Reactivation from latency of the human cytomegalovirus (CMV) continues to negatively impact hematopoietic stem cell transplant (HCT) outcomes.1 Due to treatment-related lymphopenia and T-cell dysfunction, up to 70% CMV-seropositive recipients can experience CMV reactivation, and if left untreated, end-organ disease may develop in up to 30% of these patients, leading to life-threatening complications.2,3 Current antiviral strategies to treat or prevent CMV reactivation are effective, though there are many drawbacks. Pre-emptive antiviral therapy (PET)4 is associated with drug-related organ toxicities, delays in immune reconstitution, and subsequent late-onset CMV disease. Food and Drug Administration (FDA)-approved (November 2017) 100-day letermovir prophylaxis reduces the incidence of clinically significant CMV infection (CMV disease or CMV viremia leading to PET) to 37.5% at 24 weeks in CMV-seropositive HCT recipients, and showed an acceptable safety profile.5 However, when letermovir is withdrawn, approximately 30% of patients develop CMV viremia, consistent with delayed immune reconstitution, associated with pharmacologic suppression of CMV.6 Recent data demonstrate continued efficacy and safety with extended letermovir use up to day 200 post transplant in high-risk patients, though the effect of prophylaxis was lost after the drug was stopped.7 Alternative strategies to suppress both early and late CMV reactivation and its sequelae by enhancing and sustaining protective CMV-specific cellular immune reconstitution remain of great interest.
Therapeutic vaccination of CMV seropositive HCT recipients can bolster immune control of CMV viremia post HCT, mitigating the risk of severe CMV sequelae.8 A phase I trial of PepVax,9 an investigational CMV vaccine showed safety, immunogenicity, and reduced CMV reactivation and usage of antivirals, paving the way to the current phase II trial to assess its efficacy in reducing CMV reactivation of ≥1,250 IU/mL and disease before day 100 post HCT (CMV events, primary endpoint). Developed at City of Hope, PepVax is a peptide-based vaccine, composed of the immunodominant HLA-A*0201 restricted CMV pp65495-503 CD8 T-cell epitope, linked to a tetanus T-helper epitope and co-administered with PF-03512676 adjuvant.9 The HLA A*0201 allele is most frequently represented in Caucasian individuals (approx. 46%) as well as other ethnicities, and is much less common in African and Asian Americans (approx. 16%).10 In this multicenter, placebo-controlled, randomized phase II trial (clinicaltrials.gov 02396134), patients were enrolled before HCT, reassessed for eligibility on day 28 post HCT, and randomized 1:1 to PepVax or placebo. Injections were administered subcutaneously on days 28 and 56 post HCT. Enrollment was halted when a planned interim analysis indicated that PepVax did not achieve the study primary endpoint. We herein report and discuss the complete 1-year outcomes from eligible recipients (N=61) who were randomized to PepVax (N=32) and placebo (N=29), until enrollment was terminated. There was no difference in CMV events between arms (P=0.15). PepVax was confirmed to be well tolerated and immunogenic. Significantly higher levels of pp65495-503 specific T cells were measured in non-viremic participants of the PepVax compared to the placebo arm (P=0.015). Furthermore, higher frequency of pp65495–503 CD8 T cells, displaying an effector phenotype with antiviral role was observed in PepVax compared to placebo recipients (P=0.034).
The FDA and institutional regulatory board authorities at each participating site approved the PepVax study protocol. From June 3, 2015, to November 11, 2017, 76 eligible patients were approached at 4 US cancer centers (City of Hope, Fred Hutchinson Cancer Center, The Ohio State University and University of Minnesota) for study participation (Online Supplementary Figure S1). All study patients provided written informed consent, were CMV-seropositive, HLA A*0201, 18-75 years old, and were undergoing HCT using myeloablative conditioning or reduced intensity conditioning from a CMV-seropositive or seronegative matched related or unrelated donor with 8/8 allele matching (Table 1). They were excluded from receiving the study treatment (PepVax or placebo injections) if engraftment had failed or if they relapsed, had grade III-IV acute graft-versus-host disease (aGvHD) (according to the Keystone Consensus grading system), experienced CMV reactivation ≥1250 IU/mL, had received >1 mg/kg of body weight steroids per day within seven days, or had any ongoing non-hematologic toxicity of grade ≥3 (according to the Common Terminology Criteria for Adverse Events, version 4.03). This trial was designed to have 90% power to detect a reduction of CMV events in the PepVax arm from 40% to 15%, at a one-sided 0.10 level of significance with a sample size of 48 patients randomized to each arm. With the expected dropout rate of 10-15% prior to randomization, the total accrual was expected to be of 106-115 participants. For the primary efficacy outcome, we assessed if PepVax reduced the cumulative incidence rate of CMV events compared to placebo. An interim analysis was performed per protocol once half (N=48) of the planned study participants (N=96) reached day 100 post HCT. Data analyzed showed that the futility boundary was crossed, since in the PepVax arm a higher number of CMV events (5 in 24 patients, 21%) was observed compared to the placebo arm (3 in 24 patients, 12.5%).
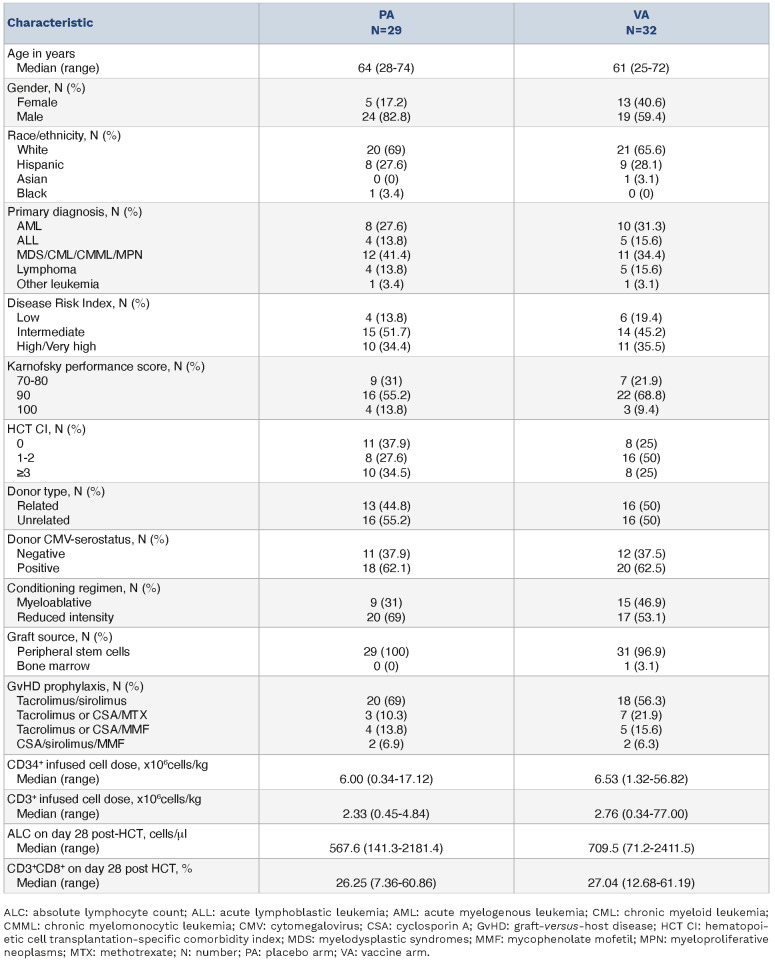
Table 1.
Patients’ characteristics at baseline.
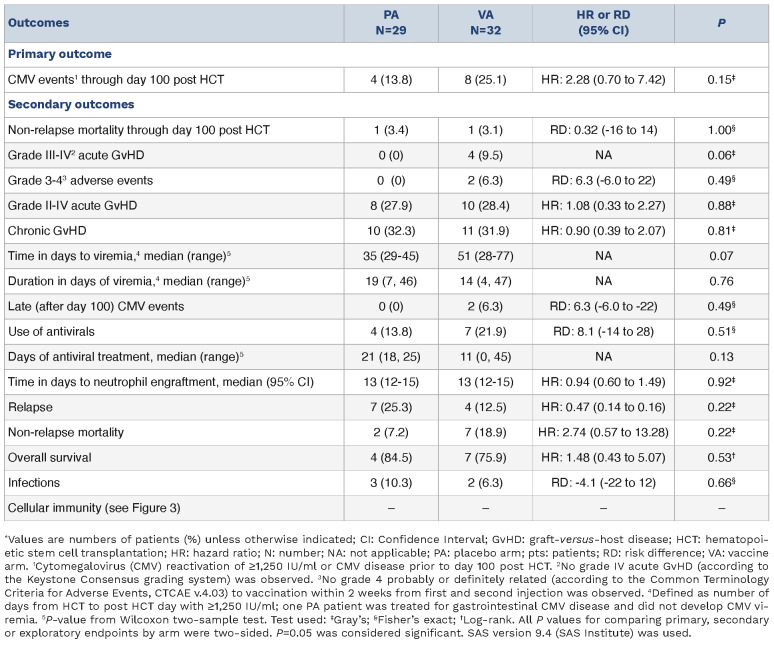
Table 1 shows characteristics of all randomized participant (N=61) until study closure to further accrual, following the interim analysis outcome. HCT variables were closely balanced between arms. The primary outcome of CMV events (Table 2, Online Supplementary Figure S2A) occurred in 8 patients of the PepVax arm (25.1%) and in 4 of the placebo arm (13.8%). By day 180, the CMV event rate increased to 33% for the PepVax arm (total, 10 CMV events), and remained at 13.8% for the placebo arm. In agreement with previous findings,2,11 a higher number of CMV events (34.8%) were observed in recipients who received an HCT from a CMV-seronegative negative donor compared to those (15.6%) who received a transplant from a CMV-seropositive donor. Also, in the CMV-serostatus subgroup analysis, CMV events rates were higher in the PepVax compared to the placebo arm (Online Supplementary Figure S2B). Secondary outcomes (Table 2) of PepVax including safety, impact on GvHD, measures of transplant success, antiviral usage, and immunogenicity were favorable.
Table 2.
Outcomes by assigned treatment arm.*
An explanation for the difference between the promising pilot trial findings,9 and the current phase II trial was terminated because lack of efficacy could not be clearly identified. PepVax was produced using the same manufacturing process and met the same release criteria. The main difference was that this phase II trial enrolled patients from 4 US cancer centers, whereas the phase I study was a single center study.9 It is possible that heterogeneity in transplant regimens (conditioning, GvHD prophylaxis), and institutional standard of care for CMV monitoring and intervention among centers may have affected the efficacy endpoint. The lack of a stratified randomization by center, which could have reduced imbalances among centers and increased statistical power,12 is a limitation of this study. Furthermore, the CMV event rate in the phase I was 38.8% for the untreated observational arm, which is within the historical CMV reactivation range.11 In contrast, in the current phase II trial, the CMV event rate in the placebo arm was 13.8% and in the PepVax arm was 25.1%. Hence, the observed rate was unexpectedly lower than that assumed in the protocol design (approx. 40%).11
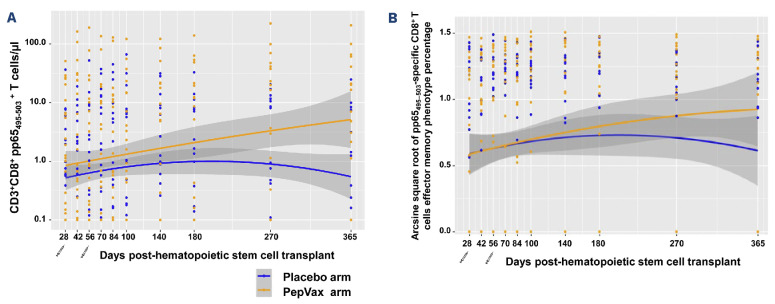
PepVax vaccinated patients reconstituted significantly higher levels of pp65495–503-specific CD8 T cells compared to the placebo arm (P=0.015) (Figure 1A) in HCT recipients who were not experiencing a CMV event. In recipients with a CMV-seropositive HCT donor, the vaccine effect in augmenting post HCT levels of pp65495–503-specific CD8 T cells was substantial (P=0.008) among PepVax vaccinated recipients compared to placebo recipients. In contrast, it was modest in HCT recipients with a CMV-seronegative donor (P=not assessable due to insufficient data) (Online Supplementary Figure S3). This interesting outcome suggests that in some viremic patients primary immune responses to other MHC class I restricted CMV epitopes could have been preferentially generated, possibly leading to the expansion of different CMV-specific T-cell subsets during viral reactivation. Several groups have reported that vaccinia virus immunized volunteers expressing the same HLA class I molecule recognized different vaccinia virus-derived epitopes, suggesting a highly variable pattern of T-cell epitope recognition in the human population.13 Hence, there is the need to re-evaluate current strategies for protective T-cell epitope discovery.
Figure 1.
Levels and memory phenotype of pp65495–503-specific CD8 T cells. pp65495–503-specific CD3+ CD8+ T cells were monitored by measuring binding to HLA-A*0201 pp65495–503 and HIVgag77-85 (control) multimers (Immudex Dextramers), as previously described. 9,14 (A) Longitudinal levels as T cells/μl and (B) memory phenotype frequency of pp65495–503-specific CD3+ CD8+ T cells were computed using the (A) LOESS scatter plot smoother providing the marginal geometric mean concentrations through time for each arm (as identified in the color legend). A 95% confidence band is shown in gray, and individual measurement trajectories are shown for each participant up to 7 days before the protocol-defined cytomegalovirus event. Logarithmic spacing of both scales is used to aid visualization. Distribution of pp65495–503 specific CD8 T-cell levels and memory phenotypes (%) were approximately normal after log 10-transformation and arcsine square root-transformation, respectively. Generalized estimating equation models were used to assess the effect of vaccines on immunological responses. All analyses were performed using SAS v.9.4 (SAS Institute). (B) pp65495–503-specific CD3+ CD8+ effectors included CD45RA- CD28- effector memory T cells (TEM) and effector 'revertant' memory T cells, re-expressing the RA isoform of the CD45 surface marker (TEMRA).14 The syringe symbol indicates the post-hematopoietic stem cell transplant day of PepVax injections.
Similarly to the phase I findings,9,1 4 PepVax not only increased frequency of pp65495–503-specific CD8 T cells, but also impacted their functional immune profiles. Effector subsets increased more vigorously during immune reconstitution in non-viremic patients from the PepVax arm compared to the placebo arm (P=0.034) (Figure 1B). Of note, the functionality and antiviral role of CMV-specific T cells have been linked to immune reconstitution profiles characterized by pools of differentiated effector memory T cells (TEM) and large subsets that, as in TEM, have lost membrane expression of the co-stimulatory molecule CD28, but also re-express the RA isoform of CD45 (TEMRA).14 T-central memory and naïve T-cell subsets were comparable between arms (data not shown). Using Fine-Gray regression model, no significant associations were found between CMV events and pp65495–503-specific CD8 T-cell levels or memory.
In the development of CMV subunit vaccines, choice of the vaccine technology can play a critical role in promoting robust immune response.8 The ASP0113 CMV vaccine, which has been stopped following the phase III failure, used a DNA-based platform. It has been shown that both DNA and peptide vaccines may induce limited immunogenicity even if used in combination with adjuvants, and to date no such vaccines have been licensed for human use.15,16 In contrast, viral vector-based vaccines, can mimic a natural infection, resulting in strong, long-lasting immune responses against the pathogen antigens.8 Triplex, a candidate CMV vaccine, uses the modified vaccinia virus Ankara (MVA) expressing pp65, IE1-exon4, and IE2-exon5 CMV genes. It is currently the only CMV vaccine for transplant indication, which met its primary endpoints in phase II trials.8
Owing to the lack of efficacy, further development of PepVax has been paused. Nonetheless, based on its favorable tolerability and immunogenicity, it could find future application as an immunotherapeutic to be used in combination with letermovir prophylaxis. Vaccinating HCT donor and/or recipient8 with PepVax in a combination regimen strategy with letermovir may overcome the immune impairment and delay in CMV-specific cellular reconstitution related to the drug-induced decrease in CMV antigen exposure. Though limited to HLA A*0201 recipients, this easy to implement combination therapy could benefit transplant patients in whom lack of CMV-specific T-cell polyfunctionality may increase the risk for late CMV infection sequalae after letermovir prophylaxis discontinuation.
Supplementary Material
Acknowledgments
We thank the patients, participating institutions and their clinical teams, research nurses and research coordinators without whose support this trial could not have been conducted. We acknowledge Dr. Thai M. Cao as a participating physician with involvement in patient trial enrollment and care, before, during and after transplant.
Funding Statement
Funding: Supported by grants from the National Cancer Institute (NCI) 1R01 CA181045; NCI-SAIC-Frederick 24XS044, and Helocyte Inc. to DJD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health (NIH).
Data-sharing statement
The clinical trial protocol and the anonymized data presented in this study can be made available upon reasonable request.
References
- 1.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25(1):151-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135(19):1619-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1687-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444. [DOI] [PubMed] [Google Scholar]
- 6.Zamora D, Duke ER, Xie H, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal R, Gordillo CA, Abramova R, et al. Extended letermovir administration, beyond day 100, is effective for CMV prophylaxis in patients with graft versus host disease. Transpl Infect Dis. 2021;23(2):e13487. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa C, Diamond DJ. Triplex, a viral vectored CMV vaccine for transplant indications: clinical trial updates. Vaccine Insights. 2023;2(7):287-308. [Google Scholar]
- 9.Nakamura R, La Rosa C, Longmate J, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol. 2016;3(2):e87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longmate J, York J, La Rosa C, et al. Population coverage by HLA class-I restricted cytotoxic T-lymphocyte epitopes. Immunogenetics. 2001;52(3-4):165-173. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Longmate J, Lacey SF, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002;359(9310):966-970. [DOI] [PubMed] [Google Scholar]
- 13.Gilchuk P, Hill TM, Wilson JT, Joyce S. Discovering protective CD8 T cell epitopes--no single immunologic property predicts it! Curr Opin Immunol. 2015;34:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Rosa C, Longmate J, Lingaraju CR, et al. Rapid acquisition of cytomegalovirus-specific T cells with a differentiated phenotype, in nonviremic hematopoietic stem transplant recipients vaccinated with CMVPepVax. Biol Blood Marrow Transplant. 2019;25(4):771-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016;15(3):313-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malonis RJ, Lai JR, Vergnolle O. Peptide-based vaccines: current progress and future challenges. Chem Rev. 2020;120(6):3210-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical trial protocol and the anonymized data presented in this study can be made available upon reasonable request.