Abstract
Thrombocytopenia occurs frequently in patients with cancer-associated thrombosis (CAT), however prospective evaluation of clinical outcomes following randomization to anticoagulants is limited. The HOKUSAI VTE Cancer study was a randomized, open-label, non-inferiority, phase III trial comparing dalteparin with edoxaban in CAT patients. This post hoc analysis of Hokusai VTE Cancer Study was performed to compare outcomes in patients with platelet count ≤100x109/L at one or more specified time points (baseline, 1-month, or 3-month) versus those without thrombocytopenia. Cumulative incidences at 180 days were calculated with death as a competing risk. The primary outcome was major bleeding; secondary outcomes were clinically relevant non-major bleeding (CRNMB), recurrent thrombosis, and survival. The analysis included 1,045 patients with primarily solid tumor malignancies (89%), median age 65 years, and 52% male. The thrombocytopenia group comprised 9.6% (N=101) of the cohort and relative to the non-thrombocytopenia cohort (N=944), experienced significantly higher major bleeding (9.0% vs. 4.0%, sub-distribution hazard ratio [SHR] =2.4; P=0.02) and CRNMB (17.9% vs. 9.6%, SHR=2.0; P=0.01). Thrombocytopenia did not impact recurrent venous thromboembolic event (VTE) (9.8% vs. 7.4%, SHR=1.3; P=0.37) nor overall mortality (21.8% vs. 26.0%, HR=0.9; P=0.48). Major bleeding was higher in patients with thrombocytopenia and gastrointestinal malignancies receiving edoxaban versus dalteparin (16.8% vs. 0; P<0.01) but similar for patients with other malignancies (P=0.30). In patients with hematologic malignances and thrombocytopenia major bleeding was higher for patients receiving dalteparin compared to edoxaban (19.0% vs. 0; P<0.01). Mild thrombocytopenia was associated with a doubling in risk of major hemorrhage in patients receiving anticoagulation for CAT. Bleeding risk for edoxaban and dalteparin varied in gastrointestinal and hematologic malignances in patients with thrombocytopenia (clinicaltrails gov. Identifier: NCT02073682).
Introduction
Thrombosis is a common complication in patients with active malignancy and has significant impact on morbidity and mortality, leading to increased health care resource utilization and financial strain.1-3 Cancer also increases the risk of bleeding which makes anticoagulation in this population challenging.4,5 Thrombocytopenia is common in patients with cancer due to either the underlying malignancy or the toxicity of cancer-directed therapies.6 Thrombocytopenia often coincides with the diagnosis of an acute venous thromboembolic event (VTE).7 Accordingly, the management of VTE in cancer patients with thrombocytopenia is challenging, as clinicians balance the benefits of anticoagulation with the likelihood of inducing a life-threatening hemorrhagic event.8 Randomized phase III trials have not been specifically conducted in patients with cancer-associated thrombosis (CAT) and thrombocytopenia such that current guidelines are largely based on retrospective cohorts.9-11
Direct oral anticoagulants (DOAC) are replacing LMWH as the primary therapy for VTE in cancer.12 These oral medications are comparably efficacious to LMWH for VTE recurrence with similar rates of hemorrhage,13-16 and have superior patient satisfaction, quality of life, and treatment adherence compared to other anticoagulation strategies.17,15 Data from prospective clinical trials on outcomes in patients with cancer-associated thrombosis and thrombocytopenia treated with direct oral anticoagulants are lacking.
In order to assess the impact of platelet counts on clinical outcomes for acute VTE in cancer, we assessed outcomes among patients enrolled in the HOKUSAI Cancer VTE trial which was a randomized phase III trial comparing edoxaban and dalteparin anticoagulation regimens in patients with acute VTE and cancer (clinicaltrails gov. Identifier: NCT02073682).
Methods
This study was a post hoc analysis utilizing de-identified clinical trial subject data from HOKUSAI Cancer VTE. The institutional review board at each participating center for the trial had previously approved the protocol and all patients were enrolled after written informed consent was obtained.13 The study team designed the analysis plan and this study was performed in collaboration with the sponsor (Daiichi Sankyo).
Hokusai VTE Cancer study was an open-label, non-inferiority trial, in which patients with cancer and acute symptomatic or incidental venous thromboembolism were randomized to receive either low-molecular-weight heparin for at least 5 days followed by oral edoxaban or subcutaneous dalteparin.13 The trial included adult patients with cancer with acute symptomatic or incidental proximal lower extremity deep vein thrombosis or pulmonary embolism (symptomatic or incidentally detected involving segmental or more proximal pulmonary arteries). Cancer diagnosis was required to be within 2 years preceding thrombotic event and either a cancer diagnosis that was recurrent, metastatic, regionally advanced, or actively receiving cancer-directed therapy (or received treatment in the last 6 months or hematologic malignancies not in remission were eligible). Relevant exclusion criteria included platelet count <50x109/L at enrollment. All patients were treated with an anticoagulant for at least 6 months and up to 1 year. The primary composite outcome included recurrent thromboembolism, bleeding, and death. Bleeding was graded in accordance with previously published criteria by the International Society of Thrombosis and Hemostasis (ISTH).18 All outcomes were adjudicated independently by a committee as per prespecified criteria outlined in the study protocol.
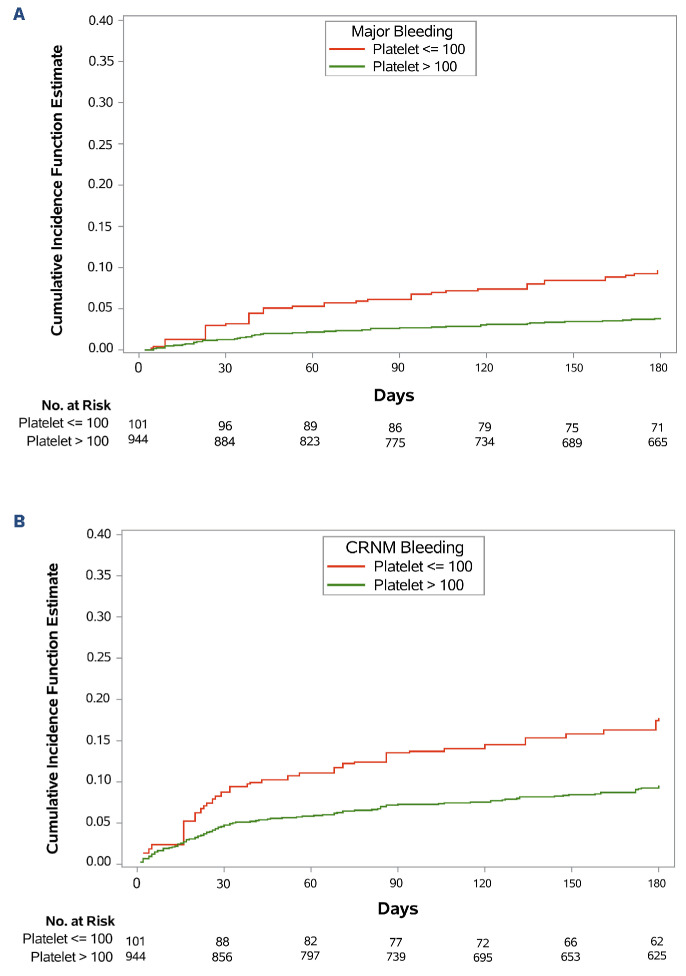
Figure 1.
Cumulative incidences of major bleeding and clinically relevant non-major bleeding. (A) Major bleeding and (B) clinically relevant non-major (CRNM) bleeding. Red line represents thrombocytopenic group (patients with platelet count ≤100x109/L at 1 or more of 3 prespecified time points: baseline, 1-month, or 3-month). Green line represents the non-thrombocytopenic group (platelet count >100x109/L at all 3 time points).
Per study protocol, for thrombocytopenia associated with chemotherapy in the first month of enrollment, if platelet count was 50-100x109/L, dalteparin dose was reduced by 2,500 IU until platelet recovery >100x109/L, and held if platelet count <50x109/L. Between 2 to 6 months, dalteparin dose was similarly adjusted or held, except in patients with body weight ≥99 kg, where dalteparin was reduced by 3,000 IU (instead of 2,500 IU). Edoxaban dose adjustment was based on body weight ≤60 kg, creatinine clearance between 30-50 mL/minute (min) inclusive, or concomitant use of P-glycoprotein (P-gp) inhibitors, without regard for platelet count. Dose interruption was allowed for any medical condition where continuing study drug may expose the subject to an increased hazard.
In order to assess the impact of thrombocytopenia on outcomes in these analyses, patients were grouped according to the first three time points in the trial that included blood counts (baseline, 1-month, 3-month). For the primary analysis, participants with platelet count ≤100x109/L at any of the three time points were included in the thrombocytopenic group and those without were in the non-thrombocytopenic group. The primary outcome for these analyses was major bleeding. Secondary outcomes included recurrent thrombosis, clinically relevant non-major bleeding (CRNMB), and mortality.
Statistical analysis
We estimated the cumulative incidence of the bleeding and thrombotic outcomes by identifying death as a competing risk.19 Statistical differences between the platelet cohorts (≤100x109/L or >100x109/L) were assessed using Gray test.20 We used the Fine-Gray method to construct time-to-event models and report the associated sub distribution function hazard ratios. Besides the platelet cohorts, dose-adjustment at randomization was included as a covariate in the model. Events occurring from randomization up to 180 days were included. For a given event in analysis if the event did not occur during this 180-day period, the subject was considered censored at 180 days. For overall survival the platelet cohorts (≤100x109/L or >100x109/L) and dose-adjustment at randomization were included in a Cox proportional hazard regression model and reported as hazard ratios with 95% confidence intervals.
Cumulative incidence (with death as a competing risk) of major bleeding and CRNMB was further estimated and compared statistically by Gray test within the thrombocytopenic cohort (≤100x109/L) based on treatment arm (edoxaban vs. dalteparin) for gastrointestinal (GI) cancers and hematologic malignancies separately.
Results
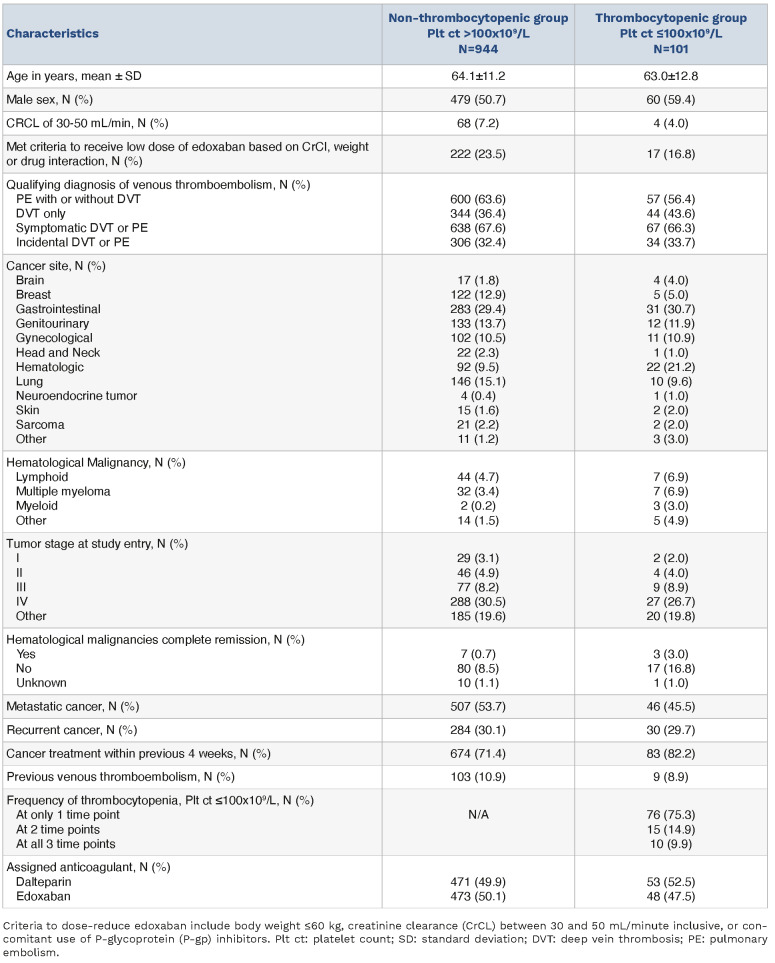
This analysis included an overall cohort of 1,045 patients, with a mean age of 64 years and 48% female. One patient from the original trial was excluded due to insufficient laboratory data. Most common sites of malignancy included gastrointestinal (30%), lung (14%), genitourinary (14%), breast (12%), and gynecological (11%). Hematologic malignancies accounted for approximately 10% of the cohort (Table 1). Of patients with solid tumor diagnoses, 53% had metastatic disease and 30% had recurrent disease at enrollment. Qualifying thrombotic events included pulmonary embolism for 63% and isolated deep vein thrombosis in 37%. Thrombocytopenia (<100x109/L) was present in 101 patients (9.6%) of the total cohort. Only 14 patients had a documented platelet count <50x109/L at any of the three time points. Of the 101 patients, 52 were first noted to have platelet count <100x109/L at baseline, 28 at 1-month and 21 at 3-months. In the thrombocytopenic group, 76 (75.3%) had thrombocytopenia at only one (of 3) time point, 15 (14.9%) at two time points, and 10 (9.9%) at all three time points. The two cohorts were comparable with respect to demographics, cancer distribution, and assignment to treatment arm (Table 1). A higher proportion of patients in the thrombocytopenic cohort had hematologic malignancies (21.8% vs. 9.8%; P<0.01).
Table 1.
Demographic and clinical characteristics of the patients at baseline by thrombocytopenia.
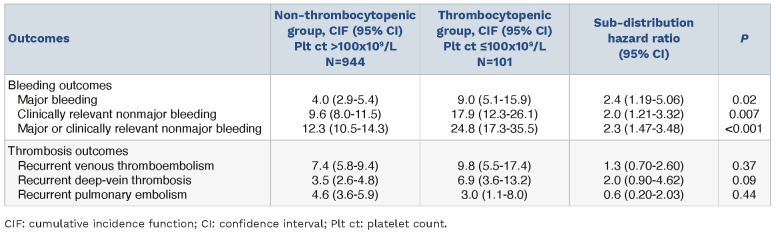
The estimated cumulative incidences at 180 days of all bleeding outcomes were higher in the thrombocytopenic group versus the non-thrombocytopenic group, including major bleeding (9.0% vs. 4.0%; SHR=2.4; P=0.02), CRNMB (17.9% vs. 9.6%; SHR=2.0; P=0.01), and major or CRNMB (24.8% vs. 12.3%; SHR=2.3; P<0.001) (Table 2). However, recurrent thrombosis were not statistically significantly different between the two groups. There was no significant difference between the two groups for death from any cause (21.8% vs. 26.0%; hazard ratio [HR]=0.9; P=0.48) or event-free survival (65.3% vs. 68.6%; HR=0.87; P=0.44) (Table 2).
Table 2.
Cumulative incidence of clinical outcomes at 180 days.
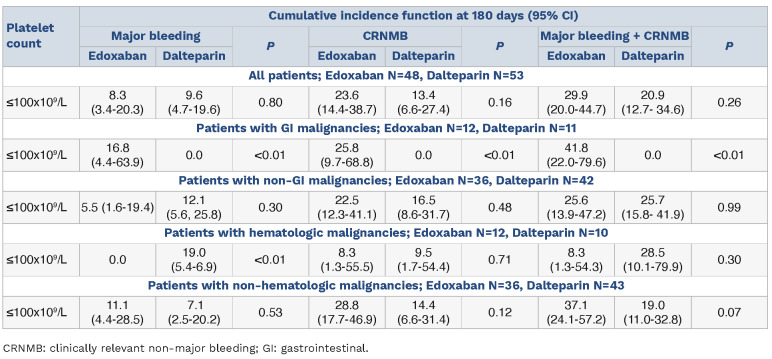
Within the thrombocytopenic group, there were 33 patients with GI malignancies and 78 patients with non-GI malignancies. The thrombocytopenic group patients with non-GI malignancies experienced similar rates of major bleeding at 180 days (5.5 vs. 12.1; P=0.30) and CRNMB (22.5 vs. 16.5; P=0.48) for those assigned to edoxaban compared to dalteparin (Table 3). Of thrombocytopenic patients with GI malignancies, edoxaban was associated with higher rates of major hemorrhage (16.8 vs. 0; P<0.01) and CRNMB (25.8 vs. 0; P<0.01). These findings are consistent with previous observations of increased risk of hemorrhage with edoxaban compared with dalteparin in patients with GI malignancies.21 In the thrombocytopenic cohort, 22 patients (21.8%) had underlying hematologic malignancies and experienced higher rates of major bleeding with dalteparin compared to edoxaban (19.0% vs. 0; P<0.01).
Table 3.
Cumulative incidence function (in percentage) of bleeding in thrombocytopenic group, by treatment drug and cancer type.
Discussion
In this post hoc analysis of the Hokusai VTE Cancer Study, thrombocytopenia was associated with a significantly increased risk of hemorrhage with an approximate doubling of major bleeding risk during the first 6 months after anticoagulation. To our knowledge this is the first analysis of a prospective, randomized trial dataset to evaluate the subgroup of patients with cancer-associated thrombosis and thrombocytopenia.
Thrombocytopenia, thrombosis, and bleeding are common complications in patients with active malignancy. Thrombocytopenia can result from underlying malignancy (commonly seen in hematologic malignancies) or emerge as a consequence of cancer-directed systemic therapies.6 In this randomized controlled study we found that thrombocytopenia was present in approximately one-tenth of enrolled patients with active malignancy and thrombosis. While this represents a clinically significant proportion, real-world evidence suggests this is an underestimation of co-occurrence of thrombocytopenia in cancer patients with acute VTE.7 A recent study found the prevalence of thrombocytopenia (platelet count <100x109/L) in CAT to be in 22% with solid tumors diagnoses and 47% with hematologic malignancies. We attribute the difference to the inherent nature of strict inclusion criteria in a prospective randomized controlled trial (i.e., patients with platelet count <50x109/L were excluded on enrollment).
Patients with malignancies that receive anticoagulation have up to a 2-fold increased risk of major bleeding compared with anticoagulated patients without cancer.5,22 Data on the estimates of bleeding in patients with cancer thrombosis and thrombocytopenia are quite limited. A systematic review identified only two retrospective cohort studies with cancer-associated thrombosis and thrombocytopenia. In a study of 128 patients with hematologic malignancies that were diagnosed with acute thrombosis the 6-month cumulative incidence rates of hemorrhage were 6.5 (95% confidence interval [CI]: 2.2-19.5) in patients with significant thrombocytopenia (<50 K/µL) versus 1.3 (95% CI: 0.2-8.9) for those without.10 In a recent multicenter prospective study in the US that enrolled 121 patients with acute CAT and platelet count <100x109/L at time of thrombosis, 19% of patients had major bleeding in the first 60 days (95% CI: 13-27). Notably, this trial enrolled only a minority of patients with solid tumors (N=36, 30%)23 In this post hoc analysis of a randomized controlled trial we found that in patients with predominantly mild thrombocytopenia, one-fourth of the patients developed clinically relevant bleeding (major bleeding or CRNMB) in the first 6 months after anticoagulation and the 180-day cumulative incidence of major bleeding was 8.9. This study represents the largest published cohort describing outcomes in patients with predominantly solid tumor diagnosis. These data highlight the importance of recognizing that even mild thrombocytopenia is a risk factor for major bleeding.
The safety of DOAC in patients with thrombocytopenia is largely unknown. In the TROVE study, three of 16 patients with CAT and thrombocytopenia treated with DOAC developed CRNMB (cumulative incidence of 20%; 95% CI: 0-40).23 According to the treatment protocol in the current study, edoxaban dosing was not held or dose adjusted during periods of thrombocytopenia. Similar to the overall study findings, increased risk of hemorrhage was associated with edoxaban compared with dalteparin in patients with GI malignancies.21 When considering major bleeding, the difference between thrombocytopenic patients with GI cancers on the edoxaban arm versus the dalteparin arm is greater (16.8 vs. 0; P<0.01). This suggests that the bleeding signal previously seen in GI cancers was influenced by the thrombocytopenic population. In contrast, we found that in patients with thrombocytopenia with underlying hematologic malignancies experienced significantly higher rates of major bleeding with dalteparin compared to edoxaban (19.0% vs. 0; P<0.01). There remains a need to conduct prospective, randomized trials to address the safety and efficacy of direct oral anticoagulants in these high-risk groups to generate quality evidence to guide clinicians.
Venous thromboembolism is associated with increased morbidity and mortality in cancer patients.3,24 The risk of recurrent VTE after a primary thrombotic event has been shown to be three to four times that of thrombosis in patients without cancer.1,5 In this study we demonstrate that although patients with thrombocytopenia had higher bleeding rates, the rate of recurrent thrombosis was not different compared to patients with platelets >100x109/L. This supports the growing evidence that thrombocytopenia is not protective for recurrent thrombosis in patients with active malignancy.25-27 Thus, the risk of hemorrhage needs to be balanced with a persistent risk of recurrent VTE when providers need to plan anticoagulation in this population. This study provides valuable insights by comparing bleeding and thrombosis rates in patients with cancer thrombosis with concomitant thrombocytopenia. The dataset is from a randomized controlled trial and thus leverages on strengths such as a prospective design, blinded validation of clinical outcomes and minimal attrition. However, we acknowledge that post hoc subgroup analyses of even high quality data has inherent limitations.28 Patients enrolled in clinical trials are often not representative of real-world scenarios.29 The HOKUSAI Cancer VTE study (as other similar trials) excluded patients with more severe thrombocytopenia at enrollment trial limiting our ability to study effects of more severe thrombocytopenia. This subgroup study had relatively few patients with thrombocytopenia (N=101). While rates of bleeding appear to be similar in the non-GI populations, we cannot conclude that outcomes are the same for DOAC versus low molecular weight heparin. In these analyses thrombocytopenia was defined based on the three earliest time points, however platelet counts in oncology populations are labile and thus we acknowledge these time points may not be reflective of platelet counts over the 180 days of the study period. Approximately half of cases were of mild-to-moderate thrombocytopenia in this study with platelet count in the 50-100x109/L range, and outcomes relative to severity of thrombocytopenia were not assessed due to limited sample sizes, and thus we are unable to make any conclusions on the safety of edoxaban in severe thrombocytopenia. These analyses were not prespecified, and patients in the two arms were not stratified by platelet count which should be recognized when evaluating the findings and the highlights the need for further prospective validation.
In conclusion, these post hoc analyses demonstrated that even mild thrombocytopenia was associated with a 2-fold risk of major bleeding. However, as consistent with prior retrospective studies, there was no concomitant decrease in recurrent thrombosis in cancer patients with thrombocytopenia. Thrombocytopenia is a frequent complication in patients with cancer and can have significant impact on outcomes of anticoagulation.
Funding Statement
Funding: No funding was received for the conduct of this study. JIZ is supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. RP is supported in part by a2021 Conquer Cancer Career Development Award. MS, MG and AD are employees of Daiichi Sankyo Pharma.
Data-sharing statement
Individual participant data that underlie the results reported in this article, after de-identification may be available subject institutional review board and sponsor approval with a data use agreement on publication. Please contact zwickerj@mskcc.org for further details.
References
- 1.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore). 1999;78(5):285-291. [DOI] [PubMed] [Google Scholar]
- 2.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164(15):1653-1661. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634. [DOI] [PubMed] [Google Scholar]
- 4.Al-Samkari H, Connors JM. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019;3(22):3770-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484-3488. [DOI] [PubMed] [Google Scholar]
- 6.Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133 Suppl 2:S63-69. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C, Patell R, Zwicker JI. The prevalence of thrombocytopenia in patients with acute cancer-associated thrombosis. Blood Adv. 2023;7(17):4721-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Samkari H, Connors JM. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Hematology Am Soc Hematol Educ Program. 2019;2019(1):71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuelson Bannow BT, Lee A, Khorana AA, et al. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(6):1246-1249. [DOI] [PubMed] [Google Scholar]
- 10.Khanal N, Bociek RG, Chen B, et al. Venous thromboembolism in patients with hematologic malignancy and thrombocytopenia. Am J Hematol. 2016;91(11):E468-E472. [DOI] [PubMed] [Google Scholar]
- 11.Kopolovic I, Lee AY, Wu C. Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol. 2015;94(2):329-336. [DOI] [PubMed] [Google Scholar]
- 12.Ay C, Beyer-Westendorf J, Pabinger I. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019;30(6):897-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. [DOI] [PubMed] [Google Scholar]
- 14.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017-2023. [DOI] [PubMed] [Google Scholar]
- 15.McBane RD, 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411-421. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599-1607. [DOI] [PubMed] [Google Scholar]
- 17.Hendriks T, McGregor S, Rakesh S, et al. Patient satisfaction after conversion from warfarin to direct oral anticoagulants for patients on extended duration of anticoagulation for venous thromboembolism - the SWAN Study. PLoS One. 2020;15(6):e0234048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C. Subcommittee on control of anticoagulation of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. [Google Scholar]
- 20.Campigotto F, Neuberg D, Zwicker JI. Accounting for death as a competing risk in cancer-associated thrombosis studies. Thromb Res. 2012;129(Suppl 1):S85-87. [DOI] [PubMed] [Google Scholar]
- 21.Kraaijpoel N, Di Nisio M, Mulder FI, et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: results from the Hokusai VTE Cancer Study. Thromb Haemost. 2018;118(8):1439-1449. [DOI] [PubMed] [Google Scholar]
- 22.Monreal M, Falga C, Valdes M, et al. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2006;4(9):1950-1956. [DOI] [PubMed] [Google Scholar]
- 23.Carney BJ, Wang TF, Ren S, et al. Anticoagulation in cancer-associated thromboembolism with thrombocytopenia: a prospective, multicenter cohort study. Blood Adv. 2021;5(24):5546-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850. [DOI] [PubMed] [Google Scholar]
- 25.Samuelson Bannow BR, Lee AYY, Khorana AA, et al. Management of anticoagulation for cancer-associated thrombosis in patients with thrombocytopenia: a systematic review. Res Pract Thromb Haemost. 2018;2(4):664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuelson Bannow BT, Walter RB, Gernsheimer TB, et al. Patients treated for acute VTE during periods of treatment-related thrombocytopenia have high rates of recurrent thrombosis and transfusion-related adverse outcomes. J Thromb Thrombolysis. 2017;44(4):442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Refaei M, Fernandes B, Brandwein J, et al. Incidence of catheter-related thrombosis in acute leukemia patients: a comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann Hematol. 2016;95(12):2057-2064. [DOI] [PubMed] [Google Scholar]
- 28.Bauchner H, Golub RM, Fontanarosa PB. Reporting and interpretation of randomized clinical trials. JAMA. 2019;322(8):732-735. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy-Martin T, Curtis S, Faries D, et al. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after de-identification may be available subject institutional review board and sponsor approval with a data use agreement on publication. Please contact zwickerj@mskcc.org for further details.