The improvements in survival probability of children with hematologic malignancies observed in the last three decades represent one of the most remarkable successes of modern medicine. Nowadays, almost 90% of children with acute lymphoblastic leukemia (ALL) and more than 70% of those with acute myeloid leukemia (AML) are cured with multiagent chemotherapy protocols, together with, in some selected subsets of patients, allogeneic hematopoietic stem cell transplantation.1,2 However, despite these impressive results, there is still a proportion of patients with acute leukemia for whom innovative approaches are desperately needed to rescue them from their relapsed/ refractory malignancies. In addition, there is growing evidence that the optimization of conventional treatments has reached its limit and we cannot further increase the intensity of chemo/radiotherapy in children with either ALL or AML, also considering that a relevant proportion of patients experience both acute and long-term severe toxicities, including life-threatening infections, pancreatitis, osteonecrosis, cardiomyopathy, neurocognitive defects, loss of fertility and endocrinopathies.3-6
Immunotherapy has emerged in the last decade as one of the most promising approaches for treating patients with relapsed/refractory hematologic malignancies and for sparing severe toxicities related to intense chemo/radiation therapy. The new immunotherapy agents that have been developed include antibodies targeting checkpoint inhibitors, naked antibodies directed against antigens expressed on tumor cells, antibody-drug conjugates,7 bispecific T-cell engagers8,9 and T cells re-directed against the tumor elements through the use of chimeric antigen receptors (CAR).10
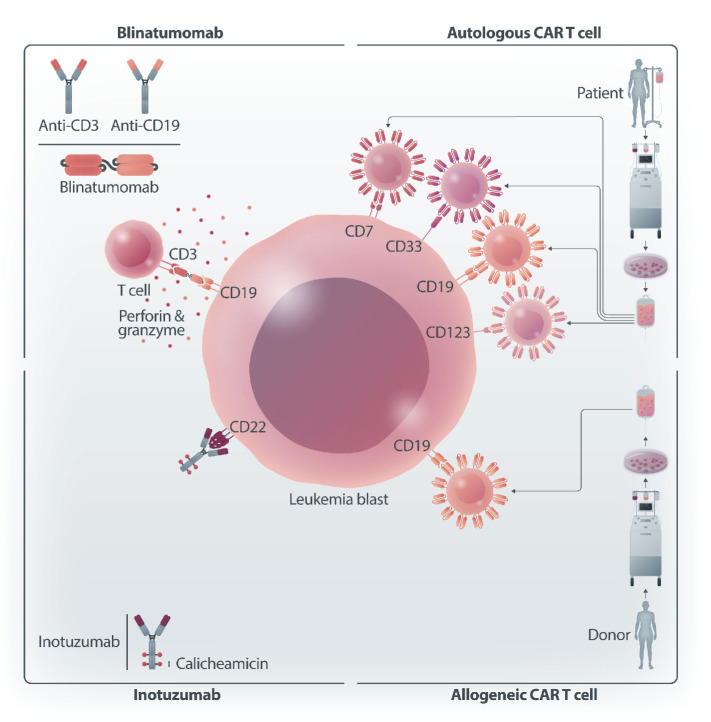
In this issue of Haematologica, five different contributions prepared by experts in the field analyze the current use and the future prospective applications of different types of immunotherapies in pediatric patients with either ALL or AML (Figure 1).11-15
Figure 1.
Schematic representation of different immunotherapy approaches for the treatment of childhood hematologic malignancies. Bispecific, CD3-CD19, T-cell engager: blinatumomab. Antibody-drug conjugate targeting CD22 and carrying calicheamicin: inotuzumab. Autologous chimeric antigen receptor (CAR) T cells directed towards CD19, CD7, CD33 or CD123. Allogeneic CAR T cells directed towards CD19. Figure created with BioRender.
Some of these immunotherapies, such as blinatumomab (the prototype of bispecific T-cell engagers) and CD19-targeting CAR T cells (tisagenlecleucel) have already been approved by the Food and Drug Administration in the USA and the European Medicines Agency in the European Union for clinical use in children with relapsed/refractory B-ALL, while others are under investigation and/or early clinical development (e.g., inotuzumab, CAR T cells for T-ALL or for AML). The studies so far conducted have clearly documented that these therapies are characterized by selective mechanisms of action, thus potentially sparing the acute and chronic toxicities associated with intensive, multiagent chemotherapy courses, which may have a particularly detrimental effect in subjects with a long expectancy of life. However, novel treatment-related toxicities have also emerged, such as the cytokine release syndrome and immune cell-associated neurotoxicity, which were unknown before the development and implementation of these treatments. Efforts aimed at properly managing these specific complications with targeted treatments, without jeopardizing the therapeutic effects of immune treatments are key to optimizing the benefit-to-risk ratio for the patients. In addition, we are still in the learning curve for already approved therapies with regard to the role played by factors influencing clinical efficacy (e.g., leukemia burden, T-cell function, downregulation or loss of the target antigen, manufacturing processes, cell doses, extramedullary leukemia relapse).16-19 Future studies will have to elucidate the best place in which these novel approaches should be inserted in the patient’s therapeutic journey and how these therapies influence each other.20 We also still have to further elaborate and refine strategies to optimize the benefit deriving from the application of these therapies, including, for example, administration of blinatumomab subcutaneously instead of by continuous intravenous infusion or the use of humanized CAR constructs potentially able to lead to longer persistence and greater efficacy.
What is certain is that, in the forthcoming years, the therapeutic scenario of childhood ALL and, it is to be hoped, AML will be marked by the introduction of several immunotherapy approaches not only in patients with relapsed/ refractory disease, but also in the treatment strategy of newly diagnosed patients, as recently demonstrated in infants with B-ALL.21 The five contributions published in this issue of Haematologica will offer readers the most recent and up-to-date information and perspective of use of these therapies which hold the promise to represent a cornerstone in the field of pediatric hematology.
References
- 1.Pieters R, Mullighan CG, Hunger SP. Advancing diagnostics and therapy to reach universal cure in childhood ALL. J Clin Oncol. 2023;41(36):5579-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33(27):2949-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231-e239. [DOI] [PubMed] [Google Scholar]
- 4.Andrés-Jensen L, Attarbaschi A, Bardi E, et al. Severe toxicity free survival: physician-derived definitions of unacceptable long-term toxicities following acute lymphocytic leukaemia. Lancet Haematol. 2021;8(7):e513-e523. [DOI] [PubMed] [Google Scholar]
- 5.Dixon SB, Liu Q, Chow EJ, et al. Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet. 2023;401(10386):1447-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennesi E, Michels N, Brivio E, et al. Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: results from a phase II trial. Leukemia. 2022;36(6):1516-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/ refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389. [DOI] [PubMed] [Google Scholar]
- 9.Locatelli F, Zugmaier G, Rizzari C, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia. A randomized clinical trial. JAMA. 2021;325(9):843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brivio E, Bautista Sirvent FJ, Zwaan CM. Naked antibodies and antibody-drug conjugates: targeted therapy for childhood acute lymphoblastic leukemia. Haematologica. 2024;109(6):1700-1712. [Google Scholar]
- 12.Naik S, Velasquez MP, Gottschalk S. Chimeric antigen receptor T-cell therapy in childhood acute myeloid leukemia: how far are we from a clinical application? Haematologica. 2024; 109(6):1656-1667. [Google Scholar]
- 13.Lyons KU, Gore L. Bispecific T-cell engagers in childhood B-acute lymphoblastic leukemia. Haematologica 2024;109(6):1668-1676. [Google Scholar]
- 14.Locatelli F, Del Bufalo F, Quintarelli C. Allogeneic chimeric antigen receptor T cells for children with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Haematologica. 2024;109(6):1689-1699. [Google Scholar]
- 15.Oh BLZ, Vinanica N, Wong DMH, Campana D. Chimeric antigen receptor T-cell therapy for T-cell acute lymphoblastic leukemia. Haematologica. 2024;109(6):1677-1688. [Google Scholar]
- 16.Queudeville M, Stein AS, Locatelli F, et al. Low leukemia burden improves blinatumomab efficacy in patients with relapsed/ refractory B-cell acute lymphoblastic leukemia. Cancer. 2023;129(9):1384-1393. [DOI] [PubMed] [Google Scholar]
- 17.Schultz LM, Baggott C, Prabhu S, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40(9):945-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotillo E, Barrett D, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacoby E, Ghorashian S, Vormoor B, et al. CD19 CAR T-cells for pediatric relapsed acute lymphoblastic leukemia with active CNS involvement: a retrospective international study. Leukemia. 2022;36(6):1525-1532. [DOI] [PubMed] [Google Scholar]
- 20.Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 2022;40(9):932-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Sluis IM, de Lorenzo P, Kotecha RS, et al. Blinatumomab added to chemotherapy in infant lymphoblastic leukemia. N Engl J Med. 2023;388(17):1572-1581. [DOI] [PubMed] [Google Scholar]