One of the main factors determining oxygen delivery to cells is the oxygen content of the blood. Blood gas tensions are measured by direct blood sampling or transcutaneous diffusion and oxygen saturation of haemoglobin from pulse oximetry. Arterial blood gas analysis is widely available in hospitals and the direct measurements (pH, Pao2, Paco2) are among the most precise in medicine. The value of such measurements, however, depends on the ability of doctors to interpret the results properly.
Arterial puncture
Arterial puncture may result in spasm, intraluminal clotting, or bleeding and haematoma formation, as well as a transient obstruction of blood flow. These factors may decrease the arterial flow in distal tissues unless adequate collateral arterial vessels are available. The brachial and femoral arteries do not have adequate collateral supplies. The radial artery at the wrist is the best site for obtaining an arterial sample because it is near the surface, relatively easy to palpate and stabilise, and usually has good collateral supply from the ulnar arteries. This can be confirmed by a modified Allen’s test.
Problems of taking arterial blood samples
Bleeding
Vessel obstruction
Infection
It is kinder to patients to use local anaesthesia over the radial artery before puncture. Analgesic patches can be used for children. Use a 20 or 21 gauge needle with a preheparinised syringe. Express the heparin from the syringe before taking the sample; adequate heparin will remain in the 0.2 ml dead space of the barrel and needle. At least 3 ml of blood is required to avoid a dilution effect from the heparin.
Any sample with more than fine air bubbles should be discarded. Air bubbles result in gas equilibration between the air and the arterial blood, lowering the arterial carbon dioxide pressure (Paco2) and increasing the arterial oxygen pressure (Pao2). The cellular constituents of blood remain metabolically active so arterial gas tensions in the sample will change over time. If the sample cannot be analysed quickly it should be cooled to 5°C immediately. The sample can then be stored for up to one hour with little clinically significant effect on the result.
Arterialised ear lobe samples provide an alternative to arterial puncture for occasional samples. A capillary tube is used to sample blood from a warmed, vasodilated earlobe. The Paco2 values agree well with those obtained from arterial samples, but accuracy of the Pao2 depends on good technique in arterialisation of the ear lobe.
Analysers
Modern blood gas analysers are automated self diagnostic instruments requiring minimal maintenance. The pH electrode measures the potential difference between a known solution within the measuring electrode and the unknown (sample) solution at 37°C.
Normal values of arterial pH and Paco2
| Mean | 1 SD | 2 SD | Acceptable | |
| pH | 7.4 | 7.38-7.42 | 7.35-7.45 | 7.3-7.5 |
| Paco2 (kPa) | 5.3 | 5.0-5.6 | 4.7-6.0 | 4-6.7 |
The Paco2 electrode measures carbon dioxide tensions by allowing carbon dioxide to undergo a chemical reaction producing hydrogen ions. The hydrogen ion production causes a difference in potential that is measured by half cells similar to those of the pH electrode. The Pao2 electrode is a polarographic device that measures oxygen tensions by oxidation-reduction reactions, a chemical process that generates measurable electric currents.
Calibration is important since the electrodes drift over time; a 1 point calibration adjustment to a single standard is made before each analysis or every 30 minutes. A 2 point calibration using two standards should be done every 2-8 hours.
Adult values for Pao2 and oxygen saturation
| Pao2 (kPa) | Sao2 (%) | |
| Normal (range) | 13 (⩾10.7) | 97 (95-100) |
| Hypoxaemia | <10.7 | <95 |
| Mild hypoxaemia | 8-10.5 | 90-94 |
| Moderate hypoxaemia | 5.3-7.9 | 75-89 |
| Severe hypoxaemia | <5.3 | <75 |
Pulse oximetry
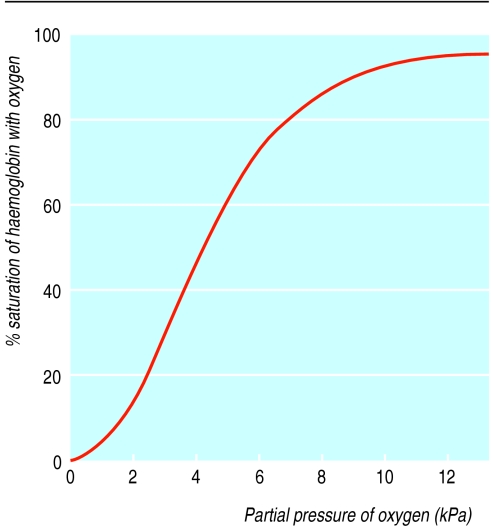
The development of simpler oximeters to measure oxygen saturation has been important in improving monitoring of oxygen therapy. The sigmoid saturation curve for haemoglobin defines the relation between saturation and Pao2. Oximeters work on the principle that desaturated haemoglobin and oxygenated haemoglobin absorb light of different wavelengths. The current devices use two wavelengths and measure the absorption in the pulsatile element of the blood flow, thus producing a measure of the oxygen saturation of arterial blood separate from the non-pulsatile venous blood.
Problems with readings of pulse oximeters
| Clinical situation | Result |
| Carboxyhaemoglobin | Falsely high saturation |
| Bilirubin | Falsely low saturation |
| Melanotic skin | Variable, reduced signal |
| Poor peripheral perfusion | Low signal, unreliable results |
The probe is applied to the finger or earlobe. The delay in registration of central changes in saturation depends on the circulation time from the lungs to the monitoring site. It is slightly greater for the finger (about 30 seconds). Oximeters tend to be less accurate with saturations below 75%, but for most clinical situations changes above this range are more important. Some clinical situations can affect the accuracy of measurement.
Transcutaneous measurements
Transcutaneous electrodes with combined sensors can be used to measure both oxygen and carbon dioxide pressures. These electrodes rely on diffusion from vasodilated vessels in heated skin. In neonates with thin, well vascularised skin the method is reliable, but it is less useful in adults. The transcutaneous carbon dioxide pressure is slightly higher than the Paco2 while the transcutaneous oxygen pressure is often around 80% of the Pao2. The results are easier to interpret if initial pressures are compared with arterial gas pressures. A delay of around 5 minutes in the signal is related to diffusion across the skin barrier. This makes the method suitable for monitoring stable measurements or slow trends rather than instantaneous changes. The electrode needs to be moved every 4-8 hours because heating of the skin may cause local burns.
Approximate relation between Paco2 and pH
| Paco2 (mmHg kPa) | pH | Plasma bicarbonate (mmol/l) |
| 80 10.6 | 7.2 | 28 |
| 60 8 | 7.3 | 26 |
| 40 5.3 | 7.4 | 24 (normal value) |
| 30 4 | 7.5 | 22 |
| 20 2.7 | 7.6 | 20 |
Disturbances in acid-base balance
Respiratory acidosis (that is, ventilatory failure)—the drop in pH is explained by the change in Paco2
Respiratory alkalosis (that is, alveolar hyperventilation)—the decreased Paco2 explains the increased pH
Metabolic acidosis—reduced pH not explained by increased Paco2. It is usually associated with an increased anion gap due to the accumulation of renal acids, lactic acids, and ketoacids (from diabetes or starvation)
Metabolic alkalosis—raised pH out of proportion to changes in Paco2. It is associated with hypokalaemia, volume contraction, or exogenous alkali administration
Relation between pH and Paco2
Acute changes in Paco2 result in predictable changes in pH (the negative log of hydrogen ion concentration) and plasma carbonic acid. This represents the respiratory acid-base change. Although the relation is not completely linear, within clinically relevant ranges it is sufficiently linear to allow the following guideline to estimate the degrees of abnormality resulting from acute changes in Paco2 :
For every increase in Paco2 of 20 mm Hg (2.6 kPa) above normal the pH falls by 0.1
For every decrease of Paco2 of 10 mm Hg (1.3 kPa) below normal the pH rises by 0.1.
Any change in pH outside these parameters is therefore metabolic in origin.
Anion gap concept
Organisms exist in a state of electroneutrality with major and minor cations balanced by similar anions. The anion gap is an artificial disparity between the concentrations of the major plasma cations and anions routinely measured—normally sodium, chloride, and bicarbonate. The anion gap is calculated as Na+−(Cl−+HCO3−).
The anion gap is normally 8-16 mmol/l, of which 11 mmol/l is typically due to albumin. A decreased anion gap is usually caused by hypoalbuminaemia or severe haemodilution. Less commonly, it occurs as a result of an increase in minor cation concentrations such as in hypercalcaemia or hypermagnesaemia. Increased anion gap acidosis is caused by dehydration and by any process that increases the concentrations of the minor anions, lactate, ketones, and renal acids, along with treatment with drugs given as organic salts such as penicillin, salicylates, and with methanol and ethylene glycol. Rarely an increased anion gap may result from decreased minor cation concentrations such as calcium and magnesium.
Minor anions and cations
| Cations | Anions |
| Potassium | Phosphates |
| Calcium | Sulphates |
| Magnesium | Organic anions such as proteins |
Metabolic acidosis without an increased anion gap is typically associated with an increase in plasma chloride concentrations; chloride ions replace plasma bicarbonate. Hyperchloraemic acidosis is usually caused by gastrointestinal loss or renal wasting of bicarbonate.
Pathophysiology of acute respiratory failure
Alveolar ventilation and carbon dioxide
Ventilation can be defined in terms of movement of a volume of air in to and out of the lungs, removing carbon dioxide from the blood and providing oxygen. Tidal volume (Vt) is the sum of alveolar volume (Va) and dead space volume (Vd). These divisions are also appropriate to tidal ventilation, which includes the component that takes part in gas exchange (alveolar ventilation) and dead space ventilation.
Assessment of alveolar ventilation is the key to determining whether a patient is getting enough oxygen
Calculation of alveolar ventilation
In a steady state:
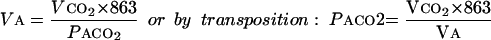 |
Where
Vco2=carbon dioxide production by the body (l/min) corrected to standard temperature and pressure dry
Va=alveolar volume per unit of time corrected to body temperature and pressure, saturated with water (BTPS)
863=conversion factor to express Va in l/min BTPS
Paco2=partial pressure of alveolar carbon dioxide (mm Hg, BTPS) which is essentially equal to arterial carbon dioxide pressure (Paco2)
Alveolar ventilation is best defined in terms of ventilation of carbon dioxide. Diffusion of carbon dioxide across the alveolocapillary membrane is more rapid than diffusion of oxygen, and the carbon dioxide dissociation curve of blood is linear over a wide range of alveolar ventilation.
Changes in alveolar ventilation (Va) are accompanied by corresponding linear changes in alveolar and thus arterial carbon dioxide pressure. Since alveolar ventilation is the important variable, measurement of tidal ventilation and respiratory rate can be misleading. A patient with hypercapnia may actually have a high tidal ventilation. For example, if Vt=0.5 l/breath; Vd=0.2 l, and respiratory rate =16 breaths/min the minute ventilation is 8 l/min (16×0.5) but alveolar ventilation is 4.8 l/min (16×(0.5−0.2)). Assuming a typical Vco2 of 0.2 l/in):
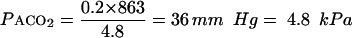 |
If Vt =0.3 l/breath, Vd=0.2 l, and respiratory rate=28 breaths/min the minute ventilation is 8.4 l/min (28×0.3) but alveolar ventilation is 2.8 l/min (28×(0.3−0.2))
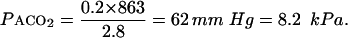 |
Thus, for a similar minute ventilation the patient is working less efficiently and the Paco2 is raised.
Oxygenation
Oxygenation is the transfer of adequate oxygen from the alveoli to the blood, where it exists mainly in the form of oxyhaemoglobin. I will consider only respiratory causes of inadequate oxygenation. However, haemoglobin may be desaturated by other causes—for example, carbon monoxide poisoning. An oxygen pressure above 8 kPa will be associated with saturation over 90% and adequate oxygen carrying capacity if the packed cell volume is normal.
Alveolar-arterial oxygen gradient
Respiratory failure is defined as type I when there is hypoxaemia without carbon dioxide retention and type II when there is hypercapnia. Calculation of the gradient between the alveolar and arterial oxygen tensions (the A-a gradient) in type II respiratory failure will help to determine whether the patient has associated lung disease or just reduced respiratory effort.
Calculation of the alveolar-arterial difference for oxygen
(A-a)Po2=Pao2−Pao2
Pao2=Pio2−Paco2 /R
Where R=respiratory quotient=volume of CO2 produced/ volume of O2 consumed
R=0.8 for a “normal” diet and approaches 1.0 as proportion of carbohydrates consumed increases
Pio2=(Pb−Ph2o) × Fio2
Pio2=partial pressure of inspired oxygen
Ph2o=water pressure
Pb= barometric pressure
Fio2=fractional concentration of oxygen in inspired gas = 0.21 breathing air
Assuming Pb=101 kPa at sea level
Ph2o= 6.2 kPa as inspired air is fully saturated by the time it reaches the carina
Assume Paco2=Paco2 because of the ease of exchange of carbon dioxide
Therefore:
Pao2=(Pb−Ph2o) × Fio2−Paco2/R
=(101−6.2) × Fio2−Paco2/0.8 (at sea level)
=94.8×Fio2−1.25× Paco2
Breathing air with a Paco2 of 8 kPa
Pao2= 94.8×0.21−(1.25×8)=10 kPa
A raised Paco2 reflects reduced alveolar ventilation. Broadly speaking, this may be produced by reduction in minute ventilation (central or pump failure), obstruction of airflow, or a mismatch with perfusion giving a relative increase in dead space ventilation and reduction in alveolar ventilation. Disorders of the lung structure reduce the efficiency of oxygen transfer and widen the A-a gradient.
The A-a gradient increases a little with age but should be less than 2.6 kPa so central respiratory depression should give a Pao2 over 7.3 kPa in this situation. A Pao2 below this signifies associated lung disease. Prolonged respiratory depression may lead to collapse of some areas of lung and an increase in the A-a gradient.
Clinical approach to blood gas interpretation
A structured approach to the interpretation of arterial blood gases helps ensure that nothing is missed. Two basic steps should be followed. Firstly, Paco2 and pH should be assessed. In essence this is the ventilatory state (respiratory acid-base balance) and will automatically lead to an assessment of the metabolic acid-base balance. Secondly, arterial oxygenation should be assessed. This includes evaluation of hypoxaemia as well as the arterial oxyhaemoglobin saturation along with the A-a gradient.
Systematic assessment of arterial blood gas measurements
Step 1a
Determine whether the Paco2 is low (<4.7 kPa) indicating alveolar hyperventilation, normal (4.7-6 kPa), or high (>6 kPa) as in ventilatory failure. Calculate the respiratory pH to determine if there is any metabolic compensation or additional disorder
Step 1b
In the presence of a metabolic acidosis, calculate the anion gap to determine whether it has increased. No increase occurs with diarrhoea or urinary loss of bicarbonate
Step 2a
Assess arterial oxygenation. Arterial hypoxaemia in adults is defined as Pao2<10.7 kPa breathing room air, although it is not usually treated as clinically important unless below 8 kPa, when oxygen saturation will be 90% or less
Step 2b
Calculate the A-a gradient to determine whether carbon dioxide retention is related to an intrapulmonary cause
Alveolar hypoventilation (raised Paco2) with a normal pH probably represents a primary ventilatory change present long enough for renal mechanisms to compensate—as in chronic ventilatory failure. A similar picture can result from carbon dioxide retention from reduced ventilation compensating for a metabolic alkalosis, although such compensation is usually only partial. In acute respiratory failure the change in pH will be accounted for by the high carbon dioxide concentration.
Alternatively if the pH is appropriately raised for the reduction in Paco2 then acute alveolar hyperventilation is present. The renal system seldom compensates completely for an alkalosis, the pH is between 7.46 and 7.50 in chronic alveolar hyperventilation.
Alveolar hyperventilation (low Paco2) with a pH of 7.35 to 7.40 indicates a primary metabolic acidosis in which the respiratory system has normalised the pH. Although not impossible, it is very unusual for either the renal or the respiratory system to overcompensate. Alveolar hyperventilation in the presence of an arterial pH<7.35 suggests a severe metabolic acidosis or some limitation on the ability of the respiratory system to compensate.
A normal Paco2 accompanied by an arterial pH >7.45 represents a primary metabolic alkalosis to which the ventilatory system has not responded. In the presence of hypoxaemia, however, this might occur when patients with chronic carbon dioxide retention increase their usual level of ventilation. This may be seen when pulmonary emboli occur in chronic lung disease such as chronic obstructive pulmonary disease.
Figure.
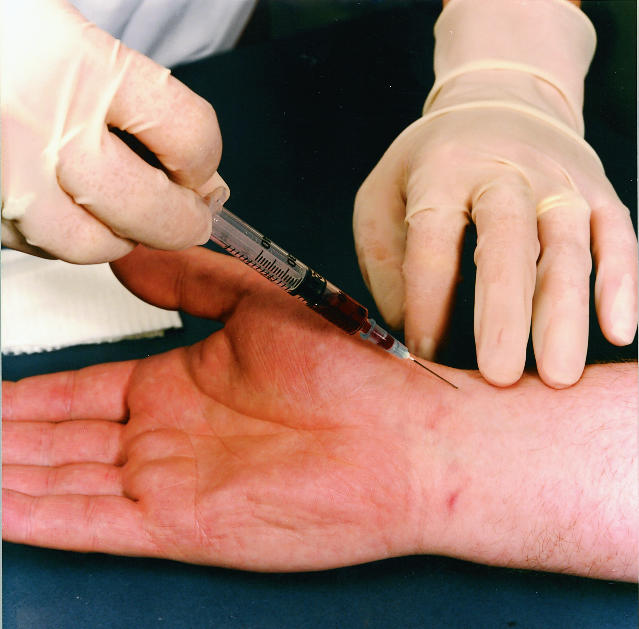
Taking arterial blood sample
Figure.
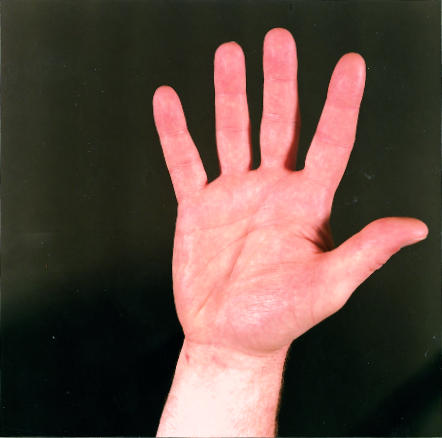
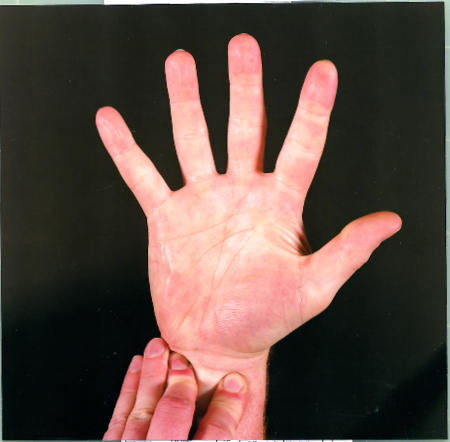
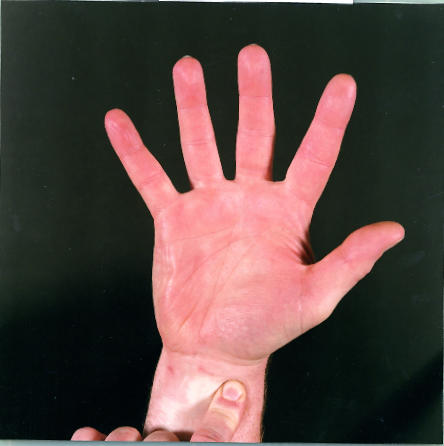
Allen’s test. The radial and ulnar arteries are occluded by firm pressure while the fist is clenched. The hand is opened and the arteries released one at a time to check their ability to return blood flow to the hand
Figure.

Transcutaneous oxygen and carbon dioxide monitor
Figure.
Oxygen saturation curve
Figure.

Results of blood gas analysis need careful interpretation
Footnotes
Adrian J Williams is director, Lane-Fox respiratory unit, St Thomas’s Hospital, London.
The ABC of Oxygen is edited by Richard M Leach, consultant physician, and P John Rees, consultant physician, Guy’s and St Thomas’s Hospital Trust, London.
The picture of the transcutaneous oxygen and carbon dioxide monitor was provided by Radiometer Medical, Copenhagen.