Abstract
Background
Ensuring the rapidity and accuracy of emergency laboratory test results is especially important to save the lives of patients with acute and critical conditions. To better meet the needs of clinicians and patients, detection efficiency can be improved by reducing extra-laboratory sample turnaround times (TATs) through the use of innovative pneumatic tube system (PTS) transport for sample transport. However, concerns remain regarding the potential compromise of sample quality during PTS transit relative to that occurring with manual transportation. This study was performed to evaluate the efficacy of an innovative PTS (Tempus600 PTS) relative to a traditional PTS in terms of sample transit time, sample quality, and the concordance of analytical results with those obtained from manually transported samples.
Methods
In total, 30 healthy volunteers aged >18 years were recruited for this study, conducted for five consecutive days. Venous blood samples were collected from six volunteers per day at fixed timepoints. From each volunteer, nine blood samples were collected into tubes with tripotassium ethylene diamine tetraacetic acid anticoagulant, tubes with 3.2 % sodium citrate, and serum tubes with separation gel (n = 3 each) and subjected to all tests conducted in the emergency laboratory in our hospital. 270 blood samples from 30 healthy volunteers were transported and analyzed, yielding 6300 test results. The blood samples were divided randomly into three groups (each containing one tube of each type) and transported to the emergency laboratory manually and with Tempus600 PTS and conventional Swisslog PTS, respectively. The extra-laboratory TATs, sample quality, and test results of the transported blood samples were compared.
Results
The sample quality and test results did not differ according to the delivery method. The TAT was much shorter with the Tempus600 than with the other two transport modes (58.40 ± 1.52 s vs. 1711.20 ± 77.56 s for manual delivery and 146.60 ± 1.82 s for the Swisslog PTS; P = 0.002).
Conclusion
Blood sample transport with the Tempus600 PTS significantly reduced the extra-laboratory TAT without compromising sample quality or test result accuracy, thereby improving the efficiency of sample analysis and the services provided to clinicians and patients.
Keywords: Emergency laboratory, Pneumatic tube system transport, Tempus600 PTS, Extra-laboratory sample turnaround times
1. Introduction
Emergency clinical laboratories are crucial components of emergency diagnosis and treatment, contributing to the rescuing of patients with acute and critical conditions. They must efficiently and rapidly provide clinicians accurate test results, as these patients require emergent treatment and diagnosis at the same time. With the development and reform of laboratory automation and artificial intelligence, the efficiency of sample detection and analysis has improved significantly, enabling the effective guaranteeing of sample turnaround times (TATs) and efficient clinical diagnosis [1,2]. However, clinicians and patients are more concerned with total turnaround times (ToTATs) from sample collection to test report issuance. The continuous evaluation of ToTATs is a fundamental requirement that provides an important indicator for hospital laboratory management [3,4], and the rapid completion of sample detection and test reporting, contributing to clinicians’ ability to resuscitate patients, make diagnoses, and establish reasonable therapeutic schedules, can be ensured only through effective ToTATs shortening. To better meet clinical needs, sample TATs outside of emergency laboratories need to be optimized.
The key to extra-laboratory TAT reduction is the improvement of specimen transport. The establishment and optimization of hospital logistics delivery systems, especially pneumatic tube systems (PTSs), are increasingly attracting attention from major hospitals [5,6]. In PTSs, compressed air is used to safely and efficiently transport samples through pipelines to laboratories, avoiding the need for manual transport and reducing sample TATs [[7], [8], [9], [10]]. For example, blood samples can be transported rapidly by a PTS from the collection location to the laboratory for testing, aiding timely diagnosis and appropriate clinical intervention, which is especially important in hospitals’ acute and critical care wards [4,7,11]. With PTS application, the average TAT for laboratory blood samples can be reduced by 16–24 %; with the added use of electronic medical orders, it can be decreased by 34–42 % [4,12]. Direct sample transfer by PTS also avoids sample loss, confusion, and other errors that can occur during manual distribution, while eliminating the need for human supervision during manual delivery [7].
In traditional PTSs, blood samples are transported to laboratories in cylindrical transfer bottles; they must be fixed in the bottles or placed with buffering liners to avoid hemolysis and loss of sample quality [[13], [14], [15], [16]]. To avoid damage caused by impact, the bottles employed at our hospital are filled with honeycomb briquette sponges [8], and blood collection nurses stuff blood samples into the sponge holes for transport to the laboratory. With the further development of hospital logistics technology, innovative PTSs have emerged. With these systems, the automated transfer of blood samples from the collection site to the laboratory occurs from a launch pad through a sealed, dedicated pipe, avoiding the need to load transfer bottles and ensuring the rapidity and safety of the process [17,18].
Blood samples transported via pneumatic tube systems (PTS) are vulnerable to physical stresses, including speed, distance, vibration, acceleration, and shock forces, with high-speed transport significantly increasing the risk of gravity acceleration and impactful shock forces that may compromise sample integrity [6,[19], [20], [21]]. PTS-based transport has been reported to increase blood sample hemolysis and affect platelet function, in turn potentially affecting the results of biochemical and coagulation tests [7,10,[22], [23], [24], [25], [26], [27]]. As PTS use in hospitals is increasing, its impacts on blood sample quality and test results must be evaluated and clarified to avoid inaccuracies.
In this study, we evaluated the distribution efficiency (TAT), sample quality, and test results obtained with the use of innovative and traditional PTSs, with manual delivery serving as the reference standard.
2. Materials and methods
2.1. Participants and procedure
In total, 30 healthy volunteers (16 [53.3 %] males, 14 [46.7 %] females) were included in this study. The mean age was 36.2 years (range, 23–45 years). The exclusion criteria were: (1) age <18 or >50 years; (2) history of a major medical condition, including cardiovascular disease, diabetes mellitus, and/or hepatic or renal impairment; (3) history of a psychiatric disorder, such as depression, anxiety, or schizophrenia; (4) history of alcohol abuse; (5) anemia; (6) concurrent participation in another clinical trial; (7) known hypersensitivity to any component of the study intervention; and (8) pregnancy or lactation. This study was approved by the ethics committee of the institutional review board of Fuwai Hospital (Approval No. 2021-1473), and all participants provided written informed consent.
For five consecutive days, six randomly selected volunteers per day underwent venipuncture for blood sample collection at a fixed timepoint (when the number of samples requiring delivery is typically largest at our hospital) using a butterfly needle connected to a vacuum device for transfer to anticoagulant tubes. Blood from each volunteer was collected into three types of Vacuette® tubes: tripotassium ethylene diamine tetraacetic acid (K3EDTA) Vacuette® tubes (2 mL; ref. 454087CN; Greiner Bio-one), Vacuette® coagulation tubes with 3.2 % sodium citrate (3 mL; ref. 454334CN; Greiner Bio-one), and Vacuette® serum tubes with separation gel (3.5 mL; ref. 454067CN; Greiner Bio-one). Samples were collected using three tubes from each type of vacutainer, following which they were randomly allocated into three distinct groups. These groups of samples were then delivered to the emergency laboratory via manual transportation, a traditional PTS (Swisslog, Germany), and an innovative PTS (Tempus600; TIMEDICO A/S, Denmark), respectively. Overall, the study encompassed the transportation and analysis of 270 blood samples from 30 volunteers, yielding 210 test results obtained for each participant, amounting to a cumulative total of 6300 results.
For each set of samples, the time taken from collection to arrival at the emergency laboratory was recorded. For the manual transportation group, timing commenced upon notification to retrieve the sample and concluded upon its arrival at the emergency laboratory. For transit using the Swisslog PTS and Tempus600 PTS, personnel at the sending and receiving stations maintained telephonic communication. The receiver-initiated timing at the moment at which the sender dispatched the specimen and stopped when the specimen arrived at the emergency laboratory.
2.2. PTS characteristics
The Swisslog pneumatic logistics system is powered by compressed air; samples are transported in transmission cylinders in a closed pipeline. The total length of the pipe is about 500 m, and it passes through four curves with acceleration of about 10 m/s. The cylinders (15-cm diameter, 35-cm length) are filled with briquette-like sponges to hold blood samples and avoid collision during transport.
The Tempus600 (TIMEDICO A/S) PTS utilizes the power generated by compressed air to automatically transport blood samples through a sealed pipeline without the need for cylinder use. The total length of the pipe is about 450 m, with three curves, and the sample transmission speed is about 600 m/s. At our hospital, the start and end points of both PTSs are in the heart failure ward and emergency laboratory, respectively.
2.3. Manual delivery
For manual transport, delivery staff members were informed after sample collection that blood samples requiring delivery to the emergency laboratory were present at the nurse's station of the heart failure ward. To represent the actual workflow as closely as possible, the samples were not identified as scientific research samples.
2.4. Sample analysis
Immediately after arrival at the emergency laboratory, the blood samples were centrifuged at 1760g at room temperature for 10 min. To comprehensively represent the extensive test repertoire performed in the emergency laboratory setting, Vacuette® tubes with separator gel were employed for biochemical assays and the assessment of the triad of myocardial infarction markers. K3EDTA Vacuette® tubes were used for the analysis of routine hematological parameters and immunological markers, including high-sensitivity cardiac troponin T (hs-cTnT), N-terminal pro–B-type natriuretic peptide (NT-proBNP), and procalcitonin. Coagulation parameters were assessed with a panel of six tests using Vacuette® tubes with 3.2 % sodium citrate (Table S1). To reduce the occurrence of random errors, the same operator used the same apparatus to test blood samples delivered in different ways. During sample analysis, visual inspection alone is inadequate to determine if transportation impacted sample quality; thus, we employed serum indices (hemolysis, icterus, and lipidemia indices) to assess sample quality in a semi-quantitative manner using the Serum Index Gen.2 assay (cat. no. 05172179190; Roche, Rotkreuz, Switzerland) via measurement of diluted sample absorbance at bichromatic wavelengths and automated calculation of indices by the instrument, providing objective quantitative data to comprehensively evaluate the effects of transportation on sample quality. We performed serum index testing for all specimen types. Biochemical analytes was performed using the Cobas 8000 c702 device (Roche, Switzerland), immunoassays(hs-cTnT/NT-proBNP/procalcitonin) were analyzed using a Cobas 8000 e801 device (Roche, Switzerland), myocardial infarction markers (high-sensitivity cardiac troponin I, creatinine kinase MB, and myoglobin) were assessed using an ARCHITECT i2000SR automatic chemiluminescence immunoassay analyzer (Abbott Laboratories, USA), coagulation parameters were measured on a STA-R MAX device (STAGO, France); and routine blood tests were performed using an XN-10 automatic blood analyzer (SYSMEX, Japan) (Table S1). The bias (%) of the analyte value is calculated by the result for manual transport (T0) and the result for PTS (TX) using the following formula:
In evaluating test results, we focused on the potassium ion and lactate dehydrogenase values, which have been reported to increase due to hemolysis with PTS transport [28].
2.5. Statistical analysis
IBM SPSS Statistics software (version 27; IBM Corporation, Armonk, NY, USA) was used to compare TATs and test results for samples transported by the three methods at the same timepoints. The normality of data distributions was assessed using the Shapiro–Wilk method (P > 0.05) and Q-Q plots. The homogeneity of variance was examined using the F test (P > 0.05). Data meeting the normality and homogeneity requirements were compared by one-way analysis of variance. Data that were not normally distributed were compared using the Kruskal–Wallis test. P values < 0.05 were considered to be significant. Centrality and dispersion are described with means and ranges. When appropriate, 95 % confidence intervals were calculated.
3. Results
In total, 270 blood samples from 30 volunteers were transported and analyzed in this study. A total of 210 test results were obtained for each volunteer (overall total, 6300 results). Recognizing that changes in sample quality during transport may not be readily apparent through visual inspection alone, we employed a semi-quantitative approach to measure the serum indices (lipemia, hemolysis, and icterus indices) of the samples. It can provide a more objective reflection of the quality variations across different transportation methods. Our investigation revealed that no significant discrepancies in serum indices of blood samples collected in serum tubes with separation gel, tubes with 3.2 % sodium citrate, tubes with K3EDTA anticoagulant, following distribution via Tempus600 PTS, Swisslog PTS, and manual transport (P > 0.05; Table 1). This finding suggests that the incidence of hemolysis in the three types of blood samples transported via PTSs is not significantly different from those transported manually.
Table 1.
Sample quality by emergency laboratory delivery method.
| Sample | Manual transport | Swisslog PTS | Tempus600 PTS | p |
|---|---|---|---|---|
| Serum tube (separation gel) | ||||
| H | 5.00 (4.25) | 4.00 (3.25) | 5.00 (5.00) | 0.249 |
| I | 1.00 (1.00) | 1.00 (1.00) | 1.00 (1.00) | 0.889 |
| L | 10.00 (15.00) | 9.00 (11.75) | 11.50 (15.25) | 0.841 |
| K3 EDTA tube | ||||
| H | 34.00 (18.25) | 29.50 (14.50) | 33.50 (16.50) | 0.228 |
| I | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | 0.837 |
| L | 31.50 (33.50) | 32.00 (27.50) | 30.00 (28.75) | 0.999 |
| Coagulant tube (3.2 % sodium citrate) | ||||
| H | 4.00 (4.00) | 4.00 (4.50) | 5.50 (4.25) | 0.236 |
| I | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | 0.692 |
| L | 15.00 (9.50) | 15.00 (10.25) | 16.00 (8.50) | 0.908 |
Data are reported as median (interquartile range).
PTS, pneumatic tube system; H, hemolysis index; I, icteric index; L, lipemic index.
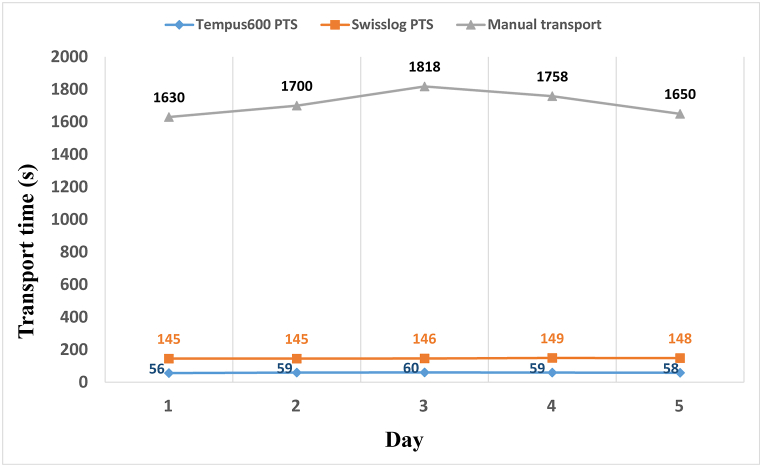
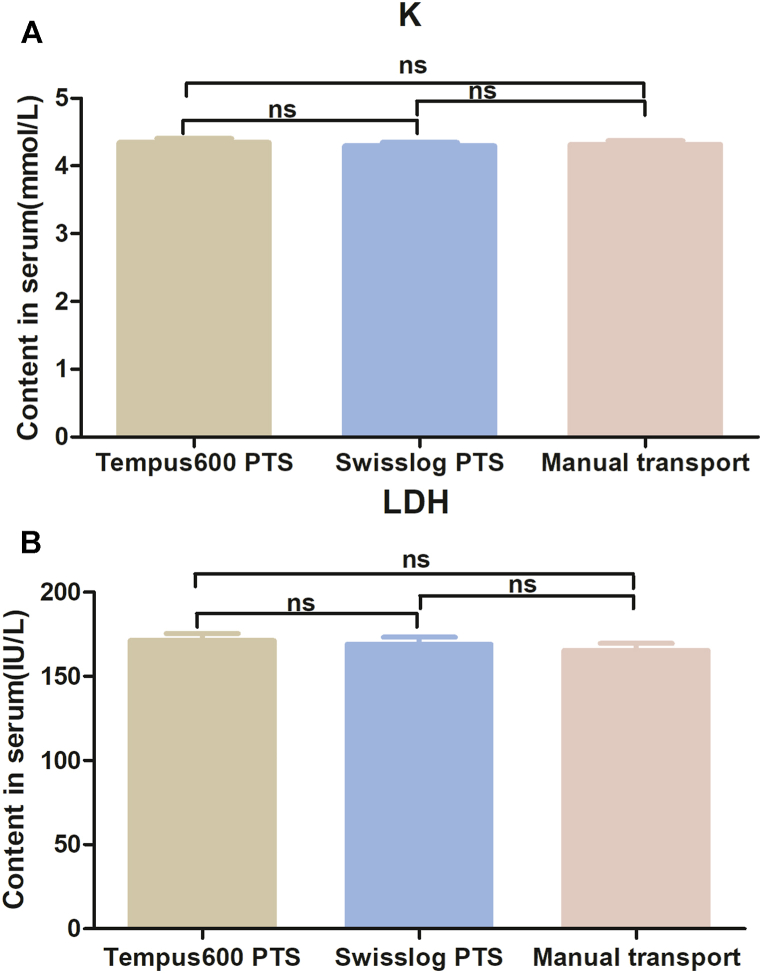
The TATs from calling (manual delivery) or sample dispatch (PTSs) to arrival at the emergency laboratory were 1711.20 ± 77.56 s for manual delivery, 146.60 ± 1.82 s for the Swisslog PTS, and 58.40 ± 1.52 s for the Tempus600 PTS (P = 0.002; Fig. 1). The TAT for the Tempus600 PTS was about 96 % shorter than that for manual delivery and >60 % shorter than that for the Swisslog PTS (Fig. 1). These results indicate that the Tempus600 PTS is the most suitable system for the transportation of samples in emergency and critical care wards. The findings revealed that there were no statistically significant differences among the test results of the samples transported by methods Tempus600 PTS, Swisslog PTS, and manual transport (p > 0.05, Table 2). This suggests that the use of PTS for blood sample transportation maintains sample stability and the accuracy of test results. Notably, the Tempus600 PTS, an innovative PTS system, not only significantly reduces the TAT but also ensures the preservation of sample quality, thereby ensuring that test results remain unaffected in our study. In particular, the results for potassium ion and lactate dehydrogenase, reported to be highly susceptible to hemolysis during PTS transport, did not differ. The mean concentrations of potassium ions were 4.32 ± 0.29 mmol/L for samples transported manually, 4.30 ± 0.29 mmol/L for those transported via the Swisslog PTS, and 4.35 ± 0.32 mmol/L for samples transported using the Tempus600 PTS, with no significant differences observed among the groups (P > 0.05; Fig. 2A). Similarly, the lactate dehydrogenase levels were 165.40 ± 23.21 IU/L for manual transport, 169.07 ± 22.63 IU/L for Swisslog PTS transport, and 171.17 ± 23.32 IU/L for Tempus600 PTS transport, respectively, again showing no statistically significant variations (P > 0.05; Fig. 2B).
Fig. 1.
The five days' transport time of samples outside the laboratory was measured by manual transport, Swisslog PTS and Tempus600 PTS. PTS, pneumatic tube system.
Table 2.
Comparison of all test results in the emergency laboratory among the Tempus600 PTS, Swisslog PTS and manual transport.
| Analytes | Units |
n |
Mean ± SD |
Median (IQR) |
Normal distribution |
p-Value |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Manual transport | Swisslog PTS | Tempus600 PTS | Manual transport | Swisslog PTS | Tempus600 PTS | |||||
| Biochemistry | ||||||||||
| PA | mg/L | 30 | 265.42 ± 54.28 | 267.82 ± 57.64 | 267.47 ± 56.61 | NA | NA | NA | Y | 0.984 |
| TP | g/L | 30 | 79.36 ± 3.72 | 79.58 ± 3.57 | 79.38 ± 3.73 | NA | NA | NA | Y | 0.968 |
| ALB | g/L | 30 | 48.97 ± 2.39 | 49.12 ± 2.40 | 49.03 ± 2.28 | NA | NA | NA | Y | 0.969 |
| ALT | IU/L | 30 | NA | NA | NA | 21.00(16.25) | 21.00(16.25) | 21.00(16.25) | N | 0.996 |
| AST | IU/L | 30 | NA | NA | NA | 20.00(7.25) | 19.50(6.00) | 21.00(8.00) | N | 0.659 |
| TBil | μmol/L | 30 | NA | NA | NA | 10.20(4.30) | 10.45(4.47) | 10.13(4.48) | N | 0.979 |
| DBil | μmol/L | 30 | NA | NA | NA | 3.74(1.65) | 3.88(1.94) | 3.65(1.65) | N | 0.553 |
| TBA | μmol/L | 30 | NA | NA | NA | 2.17(1.44) | 2.29(1.51) | 2.22(1.60) | N | 0.909 |
| Potassium | mmol/L | 30 | 4.32 ± 0.29 | 4.30 ± 0.29 | 4.35 ± 0.32 | NA | NA | NA | Y | 0.808 |
| Sodium | mmol/L | 30 | NA | NA | NA | 139.41(2.79) | 139.44(2.17) | 139.64(2.84) | N | 0.850 |
| Chloride | mmol/L | 30 | 102.73 ± 2.26 | 102.74 ± 2.26 | 102.96 ± 2.00 | NA | NA | NA | Y | 0904 |
| CO2 | mmol/L | 30 | 24.36 ± 2.73 | 24.66 ± 2.61 | 24.42 ± 2.50 | NA | NA | NA | Y | 0.897 |
| Calcium | mmol/L | 30 | 2.36 ± 0.09 | 2.38 ± 0.08 | 2.37 ± 0.09 | NA | NA | NA | Y | 0.817 |
| Glucose | mmol/L | 30 | NA | NA | NA | 5.28(0.54) | 5.25(0.64) | 5.23(0.57) | N | 0.641 |
| Creatinine | μmol/L | 30 | 62.24 ± 14.500 | 62.23 ± 14.32 | 61.90 ± 14.26 | NA | NA | NA | Y | 0.995 |
| Uric acid | μmol/L | 30 | 363.84 ± 122.94 | 362.59 ± 121.94 | 361.05 ± 120.55 | NA | NA | NA | Y | 0.996 |
| Urea nitrogen | mmol/L | 30 | 4.49 ± 1.08 | 4.48 ± 1.09 | 4.48 ± 1.08 | NA | NA | NA | Y | 0.990 |
| CK | IU/L | 30 | NA | NA | NA | 81.50(58.25) | 81.00(57.75) | 83.50(62.50) | N | 0.915 |
| CKMB-Mass | ng/mL | 30 | NA | NA | NA | 2.83(0.88) | 2.69(0.46) | 2.80(0.92) | N | 0.845 |
| LDH | IU/L | 30 | 165.40 ± 23.21 | 169.07 ± 22.63 | 171.17 ± 23.32 | NA | NA | NA | Y | 0.620 |
| Amylase | U/L | 30 | 69.50 ± 23.06 | 69.73 ± 22.85 | 69.07 ± 22.75 | NA | NA | NA | Y | 0.993 |
| HSCRP | mg/L | 30 | NA | NA | NA | 0.68(1.79) | 0.71(1.86) | 0.69(1.82) | N | 0.968 |
| Magnesium | mmol/L | 30 | 0.92 ± 0.06 | 0.92 ± 0.07 | 0.92 ± 0.06 | NA | NA | NA | Y | 0.997 |
| Cystatin C | mg/L | 30 | NA | NA | NA | 0.81(0.21) | 0.82(0.20) | 0.82(0.19) | N | 0.960 |
| eGFR | mL/min | 30 | NA | NA | NA | 120.50(12.00) | 119.50(12.50) | 119.50(14.75) | N | 0.882 |
| Coagulation | ||||||||||
| PT | s | 30 | 13.34 ± 0.53 | 13.43 ± 0.55 | 13.55 ± 0.64 | NA | NA | NA | Y | 0.380 |
| PTA | % | 30 | 101.07 ± 9.02 | 99.27 ± 9.10 | 97.53 ± 10.14 | NA | NA | NA | Y | 0.353 |
| INR | R | 30 | 1.00 ± 0.05 | 1.01 ± 0.05 | 1.02 ± 0.06 | NA | NA | NA | Y | 0.356 |
| APTT | s | 30 | 34.64 ± 3.46 | 35.04 ± 3.49 | 35.47 ± 3.39 | NA | NA | NA | Y | 0.651 |
| TT | s | 30 | 16.56 ± 0.66 | 16.52 ± 0.65 | 16.58 ± 0.64 | NA | NA | NA | Y | 0.942 |
| FIB | g/L | 30 | NA | NA | NA | 2.91(0.85) | 2.89(0.96) | 2.78(0.90) | N | 0.795 |
| D-Dimer | μg/mL | 30 | NA | NA | NA | 0.20(0.13) | 0.19(0.14) | 0.19(0.14) | N | 0.967 |
| FDP | μg/mL | 30 | NA | NA | NA | 2.50(0.00) | 2.50(0.00) | 2.50(0.00) | N | 0.356 |
| Blood Routine Tests | ||||||||||
| WBC | 109/L | 30 | 6.47 ± 2.00 | 6.56 ± 1.95 | 6.58 ± 1.97 | NA | NA | NA | Y | 0.977 |
| Neutrophil% | % | 30 | 56.65 ± 6.45 | 56.57 ± 6.91 | 56.96 ± 6.83 | NA | NA | NA | Y | 0.972 |
| Neutrophil # | 109/L | 30 | NA | NA | NA | 3.48(1.75) | 3.53(1.88) | 3.53(1.85) | N | 0.951 |
| Lymphocyte% | % | 30 | 34.30 ± 6.46 | 34.36 ± 6.88 | 33.98 ± 6.69 | NA | NA | NA | Y | 0.972 |
| Lymphocyte # | 109/L | 30 | NA | NA | NA | 2.18(0.57) | 2.23(0.67) | 2.22(0.65) | N | 0.957 |
| Monocyte% | % | 30 | NA | NA | NA | 6.15(1.58) | 6.15(1.63) | 6.00(1.80) | N | 0.998 |
| Monocyte # | 109/L | 30 | 0.40 ± 0.11 | 0.41 ± 0.11 | 0.41 ± 0.12 | NA | NA | NA | Y | 0.978 |
| Eosinophil% | % | 30 | NA | NA | NA | 1.60(1.23) | 1.65(1.23) | 1.60(1.03) | N | 0.907 |
| Eosinophil # | 109/L | 30 | NA | NA | NA | 0.09(0.09) | 0.10(0.08) | 0.09(0.07) | N | 0.900 |
| Basophil% | % | 30 | 0.57 ± 0.23 | 0.60 ± 0.23 | 0.59 ± 0.27 | NA | NA | NA | Y | 0.934 |
| Basophil# | 109/L | 30 | NA | NA | NA | 0.03(0.03) | 0.04(0.03) | 0.04(0.03) | N | 0.957 |
| RBC | 1012/L | 30 | 4.94 ± 0.50 | 4.96 ± 0.51 | 4.95 ± 0.50 | NA | NA | NA | Y | 0.995 |
| Hemoglobin | g/L | 30 | NA | NA | NA | 150.00(27.75) | 152.00(29.75) | 151.50(28.00) | N | 0.995 |
| Hematocrit | % | 30 | NA | NA | NA | 44.50(7.25) | 44.50(9.00) | 44.00(8.00) | N | 0.942 |
| MCV | fL | 30 | NA | NA | NA | 86.40(6.85) | 86.05(6.10) | 85.70(5.58) | N | 0.860 |
| MCH | pg | 30 | NA | NA | NA | 30.10(3.15) | 30.15(2.93) | 29.95(3.03) | N | 0.992 |
| MCHC | g/L | 30 | NA | NA | NA | 341.50(18.50) | 342.00(18.00) | 341.50(15.25) | N | 0.900 |
| RDW-SD | fL | 30 | 40.53 ± 3.11 | 40.33 ± 3.00 | 40.17 ± 2.90 | NA | NA | NA | Y | 0.899 |
| RDW | % | 30 | NA | NA | NA | 12.65(0.95) | 12.70(1.13) | 12.75(1.13) | N | 0.973 |
| Platelet | 109/L | 30 | 291.20 ± 72.37 | 286.00 ± 71.65 | 293.73 ± 72.46 | NA | NA | NA | Y | 0.914 |
| PDW | fL | 30 | NA | NA | NA | 11.75(2.28) | 11.50(2.38) | 11.15(2.10) | N | 0.691 |
| MPV | fL | 30 | NA | NA | NA | 10.30(1.05) | 10.00(0.93) | 10.00(1.20) | N | 0.452 |
| P-LCR | % | 30 | NA | NA | NA | 27.40(9.05) | 25.50(8.95) | 25.55(9.18) | N | 0.472 |
| Thrombocytocrit | % | 30 | 0.30 ± 0.06 | 0.28 ± 0.06 | 0.29 ± 0.06 | NA | NA | NA | Y | 0.802 |
| Immunology | ||||||||||
| hs-cTnT | ng/mL | 30 | NA | NA | NA | 0.00(0.00) | 0.00(0.00) | 0.00(0.00) | N | 0.943 |
| NT-proBNP | pg/mL | 30 | NA | NA | NA | 17.80(36.73) | 17.75(34.60) | 17.35(35.55) | N | 0.996 |
| Procalcitonin | ng/mL | 30 | NA | NA | NA | 0.05(0.04) | 0.05(0.04) | 0.05(0.04) | N | 0.850 |
| MI indicator | ||||||||||
| hs-cTnI | ng/mL | 30 | NA | NA | NA | 0.00(0.00) | 0.00(0.00) | 0.00(0.00) | N | 0.806 |
| CK-MB | ng/mL | 30 | NA | NA | NA | 0.57(0.59) | 0.58(0.56) | 0.61(0.54) | N | 0.963 |
| Myoglobin | ng/mL | 30 | NA | NA | NA | 28.06(14.02) | 28.00(15.01) | 28.02(15.43) | N | 0.956 |
NA, not applicable; IQR, interquartile range; TP, total protein; PA, prealbumin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
TBA, total bile acids; HSCRP, high sensitive creative protein; CK, creatine kinase; CKMB, creatine kinase isoenzyme; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate; PT, prothrombin time; PTA, prothrombin activation; INR, international normalized ratio; APTT, activated partial thromboplastin time; TT, thrombin time; FIB, fibrinogen; FDP, fibrinogen degradation products; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW-SD, standard deviation in red cell distribution width; PDW, platelet distribution width; MPV, mean platelet volume; P-LCR, platelet larger cell ratio; hs-cTnT/I, high sensitivity cardiac troponin T/I; NT-proBNP, N-terminal pro-B type natriuretic peptide; MI, myocardial infarction. Y indicates a normal distribution and N indicates a non-normal distribution. Difference with p-values < 0.05 were regarded as significant at a 95%-level.
Fig. 2.
Serum potassium ion (K) and lactate dehydrogenase (LDH) results by transport mode. PTS, pneumatic tube system.
Compared with the results for manual transport, we calculated the bias (%) for all test results for the Swisslog PTS and Tempus600 PTS. The bias (%) for the indicators with allowable total error in our laboratory were shown in Table 3. It was clear that the bias (%) of the test results falls within the allowable total error. We considered it acceptable as a clinically acceptable difference. Our findings further suggest that there are no clinically significant discrepancies in the test results between samples transported via the Swisslog PTS or Tempus600 PTS and those delivered manually in our study.
Table 3.
Bias% for indicators with allowable total error.
| Analytes | Units | Bias (%) |
Allowable Total Error | |
|---|---|---|---|---|
| Tempus600 PTS (Min, Max) | Swisslog PTS (Min, Max) | |||
| PA | mg/L | (-2.83 %,5.27 %) | (-2.71 %,6.70 %) | ±25 % |
| TP | g/L | (-2.24 %,4.56 %) | (-2.31 %,3.17 %) | ±5 % |
| ALB | g/L | (-4.18 %,5.31 %) | (-4.36,3.27 %) | ±6 % |
| ALT | IU/L | (-11.76 %,13.33 %) | (-11.76 %,14.29 %) | ±16 % |
| AST | IU/L | (-10.53 %,12.50 %) | (-14.29 %,6.67 %) | ±15 % |
| TBil | μmol/L | (-9.95 %,11.26 %) | (-5.52 %,9.08 %) | ±15 % |
| DBil | μmol/L | (-11.76 %,11.14 %) | (-6.56 %,10.02 %) | ±20 % |
| TBA | μmol/L | (-5.39 %,2.94 %) | (-0.47 %,9.07 %) | ±25 % |
| Potassium | mmol/L | (-4.24 %,4.39 %) | (-3.70 %,5.69 %) | ±6 % |
| Sodium | mmol/L | (-1.98 %,1.97 %) | (-1.59 %,1.69 %) | ±4 % |
| Chloride | mmol/L | (-1.68 %,2.66 %) | (-2.23 %,2.85 %) | ±4 % |
| CO2 | mmol/L | (-6.95 %,5.33 %) | (-3.69 %,6.22 %) | ±8 % |
| Calcium | mmol/L | (-2.10 %,2.62 %) | (-1.56 %,3.49 %) | ±5 % |
| Glucose | mmol/L | (-2.97 %,6.54 %) | (-1.59 %,4.88 %) | ±7 % |
| Creatinine | μmol/L | (-3.61 %,6.39 %) | (-3.32 %,4.12 %) | ±12 % |
| Uric acid | μmol/L | (-4.58 %,1.92 %) | (-4.01 %,1.60 %) | ±12 % |
| CK | IU/L | (-3.08 %,8.33 %) | (-4.62 %,5.69 %) | ±15 % |
| CKMB-Mass | ng/mL | (-10.59 %,16.41 %) | (-13.23 %,5.54 %) | ±25 % |
| LDH | IU/L | (-5.61 %,9.02 %) | (-8.67 %,9.15 %) | ±11 % |
| Amylase | U/L | (-4.00 %,3.08 %) | (-2.08 %,6.15 %) | ±18 % |
| HSCRP | mg/L | (-12.16 %,14.29 %) | (-11.87 %,7.03 %) | ±30 % |
| Magnesium | mmol/L | (-2.80 %,3.61 %) | (-3.41 %,3.61 %) | ±15 % |
| PT | s | (-5.67 %,7.69 %) | (-2.84 %,8.33 %) | ±15 % |
| APTT | s | (-1.83 %,6.99 %) | (-2.13 %,6.59 %) | ±15 % |
| TT | s | (-5.26 %,5.10 %) | (-5.85 %,4.94 %) | ±20 % |
| FIB | g/L | (-9.12 %,12.50 %) | (-9.97 %,7.62 %) | ±20 % |
| D-Dimer | μg/mL | (-10.87 %,10.71 %) | (-4.76 %,10.71 %) | ±20 % |
| WBC | 109/L | (-5.76 %,6.93 %) | (-5.24 %,8.85 %) | ±15 % |
| RBC | 1012/L | (-1.95 %,2.96 %) | (-2.14 %,3.19 %) | ±6 % |
| Hemoglobin | g/L | (-1.27 %,1.81 %) | (-3.03 %,2.52 %) | ±6 % |
| Hematocrit | % | (-3.23 %,2.70 %) | (-2.56 %,2.38 %) | ±9 % |
| MCV | fL | (-1.87 %,0.38 %) | (-1.20 %,0.76 %) | ±7 % |
| MCH | pg | (-2.63 %,2.26 %) | (-1.91 %,1.72 %) | ±7 % |
| MCHC | g/L | (-2.27 %,2.73 %) | (-2.06 %,1.85 %) | ±8 % |
| Platelet | 109/L | (-4.96 %,6.08 %) | (-7.53 %,4.56 %) | ±20 % |
| hs-cTnT | ng/mL | −0.001,0.001 | −0.001,0.001 | ±0.001 |
| N T-proBNP |
pg/mL | (-6.62 %,12.00 %) | (-13.59 %,13.24 %) | ±30 % |
| hs-cTnI | ng/mL | −0.001,0.001 | −0.001,0 | ±0.001 |
| Myoglobin | ng/mL | (-6.19 %,10.93 %) | (-5.96 %,8.19 %) | ±30 % |
4. Discussion
In this study, we compared the sample quality, test results, and transport efficiency for blood samples delivered manually (reference standard) and with the Tempus600 and Swisslog PTSs to our hospital's emergency clinical laboratory. The sample quality and test results did not differ according to the transport method. Thus, the layout, construction, and manner of use of both PTSs caused no significant sample deterioration or damage. Qavi et al. reported that insufficient sample volume was associated with an increased risk of hemolysis [29]. We carefully controlled for this preanalytical factor in this study, ensuring that minimum required volumes for analysis were met by precisely following the volume markings on the collection tubes when drawing all samples. This adherence to recommended volume guidelines effectively prevented hemolysis due to insufficient sample collection. Previous studies of the impact of PTS transport on sample quality have yielded contradictory results. Kara et al. [23] reported hemolysis in 100 % of samples transported by conventional PTS, but only 16 % of manually transported samples, whereas Fernandes et al. [4] found no significant difference in the hemolysis of samples transported by these two modes. In contrast to the methodology used in the present study, paired samples were not examined in those studies and the samples' susceptibility to hemolysis was considered to be consistent. Lorenzen et al. [30] found that transport with the Tempus600 PTS affected platelet aggregation. The effect of transport on platelet aggregation could not be assessed in the present study, as our emergency laboratory was not equipped to test platelet function. However, the transport mode did not affect the platelet count, consistent with the findings of Suchsland et al. [18].
The frequency of hemolysis occurrence with PTS transmission has also been reported to differ among blood collection vessel types, plasma samples collected into lithium-heparin tubes conferring more susceptibility than serum samples collected into serum gel separation tubes, possibly due to the more violent shaking of liquid lithium-heparin samples during rapid transit [31,32]. In contrast, the tendency of samples to be hemolytic after transport did not differ significantly among blood collection vessel types in the present study, consistent with the findings of Yurt et al. [8]. In the present study, we utilized serum separator tubes to collect blood samples and evaluated the impact of different transportation methods on the results of clinical chemistry assays. Of note, certain laboratories may routinely opt to use plasma separator tubes for these types of analyses [33]. Existing evidence suggests that compared to serum samples, plasma samples in lithium–heparin gel separator tubes may exhibit spurious elevation of patient LDH levels after undergoing PTS transportation [34]. Corroborating their findings, we employed gel separator tubes for lactate dehydrogenase measurement in this study and detected no significant difference in lactate dehydrogenase levels among samples transported via PTSs and manually. Intriguingly, Gils et al. [6] demonstrated that in lithium-heparin whole blood samples from 20 healthy volunteers, pneumatic tube transportation did not significantly affect the assays for potassium, LDH, and hemolytic index, while platelet function and activation markers remained unaltered in sodium citrate samples compared to manual transport. Moreover, reports indicate that for certain analytes, serum samples might be better suited for PTS transportation [31,32,35]. It is apparent that the use of different types of collection tubes during sample transport via pneumatic tube systems can lead to significant differences in the effects on specific laboratory indicators. Thus, for laboratories that employ specific collection tubes for the measurement of particular indicators, it is crucial to perform thorough comparative studies to confirm that the use of pneumatic tube systems does not compromise the quality of the samples, thereby ensuring the precision and dependability of the laboratory results.
In this study, we sought to exclude controllable factors to the greatest extent possible to better evaluate transport performance and effects. Our professional laboratory staff operated the PTS transmitters and receivers, and the experimenter supervised all other steps occurring outside of the laboratory. However, practical clinical applications are inevitably affected by unexpected factors that delay sample transport, such as PTS malfunction. Users should be trained to resolve common PTS faults to minimize delays in sample delivery.
In this study, the TAT was much shorter with the PTSs than with manual transport, and more than 60 % shorter with the Tempus600 PTS than with the traditional PTS. Relative to that required with manual transport, the extra-laboratory TAT with the Tempus600 PTS is almost negligible, due mainly to the lack of requirement for sample packing; samples are simply placed into the starting end of the pipeline for launch and can be transported point to point in a single channel, preventing congestion due to their transport during peak hours [18,30]. The study showed that the appropriate reduction of the transport speed with PTSs effectively avoided the alteration of platelet aggregation results obtained with a highly sensitive method. Thus, the PTS transport speed could be adjusted to meet the requirements of specific sample types, thereby improving the quality of laboratory service [36]. The transport efficiency of innovative PTSs is extremely high, and these systems can reach transport speeds that other PTSs cannot. As a result, innovative PTSs are much more expensive than conventional PTSs. We recommend their selective installation in acute and intensive care units, where rapid sample detection is highly desired. In critical cases, clinicians can quickly assess patients’ key indicators to help determine treatment directions and plans and save lives in a timely manner. However, the performance of innovative technologies, and specifically their effects on samples, must be validated before these technologies are applied in clinical laboratories. Andersen et al. [17] found that the Tempus600/GLP robot system transportation was suitable for the transport the majority of routinely used analytical tests, excepting oxygen-related parameters. In this study, we limited our analysis to laboratory-established assays, as oxygen-related testing was not available in our emergency laboratory. We evaluated the impact of the Tempus600 PTS on sample quality and test results, and utilized samples from healthy volunteers instead of patients. Future research should address these limitations to fully assess the impact of different transport methods on sample stability. Currently, the application of innovative PTSs is not widespread, and experimental data on their performance are lacking. The results of the present study provide a basis for further research.
As this study was conducted with healthy volunteers, the transport of patient samples was not fully reflected. Gils et al. [6]conducted a comparative analysis using historical laboratory test results of patients and found that there were significant differences in the test results of potassium and LDH before and after the implementation of the Tempus600 PTS system. But the patient samples were not paired in that study. Herman et al. [34]observed the spurious elevation of lactate dehydrogenase levels in plasma samples from outpatients transported via PTS, an effect that was mitigated by the use of gel separator tubes for lactate dehydrogenase analysis. Therefore, it is crucial that each laboratory meticulously validates its own system, carefully considering factors such as the types of tubes employed, the assay specifications, and the patient populations they serve, in order to ensure the preservation of sample quality.
For the first time, we report on differences in the simultaneous transport of samples with a traditional PTS, an innovative PTS (Tempus600 PTS), and manually. The extra-laboratory TAT was much shorter with the Tempus600 PTS than with the other transport modes, with no compromise of sample quality. This rapid sample transfer method can improve emergency laboratories’ detection efficiency and clinical service quality, in turn improving clinician and patient satisfaction. With the development of hospital logistics technologies, sample transport outside of emergency laboratories and detection efficiency will continue to improve. At the same time, parameter repeatability and device safety and reliability should be evaluated for each emerging technology related to more efficient extra-laboratory sample delivery to avoid inaccuracy and improve efficiency and laboratory services for clinicians and patients.
Limitations
The present study has certain limitations that should be acknowledged. First, the use of samples from healthy individuals precludes the direct extrapolation of the findings to clinical patient populations. Physiological and pathological factors inherent to patient samples may influence the observed impacts of transportation methods on sample integrity and analytical measurements. Second, although the relatively small sample size can be used for the preliminary assessment, it constrains the overall statistical power and generalizability of the conclusions. However, the results provide valuable preliminary insights that warrant further investigation with a larger, more diverse sample size to confirm and expand upon the current findings.
Compliance with ethical standards
This study was approved by the ethics committee of the institutional review board of Fuwai Hospital (Approval No. 2021-1473), and all participants provided written informed consent.
Funding statement
No funding.
Data availability statement
The datasets used and analyzed during the current study are not publicly available due to the authors do not have permission to share data, but are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Jinxing Yu: Writing – original draft, Methodology, Data curation. Guoyan Zhu: Writing – original draft, Methodology, Data curation. Kai Cui: Methodology, Data curation. Dongze Yu: Methodology, Data curation. Dabuxilite Bayartaikishigtai: Methodology, Data curation. Zixin Chen: Methodology, Data curation. Zhou Zhou: Writing – review & editing, Writing – original draft, Resources, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31511.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Tanasijevic M.J., Melanson S.E.F., Tolan N.V., Ransohoff J.R., Conrad M.J., Paik H.-I., et al. Significant operational improvements with implementation of next generation laboratory automation. Lab. Med. 2021;52:329–337. doi: 10.1093/labmed/lmaa108. [DOI] [PubMed] [Google Scholar]
- 2.Park J.Y., Kricka L.J. One hundred years of clinical laboratory automation: 1967-2067. Clin. Biochem. 2017;50:639–644. doi: 10.1016/j.clinbiochem.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Howanitz P.J., Steindel S.J., Cembrowski G.S., Long T.A. Emergency department stat test turnaround times. A College of American Pathologists' Q-Probes study for potassium and hemoglobin. Arch. Pathol. Lab Med. 1992;116:122–128. [PubMed] [Google Scholar]
- 4.Fernandes C.M.B., Worster A., Eva K., Hill S., McCallum C. Pneumatic tube delivery system for blood samples reduces turnaround times without affecting sample quality. J. Emerg. Nurs. 2006;32:139–143. doi: 10.1016/j.jen.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Lippi G., Chance J.J., Church S., Dazzi P., Fontana R., Giavarina D., et al. Preanalytical quality improvement: from dream to reality. Clin. Chem. Lab. Med. 2011;49:1113–1126. doi: 10.1515/CCLM.2011.600. [DOI] [PubMed] [Google Scholar]
- 6.Gils C., Broell F., Vinholt P.J., Nielsen C., Nybo M. Use of clinical data and acceleration profiles to validate pneumatic transportation systems. Clin. Chem. Lab. Med. 2020;58:560–568. doi: 10.1515/cclm-2019-0881. [DOI] [PubMed] [Google Scholar]
- 7.Pupek A., Matthewson B., Whitman E., Fullarton R., Chen Y. Comparison of pneumatic tube system with manual transport for routine chemistry, hematology, coagulation and blood gas tests. Clin. Chem. Lab. Med. 2017;55:1537–1544. doi: 10.1515/cclm-2016-1157. [DOI] [PubMed] [Google Scholar]
- 8.Yurt E.F., Akbiyik F., Bicer C. Investigation of the effects of pneumatic tube transport system on routine biochemistry, hematology, and coagulation tests in Ankara City Hospital. Clin. Chem. Lab. Med. 2022;60:707–713. doi: 10.1515/cclm-2021-1235. [DOI] [PubMed] [Google Scholar]
- 9.Le Quellec S., Paris M., Nougier C., Sobas F., Rugeri L., Girard S., et al. Pre-analytical effects of pneumatic tube system transport on routine haematology and coagulation tests, global coagulation assays and platelet function assays. Thromb. Res. 2017;153:7–13. doi: 10.1016/j.thromres.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Subbarayan D., Choccalingam C., Lakshmi C.K.A. The effects of sample transport by pneumatic tube system on routine hematology and coagulation tests. Adv Hematol. 2018;2018 doi: 10.1155/2018/6940152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kok J.H.J., Lam C.W.L., Lee M.Y.J. Reducing delivery times of emergency blood products through pneumatic tube systems. Asian J. Transfus. Sci. 2019;13:3–9. doi: 10.4103/ajts.AJTS_52_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guss D.A., Chan T.C., Killeen J.P. The impact of a pneumatic tube and computerized physician order management on laboratory turnaround time. Ann. Emerg. Med. 2008;51:181–185. doi: 10.1016/j.annemergmed.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Al-Riyami A.Z., Al-Khabori M., Al-Hadhrami R.M., Al-Azwani I.S., Davis H.M., Al-Farsi K.S., et al. The pneumatic tube system does not affect complete blood count results; a validation study at a tertiary care hospital. Int J Lab Hematol. 2014;36:514–520. doi: 10.1111/ijlh.12180. [DOI] [PubMed] [Google Scholar]
- 14.Pragay D.A., Edwards L., Toppin M., Palmer R.R., Chilcote M.E. Evaluation of an improved pneumatic-tube system suitable for transportation of blood specimens. Clin. Chem. 1974;20:57–60. [PubMed] [Google Scholar]
- 15.Ellis G. An episode of increased hemolysis due to a defective pneumatic air tube delivery system. Clin. Biochem. 2009;42:1265–1269. doi: 10.1016/j.clinbiochem.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Strubi-Vuillaume I., Carlier V., Obeuf C., Vasseur F., Maury J.-C., Maboudou P., et al. Gentle blood aspiration and tube cushioning reduce pneumatic tube system interference in lactate dehydrogenase assays. Ann. Clin. Biochem. 2016;53:295–297. doi: 10.1177/0004563215586600. [DOI] [PubMed] [Google Scholar]
- 17.Andersen I.B., Mogensen N., Brandslund I. Stability of biochemical components in blood samples transported by Tempus600/Sysmex GLP robot reception system. J Appl Lab Med. 2017;1:376–386. doi: 10.1373/jalm.2016.021188. [DOI] [PubMed] [Google Scholar]
- 18.Suchsland J., Winter T., Greiser A., Streichert T., Otto B., Mayerle J., et al. Extending laboratory automation to the wards: effect of an innovative pneumatic tube system on diagnostic samples and transport time. Clin. Chem. Lab. Med. 2017;55:225–230. doi: 10.1515/cclm-2016-0380. [DOI] [PubMed] [Google Scholar]
- 19.Streichert T., Otto B., Schnabel C., Nordholt G., Haddad M., Maric M., et al. Determination of hemolysis thresholds by the use of data loggers in pneumatic tube systems. Clin. Chem. 2011;57:1390–1397. doi: 10.1373/clinchem.2011.167932. [DOI] [PubMed] [Google Scholar]
- 20.Mullins G.R., Harrison J.H., Bruns D.E. Smartphone monitoring of pneumatic tube system-induced sample hemolysis. Clin. Chim. Acta. 2016;462:1–5. doi: 10.1016/j.cca.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Kapoula G.V., Kontou P.I., Bagos P.G. The impact of pneumatic tube system on routine laboratory parameters: a systematic review and meta-analysis. Clin. Chem. Lab. Med. 2017;55:1834–1844. doi: 10.1515/cclm-2017-0008. [DOI] [PubMed] [Google Scholar]
- 22.Evliyaoğlu O., Toprak G., Tekin A., Başarali M.K., Kilinç C., Colpan L. Effect of pneumatic tube delivery system rate and distance on hemolysis of blood specimens. J. Clin. Lab. Anal. 2012;26:66–69. doi: 10.1002/jcla.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kara H., Bayir A., Ak A., Degirmenci S., Akinci M., Agacayak A., et al. Hemolysis associated with pneumatic tube system transport for blood samples. Pak J Med Sci. 2014;30:50–58. doi: 10.12669/pjms.301.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calmette L., Ibrahim F., Gouin I., Horellou M.-H., Mazoyer É., Fontenay M., et al. Impact of a pneumatic tube system transport on hemostasis parameters measurement: the experiment of Cochin universitary hospital (AP-HP, Paris, France) Ann. Biol. Clin. 2017;75:93–100. doi: 10.1684/abc.2016.1215. [DOI] [PubMed] [Google Scholar]
- 25.Kratz A., Salem R.O., Van Cott E.M. Effects of a pneumatic tube system on routine and novel hematology and coagulation parameters in healthy volunteers. Arch. Pathol. Lab Med. 2007;131:293–296. doi: 10.5858/2007-131-293-EOAPTS. [DOI] [PubMed] [Google Scholar]
- 26.Nissen P.H., Wulff D.E., Tørring N., Hvas A.-M. The impact of pneumatic tube transport on whole blood coagulation and platelet function assays. Platelets. 2018;29:421–424. doi: 10.1080/09537104.2018.1430361. [DOI] [PubMed] [Google Scholar]
- 27.Koçak F.E., Yöntem M., Yücel O., Cilo M., Genç O., Meral A. The effects of transport by pneumatic tube system on blood cell count, erythrocyte sedimentation and coagulation tests. Biochem. Med. 2013;23:206–210. doi: 10.11613/bm.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lippi G., Plebani M., Di Somma S., Cervellin G. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit. Rev. Clin. Lab Sci. 2011;48:143–153. doi: 10.3109/10408363.2011.600228. [DOI] [PubMed] [Google Scholar]
- 29.Qavi A.J., Franks C.E., Grajales-Reyes G., Anderson J., Ashby L., Zohner K., et al. Increased specimen minimum volume reduces turnaround time and hemolysis. Clin. Biochem. 2023;115:137–143. doi: 10.1016/j.clinbiochem.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen H., Frøstrup A.-B., Larsen A.S., Fenger M.S., Dahdouh S., Zoel-Ghina R., et al. Pneumatic tube transport of blood samples affects global hemostasis and platelet function assays. Int J Lab Hematol. 2021;43:1207–1215. doi: 10.1111/ijlh.13470. [DOI] [PubMed] [Google Scholar]
- 31.Sodi R., Darn S.M., Stott A. Pneumatic tube system induced haemolysis: assessing sample type susceptibility to haemolysis. Ann. Clin. Biochem. 2004;41:237–240. doi: 10.1258/000456304323019631. [DOI] [PubMed] [Google Scholar]
- 32.Böckel-Frohnhöfer N., Hübner U., Hummel B., Geisel J. Pneumatic tube-transported blood samples in lithium heparinate gel separator tubes may be more susceptible to haemolysis than blood samples in serum tubes. Scand. J. Clin. Lab. Invest. 2014;74:599–602. doi: 10.3109/00365513.2014.921931. [DOI] [PubMed] [Google Scholar]
- 33.Plebani M., Banfi G., Bernardini S., Bondanini F., Conti L., Dorizzi R., et al. Serum or plasma? An old question looking for new answers. Clin. Chem. Lab. Med. 2020;58:178–187. doi: 10.1515/cclm-2019-0719. [DOI] [PubMed] [Google Scholar]
- 34.Herman D.S., Toro E., Baraban E.G., Bagg A., Wang P. Falsely increased plasma lactate dehydrogenase without hemolysis following transport through pneumatic tube system. J Appl Lab Med. 2019;4:433–438. doi: 10.1373/jalm.2018.028928. [DOI] [PubMed] [Google Scholar]
- 35.Malaeb H., Vera M.A., Sangal R.B., Venkatesh A.K., Possick S., Maciejak L., et al. Rapid serum tubes reduce transport hemolysis and false positive rates for high-sensitivity troponin T. Clin. Chim. Acta. 2023;551 doi: 10.1016/j.cca.2023.117630. [DOI] [PubMed] [Google Scholar]
- 36.Slavík L., Úlehlová J., Bradáčová P., Chasáková K., Hluší A., Palová M., et al. The modern pneumatic tube system transports with reduced speed does not affect special coagulation tests. J. Med. Syst. 2020;44:142. doi: 10.1007/s10916-020-01614-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are not publicly available due to the authors do not have permission to share data, but are available from the corresponding author on reasonable request.