Abstract
Due to the discontinuation of routine smallpox vaccination after its eradication in 1980, a large part of the human population remains naïve against smallpox and other members of the orthopoxvirus genus. As a part of biosafety personnel protection programs, laboratory workers receive prophylactic vaccinations against diverse infectious agents, including smallpox. Here, we studied the levels of cross-protecting neutralizing antibodies as well as total IgG induced by either first- or third-generation smallpox vaccines against Monkeypox virus, using a clinical isolate from the 2022 outbreak. Serum neutralization tests indicated better overall neutralization capacity after vaccination with first-generation smallpox vaccines, compared to an attenuated third-generation vaccine. Results obtained from total IgG ELISA, however, did not show higher induction of orthopoxvirus-specific IgGs in first-generation vaccine recipients. Taken together, our results indicate a lower level of cross-protecting neutralizing antibodies against Monkeypox virus in recipients of third-generation smallpox vaccine compared to first-generation vaccine recipients, although total IgG levels were comparable.
Keywords: Vaccines, Vaccinia virus, Monkeypox virus, Imvamune/Imvanex/Jynneos, Neutralizing antibodies, ELISA, serum neutralization test, Occupational biosafety
1. Introduction
In 1980, the World Health Organization (WHO) officially declared smallpox, a devastating viral disease caused by the variola virus (VARV, genus Orthopoxvirus, family Poxviridae), eradicated. The eradication of smallpox is considered the first and, as of yet, the only eradication of a human infectious disease achieved through a worldwide vaccination campaign. Before eradication, in the 20th century alone, an estimated 300 million people succumbed to the disease [[1], [2], [3]].
Smallpox vaccines used during the eradication campaign, termed first-generation vaccines, were produced through the propagation of the closely related Vaccinia virus (VACV) on the skin of calves or other animals. However, these vaccines led to rare but severe adverse events, such as post-vaccinal encephalitis, eczema vaccinatum, progressive vaccinia, and myopericarditis [4] and were frequently observed in immunocompromised individuals. To protect vulnerable populations through vaccination without risking severe vaccine-related health issues, novel vaccines were urgently needed. Second-generation vaccines were based on clones of the first-generation vaccine (Dryvax) propagated on Vero cells in vitro, whereas third-generation vaccines are based on the modified vaccinia virus Ankara (Bavarian Nordic, MVA-BN, also known as Imvamune/Imvanex/JYNNEOS™). These vaccines were developed with improved safety profiles compared to previous vaccines. The MVA-BN vaccine is a replication-restricted live VACV formulation passaged several hundred times in chicken embryo fibroblasts, resulting in increased virus attenuation and thereby leading to better tolerability [[5], [6], [7]].
In 2022, Monkeypox virus (MPXV), a close relative of VARV, emerged in several non-endemic countries in people without any travel history to endemic countries. MPXV is a double-stranded DNA orthopoxvirus, first identified in 1958 in a research facility housing a population of imported cynomolgus macaques in Copenhagen, Denmark [8]. The first human MPXV infection was identified in 1970 in the Democratic Republic of the Congo. MPXV has evolved into two different clades, both currently circulating in Africa: clade I, and clade IIa. Although the case-fatality rate (CFR) for general Mpox (as referred by the WHO [9]) is lower than observed for smallpox (30 %), clade I recorded a higher CFR (1–10 %) than clade IIa (1 %). Genome analysis comparing both clades revealed several deletions and fragmentations in clade II, leading to reduced virulence [[10], [11], [12]]. Due to a sufficient number of mutations in the MPXV causing the 2022 outbreak, it has been categorized as a new clade, IIb [13]. The observed CFR for MPXV clade IIb (January 2022 to July 2023) was 0.16 % in regions of America, Europe, Africa, Western Pacific, Eastern Mediterranean, and South-East Asia [14].
As evidenced by the MPXV outbreak in 2022, orthopoxviruses still pose a health threat to humans. During the early weeks of the 2022 MPXV outbreak, no vaccine was licensed for pre- or post-exposure prophylaxis against Mpox. However, the third-generation smallpox vaccines Imvamune/Imvanex/JYNNEOS™ obtained approval in Canada, the European Union, and the United States of America, respectively, for pre-exposure prophylaxis for people at risk, such as laboratory personnel, health care professionals or people exposed to infected patients [15].
Due to the nature of their work, laboratory workers are at increased risk of infection. The United States advisory committee on immunization practices (ACIP) has recommended the immunization of laboratory workers with vaccinia virus vaccine since 1980. Nevertheless, during 2004–2014 a total of 14 orthopoxvirus infections were reported in laboratory workers in the United States and out of these, 13 occurred in workers who had not been vaccinated according to ACIP recommendations [16]. Due to the cessation of mandatory vaccination, it is of utmost importance to administer the smallpox vaccine to those workers who risk occupational orthopoxvirus exposure, to prevent potential laboratory-acquired infections [17]. Therefore, besides strict adherence to biosafety procedures and correct usage of personal protective equipment, vaccinations of laboratory personnel should be considered essential measures to ensure personnel safety in the event of occupational exposure.
To assess neutralizing protection after vaccination, we evaluated the neutralization capacity of sera collected yearly from laboratory workers vaccinated against smallpox either with first- or third-generation smallpox vaccines against the emerging clade IIb MPXV strain from the 2022 outbreak and the Lancy vaccine strain of VACV. We also assessed the presence of orthopoxvirus-specific immunoglobulins type G (IgG) in serum samples of vaccinated people by ELISA. Results indicate low-level cross-protecting neutralizing antibodies of people immunized with third-generation vaccines against MPXV and VACV over several years. In contrast, recipients of first-generation smallpox vaccines developed a higher concentration of neutralizing antibodies (nAbs) against these viruses but not higher concentrations of VACV-specific IgGs compared to immunization with third-generation vaccine.
2. Materials and methods
2.1. Cohort
The tested cohort consisted of 132 serum samples from 29 persons, collected yearly, beginning in 2014, with a median age of 42 years (range 25–68 years). Laboratory workers must undergo annual health checks, during which the most important vaccinations are administered. In addition, a blood sample is taken during these checks to determine various parameters such as electrolytes (Sodium, Potassium), metabolites (creatinine, enhanced glomerular filtration rate), and enzymes (ASAT, ALAT, alkaline phosphatase, and G-glutamyltransferase). Furthermore, a complete blood count is generated to analyze different blood cells (leukocytes, erythrocytes, neutrophils, eosinophils, basophils, monocytes and lymphocytes). Serum samples from these individuals were stored in a biobank at the sampling hospital. These samples were made available upon request for the investigation into laboratory worker immunity against MPXV. The cohort of this study consists of current and previously employed laboratory workers, and therefore cannot be changed or standardized further. Table 1 displays an overview of the study cohort, with the geometric mean titer of reciprocal serum dilutions of VACV- and MPXV- nAbs of serum samples collected in different years, where >50 % virus neutralization was observed.
Table 1.
Study cohort of 29 laboratory workers immunized with first- or third-generation smallpox vaccines. Geometric mean titers (GMT) of reciprocal serum dilution of VACV- and MPXV nAb were calculated from two independent experiments with two replicates per dilution. Values displayed showed a viral neutralization >50 % compared to control. GMT values below 10 indicate that serum neutralization antibodies are below the limit of detection (1:10). Dash (−): no sample collected. Additional information on vaccination time, serum collections, and time between last vaccination and first serum collection are displayed in Supplementary Table S1.
| Participant | 1st or 3rd generation vaccine? | GMT VACV nAb Titer 2014 |
GMT VACV nAb titer 2015 |
GMT VACV nAb titer 2016 |
GMT VACV nAb titer 2017 |
GMT VACV nAb titer 2018 |
GMT VACV nAb titer 2019 |
GMT VACV nAb titer 2020 |
GMT VACV nAb titer 2021 |
GMT VACV nAb titer 2022 |
|---|---|---|---|---|---|---|---|---|---|---|
| GMT MPXV nAb titer 2014 | GMT MPXV nAb titer 2015 | GMT MPXV nAb titer 2016 | GMT MPXV nAb titer 2017 | GMT MPXV nAb titer 2018 | GMT MPXV nAb titer 2019 | GMT MPXV nAb titer 2020 | GMT MPXV nAb titer 2021 | GMT MPXV nAb titer 2022 | ||
| Participant 1 | First | – | 40 | 56.57 | 56.57 | – | 40 | – | 28.28 | – |
| – | 14.14 | 28.28 | 40 | – | 10 | – | 14.14 | – | ||
| Participant 2 | Third | – | 14.14 | 20 | 20 | 10 | 14.14 | 14.14 | 14.14 | 28.28 |
| – | 10 | 10 | 10 | 14.14 | 10 | 10 | 14.14 | 7.07 | ||
| Participant 3 | Third | – | – | – | – | – | – | – | – | 28.28 |
| – | – | – | – | – | – | – | – | 7.07 | ||
| Participant 4 | Third | – | – | 28.28 | 14.14 | 20 | 56.56 | 28.28 | 10 | 10 |
| – | – | 20 | 5 | 7.07 | 10 | 14.14 | 10 | 7.07 | ||
| Participant 5 | First | – | 40 | 56.56 | – | 80 | 56.56 | 28.28 | 20 | 56.56 |
| – | 20 | 80 | – | 20 | 20 | 40 | 28.28 | 28.28 | ||
| Participant 6 | First | – | – | – | 10 | 5 | – | – | – | – |
| – | – | – | 20 | 10 | – | – | – | – | ||
| Participant 7 | Third | – | – | – | – | – | 5 | 10 | 14.14 | 14.14 |
| – | – | – | – | – | 10 | 10 | 28.28 | 5 | ||
| Participant 8 | Third | – | – | – | – | – | – | – | – | 14.14 |
| – | – | – | – | – | – | – | – | 20 | ||
| Participant 9 | First | – | – | – | – | – | – | – | 28.28 | – |
| – | – | – | – | – | – | – | 20 | – | ||
| Participant 10 | Third | – | – | – | – | – | 14.14 | – | 14.14 | 28.28 |
| – | – | – | – | – | 5 | – | 10 | 14.14 | ||
| Participant 11 | Third | – | – | 80 | 10 | 14.14 | – | 28.28 | – | |
| – | – | 14.14 | 28.28 | 14.14 | – | 14.14 | – | – | ||
| Participant 12 | Third | – | – | 40 | 7.07 | 7.07 | 10 | 7.07 | – | 7.07 |
| – | – | 10 | 10 | 7.07 | 10 | 7.07 | – | 14.14 | ||
| Participant 13 | Third | – | – | 7.07 | 7.07 | 7.07 | 7.07 | 5 | 5 | 5 |
| – | – | 10 | 10 | 7.07 | 10 | 7.07 | 20 | 14.14 | ||
| Participant 14 | Third | 14.14 | – | – | – | – | – | – | – | – |
| 10 | – | – | – | – | – | – | – | – | ||
| Participant 15 | First | – | 20 | 80 | 56.57 | 20 | - | 14.14 | 14.14 | 80 |
| – | 28.28 | 56.57 | 56.57 | 28.28 | - | 28.28 | 28.28 | 80 | ||
| Participant 16 | Third | – | 7.07 | 7.07 | – | – | – | – | – | – |
| – | 14.14 | 14.14 | – | – | – | – | – | – | ||
| Participant 17 | Third | – | – | – | – | 10 | 7.07 | 20 | 14.14 | 7.07 |
| – | – | – | – | 7.07 | 5 | 7.07 | 7.07 | 10 | ||
| Participant 18 | First | – | 14.14 | 10 | 7.07 | 5 | 10 | 20 | – | – |
| – | 28.28 | 20 | 14.14 | 40 | 10 | 20 | – | – | ||
| Participant 19 | Third | – | – | – | – | – | – | 20 | 20 | 20 |
| – | – | – | – | – | – | 10 | 14.14 | 7.07 | ||
| Participant 20 | Third | – | – | 226.27 | 40 | 56.57 | 56.57 | 20 | 40 | 28.28 |
| – | – | 28.28 | 40 | 28.28 | 20 | 56.57 | 28.28 | 28.28 | ||
| Participant 21 | First | – | 56.57 | 28.28 | 56.57 | 40 | 160 | 160 | – | – |
| - | 40 | 28.28 | 40 | 28.28 | 56.57 | 80 | – | – | ||
| Participant 22 | First | – | 113.14 | 40 | 320 | 28.28 | 20 | – | – | – |
| – | 56.57 | 80 | 56.57 | 40 | 40 | – | – | – | ||
| Participant 23 | First | – | 56.57 | 160 | 56.57 | 56.57 | 28.28 | – | 40 | – |
| – | 40 | 40 | 28.28 | 40 | 20 | – | 28.28 | – | ||
| Participant 24 | Third | – | – | – | 5 | 5 | – | – | – | – |
| – | – | – | 10 | 40 | – | – | – | – | ||
| Participant 25 | Third | – | – | – | – | 7.07 | – | – | 7.07 | – |
| – | – | – | – | 5 | – | – | 10 | – | ||
| Participant 26 | Third | – | 5 | 5 | 14.14 | 7.07 | 7.07 | – | 14.14 | 10 |
| – | 7.07 | 14.14 | 10 | 20 | 14.14 | – | 20 | 14.14 | ||
| Participant 27 | Third | – | – | 10 | 5 | 10 | 10 | 10 | 20 | 20 |
| – | – | 10 | 14.14 | 7.07 | 10 | 10 | 14.14 | 10 | ||
| Participant 28 | First | – | – | 40 | 20 | 56.57 | 40 | 28.28 | 40 | 40 |
| – | – | 20 | 20 | 28.28 | 40 | 28.28 | 28.28 | 56.57 | ||
| Participant 29 | Third | – | – | – | – | – | – | 20 | 7.07 | 14.14 |
| – | – | – | – | – | – | 14.14 | 40 | 28.28 |
2.2. Sera
Human serum was collected during annual health checks of laboratory workers. Sera was obtained anonymously and stored at −20 °C until the start of experiments. Sera was thawed overnight at 4 °C and subsequently inactivated for 30 min at 56 °C in a water bath, centrifuged at 13 000 g for 10 min, and diluted 1:5 in minimum essential medium (MEM, Seraglob, M 3303) containing 2 % heat-inactivated fetal bovine serum (FBS, Biochrom GmbH, S0615). Sera were further serially two-fold diluted in 96-well plates (Techno Plastic Products (TPP), Trasadingen, Switzerland) in a final volume of 50 μl. In each plate, a serum-, cell- and virus-control were included. Serum samples used for the current study were not purposely collected for the current study on Mpox immunity. However, as part of health checks of laboratory workers, these serum samples were stored for general purposes and provided upon request to specifically study the effect on different smallpox vaccines against MPXV in laboratory workers. Therefore, we were limited to the samples stored from current and previous employees.
2.3. Vaccines
2.3.1. First-generation smallpox vaccinees
A total of 10 (52 serum samples) laboratory workers received a first-generation smallpox vaccine. Six out of these 10 workers received the vaccine before the official eradication of smallpox in 1980, whereas the remaining four study participants received an occupational vaccination at later timepoints. None of these laboratory workers received an occupational immunization with a second-generation smallpox vaccine. These vaccinations were based on live and replicative VACV, and were administered by the multiple-puncture technique. The skin of the vaccine recipient will have been pierced several times by a bifurcated needle containing a drop of 1 × 108 plaque-forming units per milliliter (PFU/ml) live virus. The vaccine is delivered through scarification [18,19]. Vaccines in this group were either the Lancy Vaxina (n = 4, 20 serum samples), the vaccine from Berna (n = 2, seven serum samples) or not determined (n.d., n = 4, 25 serum samples), due to differential documentation standards at the time of vaccination. A total of five out of 10 laboratory workers (50 %) that were first immunized with the first-generation smallpox vaccine received an additional dose of Imvanex/Imvamune. The participants immunized with a first-generation smallpox vaccine are listed in Table 2.
Table 2.
First-generation vaccine recipients. The table displays the type of vaccine received, and whether it was administered before the official eradication of smallpox, followed by the number of doses administered. If individuals were not immunized before official smallpox eradication, vaccination was obtained for occupational reason. Last column reports whether individuals received an additional dose of third-generation smallpox vaccine.
| Participant | Vaccine obtained | Year of first dose | Year of last dose | Number of first-generation vaccine doses | Vaccination rationale | Additional 3rd generation dose obtained? (year) |
|---|---|---|---|---|---|---|
| Participant 1 | Lancy Vaxina | 2007 | 2007 | 1 | Occupational | No |
| Participant 5 | n.d | 1970 | 2002 | 2 | Before official smallpox eradication | Yes (2011) |
| Participant 6 | Lancy Vaxina | 2007 | 2007 | 1 | Occupational | No |
| Participant 9 | Berna vaccine | 1956 | 1980 | 4 | Before official smallpox eradication | No |
| Participant 15 | n.d. | 1965 | 1965 | 1 | Before official smallpox eradication | Yes (2016) |
| Participant 18 | Berna vaccine | 1969 | 1969 | 1 | Before official smallpox eradication | Yes (2011) |
| Participant 21 | Lancy Vaxina | 2007 | 2007 | 1 | Occupational | No |
| Participant 22 | n.d. | 1976 | 2005 | 3 | Before official eradication smallpox | No |
| Participant 23 | n.d. | 1969 | 1969 | 1 | Before official eradication smallpox | Yes (2016) |
| Participant 28 | Lancy Vaxina | 2004 | 2004 | 1 | Occupational | Yes (2018) |
2.3.2. Third-generation smallpox vaccine: Imvamune/Imvanex
The third-generation smallpox vaccine Imvamune/Imvanex is based on an attenuated vaccinia virus (modified vaccinia virus Ankara-Bavarian Nordic, MVA-BN). Laboratory workers, without vaccination against smallpox in their childhood based on vaccination documentation (n = 19 individuals, 80 serum samples), have been immunized with the MVA-BN, in two doses with a four-week interval. Imvanex/Imvamune was not approved by the Swiss regulatory and supervisory authority for medicinal products and medical devices (Swissmedic) at the time of vaccination. Therefore, this vaccine was obtained off-label for personnel to be eligible for laboratory work as part of the personnel biosafety and protection program. Persons who received the vaccination gave their consent. Third-generation smallpox vaccines were administered at the institute of epidemiology, biostatistics and prevention at the University of Zurich, Switzerland.
2.4. Virus propagation
VACV was obtained as a vaccine ampule (lot number 17137, originally seeded as Vaccinia Lister Elstree) from Berna Biotech AG in 2004. The virus was inoculated at an MOI of 0.1 on Vero E6 cells (African green monkey kidney cells) obtained from the American Type Culture Association (ATCC CCL-81) in Minimum Essential Medium Earle, supplemented with 10 % FBS for three days at 37 °C, without CO2 buffering, and examined daily for cytopathic effect (CPE). After three days, CPE of 60–80 % was observed. Subsequently cell culture flasks underwent freeze/thaw cycles followed by centrifugation of the supernatant at 500g for 10 min at 4 °C. Finally, the supernatant was aliquoted and stored at −80 °C.
MPXV passage 1, isolated from a pustule of a patient, was obtained as a clinical isolate from the University Hospital in Geneva (Hôpitaux universitaires de Genève, HUG), Switzerland. MPXV was propagated at an MOI of 0.1 on Vero E6 cells (kindly provided by Prof. Dr. Volker Thiel, Institute of Virology and Immunology, University of Bern, Bern, Switzerland) for 3 days at 37 °C with 5 % CO2, in Minimum Essential Medium (MEM, Seraglob, M 3303) supplemented with 2 % heat-inactivated FBS (Biochrom GmbH, S0615), 2 mM l-Glutamine (Gibco, 25030-081), 0.1U penicillin and 0.1 mg/ml Streptomycin (Seraglob, A 2003), 1X non-essential amino acids (Seraglob, M 3104) and 0.86 g/l sodium bicarbonate (Seraglob, L 1601) and examined for CPE daily. Three days post infection, a CPE of 40 % was observed. The cell culture flasks underwent two freeze/thaw cycles followed by centrifugation for 10 min at 500g at 4 °C in order to remove any cell debris. Finally, the clarified supernatant was aliquoted and stored at −80 °C.
Confirmatory Mpox diagnosis was performed by real-time qPCR analysis. Patient sample was inactivated in AVL buffer (Qiagen, Hombrechtikon, Switzerland), followed by the DNA extraction using the EZ1 Virus Mini kit v2.0 (Qiagen, Hombrechtikon, Switzerland) according to the manufacturer's instructions. PCR was performed using the 1x TaqMan Fast Virus 1-step Master Mix (Applied Biosystems, Fisher Scientific AG, Reinach, Switzerland) according to the manufacturer's instruction, 0.4 μM of forward and reverse primers and 0.25 μM of probe.
The MPXV F3L and N3R genes were detected by the following MPXV-specific oligonucleotides (Microsynth, Balgach, Switzerland).
F3L: fwd: 5′-CTCATTGATTTTTCGCGGGATA-3′, rev: 5′-GACGATACTCCTCCTCGTTGGT-3′, probe: 5′-FAM-CATCAGAATCTGTAGGCCGT-MGB-3′
N3R: fwd: 5′-AACAACCGTCCTACAATTAAACAACA-3′, rev: 5′-CGCTATCGAACCATTTTTGTAGTCT-3′, probe: 5′-FAM-TATAACGGCGAAGAATATACT-MGB-3’.
Cycling parameters: 5 min at 50 °C, 20s at 95 °C, followed by 45 cycles of 3s at 95 °C and 30s at 60 °C. The real-time qPCR reactions were performed in a Light Cycler 480 instrument (Roche Diagnostics, Basel, Switzerland).
All work with infectious MPXV and VACV was performed in the biosafety-level 3 (BSL-3) containment facility at Spiez Laboratory, Spiez, Switzerland.
2.5. Serum neutralization test (SNT)
Vero E6 cells used for SNTs were cultured in MEM (Seraglob, M 3303) supplemented with 10 % heat-inactivated FBS (Biochrom GmbH, S0615), 2 mM l-Glutamine (Gibco, 25030-081), 0.1U penicillin and 0.1 mg/ml Streptomycin (Seraglob, A 2003), 1X non-essential amino acids (Seraglob, M 3104) and 0.86 g/l sodium bicarbonate (Seraglob, L 1601) at 37 °C and 5 % CO2. All neutralization tests were performed twice in duplicates and neutralization reported as the geometric mean titer of two replicates per experiment.
2.5.1. Vaccinia virus SNT
The Lancy strain of vaccinia virus (VACV, Berna Biotech AG) was diluted to 500 PFU/ml in MEM supplemented with 2 % heat-inactivated FBS and the previously listed supplements. After serum inactivation, serum dilutions were performed in separate U-bottom 96-well plates, followed by the addition of 50 μl of diluted virus. Subsequently, the serum-virus mixtures were incubated for 1 h at 37 °C with 5 % CO2, followed by transferring the mixture onto adherent Vero E6 cell monolayers. After 40-h incubation at 37 °C with 5 % CO2, the presence of nAbs was evaluated by counting plaques by eye after crystal violet staining. A reduction of plaque formation equal to or higher than 50 % relative to a non-serum containing virus control was defined as neutralizing. The range of plaques counted for the virus control, without sera, was between 20 and 25. As a negative control, serum of a laboratory worker without any history of smallpox vaccination was used. Sera that showed no neutralizing effect at the lowest dilution, were assigned the artificial value of 5 for graphical representation.
2.5.2. MPXV SNT
After serum inactivation, five-fold serum dilutions were performed in 96-well plates, followed by the addition of 50 μl of MPXV diluted to 1000 TCID50/ml, followed by an incubation for 1 h at 37 °C, with 5 % CO2. After 1 h, 100 μl of suspended Vero E6 cells (200.000 cells/ml) were added per well, and incubated for five days at 37 °C, with 5 % CO2. Five days post infection, the neutralization capacity was evaluated by crystal violet staining and plaques were counted by eye, a reduction of 50 % or more of plaque formation compared to a non-serum containing virus control was considered neutralizing. Serum of a non-immunized laboratory worker was used as a negative control. Sera that showed no neutralizing effect at the lowest dilution, were assigned the artificial value of 5, for graphical representation.
2.6. ELISA
To detect human anti-orthopoxvirus Immunoglobulin type G (IgG) in the sera of vaccinated workers, samples were analyzed using the commercially available human anti-Vaccinia virus IMV/Envelope Protein/H3L/p35 IgG ELISA kit (Alpha Diagnostics International, San Antonio, Texas 78244, USA). Human sera were prepared for analysis as described above. Anti-VACV antibodies were detected using an anti-human IgG conjugated to horse-radish peroxidase (HRP). The assay was performed according to the manufacturer's instructions and the readout performed with a GloMAX plate reader (Promega, Dübendorf, Switzerland) at 450 nm, and a reference value of 600 nm. OD450nm values were converted into units per ml (U/ml) from a standard curve.
2.7. Statistical analysis and graphical representation
Data was analyzed and visualized using GraphPad prism (GraphPad Software version 9.5.1, La Jolla, CA, USA).
3. Results
3.1. First-generation smallpox vaccines induce higher levels of VACV and MPXV neutralizing antibodies compared to third-generation smallpox vaccines
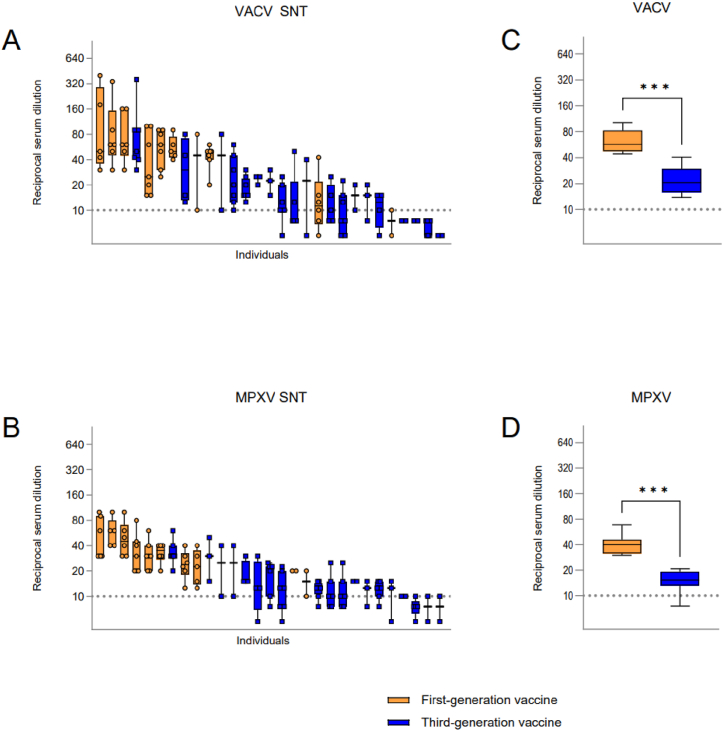
Serum samples used in the study were grouped into first- (n = 10 individuals, 52 serum samples) and third-generation smallpox vaccine (n = 19 individuals, 80 serum samples) recipients. None of the study participants has a known history of natural poxviridae infection. Laboratory workers immunized with a first-generation vaccine show higher nAb titers against VACV (Fig. 1A) and MPXV (Fig. 1B), than those immunized with the third-generation smallpox vaccine Imvamune/Imvanex, even though their immunization was several decades ago. Serum VACV nAb titers were significantly higher (***p = 0.0003) in the first-generation vaccine group, with a mean titer of 1:57.08, compared to 1:20.53 for the third-generation vaccine recipients (Fig. 1C). Similarly, first-generation vaccinees displayed significantly higher (***p = 0.0003) MPXV nAb titers with a mean of 1:40 compared to the third-generation vaccine group with an overall mean of 1:15.33 (Fig. 1D).
Fig. 1.
Serum VACV and MPXV nAb titers of immunized study participants. (A) VACV and (B) MPXV nAb titers illustrated by reciprocal serum dilution. Each individual is represented by a separate bar while each plotted value represents a different year of sampling. Serum nAbs of individuals were aligned from highest to lowest titer. Comparison of serum nAb titers of first- and third-generation vaccine recipients against (C) VACV and (D) MPXV. Statistical significance was evaluated through Mann-Whitney t-test (***p = 0.0003). Dashed lines: limit of detection, 1:10 dilution. Reciprocal serum dilution: virus neutralization >50 % compared to control.
3.2. Boosting laboratory workers immunized with a first-generation vaccine does not result in increased neutralizing antibody titers
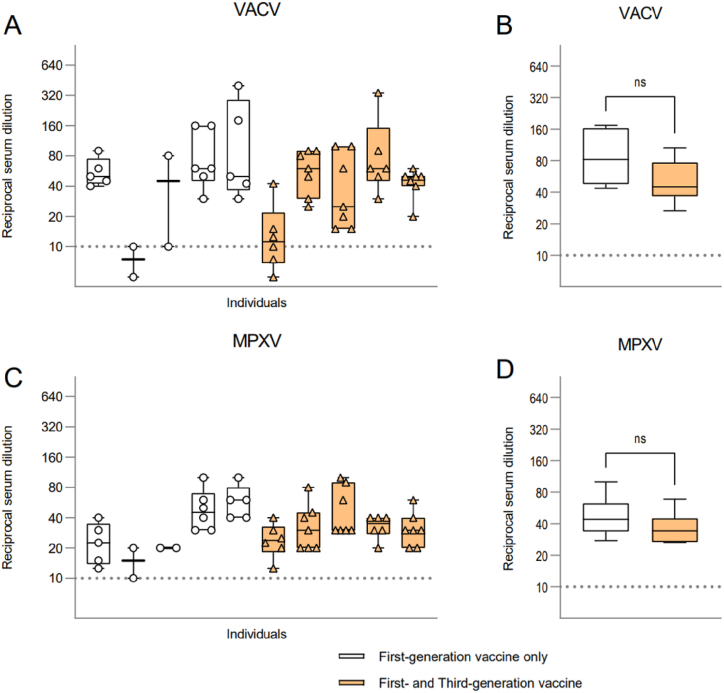
Five out of 10 (50 %) laboratory workers immunized with a first-generation vaccine received a booster dose of Imvamune/Imvanex and to determine whether the administration of this dose leads to an increase in VACV (Fig. 2A–B) and MPXV (Fig. 2C–D) nAb titers, we compared the serum of laboratory workers with and without a booster dose. Of note is, that first-generation vaccine recipients with a booster dose of Imvamune/Imvanex did not display higher nAbs compared to laboratory workers vaccinated only with the first-generation vaccine. The mean reciprocal VACV nAb titer for first-generation vaccine recipients is 1:81.96, while the mean reciprocal nAb titer for recipients of a booster dose was 1:45 (Fig. 2B).
Fig. 2.
Serum nAb titers of first-generation vaccine recipients with or without a booster dose of Imvamune/Imvanex. (A) Serum VACV nAb titers of laboratory workers vaccinated by first-generation vaccine with (orange bars) and without (white bars) a booster dose, illustrated by the reciprocal serum dilution. (B) Comparison of VACV nAb titers between individuals with and without a booster dose. (C) Serum MPXV nAb levels of laboratory workers with or without a booster dose. (D) Comparison of MPXV nAb titers of first-generation vaccine recipients and those who received a booster dose. Serum nAbs titers of individuals listed in panels (A) and (C) are in the same order, for comparison of VACV and MPXV nAb titers. Statistical significance was evaluated through Mann-Whitney t-test (ns, non-significant p = 0.1014, 0.2343, respectively) The dashed lines: limit of detection, 1:10 dilution. Reciprocal serum dilution: virus neutralization >50 % compared to control.
MPXV nAb titers between first-generation vaccine recipients and the boosted laboratory workers were even less pronounced. The mean MPXV nAb titers for the first-generation vaccine only was 1:43.99, and 1:34 for boosted laboratory workers (Fig. 2D).
3.3. All first-generation vaccine recipients displayed MPXV nAbs in 2022
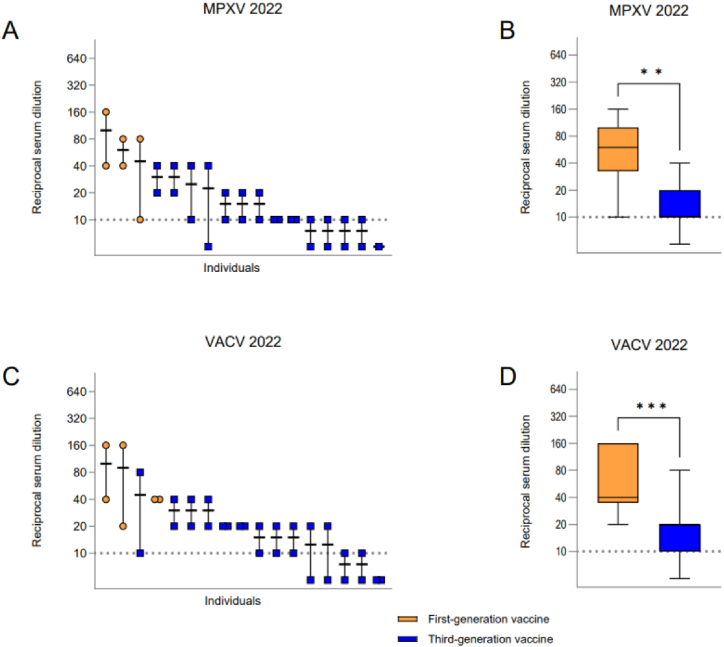
To determine whether laboratory workers had cross-protecting nAbs during the year of the worldwide Mpox outbreak in 2022, we analyzed the nAb concentration in the serum of active workers (n = 17) collected during their yearly medical check-up in 2022. While all first-generation vaccinees displayed nAb titers against MPXV, only 7 out of 14 (50 %) laboratory workers vaccinated with third-generation vaccines displayed nAb titers above the limit of detection (Fig. 3A). First-generation vaccine recipients displayed significantly higher (**p = 0.0019) MPXV nAb titers (mean 1:60) compared to the third-generation vaccine group (mean 1:10, Fig. 3B). All serum samples collected in 2022 from first-generation vaccine recipients also displayed VACV nAbs, while 11 out of 14 laboratory workers (78.57 %) of the third-generation vaccine group displayed nAb titers (Fig. 3C). Grouping serum samples according to the vaccines, we found that serum of first-generation vaccinees also had significantly higher (***p = 0.0007) VACV nAb titers (mean 1:40) compared to third-generation vaccinees (mean 1:20) (Fig. 3D).
Fig. 3.
Serum nAb titers of serum samples collected in 2022 from active laboratory workers. (A) MPXV nAb titers illustrated by the reciprocal serum dilution. Each individual is illustrated by a separate bar. Serum nAb titers of individuals are aligned from highest to lowest titer. (B) Comparison of serum MPXV nAb titers grouped in first- and third-generation vaccine recipients. (C) VACV nAb titers similarly illustrated. (D) Comparison of serum VACV nAb titers grouped in first- and third-generation vaccine recipients. Statistical significance was determined with a Mann-Whitney t-test (**p = 0.0019, ***p = 0.0007). Dashed lines: limit of detection, 1:10 dilution. Reciprocal serum dilution: virus neutralization >50 % compared to control.
3.4. First-generation smallpox vaccine administration does not result in higher orthopoxvirus-specific IgG production
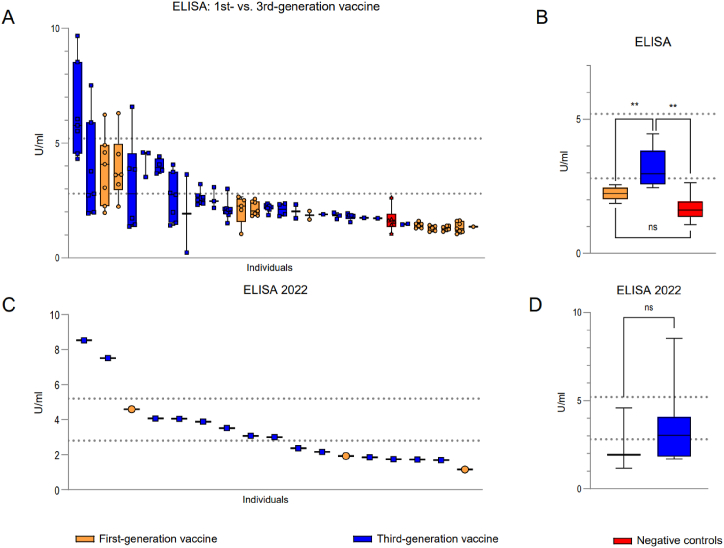
Anti-VACV IgG ELISA indicates that first-generation vaccines do not lead to higher induction of orthopoxvirus-specific IgGs in this cohort, compared to third-generation vaccine recipients. 12 out of 29 (41.38 %) study participants showed orthopoxvirus-specific IgGs within the range of the positive control (Fig. 4A). Third-generation vaccine recipients showed significantly higher IgG compared to first-generation vaccinees (**p = 0.0012). Similarly, third-generation vaccine recipients showed significantly higher IgG levels than non-immunized participants (**p = 0.0027). However, the first-generation vaccine recipients showed a trend towards higher IgG levels compared to negative controls (p = 0.0513, ns, Fig. 4B). When assessing the orthopoxvirus-specific IgGs in serum samples from study participants collected in 2022, only nine out of 17 serum samples (52.94 %) are in or above the range of the positive control supplied by the manufacturer (Fig. 4C). However, there was no statistical significance between the vaccine groups (p = 0.5912, ns, Fig. 4D).
Fig. 4.
Orthopoxvirus-specific IgGs in the serum of study participants immunized either by first- or third-generation vaccines. (A) Total orthopoxvirus-specific IgG of laboratory workers illustrated by units/ml derived from a standard curve. Serum samples included were collected from 2014 to 2021. (B) Comparison of serum orthopoxvirus-specific IgG of laboratory workers grouped according to vaccine administered. (C) Orthopoxvirus-specific IgG levels of serum samples from active laboratory workers collected in 2022. (D) Group comparison of orthopoxvirus-specific IgG levels of serum samples taken in 2022, during the active MPXV outbreak. Statistical significance was determined by Mann-Whitney t-test (**p = 0.0012,0.0027, respectively; p = 0.0513, p = 0.5912, non-significant, ns). Each bar shows an individual study participant, whereas each plotted value represents a different year of serum collection. IgG levels are aligned from highest to lowest concentration. Dashed lines: range of positive control, negative controls: sera from non-immunized laboratory workers.
4. Discussion
In the current study, we tested 132 serum samples collected yearly from 29 smallpox-vaccinated laboratory workers against the presence of VACV and MPXV cross-protecting neutralizing antibodies. We used a clinical MPXV isolate from the 2022 outbreak to test for the presence of cross-protecting nAbs in laboratory workers against this emerging strain. Our results indicate, that laboratory workers who received a first-generation smallpox vaccine, based on replicating VACV, displayed significantly higher MPXV-neutralizing capacities compared to those immunized with a replication-restricted third-generation vaccine (MVA-BN, Imvamune/Imvanex). A two-dose vaccine regimen of Imvamune/Imvanex yielded only relatively low levels of MPXV nAbs in this cohort.
Additionally, we assessed orthopoxvirus-specific IgGs in the serum of study participants. The first-generation vaccines did not induce higher concentrations of orthopoxvirus-specific IgGs, compared to third-generation smallpox vaccinees. Considering the discontinuation of routine vaccination against smallpox after its eradication in 1980, a large part of today's population remains naïve against orthopoxviruses. The proportion of non-vaccinated people in some developing countries exceeds 75 %, due to a young population [20]. Although a possible re-introduction of the variola virus or its use in bioterrorism seems unlikely, the 2022 outbreak of Mpox showed the potential impact of other orthopoxviruses on global human health. From January 1st, 2022 to September 30th, 2023, a total of 91 123 confirmed cases of human Mpox were recorded in 115 countries [21]. This represents the first worldwide outbreak of the virus, as previously mostly single cases had been observed outside of Africa. Due to potential sexual transmission and the rapid spread to multiple countries and continents in people without travel history to endemic countries, MPXV is considered an emerging condition [[22], [23], [24]].
Since Imvamune/Imvanex received approval for the prophylaxis against Mpox, it is of major interest to evaluate the efficiency of these vaccines for the prophylaxis of the disease.
A study conducted by Zaeck et al. [25] in the Netherlands demonstrated that two doses of Imvanex in non-primed health care workers resulted in the production of relatively low antibody responses. These health care workers received the Imvanex vaccines for safety purposes, due to their work in the BSL-3 laboratory. However, they detected a gradual increase in VACV-reactive IgG in individuals that received a priming and boosting dose of Imvanex. Additionally, they collected serum prior to vaccination (pre-immunization), two and four weeks after the first dose, and four weeks after the second dose. Furthermore, they showed that these recipients developed low-levels of MPXV neutralizing antibodies, which is in accordance with our findings. To investigate the effect of a booster vaccine, study participants from the Dutch group received a third dose of Imvanex one year after the second dose, which further boosted the production of VACV-reactive antibodies. So far, none of the laboratory workers in the current study received a booster vaccination one year after the two-dose Imvanex/Imvamune vaccine regimen.
A study performed in New York (N.Y., United States) investigated and compared the cellular response from vaccinees that obtained one and two doses of JYNNEOS (Imvamune/Imvanex) and convalescent patients of an authentic MPXV-infection. Antibodies elicited against orthopoxviruses (infection or vaccination) have shown a significantly better cross-neutralization capacity compared to antibodies targeting other viruses such as the human immunodeficiency virus (HIV) or influenza virus. Virus surface proteins of orthopoxviruses such as VACV, MPXV and Cowpox virus (CPXV) are abundant and show a high sequence homology (89–100 % similarity). Therefore, orthopoxvirus antibodies are able to target multiple viral surface proteins simultaneously, leading to the increased cross-neutralization [26].
Although the last case of natural smallpox infection was reported in 1977, the last fatal laboratory-associated infection occurred in 1978. This underlines the importance of biosafety measures for laboratory workers, such as appropriate handling of biohazardous material, accurate training, and immunization through various vaccinations. Also, there are several cases of laboratory-acquired VACV infections, of either vaccinated or non-vaccinated personnel. While the vaccinated persons usually developed mild disease and were released shortly upon administration to the hospital, unvaccinated persons developed a more serious disease. As of yet, there are no reports of laboratory-acquired MPXV infection [27]. Between 1985 and 2010, a total of 17 cases of laboratory-acquired VACV infections were recorded in the United States. Out of these cases, 52 % never received a VACV-based vaccine, and over 80 % never received an additional dose of vaccine during 10 years in research [28]. Furthermore, comparing Mpox in individuals receiving one-dose of Jynneos™ (≥14 days before symptoms) and unvaccinated patients, less hospitalizations occurred in the vaccinated (2 %) than unvaccinated (8 %) cohort. Furthermore, the odds of systemic signs of infection, such as fever and chills, were less frequently observed in vaccinated patients. Rash presentation in the genital and perianal areas were similar between vaccinated and non-vaccinated patients, thereby indicating a similar route of infection. However, a reduced number of rash locations was observed among vaccinated patients, indicating a possible prevention of spread through a single dose of third-generation vaccine [29]. Furthermore, according to prior reports, neutralization titers of ≥1:32 provides full protective immunity against smallpox in vaccinated people [30,31]. Our results therefore indicate, that people immunized with a first-generation smallpox vaccine likely maintained full protective immunity, while the third-generation vaccinees have a partial immunity obtained through the replication-restricted vaccine.
Vaccinations and serum collection are performed routinely during annual health check-ups in laboratory workers in the current study. However, given the unexpected emergence of Mpox in 2022, we do not have access to pre-immunization sera of laboratory workers as described in Zaeck et al., as some of the laboratory workers received the immunization prior to the first health check-up, including childhood vaccination. Furthermore, vaccination schedules differed between individual laboratory workers, based on their start of employment. As a consequence, we were unable to study (1) the effect of the two-dose vaccine regimen on the naïve immune system, and (2) the effect of the third-generation vaccine after the first dose and after the second dose. However, since some of the laboratory workers started their work several years ago, and therefore contributed several serum samples to the current study, we have been able to analyze the serum of the same persons over a time period of up to seven years. Although we were not able to compare the effect of the vaccine regimen to the naïve status, it is of great interest for a research institution to know the immune status of their workers during an active outbreak. Furthermore, since we were interested in the 2022 outbreak strain of MPXV, belonging to the clade IIb which spreads pre-dominantly through human-to-human transmission in contrast to the other two clades which are thought to be transmitted through contact with rodents [32], we did not test the sera against other MPXV isolates. Therefore, we are not able to predict the efficiency of these vaccines against isolates belonging to other clades, which may be associated with higher virulence. Since we only received serum samples stored in a biobank from current and previous employees, we were limited in experimental procedures. However, vaccine-induced neutralizing antibodies are considered a crucial factor in the protection against viral pathogens [33,34]. Furthermore, our results are in-line with previously published studies.
Taken together, our results indicate the induction of low-level nAbs in recipients of third-generation vaccines when tested against the orthopoxviruses VACV and MPXV. Also, we noted that first-generation vaccines administered several decades ago during the active smallpox eradication campaign, led to the induction of higher levels of nAbs against both VACV and MPXV. As Imvamune/Imvanex vaccination resulted in fewer side effects, and is also well tolerated in vulnerable population groups, its use for protection against Mpox is justified. Regular booster vaccinations might be necessary for maintaining protection, especially for people at increased risk of infection. For research and clinical laboratory workers, health care professionals, and designated response team members handling more virulent orthopoxviruses, such as variola virus or MPXV, it is recommended by the ACIP to administer booster vaccinations every two years after the initial two-dose immunization regimen with an additional dose of Imvamune/Imvanex/JYNNEOS. For those handling less virulent orthopoxviruses, such as various VACV strains, a booster vaccination is recommended every 10 years [35].
5. Conclusion
In conclusion, laboratory workers vaccinated with third-generation smallpox vaccines display low-level cross-protecting neutralizing antibodies against Mpox and despite the limitations of the cohort presented in this study we consider the implications and correlations to other published studies to be quite relevant to the field of vaccinology and occupational biosafety. Even with observed low baseline nAbs against MPXV in third-generation vaccinees, these vaccines are recommended for Mpox prophylaxis. Therefore, more regular booster vaccinations of laboratory workers according to the ACIP recommendations could potentially result in better occupational protection against orthopoxviruses.
Data availability statement
All data are available within the manuscript. The data are not publicly available in order to prevent the identification of individual participants. Upon reasonable request, additional data will be provided as far as the anonymity of the participants can be secured.
Ethical statement
The project was evaluated by the Swiss Association of Research Ethics Committees (swissethics) and required no specific approval, request number (BASEC Nr.) Req-2022-00882.
Funding
This research did not receive any specific funding.
CRediT authorship contribution statement
Damian Jandrasits: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation. Roland Züst: Writing – original draft, Methodology, Data curation. Denise Siegrist: Methodology. Olivier B. Engler: Writing – review & editing. Benjamin Weber: Writing – review & editing. Kristina M. Schmidt: Writing – review & editing, Conceptualization. Hulda R. Jonsdottir: Writing – original draft, Supervision, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Yelena Ruedin for supplying cells for experiments. We also thank Dr. Daniel Zysset, Beat Lörtscher and Stefan Breitenbaumer for their technical assistance. Finally, we thank Dr. med. Cornelia Staehelin and Dr. Franziska Suter-Riniker for the management of the cohort and collection of samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31490.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Strassburg M.A. The global eradication of smallpox. Am. J. Infect. Control. 1982;10(2):53–59. doi: 10.1016/0196-6553(82)90003-7. [DOI] [PubMed] [Google Scholar]
- 2.Shchelkunova G.A., Shchelkunov S.N. 40 Years without smallpox. Acta naturae. 2017;9(4):4–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson D.A. The eradication of smallpox-an overview of the past, present, and future. Vaccine. 2011;29(Suppl 4):D7–D9. doi: 10.1016/j.vaccine.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy R.B., Ovsyannikova I., Poland G.A. Smallpox vaccines for biodefense. Vaccine. 2009;27(Suppl 4):D73–D79. doi: 10.1016/j.vaccine.2009.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwar F., Haider F., Khan S., Ahmad I., Ahmed N., Imran M., Rashid S., Ren Z.G., Khattak S., Ji X.Y. Clinical Manifestation, transmission, pathogenesis, and diagnosis of monkeypox virus: a comprehensive review. Life. 2023;13(2):522. doi: 10.3390/life13020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey S.E. New smallpox vaccines for an ancient scourge. Mo. Med. 2014;111(4):332–336. [PMC free article] [PubMed] [Google Scholar]
- 7.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., Lin S., Peters E., Rhodes L., Jr., Spano Y.E., Silvera P.M., Moss B. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. U.S.A. 2008;105(31):10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8(2):129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aden D., Zaheer S., Kumar R., Ranga S. Monkeypox (Mpox) outbreak during COVID-19 pandemic-Past and the future. J. Med. Virol. 2023;95(4) doi: 10.1002/jmv.28701. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y., Mu L., Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct. Targeted Ther. 2022;7(1):373. doi: 10.1038/s41392-022-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend M.B., Keckler M.S., Patel N., Davies D.H., Felgner P., Damon I.K., Karem K.L. Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations. J. Virol. 2013;87(2):900–911. doi: 10.1128/JVI.02089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaler J., Hussain A., Flores G., Kheiri S., Desrosiers D. Monkeypox: a comprehensive review of transmission, path-ogenesis, and manifestation. Cureus. 2022;14(7) doi: 10.7759/cureus.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz D.A., Pittman P.R. Mpox (monkeypox) in pregnancy: viral clade differences and their associations with varying obstetrical and fetal outcomes. Viruses. 2023;15(8):1649. doi: 10.3390/v15081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aminul Islam Md, Mumin Jubayer, Haque Md Masudul, Azizul Haque Md, Khan Ahrar, Bhattacharya Prosun, Haque Md Atiqul. Monkeypox virus (MPXV): a Brief account of global spread, epidemiology, virology, clinical features, pathogenesis, and therapeutic interventions. Infectious Medicine. 2023 doi: 10.1016/j.imj.2023.11.001. ISSN 2772-431X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keckler M.S., Salzer J.S., Patel N., Townsend M.B., Nakazawa Y.J., Doty J.B., Gallardo-Romero N.F., Satheshkumar P.S., Carroll D.S., Karem K.L., Damon I.K. IMVAMUNE® and ACAM2000® provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines. 2020;8(3):396. doi: 10.3390/vaccines8030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen B.W., Harms T.J., Reynolds M.G., Harrison L.H. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses - recommendations of the advisory committee on immunization practices (ACIP), 2015. MMWR. Morbidity and mortality weekly report. 2016;65(10):257–262. doi: 10.15585/mmwr.mm6510a2. [DOI] [PubMed] [Google Scholar]
- 17.Lewis F.M., Chernak E., Goldman E., Li Y., Karem K., Damon I.K., Henkel R., Newbern E.C., Ross P., Johnson C.C. Ocular vaccinia infection in laboratory worker, Philadelphia, 2004. Emerg. Infect. Dis. 2006;12(1):134–137. doi: 10.3201/eid1201.051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty C., Bhattacharya M., Ranjan Sharma A., Dhama K. Monkeypox virus vaccine evolution and global preparedness for vaccination. Int. Immunopharm. 2022;113(Pt A) doi: 10.1016/j.intimp.2022.109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalca A., Zumbrun E.E. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010;4:71–79. doi: 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson K., Heymann D., Brown C.S., Edmunds W.J., Elsgaard J., Fine P., Hochrein H., Hoff N.A., Green A., Ihekweazu C., Jones T.C., Lule S., Maclennan J., McCollum A., Mühlemann B., Nightingale E., Ogoina D., Ogunleye A., Petersen B., Powell J.…Wapling A. Human monkeypox - after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) Multi-country outbreak of mpox (monkeypox) External Situation Report. October 2023;29 https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox-external-situation-report-29-20-october-2023 [Google Scholar]
- 22.Kennedy J.S., Greenberg R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expet Rev. Vaccine. 2009;8(1):13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim E.Y., Whitehorn J., Rivett L. Monkeypox: a review of the 2022 outbreak. Br. Med. Bull. 2023;145(1):17–29. doi: 10.1093/bmb/ldad002. [DOI] [PubMed] [Google Scholar]
- 24.Meyer H., Ehmann R., Smith G.L. Smallpox in the post-eradication era. Viruses. 2020;12(2):138. doi: 10.3390/v12020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaeck L.M., Lamers M.M., Verstrepen B.E., Bestebroer T.M., van Royen M.E., Götz H., Shamier M.C., van Leeuwen L.P.M., Schmitz K.S., Alblas K., van Efferen S., Bogers S., Scherbeijn S., Rimmelzwaan G.F., van Gorp E.C.M., Koopmans M.P.G., Haagmans B.L., GeurtsvanKessel C.H., de Vries R.D. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023;29(1):270–278. doi: 10.1038/s41591-022-02090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohn H., Bloom N., Cai G.Y., Clark J.J., Tarke A., Bermúdez-González M.C., Altman D.R., Lugo L.A., Lobo F.P., Marquez S., Pvi study group, Chen J.Q., Ren W., Qin L., Yates J.L., Hunt D.T., Lee W.T., Crotty S., Krammer F., Grifoni A.…Coelho C.H. Mpox vaccine and infection-driven human immune signatures: an immunological analysis of an observational study. Lancet Infect. Dis. 2023;S1473–3099(23) doi: 10.1016/S1473-3099(23)00352-3. 00352-3. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for disease Control and Prevention (CDC). Laboratory-acquired Vaccinia Exposures and Infections – United States 2005-2007 (https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5715a3.htm). [PubMed]
- 28.Wei Q., Jiang M.N., Han J., Wang Z.J. Immune control strategies for vaccinia virus-related laboratory-acquired infections. Biomed. Environ. Sci. : BES (Biomed. Environ. Sci.) 2014;27(2):142–146. doi: 10.3967/bes2014.031. [DOI] [PubMed] [Google Scholar]
- 29.Farrar J.L., Lewis N.M., Houck K., Canning M., Fothergill A., Payne A.B., Cohen A.L., Vance J., Brassil B., Youngkin E., Glenn B., Mangla A., Kupferman N., Saunders K., Meza C., Nims D., Soliva S., Blouse B., Henderson T., Banerjee E.…Mpox Cases in Vaccinated Persons Team Demographic and clinical characteristics of mpox in persons who had previously received 1 dose of JYNNEOS vaccine and in unvaccinated persons - 29 U.S. Jurisdictions, may 22-september 3, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022;71(5152):1610–1615. doi: 10.15585/mmwr.mm715152a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., Wong S.W., Yoshihara P., Hanifin J.M., Slifka M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005;11(9):1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 31.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 32.Americo J.L., Earl P.L., Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023 Feb 21;120(8) doi: 10.1073/pnas.2220415120. Epub 2023 Feb 14. PMID: 36787354; PMCID: PMC9974501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan L.N., Liu P.P., Li X.G., Zhou S.J., Li H., Wang Z.Y., Shen F., Lu B.C., Long Y., Xiao X., Wang Z.D., Li D., Han H.J., Yu H., Zhou S.H., Lv W.L., Yu X.J. Neutralizing antibodies and cellular immune responses against SARS-CoV-2 sustained one and a half years after natural infection. Front. Microbiol. 2022;12 doi: 10.3389/fmicb.2021.803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., Reimann K.A., Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 35.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., Campos-Outcalt D., Morgan R.L., Damon I., Sánchez P.J., Bell B.P. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR. Morbidity and mortality weekly report. 2022;71(22):734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within the manuscript. The data are not publicly available in order to prevent the identification of individual participants. Upon reasonable request, additional data will be provided as far as the anonymity of the participants can be secured.