority: 18–29 (74.6%) | 357 | 48.3 | Acceptance rate = 70% |
| 23. | Dabas et al. 2021 [53] | Kerala | Online | Cross-Sectional | General | April – May 2021 | Majority: ≤ 25 (64.5%) | 155 | 54.2 | Acceptance rate = 60% |
| 24. | Bansal et al. 2021 [54] | Multi-state | Online | Cross-Sectional | General | January 20 – February 28 2021 | Majority: 18–29 (66.9%) | 813 | 45.8 | Acceptance rate = 70.8% |
| 25. | Lazarus et al. 2021 [55] | National | Online | Cross-Sectional | General | Jun − 2021 | Majority: 18–29 (24.2%) | 1000 | 48 | Acceptance rate = 78% |
| 26. | Raja et al. 2021 [56] | Tamil Nadu | Off-line | Cross-Sectional | General | Dec 2020 – Jan 2021 | Majority: 18–30 (40.7%) | 140 | 60.7 | Acceptance rate = 40% |
| 27. | Sharma et al. 2022 [57] | Delhi | Off-line | Cross-Sectional | General | September 24–14 Oct 2021 | Majority: 18–49 (72.4%) | 20312 | 59.3 | Acceptance rate = 67.7% |
| 28. | Parthasarathi et al. 2022 [58] | National | Online | Cross-Sectional | General | Mid-Feb – March 2021 | Majority: 25–44 (39.3%) | 1582 | 40.3 | Willingness rate = 59.9% |
| 29. | Achrekar et al. 2022 [59] | National | Online | Cross-Sectional | General | 13 Dec 2021–10 Feb 2022 | 25.41 ± 9.1 years | 687 | 67.2 | Willingness rate = 50% |
| 30. | Gupta et al. 2022 [60] | Manipur | Off-line | Cross-Sectional | Pregnant Women | Aug − 2021 | 28.3 ± 5.5 years | 163 | 100 | Hesitancy rate = 77.9% |
| 31. | Kuchi and Parida, 2022 [61] | Odisha | Off-line | Cross-Sectional | Patients | May 25 – June 15 2021 | Majority: 18–30 (54.67%) | 450 | 42.9 | Hesitancy rate = 12.7% |
| 32. | Mohanasundaram et al. 2022 [62] | Tamil Nadu | Off-line | Cross-Sectional | Patients | 15 Sept − 14 Oct 2021 | 47.5 ± 13.17 years | 2092 | 79.2 | Hesitancy rate = 52.69% |
| 33. | Dong et al. 2022 [63] | National | Online | Cross-Sectional | General | January 29 – February 13 2021 | Majority: 26–30 (31.5%) | 406 | 40.9 | Acceptance rate = 82.5% |
| 34. | Joshi et al. 2022 [64] | Tamil Nadu | Off-line | Cross-Sectional | General | March-May 2021 | Majority: 25–54 (50%) | 3011 | 52 | Acceptance Rate = 46% |
| 35. | Romate et al. 2022 [65] | National | Online | Cross-Sectional | General | July – Sept 2021 | 18 years and above | 1006 | 63 | Willingness rate = 89% |
| 36. | Kusuma and Kant, 2022 [66] | Delhi | Off-line | Cross-Sectional | General | Sept 2020 –Jan 2021 | Majority: 30–39 (29.3%) | 1539 | 49.1 | Acceptance rate = 64.9% |
| 37. | Khan et al. 2022 [67] | Uttar Pradesh | Online | Cross-Sectional | General | 1–30 May 2021 | Majority: 18–44 (86.2%) | 254 | 45.3 | Willingness rate = 86% |
| 38. | Khan et al. 2022 [68] | Kashmir | Online | Cross-Sectional | General | 10–20 Feb2021 | Majority: 30–50 (65.1%) | 835 | 19.5 | Willingness rate = 52.7% |
| 39. | Kumari et al. 2022 [69] | North India | Online | Cross-Sectional | Pregnant Women | Nov – Dec 2021 | 28.32 ± 4.62 years | 313 | 100 | Awareness related to eligibility for vaccination = 72.2% pregnant and 65.2% lactating women |
| 40. | Jetly et al. 2022 [70] | National | Dual Mode | Cross-Sectional | General | May 1–31 August 2021 | Majority: 18–40 (70.9%) | 2024 | 49.9 | Acceptance rate = 92.8 % |
| 41. | Bansal et al. 2022 [71] | Multi-state | Off-line | Cross-Sectional | General | 28 May − 15 2021 | NA | 1428 | NA | Acceptance rate = 66 % |
| 42. | Samanta et al. 2022 [72] | West Bengal | Online | Cross-Sectional | General | February 22– March 22 2021 | Majority: <25 (66.25 %) | 803 | 45.8 | Hesitancy Rate = 12.08% |
| 43. | Hawalader et al. 2022 [73] | National | Off-line | Cross-Sectional | General | 17 Jan −2 Feb 2021 | Majority: 26–35 (65.01%) | 2891 | 40.3 | Acceptance rate = 65.7% |
| 44. | Naqvi et al. 2022 [74] | National | Off-line | Cross-sectional | Pregnant Women | Feb – Nov 2021 | Majority: 20–35 (91.7%) | 2821 | 100 | Hesitancy Rate = 12.1 % |
| 45. | Mathew et al. 2022 [75] | South India | Off-line | Cross-Sectional | General | March-May 2021 | Majority: <40 (82.8%) | 742 | 48.5 | Hesitancy rate = 20.75% |
| 46. | Ganju et al. 2022 [76] | South India | Off-line | Cross-Sectional | Patients | January 1– June 30 2021 | 30–85 years | 178 | 43 | Vaccine uptake = 70% |
Quality assessment of the studies
The quality assessment of the selected studies was carried out following the Newcastle-Ottawa scale (Table 3). The quality assessment revealed that the quality was good for 39 studies 31–46, 49–52, 54–59, 61–69, 72–74, 76] and seven were fair [47,48,53,60,70,71,75] in quality. Hence, all the studies were included for further analysis.+
Table 3.
The newcastle-ottawa scale (NOS) for quality assessment of the included studies in the meta-analysis.
| S. No. | Selection |
Comparability | Outcome |
Total Quality Score | Overall Quality Assessment (AHRQ standards) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Representativeness of the sample | Sample Size | Non-respondents | Ascertainment of the exposure (risk factor) | Outcome groups are comparable | Assessment of outcomes | Statistical test | |||
| [31] | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 6 | Good | |
| 1. | [32] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | Good |
| 2. | [33] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Good |
| 3. | [34] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | Good |
| 4. | [35] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 5. | [36] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 6. | [37] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 7. | [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | Good |
| 8. | [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | Good |
| 9. | [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | Good |
| 10. | [41] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 | Good |
| 11. | [42] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 12. | [43] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Good |
| 13. | [44] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 14. | [45] | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 8 | Good |
| 15. | [46] | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | Good |
| 16. | [47] | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 5 | Fair |
| 17. | [48] | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 5 | Fair |
| 18. | [49] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 19. | [50] | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 7 | Good |
| 20. | [51] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 21. | [52] | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 6 | Good |
| 22. | [53] | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 7 | Good |
| 23. | [54] | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 4 | Fair |
| 24. | [55] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 25. | [56] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 26. | [57] | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 7 | Good |
| 27. | [58] | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | Good |
| 28. | [59] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 29. | [60] | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 5 | Fair |
| 30. | [61] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Good |
| 31. | [62] | 1 | 0 | 0 | 2 | 2 | 1 | 1 | 7 | Good |
| 32. | [63] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 33. | [64] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Good |
| 34. | [65] | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 7 | Good |
| 35. | [66] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 36. | [67] | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 8 | Good |
| 37. | [68] | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 | Good |
| 38. | [69] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 39. | [70] | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 5 | Fair |
| 40. | [71] | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 4 | Fair |
| 41. | [72] | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 6 | Good |
| 42. | [73] | 1 | 0 | 0 | 2 | 2 | 1 | 1 | 7 | Good |
| 43. | [74] | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 | Good |
| 44. | [75] | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 5 | Fair |
| 45. | [76] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | Good |
Data synthesis
The extracted data were managed in Microsoft Excel for all analyses and exported into StatsDirect Statistical Analysis Software for meta-analysis. Meta-analysis was performed considering the presence of heterogeneity among the studies and was quantified by estimating the variance using the I2 Statistics. I2 heterogeneity was interpreted based on Higgins and Thompson’s classification [77]. It takes the value between 0–100%, and percentages of 0%, 25%, 50%, and 75% were considered as the absence of heterogeneity, low, moderate and high heterogeneity, respectively. The random effect model was utilized to estimate the pooled COVID-19 vaccine hesitancy rate among the Indian populations at a 95% confidence interval (CI) and presented in a forest plot. Publication bias was checked by the funnel plot and Egger’s test.
Results
Search results
A total of 3,339 articles were identified by searching three databases (Figure 1). In the first step, the titles of the retrieved studies were examined for eligibility, and 1181 articles were identified. The identified articles were checked for duplication, and 242 remained after removing the duplicates. In the second step, abstracts were read, based on which 60 articles were included. The full text of these 60 articles were read, and 14 articles were not considered as they did not report vaccine acceptance or hesitancy data. Thus, finally, 46 articles were included in the present review.
Figure 1.
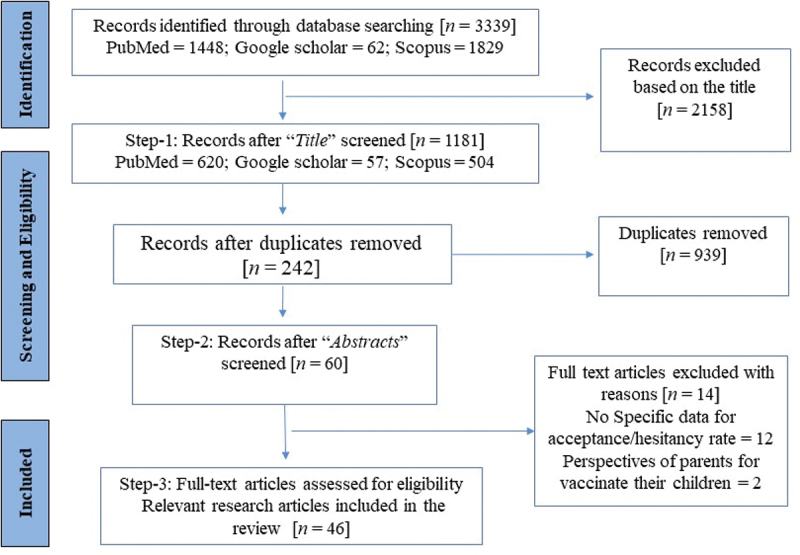
PRISMA flow diagram of the selection of the articles.
Characteristics of the included studies
Table 2 presents the studies included in the current review along with vaccine acceptance/hesitancy/uptake rate, and Table 4 presents the descriptive analysis of the included studies.
Table 4.
Descriptive analysis of the included studies.
| Study characteristics | Study attributes | Number of studies (%) | Study ID |
|---|---|---|---|
| Year of Publication | 2020 | 2 (4%) | 31,32 |
| 2021 | 24 (52%) | 33–56 | |
| 2022 | 20 (43%) | 57–76 | |
| Geographical Coverage | National Level | 17 (37%) | 31,40–41, 44–45, 47, 49, 51–52, 55, 58–59, 63, 65, 70, 73–74 |
| Multi-state Level | 5 (11%) | 39, 54, 71, 75–76 | |
| State Level | 22 (48%) | 32–35, 37–38, 42–43, 46, 48, 50, 53, 57, 60–62, 64, 66–69, 72 | |
| District Level | 2 (4%) | 36, 56 | |
| Mode of data collection | Online | 26 (57%) | 31–34, 37, 39–41, 44–45, 47, 49–55, 58–59, 63, 65, 67–69, 72 |
| Off-line | 18 (39%) | 35–36, 38, 42–43, 48, 56–57, 60–62, 64, 66, 71, 73–76 | |
| Dual mode | 2 (4%) | 46, 70 | |
| Study design | Cross-Sectional | 43 (93%) | 31–35, 38, 40–76 |
| Longitudinal | 3 (7%) | 36–37, 39 | |
| Survey duration | ≤1 Month | 27 (59%) | 31, 34, 37–38, 40–41, 43–45, 48, 50–53, 55–57, 60–63, 67–69, 71–73 |
| >1 & ≤ 2 Month | 9 (20%) | 32–33, 35, 54, 58–59, 64–65, 75 | |
| ≥3 Month | 5 (11%) | 46, 66, 70, 74, 76 | |
| Separate Rounds (1 Month Each) | 2 (4%) | 36, 39 | |
| Not mentioned | 3 (7%) | 42, 47, 49 | |
| Age | 18 years and above | 34 (74%) | 31, 33, 35–37, 40–46, 48–50, 52–59, 62–68, 70, 72–73, 75 |
| Age range mentioned | 4 (9%) | 38, 51, 74, 76 | |
| Mean age mentioned | 5 (11%) | 34, 39, 60–61, 69 | |
| Not mentioned | 3 (7%) | 32, 47, 71 | |
| Sample Size | Sum | 65551 | - |
| Average | 1425 | - | |
| <1000 | 28 (61%) | 31, 34–38, 41–43, 46–50, 52–54, 56, 59–61, 63, 67–69, 72, 75–76 | |
| 1000–2000 | 9 (20%) | 32–33, 40, 45, 55, 58, 65–66, 71 | |
| 2000–3000 | 7 (15%) | 39, 44,51,62, 70, 73–74 | |
| >3000 | 2 (4%) | 57, 64 | |
| Gender | Female | 35906 (55%) | - |
| Male | 25723 (39%) | - | |
| Gender distribution is not mentioned | 6 (13%) | 32, 41, 43, 47–48, 71 | |
| Target Population | General | 37 (80%) | 31–36, 39–42, 44–59, 63–68, 70–73, 75 |
| Vulnerable (Patients with HIV; Psychiatric disorders; systemic autoimmune rheumatic disease; individuals with co-morbidities; multiple myeloma and AL amyloidosis | 6 (13%) | 37–38, 4, 61–62, 76 | |
| Pregnant Women | 3 (7%) | 60, 69, 74 | |
| Vaccine Outcomes | Vaccine acceptance | 21 (46%) | 32–35, 43–46, 51–57, 63–64, 66, 70–71, 73 |
| Vaccine hesitancy | 16 (35%) | 31, 36–40, 42, 47, 49–50, 60–62, 72, 74–75 | |
| Vaccine willingness/awareness | 8 (17%) | 41, 48, 58–59,65, 67–69 | |
| Vaccine uptake | 1 (2%) | 76 | |
| Key Considerations | Survey duration – not mentioned | 3 (7%) | 42, 47, 49 |
| Age distribution – not mentioned | 3 (7%) | 32, 47, 71 | |
| Gender distribution – not mentioned | 6 (13%) | 32, 41, 43, 47–48, 71 |
Out of the 46 studies included, 24 (52%) were published in the year 2021, and the rest were published in 2022 (20 articles) and 2020 (2 articles). Forty three studies followed a cross-sectional design, and three were longitudinal. Twenty-six studies collected data online, eighteen collected through interviewer-administered face-to-face interviews, and two collected data through both modes. All surveys included in this review were conducted between July 2020 and February 2022. The survey duration was ≤1 Month for 59% of the studies. Most studies (74%) included participants aged 18 years or above. The total sample of the included studies was 65,551, ranging from 25 [48] to 20,312 respondents [57] in individual studies.
Vaccine acceptance and hesitancy rate
Twenty one out of the 46 studies reported the vaccine acceptance rate, and 16 studies reported the rate of vaccine hesitancy. The studies assessed vaccine acceptance or hesitancy of the participants through questionnaire-based surveys (either online, face-to-face or both). Various studies asked whether they would be willing to take/accept the COVID-19 vaccine (yes/no) [32,44,45,56,63,64,66]; intent to vaccinate against COVID-19 (yes/no/not sure) [35,51,73]; willing to accept the COVID −19 vaccine when available (yes/no/maybe) [34,46,55] and plan on COVID −19 vaccination (as soon as possible/delay) [53]. Vaccine hesitancy was assessed based on uncertainty regarding vaccine uptake and complete denial of vaccination among the respondents. The studies assessed the vaccine hesitancy rate as the percentage of the respondents not intending to get vaccinated’ [31,36,61], ‘not sure’ [40], ‘probably’ or ‘no’ [39,42,49], ‘extremely unlikely’ or ‘never’ [37], ‘not necessary’ [72], ‘delay the vaccination’ [75]. Some studies reported vaccine hesitancy, while others reported vaccine acceptance. For the present review, vaccine hesitancy was derived based on the acceptance rate for those studies reporting vaccine acceptance. The vaccine hesitancy ranged from 7% [70] to 59% [39] among the general populations of India, and a higher hesitancy rate of 77.9% was reported among pregnant women from the state of Manipur [60].
Factors associated with vaccine hesitancy
This systematic review identified several factors associated with COVID-19 vaccine hesitancy (Figure 2). Most of the studies highlighted the fear of the vaccine’s side effects (37%) as a major barrier to vaccine acceptance, followed by concerns regarding the efficacy (30%) and safety (30%) of the vaccine.
Figure 2.
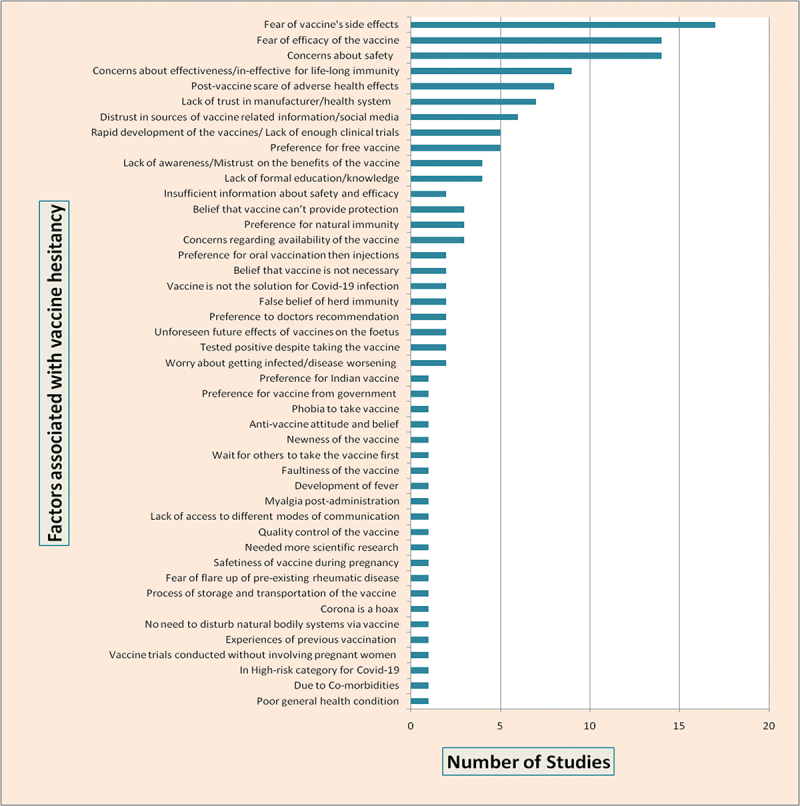
Studies reported COVID-19 vaccine hesitancy associated factors.
Pooled COVID-19 vaccine hesitancy rate among general population in India
The COVID-19 vaccine hesitancy rate was calculated using data from forty-six (46) studies in India. Based on DerSimonian and Laird’s random effects model, the meta-analysis revealed a pooled COVID-19 vaccine hesitancy rate of 31% [95% CI: 27% − 36%] (Figure 3). The highest hesitancy rate was 78% (95% CI: 71% − 84%) in Manipur [60], and the lowest rate was 7% (95% CI: 6% − 8%) reported in a study covering the general population across India [70]. However, there was significant variability among the studies [I2 = 99.3%, p ≤ 0.0001].
Figure 3.
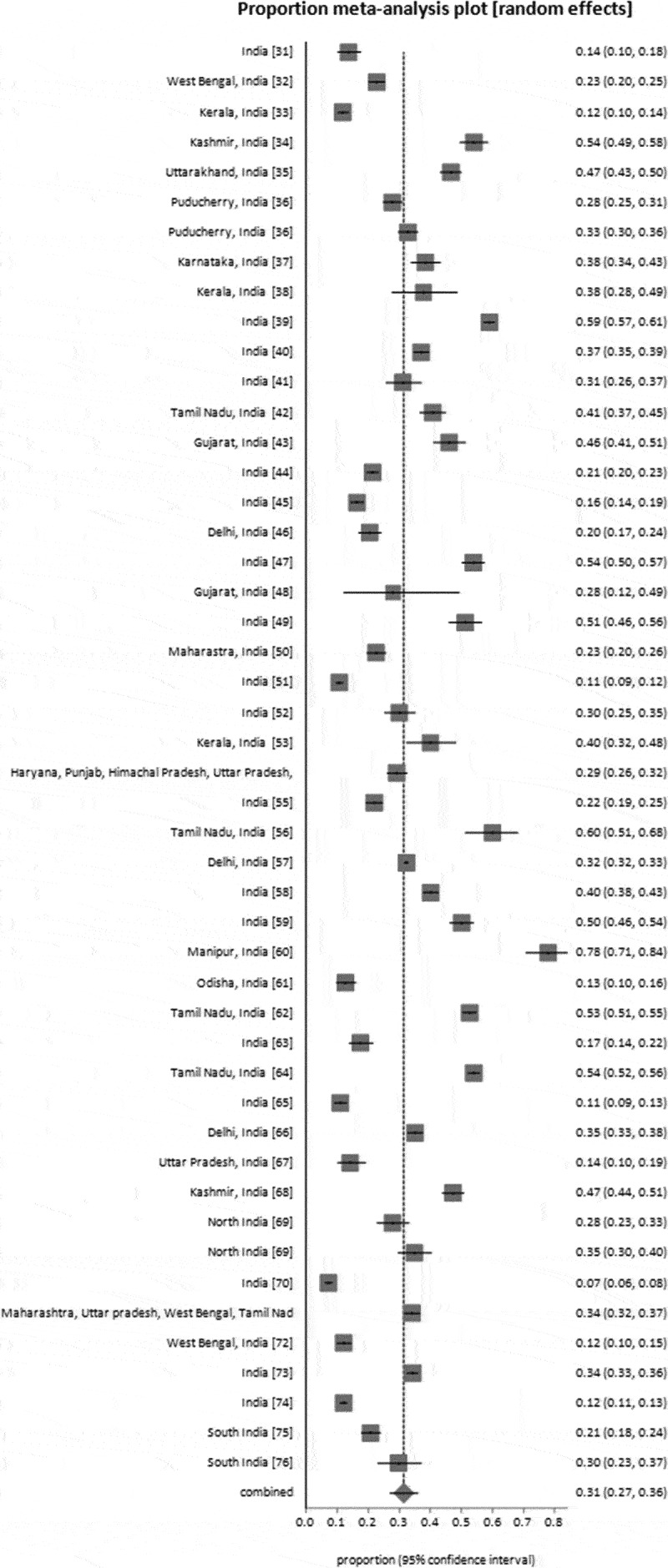
Forest plot of COVID-19 vaccine hesitancy rate.
Publication bias assessment
The funnel plot shows no evidence of publication bias (Figure 4). Eggers’s test for a regression intercept gave a p-value of 0.138, indicating no evidence of publication bias.
Figure 4.
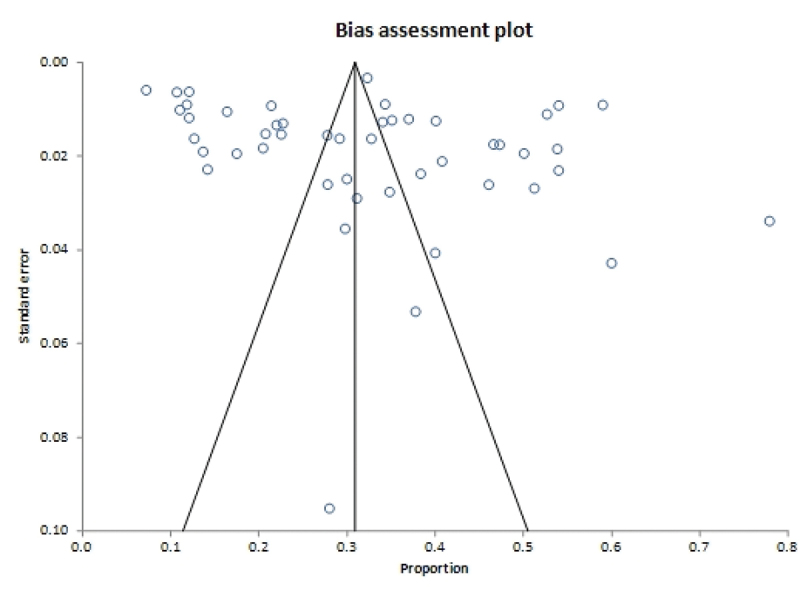
Assessment of publication bias.
Discussion
This systematic review was conducted to ascertain India’s COVID-19 vaccine acceptance and hesitancy rate, and possible reasons for vaccine hesitancy. In a country like India, with an amalgam of various population groups, based on socioeconomic disparities, ethnicity, religion, etc., the success of the vaccination programme depends mainly on how people perceive and accept the vaccine, which may vary according to time, place, and socio-cultural diversity. Evidence from the current systematic review depicts that vaccine hesitancy is considerable in the country and may influence future vaccine uptake. A higher vaccine hesitancy (77.9%) rate was reported by a study from Manipur [60], followed by 59%, as reported in a longitudinal study that included participants from various Indian states [39]. The country-level studies from India reported a COVID-19 vaccine acceptance rate of 65.7% in January-February 2021 [73], which increased to 92.8% in May-August 2021 [70]. Similarly, the rate of vaccine hesitancy in December 2020 was 37% [40] and dropped to 12.1% through November 2021 [74]. Thus, the review reveals that vaccine hesitancy and acceptance are dynamic and will change over time depending on various factors.
The overall hesitancy rate for the COVID-19 vaccination was 31% [95% CI: 27% − 36%, I2 = 99.3%, 46 studies]. An earlier review reported a pooled hesitancy rate of 23.3% based on the studies conducted till 2022 [3]. In the current review, the hesitancy rate was higher in Manipur (78%; 95% CI: 71% − 84%). The probable reason could be that the population sub-group included pregnant women, who may have concerns about vaccine safety during pregnancy and vaccines’ effects on the baby [60]. Jetly et al. [70] highlighted the lowest hesitancy rate of 7% (95% CI: 6% − 8%) among the general population of India; this low hesitancy might be attributed to the data collection phase (May-August, 2021). It coincided with the second wave of the pandemic in India, which led to fear among the population about the disease and scarcity of vaccines, increasing vaccine eagerness and acceptance.
This review identified various factors associated with COVID-19 vaccine hesitancy. Fear of the vaccine’s side effects, efficacy and safety surfaced as major factors associated with COVID-19 vaccine hesitancy. Initially, people’s approach is to wait and see before making vaccine decisions based on others’ vaccine experiences. Vulnerable people, like the elderly and those with co-morbidities, have major concerns about adverse side effects of the vaccine and fear that their present condition may worsen. Pregnant and lactating women were concerned about the lack of specific vaccine trials involving pregnant women, the unforeseen future effects of the vaccine on the fetus, and vaccines’ safety during pregnancy. The effectiveness of the vaccine in providing life-long immunity is another concern. The rapid development of the vaccine led to dilemmas with vaccine decisions. Lack of awareness about the benefits of the vaccine, and previous vaccine experiences, contribute to vaccine hesitancy. There is a need to address certain existing beliefs surrounding the vaccines, such as the belief in natural immunity, that vaccination is not necessary for dealing with COVID-19, testing positive even after taking the vaccine, and that the COVID-19 is a hoax. Also, the review reveals that in situations like COVID-19 and the introduction of new vaccines, people look for doctors’ advice for making vaccine decisions. The review also identified peoples’ preference for oral vaccines and the expectation that the vaccines are to be provided by the government due to affordability issues. Thus, the review identified various factors that interfere with vaccine decisions. We opine that all those factors and concerns should be given importance to deal with vaccine hesitancy.
Some studies identified gender, formal education, occupation, religion, and monthly income as associated with attitudes toward vaccination [33,42,44,48,51,58,59,71]. Low educational attainment, mainly among women, surfaced as a barrier to vaccine acceptance. They often relied on family members and healthcare providers for advice and considered them the trusted source of information related to COVID-19 vaccinations [74]. Similar findings were reported by Joshi et al. that COVID-19 vaccine acceptance was significantly higher among men and those with professional education and higher income levels [64]. In India, hesitancy and refusal of vaccination can also be correlated with vulnerable sections of society, such as the rural elderly population belonging to low-income groups [36,42], and [48]. However, few studies found that urban, highly educated individuals were also likely to say ‘no or not sure’ about the COVID-19 vaccination [34,40].
Misinformation generates panic and fear among the population, especially during a crisis like the COVID-19 outbreak. During such scenarios, opinion makers from the community, such as local leaders and village headmen, should be involved to clarify information about disease transmission, correct prevention methods and control measures so that messages are adequately conveyed to the population. ‘Infodemic is worse than an epidemic because it causes fear and rumours to spread multiple times faster than the disease-causing agent’ [78]. However, the government took initiatives to accelerate vaccine coverage through the ‘Har Ghar Dastak’ (meaning knocking on every door/house) campaign to reach all beneficiaries; and frontline health workers conducted house-to-house visits to promote COVID-19 vaccination [79,80]. It may be more beneficial to disseminate information that the infection rate in unvaccinated individuals was significantly higher than those who had been completely vaccinated [81]. This should be an impetus of paramount importance for the population to get vaccinated, as viral load has been identified as a critical driver of transmission [82]. Furthermore, it is crucial to effectively communicate the existing scientific knowledge to communities, empowering them to make well-informed decisions regarding vaccines. This entails the responsible dissemination of scientific knowledge to communities through reliable channels, including government and private healthcare institutions and providers. By doing so, we can counteract misinformation and foster trust in vaccines, ultimately empowering individuals, their families, and communities to make informed and responsible choices regarding vaccination. The vaccine hesitancy/acceptance rates highlighted in the current review can help plan and implement targeted actions, awareness programs, initiatives and health education materials needed to generate the knowledge and critical understanding about the pandemic. There is an urgent need to identify pockets of vaccine hesitancy and targeted intervention strategies. Effective communication and appropriate health campaigns are to be planned by understanding community members’ specific issues and perspectives. Given the upcoming vaccinations for younger age groups of below 12 years, it is crucial to improve vaccine confidence among parents [49].
Given the emergence of new variants of COVID-19 and the potential for new infections, it is essential to study vaccine acceptance and hesitancy. In India, the data on COVID-19 vaccine uptake is significant, with 2.2 billion vaccine doses administered [83]. Our World in Data [84] corroborates this, reporting that 1.03 billion individuals in India have received at least one dose of the COVID-19 vaccine, 952 million (67%) have received both doses, and 75.4 million (5.3%) have received a partial vaccination. Despite the substantial uptake of the first and second vaccine doses, there has been a sudden decline in the uptake of precautionary doses. This decline warrants investigation into the factors contributing to this reduced uptake. Therefore, it is imperative to conduct further research on vaccine hesitancy to support public health efforts. For instance, as COVID-19 continues to evolve with new variants, addressing hesitancy and uncertainty surrounding newer vaccines becomes crucial. Research should delve into socio-behavioral aspects, focusing on psychological and socio-demographic factors, community-level influences on vaccine acceptance, and health system-level factors such as access to vaccines. Understanding the impact of various sources of information, including misinformation, and developing effective communication strategies is another critical area for future vaccine hesitancy research. Above all, establishing trust in vaccines and the healthcare system is essential for the success of vaccination programs. Continued research on vaccine hesitancy, and strategies to combat it, can provide valuable insights for evidence-based approaches. Additionally, research into vaccine side effects and the long-term effects of COVID-19 and COVID-19 vaccines is essential for developing appropriate policies.
There are certain limitations of this study. We only considered the general population’s perspectives, and the views of healthcare workers are not covered. Some studies might have been missed during the search process, such that studies published in languages other than English, and if they are not indexed in the selected databases. The studies mainly had a cross-sectional design, with the data collected via online surveys due to COVID restrictions, hence subject to self-selection bias. Moreover, the findings of this study are specific to the Indian context, which inadvertently overlooks pertinent research conducted in other regions, potentially introducing bias. It’s worth noting that newer research may emerge after the completion of this review, potentially influencing the applicability of these findings. Despite these limitations, the current study offers valuable insights into the evolving landscape of COVID-19 vaccine hesitancy and acceptance within India.
Conclusions
This review revealed that COVID-19 vaccine hesitancy is considerable in Indian communities, with nearly one-third hesitant to accept the vaccine. This review identified various factors associated with COVID-19 vaccine hesitancy and captured various concerns about COVID-19 vaccines. To address vaccine hesitancy, we suggest implementing health awareness programmes and educational campaigns that disseminate transparent information through the government and media. These efforts can help promote proactive vaccination behaviors, enhance trust in government agencies, and underscore the importance of vaccine acceptance, not only for COVID-19 but also for forthcoming vaccines. It is crucial to prioritize addressing citizens’ concerns and creating public awareness about COVID-19 vaccines through campaigns.
Funding Statement
The work was supported by the Indian Council of Medical Research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Ackah M, Ameyaw L, Gazali Salifu M, et al. COVID-19 vaccine acceptance among health care workers in Africa: a systematic review and meta-analysis. Plos One. 2022; 17(5): e0268711. doi: 10.1371/journal.pone.0268711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229): 1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Patwary MM, Alam MA, Bardhan M, et al. COVID-19 vaccine acceptance among low- and lower-middle-income countries: a rapid systematic review and meta-analysis. Vaccines. 2022; 10(3): 427–447. doi: 10.3390/vaccines10030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020; 92(4): 401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Andrews MA, Areekal B, Rajesh KR, et al. First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res. 2020; 151(5): 490–492. doi: 10.4103/ijmr.IJMR_2131_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cucinotta D, Vanelli M.. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zirpe KG, Dixit S, Kulkarni AP, et al. The second- vs first-wave COVID-19: more of the same or a lot worse? a comparison of mortality between the two waves in patients admitted to intensive care units in nine hospitals in Western Maharashtra. Indian J Crit Care Med. 2021; 25(12): 1343–1348. doi: 10.5005/jp-journals-10071-24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lauring AS, Malani PN. Variants of SARS-CoV-2. JAMA. 2021; 326(9): 880. 10.1001/jama.2021.14181 [DOI] [PubMed] [Google Scholar]
- [9].Ranjan R, Sharma A, Verma MK. Characterisation of the second wave of COVID-19 in India. MedRxiv. 2021. https://www.medrxiv.org/content/10.1101/2021.04.17.21255665v2.full.pdf [Google Scholar]
- [10].Mohapatra RK, Sarangi AK, Kandi V, et al. Omicron (B.1.1.529 variant of SARS-CoV-2); an emerging threat: current global scenario. J Med Virol. 2022; 94(5): 1780–1783. doi: 10.1002/jmv.27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Joshi A, Kaur M, Kaur R, et al. Predictors of COVID-19 vaccine acceptance, intention, and hesitancy: a scoping review. Front Public Health. 2021;9:698111. doi: 10.3389/fpubh.2021.698111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li H, Liu SM, Yu XH, et al. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020; 55(5): 105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Worldometer . India population. [Cited 2023 Jan 3]. Available from: https://www.worldometers.info/world-population/india-population/
- [14].Marzo RR, Ahmad A, Islam MS, et al. Perceived COVID-19 vaccine effectiveness, acceptance, and drivers of vaccination decision-making among the general adult population: a global survey of 20 countries. PLoS Negl Trop Dis. 2022. 28;16(1):e0010103. doi: 10.1371/journal.pntd.0010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Askarian M, Semenov A, Llopis F, et al. The COVID-19 vaccination acceptance/hesitancy rate and its determinants among healthcare workers of 91 countries: a multicenter cross-sectional study. EXCLI J. 2022;21:93–103. doi: 10.17179/excli2021-4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Government of India . Ministry of Health and family Welfare: covid-19 statistics. [cited 2023 Jan 4]. Available from: https://www.mohfw.gov.in/
- [17].Truong J, Bakshi S, Wasim A, et al. What factors promote vaccine hesitancy or acceptance during pandemics? A systematic review and thematic analysis. Health Promot Int. 2022; 37(1):daab105. doi: 10.1093/heapro/daab105. [DOI] [PubMed] [Google Scholar]
- [18].Sachdeva S, Saluja H, Mani A. Cognizance, adverse effects and motivation regarding COVID-19 vaccination amongst health care professionals: a cross-sectional study. Dent Med Probl. 2022; 59(1): 13–19. doi: 10.17219/dmp/145757. [DOI] [PubMed] [Google Scholar]
- [19].Kashte S, Gulbake A, El-Amin SF III, et al. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021. May;34(3):711–733. doi: 10.1007/s13577-021-00512-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].COVID-19 vaccine tracker and landscape. Geneva: World Health Organization; 2021. [cited 2022 Oct 21]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. [Google Scholar]
- [21].Bharat Biotech and ICMR Announce interim results from phase 3 trials of COVAXIN®. Hyderabad: Bharat Biotech. 2021. Apr 21. [cited 2022 25 Oct]. Available from: https://www.bharatbiotech.com/images/press/covaxin-phase3-clinical-trials-interim-results.pdf. [Google Scholar]
- [22].Government of India, Ministry of Health and family Welfare. FAQs on COVID-19 vaccines and vaccination program. https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload27Sep.pdf
- [23].Purohit N, Chugh Y, Bahuguna P, et al. COVID-19 management: the vaccination drive in India. Health Policy Technol. 2022;11(2):100636. doi: 10.1016/j.hlpt.2022.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].World Health Organization . (2019). Ten Threats to global health in 2019. [cited 2022 May 11]. Ten threats to global health in 2019 (who.int).
- [25].MacDonald NE, SAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036 [DOI] [PubMed] [Google Scholar]
- [26].Government of India . Ministry of Health and family Welfare: cumulative coverage report of COVID-19 vaccination. [cited Jan 4, 2023]. Available from: https://www.mohfw.gov.in/pdf/CummulativeCovidVaccinationReport04Jan2023.pdf
- [27].Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Centre for Reviews and Dissemination . York Publ Services. 2009. (no.4). https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- [29].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4): 264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- [30].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021; 10(1): 89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sharun K, Rahman CF, Haritha CV, et al. COVID-19 vaccine acceptance: beliefs and barriers associated with vaccination among the general population in India. J Exp Biol Agric Sci. 2020; 8(Spl–1–SARS–CoV–2): S210–18. 10.18006/2020.8(Spl-1-SARS-CoV-2).S210.S218 [DOI] [Google Scholar]
- [32].Gautam A, Dhara B, Mukherjee D, et al. A digital survey on the acceptance and affordability of COVID-19 vaccine among the people of West Bengal, India - a survey based study. MedRxiv. 2020. https://www.medrxiv.org/content/medrxiv/early/2020/11/16/2020.11.13.20229534.full.pdf [Google Scholar]
- [33].Leelavathy M, Messaline S, Ramachandran D, et al. Attitude towards COVID-19 vaccination among the public in Kerala: a cross sectional study. J Family Med Prim Care. 2021; 10(11): 4147–4152. doi: 10.4103/jfmpc.jfmpc_583_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ain SN, Ahmad R, Qulsum R, et al. Potential vaccine hesitancy regarding COVID-19 vaccines in Kashmiri population. J Educ Health Promot. 2021;10:436. doi: 10.4103/jehp.jehp_40_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kumar R, Bairwa M, Beniwal K, et al. COVID-19 vaccine acceptability, determinants of potential vaccination, and hesitancy in public: a call for effective health communication. J Educ Health Promot. 2021;10:392. doi: 10.4103/jehp.jehp_327_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Anandraj J, Krishnamoorthy Y, Sivanantham P, et al. Impact of second wave of COVID-19 pandemic on the hesitancy and refusal of COVID-19 vaccination in Puducherry, India: a longitudinal study. Hum Vaccin Immunother. 2021; 17(12): 5024–5029. doi: 10.1080/21645515.2021.2000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ekstrand ML, Heylen E, Gandhi M, et al. COVID-19 vaccine hesitancy among PLWH in South India: implications for vaccination campaigns. J Acquir Immune Defic Syndr. 2021; 88(5): 421–425. doi: 10.1097/QAI.0000000000002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Uvais NA COVID-19 vaccine hesitancy among patients with psychiatric disorders. Prim Care Companion CNS Disord. 2021; 23(6): 21br03028. doi: 10.4088/PCC.21br03028. [DOI] [PubMed] [Google Scholar]
- [39].Umakanthan S, Patil S, Subramaniam N, et al. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines (Basel). 2021; 9(10): 1064. doi: 10.3390/vaccines9101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chandani S, Jani D, Sahu PK, et al. COVID-19 vaccination hesitancy in India: state of the nation and priorities for research. Brain Behav Immun Health. 2021;18:100375. doi: 10.1016/j.bbih.2021.100375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kishore J, Venkatesh U, Ghai G, et al. Perception and attitude towards COVID-19 vaccination: a preliminary online survey from India. J Family Med Prim Care. 2021;10(8):3116–3121. doi: 10.4103/jfmpc.jfmpc_2530_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Danabal KGM, Magesh SS, Saravanan S, et al. Attitude towards COVID-19 vaccines and vaccine hesitancy in urban and rural communities in Tamil Nadu, India - a community based survey. BMC Health Serv Res. 2021; 21(1): 994. doi: 10.1186/s12913-021-07037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gaur P, Agrawat H, Shukla A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int. 2021;41(9):1601–1605. doi: 10.1007/s00296-021-04938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jacob J, Stephen S, Issac A, et al. Determinants of willingness for COVID-19 vaccine: implications for enhancing the proportion of vaccination among Indians. Cureus. 2021; 13(5): e15271. doi: 10.7759/cureus.15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kumari A, Ranjan P, Chopra S, et al. Knowledge, barriers and facilitators regarding COVID-19 vaccine and vaccination programme among the general population: a cross-sectional survey from one thousand two hundred and forty-nine participants. Diabetes Metab Syndr. 2021; 15(3): 987–992. doi: 10.1016/j.dsx.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Islam F, Agarwalla R, Panda M, et al. Assessment of the knowledge, preferences and concern regarding the prospective COVID-19 vaccine among adults residing in New Delhi, India - a cross-sectional study. J Family Med Prim Care. 2021; 10(6): 2369–2375. doi: 10.4103/jfmpc.jfmpc_2437_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bharadwaj AH, Ramachandra SC, Devaraju A, et al. Perception of indian citizens toward the available COVID-19 vaccines: need to create increased awareness. Perspect Clin Res. 2021;12(4):236–237. doi: 10.4103/picr.picr_97_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shah SN, Shah D, Desai N, et al. Analysis of change in knowledge, attitude, and practices about COVID-19 following and awareness session in rural population of Western India. Ind Psychiatry J. 2021; 30(Suppl 1): S35–S40. doi: 10.4103/0972-6748.328786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bhondve AS, Ingawale SM, Rajapurkar ND. COVID19 vaccine hesitancy and its determinants among healthcare workers and nonhealthcare workers: an online survey in India. J Clin Sci Res. 2021;10:212–220. doi: 10.4103/jcsr.jcsr_38_21 [DOI] [Google Scholar]
- [50].Bhangale C, TSES I, Ramanand J, et al. Assessment of vaccine hesitancy for COVID-19 vaccines and evaluation of factors associated with it, among the residents of North Maharashtra Region, India. Natl J Physiol Pharm Pharmacol 2022; 12(4): 462–467. 10.5455/njppp.2022.12.09352202106102021 [DOI] [Google Scholar]
- [51].Goruntla N, Chintamani SH, Bhanu P, et al. Predictors of acceptance and willingness to pay for the COVID-19 vaccine in the general public of India: a health belief model approach. Asian Pac J Trop Med. 2021; 14(4): 165–175. doi: 10.4103/1995-7645.312512. [DOI] [Google Scholar]
- [52].Suresh A, Konwarh R, Singh AP, et al. Public awareness and acceptance of COVID-19 vaccine: an online cross-sectional survey, conducted in the first phase of vaccination drive in India. Res Square. 2021. doi: 10.21203/rs.3.rs-324238/v1. [DOI] [Google Scholar]
- [53].Dabas P, Raj KJ, John J, et al. Covid-19 vaccine acceptance and determinants–an online cross-sectional survey in Kerala, India. Natl J Comm Med. 2021; 12(12): 432–438. doi: 10.5455/njcm.20211021095953 [DOI] [Google Scholar]
- [54].Bansal S, Gangwar AK, Sharma A, et al. Knowledge, attitude, practice, and post-infection effects regarding COVID-19, and vaccine acceptance among general people of North India-a multicentred cross-sectional study. Europe PMC. 2021. doi: 10.22541/au.162289618.82152279/v1. [DOI] [Google Scholar]
- [55].Lazarus JV, Wyka K, White TM, et al. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun. 2022;13(1):3801. doi: 10.1038/s41467-022-31441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Raja D, Sasithra S .COVID-19 vaccine acceptance and its determinants among adult population of Chengalpattu district: a mixed-method study. J Comm Dis. 2022:68–74. Special Issue - COVID-19 & Other Communicable Disease. doi: 10.24321/0019.5138.202211. [DOI] [Google Scholar]
- [57].Sharma P, Basu S, Mishra S, et al. COVID-19 vaccine acceptance and its determinants in the general population of Delhi, India: a state level cross-sectional survey. Cureus. 2022; 14(7): e26936. doi: 10.7759/cureus.26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Parthasarathi A, Puvvada RK, Shankar M, et al. Willingness to accept the COVID-19 vaccine and related factors among Indian adults: a cross-sectional study. Vaccines (Basel). 2022;10(7):1095. doi: 10.3390/vaccines10071095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Achrekar GC, Batra K, Urankar Y, et al. Assessing COVID-19 booster hesitancy and its correlates: an early evidence from India. Vaccines (Basel). 2022. Jun;10(7):1048. doi: 10.3390/vaccines10071048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gupta A, Christina S, Umar AY, et al. COVID-19 vaccine hesitancy among pregnant women: a facility-based cross-sectional study in Imphal, Manipur. Indian J Public Health. 2022;66(2):98–103. doi: 10.4103/ijph.ijph_1826_21 [DOI] [PubMed] [Google Scholar]
- [61].Kuchi S, Parida SP. COVID-19 vaccination hesitancy and attitude post-initiation of vaccination drive, a cross-sectional study across odisha. J Family Med Prim Care. 2022;11(5):1996–2001. doi: 10.4103/jfmpc.jfmpc_1862_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mohanasundaram K, Santhanam S, Natarajan R, et al. Covid-19 vaccination in autoimmune rheumatic diseases: a multi-center survey from Southern India. Int J Of Rheum Dis. 2022; 25(9):1046–1052. doi: 10.1111/1756-185X.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sengupta M, Dutta S, Roy A, et al. Knowledge, attitude and practice survey towards COVID-19 vaccination: a mediation analysis. Int J Health Plann Manage. 2022; 37(4):2063–2080. doi: 10.1002/hpm.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Joshi A, Surapaneni KM, Kaur M, et al. A cross sectional study to examine factors influencing COVID-19 vaccine acceptance, hesitancy and refusal in urban and rural settings in Tamil Nadu, India. Plos One. 2022; 17(6):e0269299. doi: 10.1371/journal.pone.0269299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Romate J, Rajkumar E, Greeshma R. Using the integrative model of behavioural prediction to understand COVID-19 vaccine hesitancy behaviour. Sci Rep. 2022;12(1):9344. doi: 10.1038/s41598-022-12466-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kusuma YS, Kant S. COVID-19 vaccine acceptance and its determinants: a cross-sectional study among the socioeconomically disadvantaged communities living in Delhi, India. Vaccine: X. 2022;11:100171. doi: 10.1016/j.jvacx.2022.100171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Khan IA, Bashar A, Tewari HC. Attitude, perceptions and willingness to receive COVID-19 vaccine and its associated factors among general population of Uttar Pradesh, Northern India. Clin Epidemiol Glob Health. 2022:101040. doi: 10.1016/j.cegh.2022.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Khan Z, Khursheed SQ, Dar SA, et al. Vaccine hesitancy and Coronavirus disease-19: where do we stand? J Educ Health Promot. 2022;11:59. doi: 10.4103/jehp.jehp_642_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kumari A, Mahey R, Kachhawa G, et al. Knowledge, attitude, perceptions, and concerns of pregnant and lactating women regarding COVID-19 vaccination: a cross-sectional survey of 313 participants from a tertiary care centre of North India. Diabetes Metab Syndr. 2022; 16(3):102449. doi: 10.1016/j.dsx.2022.102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jetly S, Bhardwaj P, Arora G, et al. Hesitancy and acceptance of COVID-19 vaccination amidst the second wave of pandemic in India: a general population study. Asia Pac J Public Health. 2022; 34(4):446–449. doi: 10.1177/10105395221077062. [DOI] [PubMed] [Google Scholar]
- [71].Bansal P, Raj A, Sinha RK. Correlates of the COVID-19 vaccine hesitancy among Indians. Asia Pac J Public Health. 2022;34(5):583–585. doi: 10.1177/10105395221077065 [DOI] [PubMed] [Google Scholar]
- [72].Samanta S, Banerjee J, Kar SS, et al. Awareness, knowledge and acceptance of COVID-19 vaccine among the people of West Bengal, India: a web-based survey. Vacunas. 2022;23:S46–S55. doi: 10.1016/j.vacun.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hawlader MDH, Rahman ML, Nazir A, et al. COVID-19 vaccine acceptance in South Asia: a multi-country study. Int J Infect Dis. 2022. [cited 2021 Sep 28];114:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Naqvi S, Saleem S, Naqvi F, et al. Knowledge, attitudes, and practices of pregnant women regarding COVID-19 vaccination in pregnancy in 7 low- and middle-income countries: an observational trial from the global network for women and children’s health research. BJOG. 2022; 129(12):2002–2009. doi: 10.1111/1471-0528.17226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mathew B, George D, Thomas S, et al. Knowledge, attitude and acceptance of COVID-19 vaccine among general population in South India. J Phar Pract. 2022;15(1):36–39. doi: 10.5530/ijopp.15.1.7 [DOI] [Google Scholar]
- [76].Ganju P, Jayachandran PK, Karunakaran P, et al. P-137: COVID – 19 vaccine uptake in patients with multiple myeloma and AL amyloidosis: a cross-sectional observational study from India. Clin Lymphoma Myeloma Leuk. 2022;22:S110. doi: 10.1016/S2152-2650(22)00467-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- [78].Sonekar HB, Ponnaiah M. Emergence of Coronavirus (COVID-19) outbreak: anthropological and social science perspectives. Disaster Med Public Health Prep. 2020; 14(6):759–761. doi: 10.1017/dmp.2020.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].The economic times: covid vaccination: ‘har ghar dastak’ campaign begins in Delhi. (2021). https://economictimes.indiatimes.com/news/india/covid-vaccination-har-ghar-dastakcampaign-begins-in-delhi/articleshow/87665722.cms
- [80].Ministry of Health and family Welfare: Union Health Ministry reviews status and progress under “har ghar dastak” campaign with States/UTs. 2021.https://pib.gov.in/PressReleasePage.aspx?PRID=1777164
- [81].Narayan P, Ts SK, Bv MM, et al. Uptake and impact of vaccination against COVID-19 among healthcare workers-evidence from a multicentre study. Am J Infect Control. 2022; 50(3):361–363. doi: 10.1016/j.ajic.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Centre for Disease Control . The importance of COVID-19 vaccination for healthcare personnel. Centers For Disease Control And Prevention; 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/hcp.html.
- [83].Government of India . Ministry of Health and family Welfare: covid-19 statistics. COVID-19 Vaccination As On: 19 September 2023, 08: 00 IST (GMT+5: 30). [cited 2023 Sept 19]. Available from: https://www.mohfw.gov.in/
- [84].Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Human Behav. 2021. Jul [cited 2023 19, Sept];5(7):947–953. doi: 10.1038/s41562-021-01122-8 [DOI] [PubMed] [Google Scholar]


