Abstract
Background
En‐Bloc transurethral resection of bladder tumor (ERBT) was clinically used to resect non‐muscle‐invasive bladder cancer (NMIBC). However, discrepancies persist regarding the comparisons between ERBT and conventional transurethral resection of bladder tumor (cTURBT).
Methods
We conducted a comprehensive search in PubMed, Embase, Web of Science, Cochrane Database of Systematic Reviews, and performed manual searches of reference lists to collect and extract data. Data evaluation was carried out using Review Manager 5.4.0, Rx64 4.1.3, and relevant packages.
Results
There were nine eligible meta‐analyses and nine eligible RCTs in our study. NMIBC patients undergoing ERBT were significant associated with a lower rate of bladder perforation and obturator nerve reflex compared to those receiving cTURBT. Our pooled result indicated that ERBT and cTURBT required similar operation time. Regarding postoperative outcomes, ERBT demonstrated superior performance compared to cTURBT in terms of detrusor muscle presence, catheterization time, and residual tumor. ERBT exhibited a higher rate of three‐month recurrence‐free survival (RFS) compared to those receiving cTURBT (p < 0.05; I2 = 0%). In bipolar subgroup, ERBT had a significant better 12‐month RFS than cTURBT (p < 0.05; I2 = 0%). Simultaneously, the exclusion of Hybrid Knife data revealed a significant improvement in 12‐month RFS associated with ERBT (p < 0.05; I2 = 50%).
Conclusion
Using a combination of umbrella review and meta‐analysis, we demonstrated that ERBT had better or comparable perioperative outcome and improved 3 and 12 month RFS than cTURBT. We suggest that ERBT maybe a better surgical method for patients with NMIBC compared with cTURBT.
Keywords: bladder cancer, En bloc resection of bladder tumor, transurethral resection of bladder tumor, urothelial carcinoma
1. INTRODUCTION
Ranked as the 6th most prevalent cancer, bladder cancer (BCa) stands as the 9th leading cause of cancer‐related deaths in men. 1 Around 70% of newly diagnosed BCa patients present with non‐muscle‐invasive bladder cancer (NMIBC), known for its high recurrence and progression rates. 2 , 3 Various approaches have been employed to enhance the perioperative and survival outcomes of NMIBC patients. 4 , 5 Approximately 30% of patients diagnosed with NMIBC experience recurrence within 12 months after conventional transurethral resection of bladder tumor (cTURBT) and intravesical instillation. 6 , 7 The need for repeated cTURBT places a significant economic and physical burden on these patients. Additionally, a notable percentage of these patients may advance to muscle‐invasive BCa, which is linked to poor overall survival rates despite undergoing radical cystectomy and adjuvant therapy. 8 , 9 Thus, many treatments are exploring to improve the prognosis of patients with NMIBC. 4 , 10 , 11
En‐Bloc transurethral resection of bladder tumor (ERBT) was initially reported in 1997. 29 However, this technique did not attract the attention of most of researchers until 2011. Herrmann et al. 12 improved the technique of ERBT and reported the positive outcomes of six NMIBC patients. Meanwhile, ERBT can offer a complete specimen, thereby facilitating the investigation of the tumor microenvironment. 13 , 14 These outstanding findings have prompted further studies on ERBT in many teams. Multiple studies have identified ERBT as a developing alternative to cTURBT. In fact, the European Association of Urology recommends the utilization of ERBT for resecting NMIBC. 15 However, discrepancies persist regarding the comparisons between ERBT and cTURBT. In 2020, Teoh et al. 16 reported no statistically significant difference in recurrence‐free survival (RFS) between ERBT and cTURBT based on pooled data up until June 2019. In 2023, Teoh et al. 17 demonstrated NMIBC patients undergoing ERBT were significantly associated with longer RFS than those receiving cTURBT based on the results of a multicenter randomized trial (EB‐STAR study). Furthermore, surrounding perioperative outcomes, there are still some controversies between ERBT and cTURBT. For instance, most meta‐analyses 18 , 19 , 20 , 21 , 22 indicated that ERBT and cTURBT required similar operation times. However, one meta‐analysis reported that ERBT would require more time for tumor resection. 16
In this study, our objective was to tackle these issues by conducting an umbrella review of ERBT in NMIBC. Additionally, we carried out a pooled analysis using data from randomized controlled trials (RCTs) to reconcile discrepancies noted in various meta‐analyses.
2. MATERIALS AND METHODS
We conducted an umbrella review of early recurrence bladder tumors in NMIBC following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines. 23
2.1. Literature search
In May 2023 (last update), we systematically searched PubMed, Embase, Web of Science, and the Cochrane Database of Systematic Reviews to identify relevant systematic reviews, meta‐analyses, and RCTs. Referring to the Scottish Intercollegiate Guidelines Network”s guidance, 24 a comprehensive literature search on ERBT was conducted using a combination of Medical Subject Headings terms, keywords, and various text word variations across multiple databases. The search terms included (en‐bloc resection OR ERBT OR ETURBT) AND (bladder tumor). Initially, two authors (DXL and DCF) independently screened titles and abstracts retrieved from the databases. Subsequently, meta‐analyses and RCTs meeting the inclusion criteria were identified through full‐text reading by the two authors. In cases of discrepancies, a third author (RCW) resolved the differences in literature screening. Additionally, a manual search was performed to review the meta‐analyses, reviews, and RCTs cited in the references of selected articles.
2.2. Study selection
We examined the efficiency of ERBT and cTURBT in terms of perioperative outcomes and survival benefits. The systematic reviews and meta‐analyses included in the analysis had to meet specific criteria: they had to be systematic reviews of RCTs or cohort studies, case–control studies, or cross‐sectional studies comparing the efficiency of ERBT and cTURBT. The RCTs included in the analysis had to meet certain criteria as well: they had to compare ERBT and cTURBT, have accurate and available data on perioperative outcomes and survival benefits. Studies in languages other than English, as well as animal and cell culture studies, were excluded.
2.3. Data extraction
The following information was independently extracted from each included meta‐analysis by two reviewers (DXL and DCF): (1) first author”s name, (2) publication year, number of included studies and patients, (3) perioperative outcomes and (4) RFS. Any disagreement was determined by a third author (RCW).
The information extracted from each RCT included the first author”s name and publication year, country of the study, energy for ERBT and cTURBT, patient numbers, tumor size, tumor number, T stage, World Health Organization (WHO) grade, inclusion of carcinoma in situ (CIS), adjuvant medicine type, estimated summary effect (risk ratio (RR), odds ratio (OR), mean difference (MD), standardized summary effect (SMD) with 95% confidence intervals (CI)), heterogeneity (I2), perioperative outcomes (operation time (ORT), bladder irrigation), and RFS at 3 and 12 months. In cases where a RCT was published as both an article and conference paper, data from the most recent study was prioritized. Any disagreements were resolved by a third author.
2.4. Quality assessment of methods and evidence
ROBIS 25 was used to evaluate methodological quality of the included meta‐analyses by two reviewers (DXL and DCF). ROBIS consisted of three phases, with results being rated as low, high, or unclear. Additionally, each health outcome underwent evidence evaluation and was assigned a quality grade of “high,” “moderate,” “low,” or “very low” based on the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). 26
Two reviewers (DXL and DCF) independently assessed the methodological quality of the RCTs included in our meta‐analysis following the guidelines outlined in the Cochrane Handbook. 27 According to the result, we classified the studies into one of the three levels: low risk of bias, unclear risk of bias, or high risk of bias. Any disagreement was determined by a third author (RCW).
2.5. Statistical analysis
The data was evaluated using Review Manager 5.4.0, Rx64 4.1.3, and their respective packages. Continuous outcomes were assessed using MD with 95% CI, while dichotomous outcomes were assessed using OR with 95% CI. A random‐effects model was applied for data analysis in the presence of significant heterogeneity (p < 0.05), with heterogeneity evaluated using the I2 statistic where I2 >50% indicated high heterogeneity. The statistical significance level was set at p <0.05.
3. RESULTS
A total of 1295 studies were initially identified through database searches. After removing duplicates, 797 studies were screened, resulting in the selection of 21 studies for potential inclusion in meta‐analyses and 54 studies for potential inclusion in RCTs. Ultimately, nine meta‐analyses and nine RCTs met the eligibility criteria for our study (Figure 1). The studies included in each meta‐analysis are presented in Table S1.
FIGURE 1.
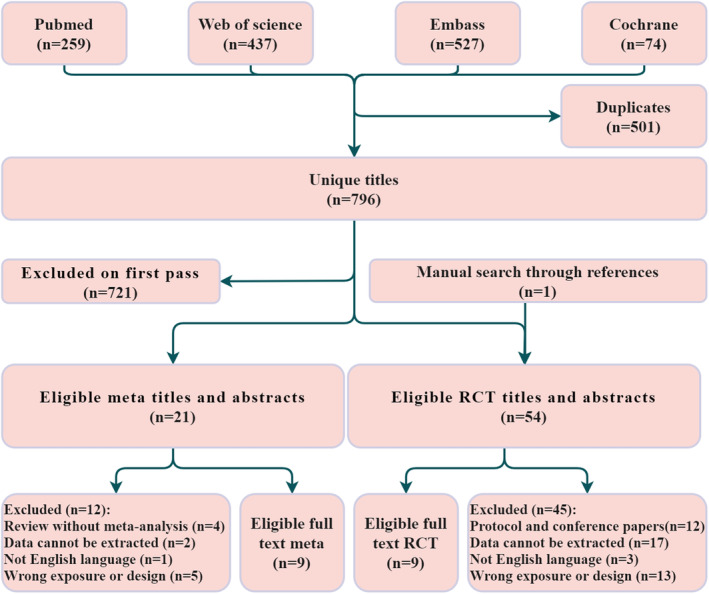
Work flow diagram.
3.1. The characteristics of eligible studies and risk of bias assessment
Table 1 showed the characteristics of eligible meta‐analyses. 16 , 18 , 19 , 20 , 21 , 22 , 28 , 29 , 30 Out of the eligible meta‐analyses, two studies exclusively incorporated RCTs. 16 , 20 The latest search day was January 2022. 20 Table 2 contained the characteristics of eligible RCTs. 17 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Four of these RCTs came from China, two from Egypt, one from Romania, one from Germany and one from Spain. Five RCTs selected holmium laser as energy for ERBT group, two selected bipolar, one RCT selected green‐light laser and one selected Hybrid Knife.
TABLE 1.
Summary of included meta‐analyses and outcomes.
| Author (Year) | Last research | Included studies | Type | No. of EBRT | No. of cTURBT | Outcomes |
|---|---|---|---|---|---|---|
| Wang_CW (2023) | April 2021 | 31 | RCT/NRCT | 2024 | 2171 | RTR, detrusor muscle, RFS (same‐site, 3, 12, 24 months), ORT, HPT, CTT, bladder perforation, ONR, bladder irritation |
| Yanagisawa_T (2022) | August 2021 | 29 | RCT/NRCT | 4484 | RTR, RFS (12, 24 months), ORT, CTT, bladder perforation, detrusor muscle, muscularis mucosa, CIS | |
| Motlagh_RS (2022) | June 2021 | 14 | RCT/NRCT | 2092 | RFS (3 and 12 months), detrusor muscle, SAER | |
| Li_ZY (2022) | January 2022 | 7 | RCT | 1870 | 1844 | RTR, ORT, HPT, CTT, Re‐TURBT, ONR, bladder perforation, Hemoglobin deficit, Detrusor muscle, Urethral stricture, RFS (3, 12, 24 and 36 months) |
| Di_Y (2022) | January 2022 | 28 | RCT/NRCT | 1142 | RFS (12 and 24 months), HPT, CTT, bladder irritation | |
| Zhang_D (2020) | November 2019 | 19 | RCT/NRCT | 1870 | 1844 | RFS (12 and 24 months) |
| Yang_H (2020) | April 2019 | 9 | RCT/NRCT | 1020 | ORT, HPT, CTT, bladder irrigation, RFS (24 months), bladder perforation, ONR, Urethral stricture, postoperative adjuvant intravesical chemotherapy | |
| Wu_YP (2016) | September 2016 | 7 | RCT/NRCT | 438 | 448 | ORT, HPT, CTT, RFS (24 months), ONR, bladder perforation, bladder irritation, urethral stricture, postoperative adjuvant intravesical chemotherapy |
| Teoh_YJ (2020) | June 2019 | 13 | RCT | 586 | 569 | ORT, bladder irritation, CTT, HPT, ONR, bladder perforation, detrusor muscle, RFS (12, 24 and 36 months) |
Abbreviations: CTT, catheterization time; CTURBT, conventional transurethral resection of bladder tumor (CTURBT); EBTR, En‐Bloc transurethral resection of bladder tumor; HPT, hospitalization time; NRCT, non‐randomized controlled trial; ONR: obturator nerve reflex; ORT: operation time; PFS, Progression‐free survival; RTR, residual tumor rate; RFS, recurrence‐free survival; SAER: serious adverse event rates; RCT, randomized controlled trial.
TABLE 2.
The characteristics of included RCTs.
| Author | Year | Country | Energy | No. sample (EBRT/cTURBT) | Tumor size | No. tumor | T stage | WHO grade | CIS | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| BĂLAN_GX | 2018 | Romania | Bipolar vs. monopolar | 45/45 | <=3 cm | NA | Ta/T1 | G1_3 | NA | Epirubicin/BCG |
| Liu_H | 2013 | China | 2‐mm (thulium) laser vs. monopolar | 64/56 | NA | NA | Ta/T1 | PUNLM, low, and high | NA | Epirubicin |
| Chen_X | 2014 | China | 2‐mm (thulium) laser vs. monopolar | 71/71 | NA | NA | Ta/T1 | PUNLM, low, and high | Yes | Epirubicin |
| Gakis_G | 2020 | Germany | Hybrid Knife vs. monopolar | 56/59 | >0.5 cm | <=5 | Ta/T1 | G1_3 | Yes | Mitomycin C/BCG |
| Hashem_A | 2021 | Egypt | Holmium Laser vs. monopolar | 42/49 | <=5 cm | <=5 | Ta/T1 | G1_3 | Yes | Epirubicin |
| Fan_JH | 2021 | China | Green‐light laser vs. monopolar | 116/117 | <=3 cm | NA | Ta/T1 | PUNLM, low, and high | Yes | Pirarubicin |
| Teoh_JY | 2023 | China | Bipolar vs. bipolar | 143/133 | <=3 cm | NA | Ta/T1 | PUNLM, low, and high | Yes | Mitomycin C |
| Gallioli_A | 2022 | Spain | Holmium Laser/monopolar/bipolar vs. monopolar/bipolar | 140/108 | <=3 cm | <=3 | Ta/T1 | PUNLM, low, and high | Yes | Mitomycin C/Epirubicin |
| Badawy_A | 2022 | Egypt | 2‐mm (thulium) laser vs. monopolar | 60/60 | NA | <=2 | Ta/T1 | Low and high | NA | Doxorubicin |
Abbreviations: No, number of; CIS, carcinoma in situ; BCG: Bacillus Calmette‐Guerin; NA, no data; WHO, World Health Organization.
According to the results of risk of bias assessment, only two of nine (22.2%) meta‐analyses were low risk (Table S2). Four of nine (44.4%) were classified as high risk due to English limit in searching section. For RCTs, six of nine (66.7%) had performance bias due to the surgery was hardly to performed blind method (Figure 2A).
FIGURE 2.
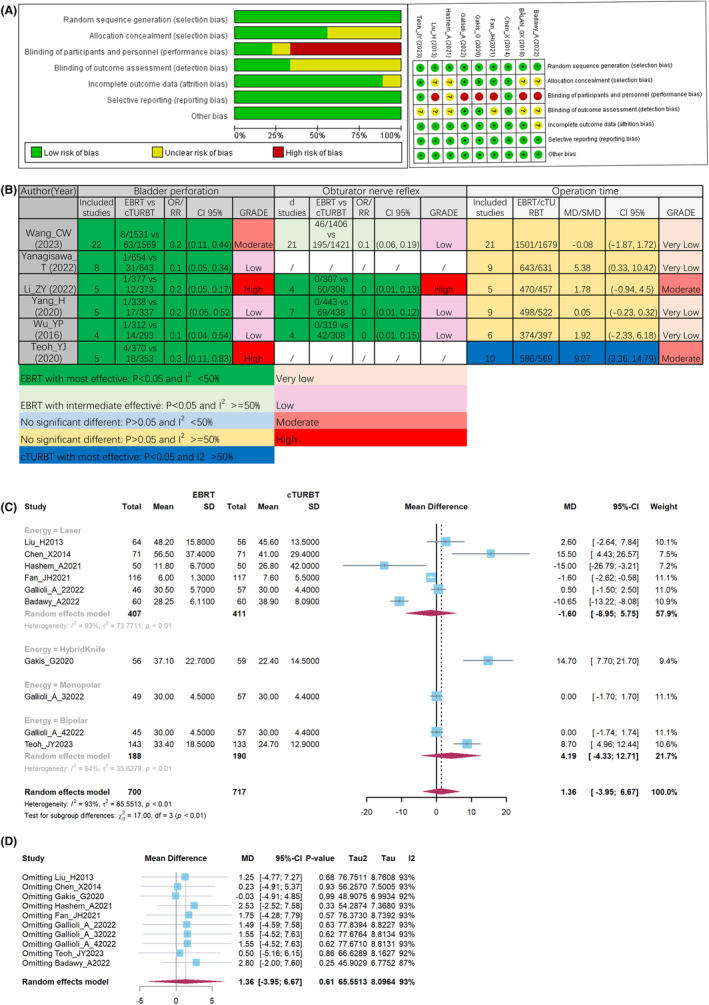
Quality assessment of included RCTs (A), outcomes during operation from meta‐analyses (B), pooled result (C), and sensitivity analysis, (D) of operation time.
3.2. ERBT has comparable results during operation
All identified meta‐analyses consistently reported a significantly lower incidence of bladder perforation in patients who underwent ERBT compared to those who underwent cTURBT (Figure 2B). Table S3 contained the detail of GRADE assessment. Similarly, patients received ERBT was statistically associated with lower rate of obturator nerve reflex (ONR) than those accepted cTURBT (Figure 2B). In term of ORT, there was no difference between ERBT and cTURBT in five meta‐analyses. 18 , 19 , 20 , 21 , 22 Only Teoh et al 16 found that ERBT had longer ORT. Consequently, we synthesized the data from eight RCTs pertaining to ORT. Among these RCTs, Gallioli et al. 36 conducted four separate comparisons based on variations in energy levels. From Figure 2C, no significant difference was observed between ERBT and cTURBT in terms of ORT (MD: 1.36; 95% CI: −3.95, 6.67; p > 0.05). However, substantial heterogeneity was observed (I2 = 93%), which was consistence with previous meta‐analyses. 18 , 19 , 20 , 21 , 22 We were failed to find out the cause of heterogeneity, despite conducting subgroup analyses based on energy source and country. In further sensitivity analysis, no significant decrease of heterogeneity was observed (Figure 2D).
3.3. ERBT has better postoperative results than cTURBT
In postoperative outcomes, four meta‐analyses with six separate comparisons consistently identified that ERBT yielded a higher rate of detrusor muscle acquisition compared to cTURBT (Figure 3A). Similarly, NMIBC patients received ERBT had shorter catheterization time (CTT) and a lower rate of residual tumor than those accepted cTURBT. Regarding bladder irrigation, five meta‐analyses consistently reported that ERBT was associated with either a shorter duration or a lower rate of bladder irritation compared to cTURBT. However, Li et al. 20 did not find significant difference in bladder irritation between these two groups. Thus, we tried to synthesized the data from RCTs pertaining to bladder irritation. Unfortunately, only two RCTs provided data and one of them could not be calculated (Figure 3B). At least, ERBT and cTURBT exhibited comparable outcomes of bladder irritation.
FIGURE 3.
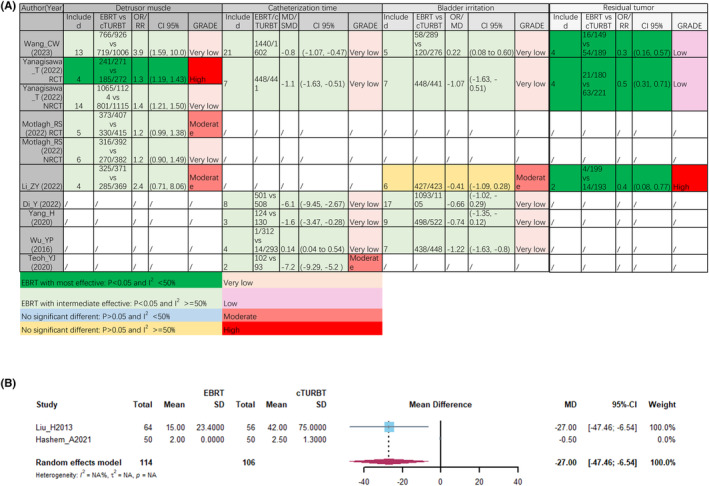
Postoperative outcomes from meta‐analyses, (A) pooled result of bladder irritation (B).
3.4. ERBT may bring survival benefits to NMIBC patients
In a 3‐month period, two comparisons showed positive outcomes for patients with NMIBC treated with ERBT, while two other comparisons found no significant difference between ERBT and cTURBT (Figure 4). When looking at 12‐month RFS, only one out of eight comparisons showed a survival benefit with ERBT, while the remaining seven comparisons found no significant difference in RFS between ERBT and cTURBT. For 24‐month RFS, half of the meta‐analyses indicated a significant survival benefit with ERBT, while the other four did not find statistical survival benefits. These combined results highlight a debate over whether ERBT can offer significant survival advantages to NMIBC patients.
FIGURE 4.
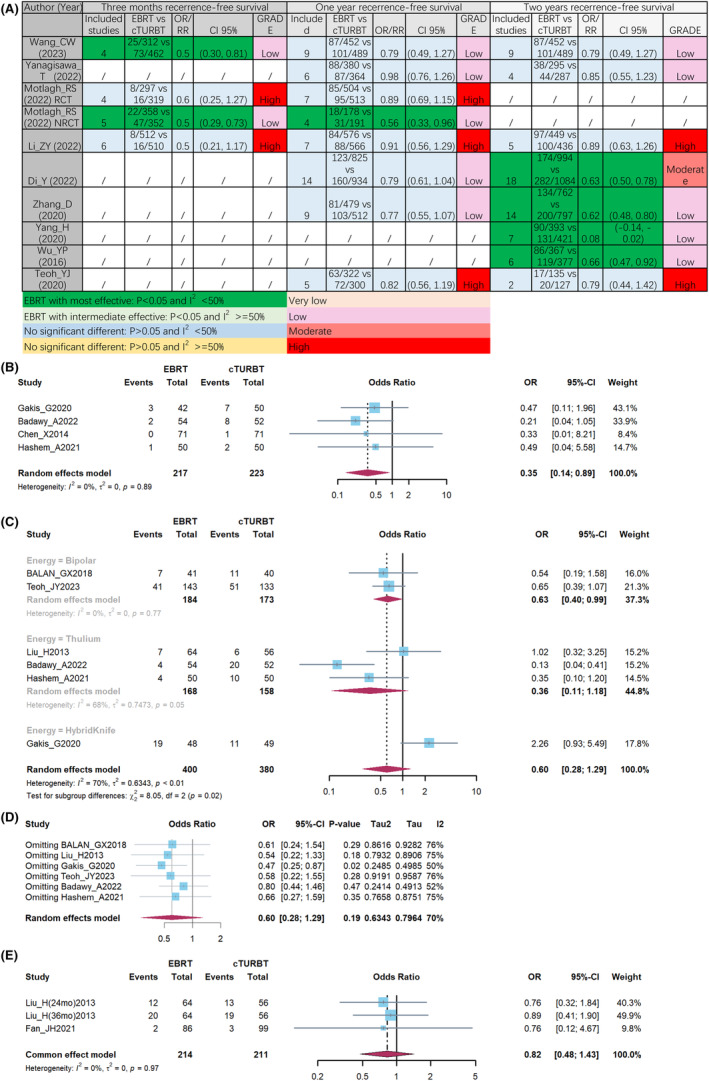
Recurrence‐free survival (RFS) outcomes from meta‐analyses (A), pooled result of 3‐month RFS (B), pooled result (C), and sensitivity analysis (D), of 12‐month RFS, pooled result of more than 12‐month RFS (E).
Consequently, pooling the results of four RCTs, we observed that patients undergoing ERBT exhibited a higher rate of 3‐month RFS compared to those receiving cTURBT (Figure 4B; OR: 0.35; 95% CI: 0.17, 0.89; p < 0.05; I2 = 0%). In terms of 12 months RFS, there was no significant difference between ERBT and cTURBT based on all meta‐analyses (Figure 4C; OR: 0.6; 95% CI: 0.28, 1.29; p > 0.05; I2 = 70%). To find the cause of heterogeneity, we conducted subgroup analyses based on energy source. Then, in bipolar subgroup, ERBT had a significant better 12‐month RFS than cTURBT (Figure 4C; OR: 0.63; 95% CI: 0.4, 0.99; p < 0.05; I2 = 0%). During the sensitivity analysis, the exclusion of Gakis_G et al.”s 35 data resulted in a decrease in I2 to 50%. Simultaneously, the omission of data from the Hybrid Knife revealed a significant improvement in 12‐month RFS associated with ERBT (Figure 4D; OR: 0.47; 95% CI: 0.25, 0.87; p < 0.05; I2 = 50%). Therefore, based on our findings, we propose that NMIBC patients who undergo ERBT, particularly with the exclusion of Hybrid Knife energy, exhibit improved 3 and 12 month RFS, especially when utilizing bipolar energy. In RFS longer than 12 months, there was no significantly difference between ERBT and cTURBT (Figure 4E, OR: 0.82; 95% CI: 0.48, 1.43; p = 0.97; I2 = 0%).
4. DISCUSSION
In 1997, Kawada et al. 39 initially reported the clinical application of ERBT with monopolar arched electrode. However, some controversies remain unresolved and require further investigation. Recently, these problems are discussed in several studies (including meta‐analysis and RCT). 17 , 22 , 31 Using a combination of umbrella review and meta‐analysis, we demonstrated that ERBT had a better or comparable perioperative outcome than cTURBT. Furthermore, NMIBC patients undergoing ERBT exhibited improved 3 and 12 month RFS compared to those receiving cTURBT. We suggest that ERBT maybe a better surgical method for patients with NMIBC compared with cTURBT.
When compared to cTURBT, ERBT has shown a notable association with reduced rates of bladder perforation and ONR, suggesting that it may offer a safer surgical alternative for NMIBC patients. 16 , 18 , 19 , 20 , 21 , 22 The pooled ORT result reported by Teoh et al. 16 exhibited disparities from the findings of other five meta‐analyses. 18 , 19 , 20 , 21 , 22 In our meta‐analysis, comprised of five studies, ERBT and cTURBT required a similar amount of time to complete the surgery. Surgeons may need to invest time in learning a new surgical technique, which could explain the significant heterogeneity observed across different meta‐analyses. Considering these results, we tentatively suggest that ERBT is a safe surgical option.
Invasive detrusor muscle is the diagnostic criterion for NMIBC and MIBC. 15 Simultaneously, obtaining the entire tumor specimen could assist pathologists in making more accurate diagnoses and facilitate the identification of histological variants that had a significant impact on the prognosis of patients with NMIBC. 40 , 41 , 42 Moreover, all pooled meta‐analyses demonstrated that ERBT was associated with a lower incidence of residual tumor. These two pieces of evidence confirmed the sufficient efficacy of ERBT in managing NMIBC. In term of postoperative outcomes, patients undergoing ERBT had statistically shorter CTT based on six meta‐analyses. 16 , 18 , 19 , 21 , 29 , 43 Li et al. 20 did not find significant difference in bladder irritation between these two groups, while other five meta‐analyses identified a significant association between ERBT and shorter duration of bladder irritation. 16 , 18 , 19 , 21 , 22 Consistent to the five meta‐analyses, Liu et al. 38 also reported a significant correlation between ERBT and shorter bladder irritation. Hashem et al. 37 found two patients in cTURBT group had bladder irritation, while no patients in ERBT group diagnosed bladder irritation. These results revealed that ERBT offered a comparable or even better performance of bladder irritation.
Almost 30% of NMIBC patients would experience recurrence even after accepting BCG. 15 , 44 Therefore, researchers strived efforts to find powerful biomarkers and various new therapies to improve the prognosis of patients with NMIBC. 45 , 46 Urological surgeons have widely deliberated on the potential of ERBT to enhance RFS in patients with NMIBC. 47 However, no consensus has been reached on this matter now. Controversies arose regarding the 3, 12, and 24‐month RFS based on the pooled results of the aforementioned meta‐analyses. Thus, we collected and pooled the data on 3‐month RFS, which revealed that patients in the ERBT group exhibited superior RFS compared to those in the cTURBT group. In the result of 12‐month RFS, ERBT with bipolar had significant better RFS. After excluding the study with Hybrid Knife, the heterogeneity decreased to 50% and the new pooled outcome revealed a significant improvement in 12‐month RFS associated with ERBT. Different energy could bring different outcomes in ERBT. 48 Based on these findings, we concluded that ERBT was significantly associated with an improved 12‐month RFS. Based on the combined analysis of perioperative and survival outcomes, we suggest that ERBT is a safe surgical approach that may offer postoperative and RFS benefits for patients with NMIBC.
There were some limitations should be noticed. First, the 24‐month RFS did not pooled due to lack the data of RCTs. However, the 24‐month RFS outcomes of ERBT and cTURBT were found to be comparable, indicating that ERBT did not yield worse results in terms of 24‐month RFS. Second, we did not compare the RFS between laser and bipolar due to lack the data. This limitation maybe discussed in future when data is enough.
5. CONCLUSION
Using a combination of umbrella review and meta‐analysis, we demonstrated that ERBT had better or comparable perioperative outcome than cTURBT. Furthermore, NMIBC patients undergoing ERBT exhibited improved 3 and 12‐month RFS compared to those receiving cTURBT. We suggest that ERBT maybe a better surgical method for patients with NMIBC compared with cTURBT.
AUTHOR CONTRIBUTIONS
Deng‐xiong Li: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (equal); methodology (lead); software (equal); visualization (equal); writing – original draft (equal). Qing‐xin Yu: Project administration (lead); resources (equal); supervision (equal); validation (equal). Rui‐cheng Wu: Formal analysis (equal); investigation (lead); methodology (equal); software (lead). Jie Wang: Validation (lead). De‐chao Feng: Conceptualization (equal); data curation (equal); formal analysis (lead); methodology (lead); visualization (equal); writing – original draft (equal). Shi Deng: Project administration (lead); resources (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing (lead).
FUNDING INFORMATION
This research was funded by Chinese Scholarship Council (grant no. 202206240086). The funder had no role in the study design, data collection or analysis, preparation of the manuscript, or the decision to publish.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
This study is an umbrella review and meta‐analysis. Therefore, it does not require ethical review and approval.
Supporting information
Table S1.
Table S2.
Table S3.
ACKNOWLEDGMENTS
We appreciated the Figdraw (www.figdraw.com) for their assistance in drawing process.
Li D‐x, Yu Q‐x, Wu R‐c, Wang J, Feng D‐c, Deng S. Efficiency of transurethral en‐bloc resection vs. conventional transurethral resection for non‐muscle‐invasive bladder cancer: An umbrella review. Cancer Med. 2024;13:e7323. doi: 10.1002/cam4.7323
Deng‐xiong Li, Qing‐xin Yu, and Rui‐cheng Wu were contributed equally to this work and were listed as co‐first authors.
Contributor Information
De‐chao Feng, Email: fdcfenix@stu.scu.edu.cn.
Shi Deng, Email: dengshidiywchsj@163.com.
DATA AVAILABILITY STATEMENT
All data from this study were downloaded from an online database. Therefore, everyone can get the data online. Further inquiries can be directed to the corresponding author.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72:7‐33. [DOI] [PubMed] [Google Scholar]
- 2. Deng‐Xiong L, Qing‐Xin Y, De‐Chao F, et al. Systemic immune‐inflammation index (SII) during induction has higher predictive value than preoperative SII in non‐muscle‐invasive bladder cancer patients receiving Intravesical bacillus Calmette ‐Guerin. Clin Genitourin Cancer. 2023;21:e145‐e152. [DOI] [PubMed] [Google Scholar]
- 3. Jin YH, Zeng XT, Liu TZ, et al. Treatment and surveillance for non‐muscle‐invasive bladder cancer: a clinical practice guideline. Mil Med Res. 2022;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng D, Li D, Wu R, Han P. Scientific advancements in drug development and trials for urothelial carcinoma: insights from the 2023 ASCOGU cancers symposium. Aging Dis. 2023;14:1953‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan Z, Shi H, Luo J, et al. Diagnostic and therapeutic effects of fluorescence cystoscopy and narrow‐band imaging in bladder cancer: a systematic review and network meta‐analysis. Int J Surg. 2023;109:3169‐3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu R, Li D, Zhang F, Bai Y, Wang X, Han P. Prognostic value of platelet‐to‐lymphocyte ratio in non‐muscle invasive bladder cancer patients: Intravesical bacillus Calmette‐Guerin treatment after transurethral resection of bladder tumor. Front Surg. 2022;9:907485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng Y, Ye Y, Chen J, et al. Prevalence and outcomes of transurethral resection versus radical cystectomy for muscle‐infiltrating bladder cancer in the United States: a population‐based cohort study. Int J Surg. 2022;103:106693. [DOI] [PubMed] [Google Scholar]
- 8. Feng D, Wang Z, Yang Y, Li D, Wei W, Li L. Incidence and risk factors of parastomal hernia after radical cystectomy and ileal conduit diversion: a systematic review and meta‐analysis. Transl Cancer Res. 2021;10:1389‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li D, Wu R, Wang J, et al. A prognostic index derived from LASSO‐selected preoperative inflammation and nutritional markers for non‐muscle‐invasive bladder cancer. Clin Genitourin Cancer. 2024;102061. [DOI] [PubMed] [Google Scholar]
- 10. Ruiz de Porras V. Natural bioactive compounds: a potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment? Cancer Drug Resist. 2022;5:339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng L, Yang F, Tang L, et al. Electrochemical evaluation of tumor development via cellular Interface supported CRISPR/Cas trans‐cleavage. Research. 2022;2022:9826484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolters M, Kramer MW, Becker JU, et al. Tm:YAG laser en bloc mucosectomy for accurate staging of primary bladder cancer: early experience. World J Urol. 2011;29:429‐432. [DOI] [PubMed] [Google Scholar]
- 13. Li DX, Feng DC, Shi X, Wu RC, Chen K, Han P. Identification of endothelial‐related molecular subtypes for bladder cancer patients. Front Oncol. 2023;13:1101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li PH, Kong XY, He YZ, et al. Recent developments in application of single‐cell RNA sequencing in the tumour immune microenvironment and cancer therapy. Mil Med Res. 2022;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. EAU Guidelines . Edn. presented at the EAU Annual Congress Milan. 2023. ISBN 978‐94‐92671‐19‐6.
- 16. Teoh JY‐C, MacLennan S, Chan VW‐S, et al. An international collaborative consensus statement on En bloc resection of bladder tumour incorporating two systematic reviews, a two‐round Delphi survey, and a consensus meeting. Europ Urol. 2020;78:546‐569. [DOI] [PubMed] [Google Scholar]
- 17. Transurethral en bloc resection versus standard resection of bladder tumor: a multi‐center randomized trial (eb‐star study).
- 18. Wu Y‐P, Lin T‐T, Chen S‐H, et al. Comparison of the efficacy and feasibility of en bloc transurethral resection of bladder tumor versus conventional transurethral resection of bladder tumor a meta‐analysis. Medicine. 2016;95(e5372):e5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang H, Lin J, Gao P, et al. Is the En bloc transurethral resection more effective than conventional transurethral resection for non‐muscle‐invasive bladder cancer? A systematic review and meta‐analysis. Urol Inter. 2020;104:402‐409. [DOI] [PubMed] [Google Scholar]
- 20. Li Z, Zhou Z, Cui Y, Zhang Y. Systematic review and meta‐analysis of randomized controlled trials of perioperative outcomes and prognosis of transurethral en‐bloc resection vs. conventional transurethral resection for non‐muscle‐invasive bladder cancer. Int J Surg. 2022;104:106777. [DOI] [PubMed] [Google Scholar]
- 21. Yanagisawa T, Mori K, Motlagh RS, et al. En bloc resection for bladder tumors: an updated systematic review and meta‐analysis of its differential effect on safety recurrence and histopathology. J Urol. 2022;207:754‐768. [DOI] [PubMed] [Google Scholar]
- 22. Wang C‐W, Lee P‐J, Wu C‐W, Ho C‐H. Comparison of pathological outcome and recurrence rate between En bloc transurethral resection of bladder tumor and conventional transurethral resection: a meta‐analysis. Cancer. 2023;15(7):2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scottish Intercollegiate Guidelines Network . Search Filters. https://www.sign.ac.uk/what‐we‐do/methodology/search‐filters/
- 25. Whiting P, Savović J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane. 2011. [Google Scholar]
- 28. Sari Motlagh R, Rajwa P, Mori K, et al. Comparison of Clinicopathologic and oncological outcomes between transurethral En bloc resection and conventional transurethral resection of bladder tumor: a systematic review, meta‐analysis and network meta‐analysis with focus on different energy sources. J endourol. 2022;36:535‐547. [DOI] [PubMed] [Google Scholar]
- 29. Di Y, Li H, He C, Peng H. En‐bloc transurethral resection vs. conventional transurethral resection for primary non‐muscle invasive bladder cancer: a meta‐analysis. Actas Urol Esp. 2022;47:309‐316. [DOI] [PubMed] [Google Scholar]
- 30. Zhang D, Yao L, Yu S, et al. Safety and efficacy of en bloc transurethral resection versus conventional transurethral resection for primary nonmuscle‐invasive bladder cancer: a meta‐analysis. World J Surg Oncol. 2020;18(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Badawy A, Sultan SM, Marzouk A, El‐Sherif E. Thulium laser en bloc resection versus conventional transurethral resection of urinary bladder tumor: a comparative prospective study. Urology Annals. 2023;15:88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bălan GX, Geavlete PA, Georgescu DA, et al. Bipolar en bloc tumor resection versus standard monopolar TURBT ‐ which is the best way to go in non‐invasive bladder cancer? Romanian J Morphol Embryol. 2018;59:773‐780. [PubMed] [Google Scholar]
- 33. Chen X, Liao J, Chen L, et al. En bloc transurethral resection with 2‐micron continuous‐wave laser for primary non‐muscle‐invasive bladder cancer: a randomized controlled trial. World J Urol. 2015;33:989‐995. [DOI] [PubMed] [Google Scholar]
- 34. Fan J, Wu K, Zhang N, et al. Green‐light laser en bloc resection versus conventional transurethral resection for initial non‐muscle‐invasive bladder cancer: a randomized controlled trial. Int J Urol. 2021;28:855‐860. [DOI] [PubMed] [Google Scholar]
- 35. Gakis G, Karl A, Bertz S, et al. Transurethral en bloc submucosal hydrodissection vs conventional resection for resection of non‐muscle‐invasive bladder cancer (HYBRIDBLUE): a randomised, multicentre trial. BJU Int. 2020;126:509‐519. [DOI] [PubMed] [Google Scholar]
- 36. Gallioli A, Diana P, Fontana M, et al. En bloc versus conventional transurethral resection of bladder tumors: a single‐center prospective randomized noninferiority trial. Eur Urol Oncol. 2022;5:440‐448. [DOI] [PubMed] [Google Scholar]
- 37. Hashem A, Mosbah A, El‐Tabey NA, et al. Holmium laser En‐bloc resection versus conventional transurethral resection of bladder tumors for treatment of non–muscle‐invasive bladder cancer: a randomized clinical trial. Eur Urol Focus. 2021;7:1035‐1043. [DOI] [PubMed] [Google Scholar]
- 38. Liu H, Wu J, Xue S, et al. Comparison of the safety and efficacy of conventional monopolar and 2‐micron laser transurethral resection in the management of multiple nonmuscle‐invasive bladder cancer. J Int Med Res. 2013;41:984‐992. [DOI] [PubMed] [Google Scholar]
- 39. Kawada T, Ebihara K, Suzuki T, Imai K, Yamanaka H. A new technique for transurethral resection of bladder tumors: rotational tumor resection using a new arched electrode. J Urol. 1997;157:2225‐2226. [PubMed] [Google Scholar]
- 40. Li D, Li A, Yang Y, et al. Clinical characteristics and prognosis of rare histological variants of bladder cancer: a single‐center retrospective study from China. Cancer Manag Res. 2020;12:9635‐9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li DX, Wang XM, Tang Y, et al. Prognostic value of preoperative neutrophil‐to‐lymphocyte ratio in histological variants of non‐muscle‐invasive bladder cancer. Investig Clin Urol. 2021;62:641‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Villoldo GM, Pombo MT, Aris M, et al. A Th2‐score in the tumor microenvironment as a predictive biomarker of response to bacillus Calmette Guérin in patients with non‐muscle invasive bladder carcinoma: a retrospective study. Oncol Res. 2023;31:207‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu C, Wang S, Lai WF, Zhang D. The Progress of chitosan‐based nanoparticles for Intravesical bladder cancer treatment. Pharmaceutics. 2023;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li DX, Wang XM, Feng DC, et al. Lymphocyte‐to‐monocyte ratio (LMR) during induction is a better predictor than preoperative LMR in patients receiving Intravesical bacillus Calmette ‐Guerin for non‐muscle‐invasive bladder cancer. Front Oncol. 2022;12:937638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li DX, Feng DC, Wang XM, et al. M7G‐related molecular subtypes can predict the prognosis and correlate with immunotherapy and chemotherapy responses in bladder cancer patients. Eur J Med Res. 2023;28:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li DX, Wang XM, Feng DC, Han P. Neutrophil‐to‐lymphocyte ratio (NLR) during induction is a better predictor than preoperative NLR in non‐muscle‐invasive bladder cancer receiving bacillus Calmette‐GuÉRin. Asian J Surg. 2023;46:1348‐1351. [DOI] [PubMed] [Google Scholar]
- 47. Enikeev D, Morozov A, Shpikina A, Fajkovic H, Baniel J, Herrmann TRW. A 10‐year renaissance of en bloc resection of bladder tumors (ERBT): are we approaching the peak or is it back to the trough? World J Urol. 2023;41:2607‐2615. [DOI] [PubMed] [Google Scholar]
- 48. Diana P, Gallioli A, Fontana M, et al. Energy source comparison in en‐bloc resection of bladder tumors: subanalysis of a single‐center prospective randomized study. World J Urol. 2022;41(10):2591‐2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Data Availability Statement
All data from this study were downloaded from an online database. Therefore, everyone can get the data online. Further inquiries can be directed to the corresponding author.


