A slight mystery surrounds the clinical trial that this special issue of the BMJ commemorates.1 It is now widely recalled that pulmonary tuberculosis patients in the Medical Research Council (MRC) trial were allocated to a streptomycin treatment group and a control group by a process using random sampling numbers and sealed envelopes (box). The editorial that introduced the MRC’s report to BMJ readers on 30 October 1948 called attention to this new scheme, distinguishing it from the older practice of taking alternate cases in order of admission to hospital as the method of creating a control group.2 The statistician involved, Professor (later Sir) Austin Bradford Hill, had been promoting the use of random allotment since before the second world war.3 Remarkably, however, the word “random” appeared nowhere in the MRC’s files on streptomycin for 1946. During that year, the now famous scheme was explicitly mentioned in a single letter. So why did the MRC use randomisation in this clinical trial?
Summary points
Randomised allocation of patients is rarely mentioned in the Medical Research Council’s documents on streptomycin clinical trials
The meaning of the term “randomisation” has shifted over time; justifications for using randomisation have also changed
Streptomycin was isolated in 1943 at Rutgers University in New Jersey; the MRC began planning clinical trials in 1946; the first patients with tuberculosis entered the trials in 1947
The British government initially purchased 50 kg of American streptomycin for the MRC; most of the supply went to the clinical trial in pulmonary tuberculosis
Public demand for streptomycin was far in excess of supplies in Britain. Randomisation relieved the MRC’s clinicians of responsibility for deciding who would be treated
Any answer is tentative as contemporary evidence about the reasoning of the members of the MRC’s committee is thin. We can make some inferences from the arguments they used after the fact to justify the random allocation scheme to the medical profession. Their design, and how it was presented, should be understood in the context of the medical culture of Britain just after the war. This goes beyond the often repeated argument that a shortage of streptomycin made it ethically permissible to leave some patients untreated,1,4–7 which, as we shall see below, addresses a different point from the method of allotment. It seems that methodological ends motivated Bradford Hill to conceive his technique, and pressure to distribute streptomycin in a fair manner enabled its implementation.
Randomised allocation method used in the MRC’s clinical trial of streptomycin (taken from Bradford Hill’s original paper1)
The Control Scheme
Determination of whether a patient would be treated by streptomycin and bed-rest (S case) or by bed-rest alone (C case) was made by reference to a statistical series based on random sampling numbers drawn up for each sex at each centre by Professor Bradford Hill; the details of the series were unknown to any of the investigators or to the co-ordinator and were contained in a set of sealed envelopes, each bearing on the outside only the name of the hospital and a number. After acceptance of a patient by the panel, and before admission to the streptomycin centre, the appropriate numbered envelope was opened at the central office: the card inside told if the patient was to be an S or C case, and this information was then given to the medical officer of the centre. Patients were not told before admission that they were to get special treatment; C patients did not know throughout their stay in hospital that they were control patients in a special study; they were in fact treated as they would have been in the past, the sole difference being that they had been admitted to the centre more rapidly than was normal. Usually they were not in the same wards as S patients, but the same regimen was maintained.
Controls and random allocation
Medical researchers had a long tradition of making comparisons between groups of patients. One group would be treated with a particular substance and the second group, the controls, would receive a different treatment or sometimes no treatment at all. Without the use of controls, critics might have objected that the results attributed to treatment would have occurred by letting nature take its course.
Professor Bradford Hill presented basic principles of experimental design in a series of articles for the Lancet in 1937, which were soon reprinted in the form of a textbook on medical statistics. Bradford Hill argued that the essence of the experimenter’s task was “to ensure beforehand that, as far as possible, the control and treated groups are the same in all relevant respects,” although he admitted that there was no way to be certain that some factor had not been overlooked.3 The MRC’s therapeutic trials committee had used alternating controls—for example, in its study of serum treatment of lobar pneumonia. Bradford Hill argued that alternation was often acceptable because, as a method of random allotment, it protected against suspicion of bias and could fairly reliably achieve similarity between the groups.3 Neither of these justifications had much connection with randomisation as discussed in the statistical theory of R A Fisher.8
Shortly before his death Bradford Hill suggested that in 1937 he had already been thinking about random sampling numbers but had not wanted presentation of complicated concepts to scare off the average practitioner.4 His early articles show no signs of his later qualms about whether alternate assignment would actually achieve randomness. During the second world war, alternation continued to be considered a satisfactory form of treatment allocation—as in, for example, an MRC study of the antibiotic patulin in treatment of the common cold.9
The field of tuberculosis research has had a special role in the development of experimental methodology, partly because the disease, especially in its pulmonary forms, exhibited spontaneous recoveries. In 1944 the leading tuberculosis researchers at the Mayo Clinic in Minnesota, William Feldman and Corwin Hinshaw, presented numerous techniques to reduce the possibility of erroneous claims from antituberculosis trials in human patients. Their concepts of clinical trial design attempted to extend controlled laboratory conditions to the bedside. Their guidelines included careful definition of eligible cases to ensure a homogeneous group of cases, roentgenological interpretation blinded as to whether patients had received treatment, and “some procedure of chance” in allocating patients10—ideas all implemented in the MRC’s trial. Feldman and Hinshaw’s study, then under way, used the toss of a coin to select one member from each of several pairs of patients who had been matched for clinical condition.
A historical argument for using controls blamed poorly controlled studies for the adoption of numerous treatments that were later discredited.10–12 Most notorious of these was sanocrysin, a gold compound that was popular for about a decade from 1925. In 1931 a team in Detroit divided 24 patients into two groups, with patients paired as closely as possible according to criteria such as age and severity of disease. A single flip of a coin decided which group would receive sanocrysin and which group injections of distilled water. The control group fared better. 8,13
Streptomycin
The antibiotic streptomycin, like penicillin before it, was subject to extraordinary commercial and public pressures. Streptomycin was isolated around November 1943 by Albert Schatz, a PhD student in Professor Selman Waksman’s department at Rutgers University in New Jersey.5,14 The drug was developed by a large American pharmaceutical firm, Merck, with which Waksman had had a commercial agreement since 1940.15 Feldman and Hinshaw showed streptomycin to have a definite inhibitory effect on tuberculosis in guinea pigs, which were highly susceptible to the disease.16 In 1945 Merck invested $3.5m (£875 000) in a new plant in Virginia (figures 1 and 2).17 Ten other firms tried to produce the drug, with varying degrees of success. Preliminary clinical trials were conducted at the Mayo Clinic, with cautiously optimistic conclusions.
Figure 1.

Production of streptomycin was technically difficult18
Figure 2.
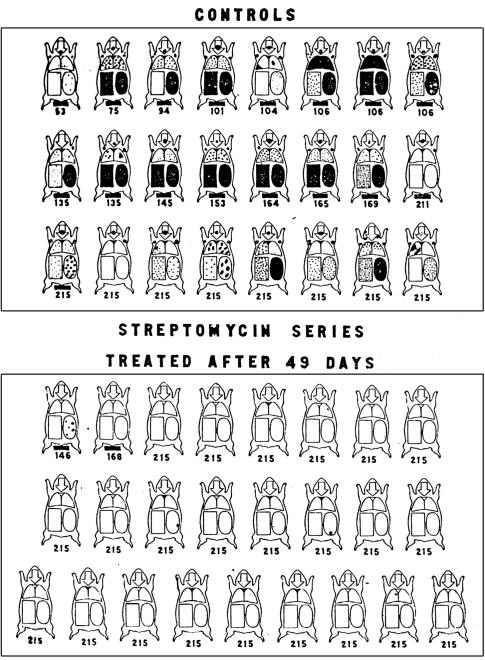
Controlled study of streptomycin treatment of tuberculous guinea pigs16
In July 1946 Feldman was brought on a tour of Britain—originally at the instigation of the MRC. The Ministry of Health privately criticised the council for having added further to the publicity, and it succeeded in curbing the council’s initial enthusiasm. Feldman’s lectures in Oxford and London included a highly persuasive graphical presentation of the laboratory evidence. A few days later a representative of the Ministry of Supply asked the MRC to plan clinical trials to test streptomycin that was expected shortly from Boots, Glaxo, and Distillers. Geoffrey Marshall, a respected consultant at London’s Brompton Hospital, Britain’s most prestigious institution for treatment of tuberculosis, chaired a hastily assembled conference of clinicians. They decided in the summer that the main trial would focus on pulmonary tuberculosis and would require untreated controls.
American production of streptomycin increased rapidly in the autumn of 1946. Once patients in the United States could readily obtain the drug on prescription without having to enter a research programme, large scale clinical trials in that country maintained controlled designs with some difficulty.18 Export quotas determined the quantity of American streptomycin that could be made available to the MRC, which was substantial: 50 kg were offered to the British government in November, at a cost of $320 000. Domestic production of streptomycin did not get beyond pilot scale for several more years.19 Meanwhile a flow of private supplies to Britain raised sensitive issues about distribution of the potentially lifesaving drug.
A “right to treatment” was not part of patients’ vocabulary, even after the passage of the National Health Service Act. Nevertheless, they and their doctors besieged the government with requests for the drug—in the vast majority of cases for treatment of tuberculosis, which was responsible for some 25 000 deaths annually in Britain. The BBC broadcast many emergency appeals for the drug, and a black market emerged.7,15,20 Most patients’ only hope of obtaining the drug was through the MRC’s research programme.
Bradford Hill’s scheme
The Streptomycin Clinical Trials (Tuberculosis) Committee, as it was formally known, was created in October, with Marshall as chairman and Philip Hart, who later directed the MRC’s tuberculosis research unit, as secretary. The minutes of the committee’s first meeting, on 21 November 1946, recorded enigmatically, “the use of control-cases was fully discussed,” without saying a word about the method of allocating patients.21
The 1948 report in the BMJ took pains to defend the use of an untreated control group, a familiar but still ethically contentious research design. It argued, understandably, that the effectiveness of the drug was still uncertain, and that in any event the shortage had made it impossible to treat all the patients with pulmonary disease. The rest of the MRC’s supplies, it said, went to the trials researching two uniformly fatal forms of tuberculosis (in which concurrent controls were not used). This was not strictly accurate, as a small programme under Sir Alexander Fleming tested streptomycin in various non-tuberculous conditions.22
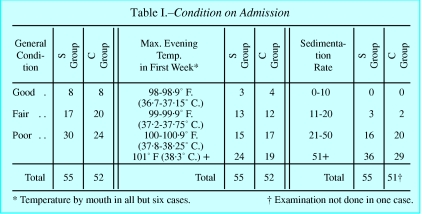
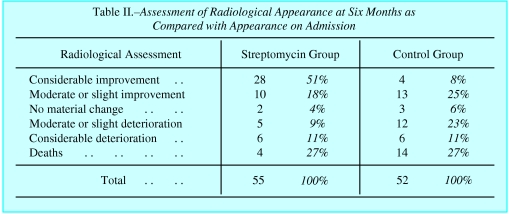
The report pointed to the fact that random allocation yielded groups that started with almost equally severe disease (fig 3). It showed conclusively that the drug was useful, by comparing outcomes between these randomly allocated groups (fig 4). The accompanying editorial noted that in a trial using allotment by alternation, the clinicians would be certain that the next patient to be admitted would, say, receive the treatment, and their decision whether to accept this patient might consequently be affected. In the era of the slogan, “fair shares for all,” suspicion of favouritism was to be avoided. Under Bradford Hill’s scheme, there could be no such worries, as the decision whether to include the patient in the trial was made in complete ignorance of which group the patient would join. The new method of random allocation “removed personal responsibility from the clinician,” the BMJ noted.2 Hart’s secretary once advised about a patient with pulmonary disease whose physician was seeking to enrol him in the trial, “the strongest point against any possible acceptance of his case is that with the control system we dare not take isolated cases of this kind—we don’t decide whether the case is to be a treated one or a control case.”23 When one senior physician contracted tuberculosis, the MRC obtained supplies for him outside the trial, rather than compromise the integrity of this admission system.7
Figure 3.
Randomisation was intended to make streptomycin and control groups comparable at the outset (table taken from Bradford Hill’s original paper1)
Figure 4.
An independent panel found a clear difference between streptomycin treatment and control groups (table taken from Bradford Hill’s original paper1)
As in numerous other contemporary projects, such as the wartime penicillin studies in the United States, central control enabled researchers to follow their methodological predispositions.18 Alternation was used under the MRC’s auspices in certain later streptomycin trials. Bradford Hill’s longstanding commitment to ensuring comparability between groups was only one factor in the pulmonary tuberculosis trial; equally important was the protection of the admission process from external pressure.
Conclusion
The MRC’s clinical trial has rightly been recognised for its careful design and implementation, although it was not as novel as it is usually portrayed. The innovation of centrally controlled randomisation can be attributed to a combination of scientific logic and political and social pressures on a medical bureaucracy.
Figure.

Professor Austin Bradford Hill was one of 15 members of the MRC’s committee that planned the tuberculosis trials
Footnotes
Funding: The research was partially funded by the Wellcome Trust.
Competing interest: Merck provided several hundred photocopies free of charge.
References
- 1.Medical Research Council. Streptomycin treatment of pulmonary tuberculosis. BMJ. 1948;2:769–782. [PMC free article] [PubMed] [Google Scholar]
- 2.The controlled therapeutic trial [editorial] BMJ. 1948;2:791–792. [PMC free article] [PubMed] [Google Scholar]
- 3.Hill AB. Principles of medical statistics:he aim of the statistical method. Lancet. 1937;1:41–43. [Google Scholar]
- 4.Hill AB. Memories of the British streptomycin trial in tuberculosis. Controlled Clinical Trials. 1990;11:77–79. doi: 10.1016/0197-2456(90)90001-i. [DOI] [PubMed] [Google Scholar]
- 5.Ryan F. The greatest story never told: the human story of the search for the cure for tuberculosis and the new global threat. Bromsgrove: Swift; 1992. [Google Scholar]
- 6.Doll R. Development of controlled trials in preventive and therapeutic medicine. J Biosoc Sci. 1991;23:365–378. doi: 10.1017/s002193200001943x. [DOI] [PubMed] [Google Scholar]
- 7.Holme CI. Trial by TB: a study into current attempts to control the international upsurge in tuberculosis. Proc R Soc Coll Phys Edin 1997;27(suppl 4).
- 8.Armitage P. The role of randomisation in clinical trials. Stat Med. 1982;1:345–352. doi: 10.1002/sim.4780010412. [DOI] [PubMed] [Google Scholar]
- 9.MRC Patulin Clinical Trials Committee. Clinical trial of patulin in the common cold. Lancet. 1944;2:373–375. [Google Scholar]
- 10.Hinshaw HC, Feldman WH. Evaluation of chemotherapeutic agents in clinical tuberculosis: a suggested procedure. Am Rev Tuberc. 1944;50:202–213. [Google Scholar]
- 11.Hart PD’A. Chemotherapy of tuberculosis: research during the past 100 years. Part 1. BMJ. 1946;2:805–810. doi: 10.1136/bmj.2.4482.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart PD’A. Chemotherapy of tuberculosis: research during the past 100 years. Part II. BMJ. 1946;2:849–855. [PubMed] [Google Scholar]
- 13.Amberson JB, McMahon BT, Pinner M. A clinical trial of sanocrysin in pulmonary tuberculosis. Am Rev Tuberc. 1931;24:401–435. [Google Scholar]
- 14.Wainwright M. Miracle cure: the story of penicillin and the golden age of antibiotics. Oxford: Blackwell; 1990. [Google Scholar]
- 15.Waksman SA. Berkeley: University of California Press; 1964. The conquest of tuberculosis. [Google Scholar]
- 16.Feldman WH, Hinshaw HC, Mann FC. Streptomycin in experimental tuberculosis. Am Rev Tuberc. 1945;52:269–298. [PubMed] [Google Scholar]
- 17.Porter RW. Streptomycin: engineered into commercial production. Chemical Engineering 1946 (Oct).
- 18.Marks HM. The progress of experiment: science and therapeutic reform in the United States, 1900-1990. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 19.Davenport-Hines RPT, Slinn J. Glaxo: A history to 1962. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 20.Bryder L. Below the magic mountain: a social history of tuberculosis in twentieth-century Britain. Oxford: Clarendon Press; 1988. [Google Scholar]
- 21.Minutes of Streptomycin Clinical Trials (Tuberculosis) Committee, 21 November 1946. Public Record Office, London; FD1/6756.
- 22.Wilson C. Streptomycin in non-tuberculous conditions. BMJ. 1948;2:552–553. doi: 10.1136/bmj.2.4576.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlene Agnew to Dr F H K Green, 19 Jun 1947. Public Record Office, London; FD1/6756.