Abstract
We isolated several new mutator mutations of the Escherichia coli replicative polymerase dnaE subunit alpha and used them and a previously reported dnaE mutation to study spontaneous frameshift and base substitution mutations. Two of these dnaE strains produce many more mutants when grown on rich (Luria-Bertani) than on minimal medium. A differential effect of the medium was not observed when these dnaE mutations were combined with a mismatch repair mutation. The selection scheme for the dnaE mutations required that they be able to complement a temperature-sensitive strain. However, the ability to complement is not related to the mutator effect for at least one of the mutants. Comparison of the mutation rates for frameshift and base substitution mutations in mutS and dnaE mutS strains suggests that the mismatch repair proteins respond differently to the two types of change. Deletion of dinB from both chromosome and plasmid resulted in a four- to fivefold decrease in the rate of frameshift and base substitution mutations in a dnaE mutS double mutant background. This reduction indicates that most mistakes in replication occur as a result of the action of the auxiliary rather than the replicative polymerase in this dnaE mutant. Deletion of dinB from strains carrying a wild-type dnaE had a measurable effect, suggesting that a fraction of spontaneous mutations occur as a result of dinB polymerase action even in cells with a normal replicative polymerase.
Mutation is a characteristic of all living systems and provides the material for natural selection (43, 48). However, most mutations are deleterious, and organisms have evolved mechanisms to protect themselves from excessive mutation rates. These protective mechanisms recognize and correct mismatches that have occurred in DNA as a result of replication or spontaneous deamination and recognize and remove potentially mutagenic changes that have occurred as a result of the reaction of the DNA with endogenous or exogenous mutagens (21). The activity of these repair systems can be modulated, and one method of increasing the rate of mutations either permanently or transiently is to decrease the activity of the repair systems, e.g., mismatch repair (14). It has generally been assumed that the interaction of the error repair systems with the natural error rate of the replicative DNA polymerases satisfactorily accounts for mutation rates and their modulation (7). However, among the characteristics of biological systems are their complexity and the multilevels of control that they employ. Regulation, for example, is characterized by both accelerating and inhibiting or braking features, and this principle can be seen in systems as diverse as the regulation of the rate of the heartbeat in vertebrates (38) and the regulation of lactose utilization in bacteria (31). The recent discoveries of DNA polymerases which seem designed to produce a high frequency of errors should therefore not be viewed with surprise, but rather as another illustration of the redundancy with which organisms manage vital processes. What these recent discoveries do show is that the production of mutations by organisms is much more complex than might be supposed from in vitro models. The availability of variants of the various components of the mutational system makes it possible to understand something of the interactions of these components. In this study we have attempted to understand the interactions of the mismatch repair system, the replicative polymerase, and one of the newly discovered polymerases, DNA polymerase IV (pol IV), coded for by the dinB gene (49).
We originally assumed that Escherichia coli mutations on an ostensibly undamaged template occurred as a result of errors made by the replicative DNA polymerase III complex. It had already been demonstrated that mutations of the α subunit coded for by dnaE could have either mutator (22) or even antimutator (9, 10) effects. A major mutator effect is related to the interaction of the α subunit with the proofreading subunit ɛ, coded for by dnaQ. Maki et al. supposed that their polymerase mutator was deficient in its ability to complex with and activate the proofreader (22, 26), and Schaaper and his colleagues have shown that the products of certain dnaQ mutants are unable to interact properly with the polymerase (18, 41). While proofreading is certainly a key element, the fact that it plays a minor role in the surveillance of longer repeats (40, 46) prompted us to search for mutators with a different mode of action. We were guided in this search by the demonstration that mutations in two other polymerases, reverse transcriptase and DNA polymerase I, located away from the active site in the “thumb” domain (1, 25), could result in frameshifts in an in vitro system, presumably because of the loss of contacts which hold the to-be-replicated sequences in place and prevent folding. We therefore attempted to isolate dnaE mutants which produced excessive numbers of frameshifts. In our previous attempts to isolate such mutators (40) we treated P1 transducing phage with hydroxylamine and selected transductants at the closely linked metD marker, scoring for transversion mutators. We isolated 30 such mutants and sequenced 15; all turned out to have mutations of the proofreading subunit dnaQ, which is adjacent to and on the other side of the selected metD marker. In the present study we mutagenized a cloned dnaE located on a pBR322-derived plasmid by passage through a dnaQ mutS double mutant. The mutagenized dnaE was then forced to complement a temperature-sensitive chromosomal dnaE, and the resulting colonies were screened for mutator properties. Identified mutators were confirmed by site-directed mutagenesis and transferred to the chromosome for further tests. Since all the mutations made by mutant polymerases are presumably checked by the mismatch repair system, we thought it necessary to determine the spontaneous mutation rates of the mutators in a mismatch repair-deficient background. While this work was in progress, it was reported that E. coli produced auxiliary polymerases which were involved in frameshift mutation (20, 42, 49). We therefore studied the effect of deletion of one of these polymerases, the dinB polymerase (12, 17), on mutations in dnaE mutator strains. In this paper we show that mutations of the dnaE gene leading to mutator properties interact differently with mutS for the production of base substitutions and of frameshifts. We also show that deletion of the dinB gene results in about a 75% decrease in spontaneous frameshift and base substitution mutations in a dnaE mutS double mutant and a similar decrease in a dnaE+ strain. Our findings suggest a general role for the auxiliary DNA polymerase in spontaneous mutation.
MATERIALS AND METHODS
Strains.
The bacterial strains used in this study are shown in Table 1. The temperature-sensitive dnaE74 allele (formerly called polC74 [3]) was obtained from R. Moses. This allele is the result of a GGA (Gly)→AGA (Arg) substitution at codon 134 (R. Woodgate, personal communication). dnaE74 was transferred by transduction using the closely linked metD mutation for selection (40). Plasmid pDDS7-11, containing a cloned dnaE gene (44), was provided by Charles McHenry. dnaE mutations were transferred from plasmid to chromosome as described by Murphy (27); plasmid isolated by site-directed mutagenesis of pDDS7-11 (44) was restricted with EcoRI (New England BioLabs) following the directions of the manufacturer, and the 4.2-kb linear fragment containing the dnaE gene was isolated and transformed by electroporation into BS40dnaE74(Ts)(G134R)/F′CC107 carrying the pTP223 plasmid (27, 29). Transformants were selected by incubation at 40°C on tetracycline-containing medium. Purified clones were checked for ampicillin sensitivity and for mutability by spot tests on rifampin-containing medium. DNA from putative mutators was isolated, checked for the absence of detectable plasmid by electrophoresis, and amplified using the F2 and B4 primers (see below). This amplified DNA was gel extracted and sequenced to confirm the putative mutation and to make sure that no wild-type allele was present. The pTP223 Tetr plasmid was removed by counterselection on fusaric acid plates (23) or, more commonly, the chromosomal dnaE was transferred to BS40 by P1 transduction, selecting for metD+. recA-deficient derivatives of dnaE74 were prepared by transduction with P1 phage grown on a recA938(Camr) strain (52).
TABLE 1.
Bacterial strains used
| Strain | Genotype | Reference(s) or source |
|---|---|---|
| dnaE74 (formerly called polC74) | dnaE74(Ts) (G134R) | R. Moses (3, 37) |
| BS40 | metB metD Δ(proAB lac) rpsL (Smr) | 40 |
| BS40metD+ | metB Δ(proAB lac) rpsL (Smr) | From BS40 by transduction |
| CC107 | ara Δ(lac proB) XIII/F′ lacIZ proB+ | 5 |
| CSH143 | ara Δ(gpt-lac)5 gyrA/F′ lac proA+B+ | 24 |
| MutS | lacZ53(Am) λ−mutS215::Tn10 (Tetr) thyA36 IN(rrnD-rrnE)1 rha-5 metB1 deoC2 | E. coli Genetic Stock Center (39) |
| FC755 | araD Δ(lac-proB)XIIIthi/F′ Δ (lacIZ) pro+ | R. Schaaper and P. Foster |
| DB1319 | λ−tyrT58(AS)trp-89::Tn5 recA938 (Camr)::Tn9-200 recD104(Nuc−) hsdR2 mdoB202::Tn10 | E. coli Genetic Stock Center (52) |
| S90C | ara Δ(lac proB)XIIIrpsL(Smr) | J. Miller |
| BS40dnaE74 | metB Δ(proAB lac) dnaE74(Ts) rpsL | This study |
| BS40dnaE173 | metB Δ(proAB lac) dnaE173 (E612K) rpsL | 22, 26 |
| BS40dnaE1336 | metB Δ(proAB lac) dnaE1336 (F347I) rpsL | |
| BS40dnaE1337 | metB Δ(proAB lac) dnaE1337 (D630G) rpsL | |
| BS40dnaE1338 | metB Δ(proAB lac) dnaE1338 (K655R) rpsL | |
| BS40dnaEn/F′CC107 | metB Δ(proAB lac) dnaEn rpsL/F′ lacIZ proB+ | |
| BS40dnaEn/F′CSH143 | metB Δ(proAB lac) dnaEn rpsL/F′ lac proA+B+ | |
| BS40mutSdnaEn/F′CC107 | metB Δ(proAB lac) mutS215::Tn10 dnaEn rpsL/F′ lacIZ proB+ | This study, by transduction |
| BS40mutSdnaEn/F′CSH143 | metB Δ(proAB lac) mutS215::Tn10 dnaEn rpsL/F′ lac proA+B+ | This study, by transduction |
| BS40dnaE74(Ts) ΔrecA::cam/F′CC107 | metB Δ(proAB lac) dnaE74(Ts) rpsL recA::cam/F′ lacIZ proB+ | This study, by transduction from DB1319 |
| YG7207 (PF2145) | AB1157 ΔdinB::kan | 20 |
| FC1237 | ara Δ(lac proB) XIII ΔdinB::kan | P. Foster |
| PFI173 CC107ΔdinB | ara Δ(lac proB) XIII Δ dinB::kan/F′ lacIZ proB+ ΔdinB::kan | P. Foster |
| BS40ΔdinB/F′CC107ΔdinB | metB Δ(proAB lac) rpsL ΔdinB::kan/F′ lacIZ proB+ ΔdinB::kan | This study |
| BS40ΔdinB/F′CSH143ΔdinB | metB Δ(proAB lac) dnaE630 rpsL ΔdinB::kan/F′ lac proA+B+ ΔdinB::kan | |
| BS40mutSΔdinB/F′CC107ΔdinB | metB Δ(proAB lac) rpsL ΔdinB::kan/F′ lacIZ proB+ ΔdinB::kan | |
| BS40mutSΔdinB/F′CSH143ΔdinB | metB Δ(proAB lac) dnaE630 rpsL ΔdinB::kan/F′ lac proA+B+ ΔdinB::kan | |
| BS40dnaE1336ΔdinB/F′CC107ΔdinB | metB Δ(proAB lac) dnaE1336 rpsL ΔdinB::kan/F′ lacIZ proB+ ΔdinB::kan | |
| BS40dnaE1336ΔdinB/F′CSH143ΔdinB | metB Δ(proAB lac) dnaE1336 rpsL ΔdinB::kan/F′ lac proA+B+ ΔdinB::kan | |
| BS40mutSdnaE347ΔdinB/F′CC107ΔdinB | metB Δ(proAB lac) dnaE1336 rpsL ΔdinB::kan/F′ lac proA+B+ ΔdinB::kan | This study |
| BS40mutSdnaE347ΔdinB/F′CSH143ΔdinB | metB Δ(proAB lac) dnaE1336 rpsL ΔdinB::kan/F′ lac proA+B+ ΔdinB::kan |
The CSH143 F′ factor with dinB deleted was constructed following the directions of Pat Foster (personal communication). This method requires transduction of a strain containing the F′ factor from which dinB is to be deleted with P1 phage grown on a ΔdinB::kan strain and selecting for kanamycin resistance. Resistant transductants are gridded and “plate mated” to a recipient strain on a medium which selects recipients into which kanamycin resistance has been transferred. The ΔdinB F′ plasmid is then transferred by a second conjugation into the desired strain. We used PCR with dinB primers located either within the deleted region or just proximal (see below) to demonstrate the presence of the dinB gene in the original strain, its absence in the ΔdinB strains, and the presence of the kan insert in the deleted strain (data not shown).
Media.
The media and general methods used are as described by Miller (24). The medium for the detection of dnaE mutators contained M63 salts, MgSO4 (1 mM), 0.2% glucose, 0.2% lactose, thiamine hydrochloride (100 μg/ml), d-methionine (20 μg/ml), streptomycin (100 μg/ml), ampicillin (100 μg/ml), and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, 40 μg/ml). Plates for the determination of resistance to rifampin contained 100 μg of rifampin per ml in Luria-Bertani (LB) medium buffered by the addition of 1× A salts (24). Plates for the determination of reversion from Lac− to Lac+ contained M63 minimal salts supplemented with MgSO4, thiamine hydrochloride (100 μg/ml), and d-methionine (10 μg/ml) and with filter-sterilized lactose (0.2%) as the sole sugar.
Site-directed mutagenesis.
Site-directed mutagenesis was accomplished using the Stratagene QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). Primers (Table 2) were designed to incorporate the desired mutation and a nearby silent mutation creating a new restriction site so that the presence of a mutagenized dnaE could be quickly detected. Sample mixes included 5 μl of 10× reaction buffer, 5 to 50 ng of pDDS711 as a double-stranded DNA template, 125 ng of each primer, and its complement, 1 μl of deoxynucleoside triphosphate (dNTP) mix, double-distilled H2O to a final volume of 50 μl, and 2.5 U of PfuTurbo DNA polymerase, all overlaid with 50 μl of mineral oil as described in the protocol provided. Thermal cycling included an initial denaturation step of 95°C for 30 s, followed by 18 cycles of 30 s at 95°C, 1 min at 55°C, and 12.5 min at 68°C. Samples were kept at 4°C until the addition of DpnI. Digestion of the methylated strand with DpnI and transformation into E. coli XL1-Blue supercompetent cells were performed as described by the manufacturer.
TABLE 2.
dnaE mutations and primers for site-directed mutagenesis
| Mutation | Change(s) | Primera | Silent restriction site |
|---|---|---|---|
| dnaE1336 | 347Phe→Ile (TTT→ATT) | 5′-GGC TAC TTC CTC ATC GTT ATG GAA ATT ATT CAG TGG TCG AAA GAT AAC GGC-3′ | XmnI |
| dnaE1337 | 630Asp→Gly (GAT→GGT), 159Glu→Glu | 5′-GAC TGC TTC GAA GGT ATG ATC GCA CTA GTG GCA CTG-3′ | SpeI |
| dnaE1338 | 655Lys→Arg (AAA→AGA) | 5′-GAT AAC TTT ATC GAC CGT AGA CAC GGC CGT GAA GAG ATC TCC-3′ | EagI |
| 655Lys→Gly (AAA→GGC) | 5′-G GAT AAC TTT ATC GAC CGT GGC CAT GGT CGT GAA GAG ATC TCC-3′ | NcoI | |
| dnaE173 | 612Glu→Lys (GAA→AAA) | 5′-G GTA TTC CAG CTT AAA TCA CGT GGC ATG AAG GAC CTG ATC AAG CG-3′ | Eco721 |
One member of a complementary primer pair is shown.
Sequencing.
Sequencing was done by the University of Chicago Cancer Research Center DNA sequencing facility using an ABI Prism 377 DNA sequencer. Primers used for sequencing include 5′-ATACAGGATAATGCCGTAGGT-3′ for the dnaE1338 and dnaE1337 mutations, 5′-ACCCTCGACGATCCTAAA-3′ for the dnaE1336 mutations, 5′-CGGTGGGGTGGTTATCGC-3′ for the dnaE173 mutations, and 5′-TTGTGGTCTGGTGAAGTTCTA-3′ for dnaE-74(Ts).
PCR.
Amplification of the dnaE gene was performed using primers F2 (5′-GGA AAA ACT GGC TGA ACA CGC-3′, starting 121 bases 5′ to the starting ATG of dnaE) and B4 (5′-AGC TCT GCA ATC GGC TGT TC-3′, starting 58 bases 3′ to the last codon of dnaE). Primers for dinB were for uninterrupted genes 5′-TTATGCCCACATCTCACCTTGC-3′ (starting 193 bases 3′ to the starting ATG and proceeding into the gene) and 5′-AATCCCAGCACCAGTTGTCTTTC-3′ (starting 4 bases 5′ to the terminating TGA and proceeding into the gene). For deletions of dinB by the addition of Kanr, we used the primers 5′-TTTTTCGCCGCAGTGGAGA-3′ (starting 34 bases 3′ to the starting ATG and proceeding into the gene) and 5′-AAGCATGTCCATTAACGCTTCG-3′ (starting 219 bases 3′ to the dinB termination codon and heading into the dinB gene. The primer is in an intergenic region with the next upstream gene being yafN, which starts 138 bases from the 5′ end of the primer. PCR was carried out in a 50-μl reaction mixture using 1 U of Platinum Taq DNA polymerase high fidelity (Life Technologies), samples, 5 μl of 10× buffer, 1 μl of 10 mM dNTP mix, 2 μl of 50 mM MgSO4, 125 ng of each primer, double-distilled H2O to 50 μl, and 50 μl of mineral oil overlay. Thermal cycling included an initial denaturation step of 95°C for 120 s, followed by 35 cycles of 40 s at 95°C, 40 s at 50°C, and 4 min at 68°C. Following thermal cycling, the samples were kept at 4°C.
Determination of mutation rates.
Individual cultures in 1.5 ml of LB or minimal medium were incubated overnight with shaking. The cultures were then plated directly or after dilution in phosphate-buffered saline onto plates containing rifampin (for resistance determination) or onto minimal plates with lactose as a sugar. All tests for reversion to Lac+ were carried out in the presence of FC755 scavenger cells, and the cultures were incubated for 2 days before counting (32). We determined that 10-fold dilutions of the cultures resulted in 10-fold decreases in the number of revertants obtained. The mutation frequencies were determined, and a mutation rate was calculated by reiteratively solving the equation μ = 0.4343f/log(Nμ), where f is the median mutation frequency from at least 16 replicates (28 replicates in several of the ΔdinB comparisons) and N is the population size (6). The data were analyzed by applying the nonparametric Wilcoxon rank sum test to the experimentally determined mutation frequencies. An estimate of the ratio of the mutation frequencies (not rates) was determined by calculating the difference in the logarithm of the geometric means and transforming back to the original scale.
RESULTS
In order to isolate mutator mutants of the dnaE gene, we transformed a dnaQ mutS strain of E. coli (40) with a plasmid which carries a cloned dnaE within EcoRI restriction sites. After growth, typically 2 days for these very slow growing strains, the mutagenized plasmid was harvested and used for the transformation of strain BS40dnaE74(Ts)/F′CC107, a strain carrying a temperature-sensitive dnaE allele and containing the CC107 F′ factor designed by Cupples et al. for the detection of +1G frameshift mutations (5). Transformants were plated on medium with ampicillin and X-Gal at 37°C and incubated long enough for the appearance of blue papillae (about 3 days). Putative mutators were recognized by the appearance of multiple papillae in a short time, and such colonies were picked and purified. The mutator activity was checked by measurement of reversion to Lac+ and by transfer of mutator activity with plasmid isolated from the mutator strain. We sequenced the putative mutators and in each case found multiple changes, including silent mutations. In addition, some of the mutant plasmids appeared to be heterozygotes; that is, we found both wild-type and mutant sequences. We had used a Rec+ strain to detect mutation because of the unknown effects on mutation that might result from the use of a Rec− strain. Probably as a result of our strains being Rec+ (15), it appeared on gel analysis that the plasmid had dimerized, accounting for the simultaneous presence of two alleles. Restriction of plasmid with an enzyme making a single cut in a monomer followed by ligation permitted us to isolate homozygous plasmids which transferred mutator activity. We next introduced each of the identified changes separately by site-directed mutagenesis into the wild-type dnaE allele carried in the pDDS7-11 plasmid. Such syntheses permitted us to identify a single change responsible for the mutator effect. Reconstruction of the mutation by site-directed mutagenesis also permitted us to insert a nearby silent mutation, creating a new restriction site as an aid to recognition of the allele in future gene transfer experiments (Table 2). We identified three new dnaE mutator alleles: dnaE1336 (F347I), dnaE1337 (D630G), and dnaE1338 (K655R) (allele numbers have been assigned by M. Berlyn of the E. coli Genetic Stock Center).
Characteristics of dnaE mutants.
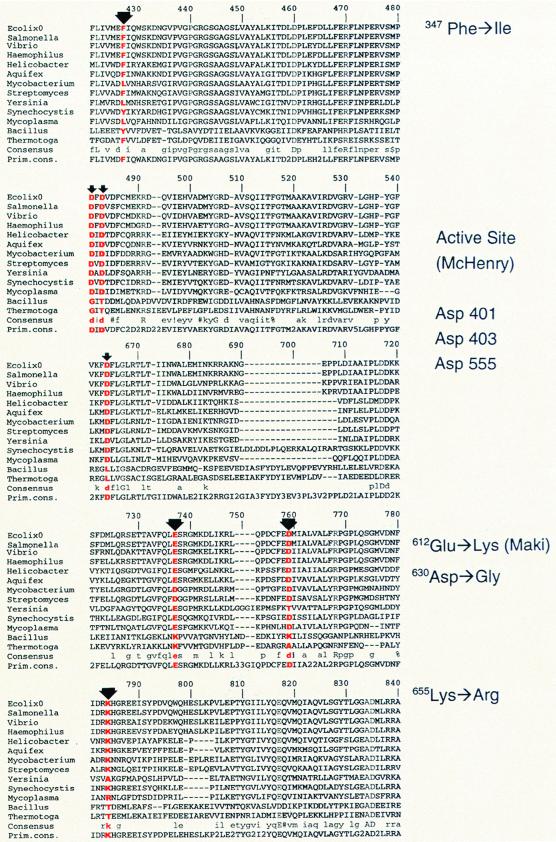
Until the recent discovery of auxiliary polymerases, the DNA polymerases were classified into four families (30): polymerases homologous to E. coli pol I, eukaryotic pol alpha, eukaryotic pol beta and terminal transferase, and E. coli pol III. The pol III family is the sole member without a crystallized representative and until recently had few if any recognized homologs. The rapid progress in whole-genome sequencing has resulted in the identification of numerous microbial homologs (30). We used the Multalin program (4) to align 13 polymerases in order to ascertain the possible importance of the mutator sites and to compare their locations with the active-site aspartic acid residues identified at E. coli dnaE codons 401, 403, and 555 (30). The three mutators that we identified and the previously reported dnaE173 (E612K) (22) were all located by the program at highly conserved sites (Fig. 1). The active sites are similarly conserved and anchor the alignment. (Because the alignment creates gaps, the numbers assigned to sites by the program [Fig. 1] are not related to the numbers of E. coli dnaE codons.) Since the crystal structure of the alpha subunit is not yet available, it is not possible to say anything about the structure or about the possible binding sites to the proofreading subunit. Antimutator sites are present at E. coli dnaE codons 357, 395, 464, 498, 603, 703, and 752, and these have been suggested as possible sites for interaction with the ɛ (proofreading) subunit (9, 10, 28).
FIG. 1.
Alignment of the E. coli DNA pol III alpha subunit structure with 12 other prokaryotic sequences. Only that portion of the sequence surrounding the sites of the mutator mutants and the active sites (30) is shown. Alignment was done by the Multalin program (4), accessed at http://pbil.ibcp.fr/NPSA. Alignment numbers at the top of the sequence do not refer to E. coli dnaE codons but were assigned by the program to include the gaps required to align all the sequences. The thicker arrows mark the positions of the mutator dnaE alleles used in this study: dnaE1336 (F347I), dnaE1337 (D630G), dnaE1338 (K655R), and dnaE173 (E612K). Thin arrows indicate the position of the active-site aspartic acid residues as defined by Pritchard and McHenry (30). The alleles indicated by the arrows are listed in the margins, with the E. coli dnaE codon numbers given.
Complementation and the mutator effect.
Because dnaE1338 had the greatest mutator effect, we wanted to know whether its arginine substitution for lysine was unique in its ability to produce a mutator effect at position 655 while still complementing the temperature sensitivity of dnaE74. We therefore prepared a set of pDDS7-11 derivatives with different amino acids (and a different neighboring restriction site, NcoI) at this position (Table 2). These plasmids were transformed into BS40dnaE74(Ts)/F′CC107 and BS40dnaE74(Ts)ΔrecA(Camr)/F′CC107, and the transformants were tested for growth at 30 and 40°C (Table 3). We constructed strains with a deletion in the recA gene to inhibit recombination between chromosome and plasmid and to make sure that the mutations we measured were not SOS dependent. The recA phenotype was accompanied, as expected, by extreme UV radiation sensitivity. We found that plasmids carrying dnaE with either alanine or arginine instead of lysine at position 655 were able to complement the dnaE74(Ts) mutation in either the rec+ or ΔrecA background. Plasmids carrying dnaE with asparagine, glycine, or tyrosine instead of lysine at position 655 gave strains with about 1% survival in a recA+ strain and 0.01% or less in a recipient with the recA function deleted. We assume that the survivors in the recA+ strains are the result of recombination between the dnaE mutation carried on the plasmid and the dnaE74 on the chromosome to produce a chromosomal wild type. The difference between recA-proficient and -deficient strains carrying a plasmid encoding Asn, Gly, or Tyr substitutions implies that the polymerases with these substitutions at position 655 are unable to function at 40°C. We do not know whether they can function at 30°C. Subcultures of the survivors at 40°C gave cultures which grew as well at 40 as at 30°C, indicating their probable origin as recombination products.
TABLE 3.
Mutations induced by different amino acid substitutions in dnaE codon 655a
| Strain | recA | Plasmid | Survival (N40°C/N30°C) | Lac−→Lac+ (f× 106) | Rifs→Rifr (f× 106) |
|---|---|---|---|---|---|
| BS40dnaE74/F′CC107 | + | None | 2 × 10−5 | 0.07 | 0.18 |
| − | 8 × 10−6 | 0.04 | 0.52 | ||
| BS40dnaE74/F′CC107 | + | Lys (WT) | 1.07 | 0.092 | 0.017 |
| − | 1.12 | 0.28 | 0.007 | ||
| BS40dnaE74/F′CC107 | + | Arg | 1.33 | 24.2 | 8.4 |
| − | 0.61 | 92 | 4.5 | ||
| BS40dnaE74/F′CC107 | + | Ala | 0.84 | 4.4 | 0.87 |
| − | 0.85 | 11.5 | 1.0 | ||
| BS40dnaE74/F′CC107 | + | Asn | 0.027 | 96 | 42.5 |
| − | 9.5 × 10−5 | 29 | 32.3 | ||
| BS40dnaE74/F′CC107 | + | Gly | 0.011 | 58 | 18 |
| − | 5.8 × 10−5 | 52 | 14.9 | ||
| BS40dnaE74/F′CC107 | + | Tyr | 2.2 × 10−4 | 33 | 20.1 |
| − | 6.2 × 10−7 | — | 8.2 |
Cultures were transformed with mutagenized plasmids and selected on ampicillin-containing medium at 30°C. Transformants were grown overnight at 30°C in 1.5 ml of LB medium and then plated on LB plates for viable-cell count determination and on minimal/lactose or rifampin plates for determination of mutation frequency. Plates for viable-cell count were incubated at 30 and 40°C, and the surviving fraction (N40°C/N30°C) was calculated from these counts. Plates for the determination of mutation frequency were incubated at 30°C.
We determined the mutation frequency to rifampin resistance (base substitution) and from Lac− to Lac+ (frameshifts) in cultures carrying the dnaE-substituted plasmids in a dnaE74(Ts) chromosomal background incubated overnight in LB medium at 30°C and then plated at 30°C (Table 3). We observed a large increase in mutation frequency in strains carrying Arg, Ala, Asn, Gly, or Tyr substitutions at position 655. The increase in mutation frequency was even greater in cultures carrying the noncomplementing Asn, Gly, or Tyr dnaE substitutions than in the complementing Arg and Ala substitutions. We also grew the set of BS40/F′CC107/pDDS7-11 dnaE (Ampr) (both recA proficient and deficient) strains overnight at 37°C and then determined mutation frequency by plating at 40°C (Table 4). Plasmids carrying dnaE with an arginine substitution had a slight dominant effect, but only plasmids carrying a tyrosine substitution at dnaE position 655 produced large numbers of mutations. In contrast to plasmids carrying dnaE with the other substitutions, cells with either a wild-type or dnaE74(Ts) chromosomal constitution carrying the tyrosine substitution gave smaller colonies and produced only about 30% as many cells as did control or cells carrying the wild-type plasmid after overnight growth on LB medium.
TABLE 4.
Mutator effect of amino acid substitutions in dnaE codon 655 carried on a plasmid in a dnaE+ hosta
| Substitution encoded by plasmid | Mutation frequency (106)
|
|
|---|---|---|
| Lac−→Lac+ | Rifs→Rifr | |
| None (no plasmid) | 0.24 | 0.01 |
| None (Lys) | 0.25 | 0.02 |
| Arg | 1.88 | 0.3 |
| Ala | 0.76 | 0.14 |
| Asn | 1.3 | 1.34 |
| Asp | 0.23 | 0.02 |
| Gly | 0.33 | 0.021 |
| Tyr | 21.9 | 8.45 |
Plasmids containing mutant dnaE1338 alleles were transformed into BS40/F′CC107 and selected for ampicillin resistance. Purified cultures were grown overnight in 1.5 ml of LB medium at 37°C and plated at 40°C for the determination of mutation.
Interaction of dnaE and mutS.
Given the nature of the pol III holoenzyme with multiple polymerase subunits (19), any studies on mutation rate using a mutator carried on a plasmid are compromised by the inability to separate possible effects resulting from interaction of chromosomal subunits with mutator polymerase coded for by the plasmid, much as shown above. This is especially so because dnaE74 was initially identified as a mutator (3, 37) even though in our hands mutator effects were minimal. We therefore transferred the mutators to the chromosome as described in Materials and Methods. The resulting strains carried no detectable plasmid and were ampicillin sensitive, and PCR amplification gave a single band of the expected size, indicating that the allele had been integrated properly at the dnaE locus (data not shown).
The dnaE mutators present on the chromosome were divided into two classes on the basis of their production of mutations when grown on different media (Table 5). Compared to growth on minimal medium, growth on rich (LB) medium produces at most a twofold increase in mutation frequency for dnaE1336 and dnaE1337. In contrast, dnaE1338 and the Maki mutant dnaE173 produce 25 to 90 times more mutants on rich than on minimal medium. This behavior is reminiscent of some dnaQ proofreading mutations, which make large numbers of mutations only when grown on rich medium. Schaaper and Radman suggest that the rapid growth on rich medium compared to minimal medium results in the production of such large numbers of mutants in the proofreading-defective strains that mismatch repair becomes saturated (35). We therefore prepared the mutS double mutants and compared the dnaE1338 and dnaE173 mutants with the dnaE1338 mutS and dnaE173 mutS double mutants after growth on minimal and LB media. Many more mutants were produced by the mutS dnaE1338 double mutants than by the dnaE1338 mutant, but the growth medium did not have the same effect (Table 5). The behavior of the dnaE173 mutS is harder to understand. The frequency of +1G frameshifts in a run of six G's (F′CC107 Lac−→Lac+) is more than 10 times lower in the mutS dnaE173 double mutants after growth in LB than in minimal medium. The results with base substitution mutants, whether on the chromosome (Rifs→Rifr) or on an episome (F′CSH143 Lac−→Lac+) are simpler to summarize. Mutation frequency is much higher for dnaE173 (30 times) and dna1338 (100 times) on rich medium (LB) than on minimal medium. The mutS dnaE double mutants have mutation rates that are modestly higher, but there is not a large difference between the results on minimal and on LB medium. The observations on base substitution mutations suggest that the dnaE173 strain is functionally mismatch repair deficient and the dnaE1338 strain somewhat less so when grown on rich (LB) but not on minimal medium.
TABLE 5.
Effect of growth medium on mutationa
| Median mutation frequency (106)
|
||||
|---|---|---|---|---|
| Strain | Lac−→Lac+
|
Rifs→Rifr
|
||
| Minimal | LB | Minimal | LB | |
| BS40/F′CC107 | 0.38 | 0.38 | 0.067 | 0.024 |
| BS40mutS/F′CC107 | 240 | 380 | 0.67 | 1.1 |
| BS40dnaE1336/F′CC107 | 0.63 | 1.0 | 0.23 | 0.42 |
| BS40dnaE1337/F′CC107 | 0.83 | 0.97 | 0.047 | 0.094 |
| BS40dnaE1338/F′CC107 | 1.6 | 39 | 0.05 | 4.8 |
| BS40dnaE1338mutS/F′CC107 | 1,100 | 850 | 13 | 47 |
| BS40dnaE1338mutS/F′CSH143 | 28 | 18 | 43 | 58 |
| BS40dnaE173/F′CC107 | 15 | 1,300 | 0.92 | 26 |
| BS40dnaE173mutS/F′CC107 | 6,500 | 470 | 56 | 70 |
| BS40dnaE173mutS/F′CSH143 | 2.5 | 1.8 | 19 | 33 |
Recorded median of three values for each entry except BS40dnaE1338mutS/F′CC107 and BS40dnaE173mutS/F′CC107, for which the value is the median of seven replicates.
We determined the spontaneous mutation rates for both base substitutions and frameshifts in both dnaE and mutS dnaE double mutants (Table 6). Although mutation from Rifs to Rifr occurs by base substitution as the result of a change in the chromosomal rpoB gene (24), there is the possibility that mutagenesis on an F factor is not identical to the process on the chromosome. We therefore decided to compare base substitution and frameshift mutations in genes which were both located on similar F factors. Reversion to Lac+ of strains carrying the CC107 F′ factor occurs by addition of 1 G to a run of six G's (5). Reversion to Lac+ of strains carrying the CSH143 F′ factor occurs by one of at least eight base changes (24). We therefore used strains isogenic except for the different F′ factors for these experiments.
TABLE 6.
Spontaneous mutation rates for dnaE alleles
| Strain | Mutation rate (106)
|
|||
|---|---|---|---|---|
| Base substitutions
|
Frameshifts
|
|||
| mutS+ | mutS | mutS+ | mutS | |
| BS40 | 0.011 | 0.68 | 0.048 | 34.4 |
| BS40dnaE1336 | 0.25 | 7.6 | 0.34 | 61.0 |
| BS40dnaE1337 | 0.077 | 5.2 | 0.20 | 81.1 |
| BS40dnaE1338 | 6.0 | 8.8 | 20 | 137 |
| BS40dnaE173 | 3.2 | 0.44 | 120 | 217 |
Cultures were incubated overnight at 37°C in 1.5 ml of LB medium and then plated for viability and mutation determinations. At least 16 independent mutation frequencies were determined for each. Mutation rates were calculated by the method of Drake (6).
Introduction of a mutS allele into either of the two dnaE mutants sensitive to rich medium, the dnaE173 and dnaE1338 mutants, does not increase the base substitution mutation rate (Table 6). For dnaE1338, the rates for the single and double mutants were almost indistinguishable. Using dnaE173, the mutation rate for base substitution appeared, in this series of 24 replicate cultures each, to be much lower in the mutS dnaE double mutant than in the dnaE mutant. Since the previous experiments (Table 5) had indicated that these dnaE mutants were functionally mismatch repair deficient for base substitutions, this result is not surprising, although we do not understand why dnaE173 mutS should have a lower mutation rate than dnaE173. In contrast, introduction of a mutS allele into the wild type or either the dnaE1336 or dnaE1337 mutant results in a 30- to 67-fold increase in the mutation rate for base substitution mutations, as would be expected if mismatch repair corrects errors which have escaped proofreading (Table 6).
Introducing a genetic mismatch repair deficiency produces a different result when considering frameshifts. Most obviously, introduction of a mismatch repair deficiency increases the frequency of frameshifts 5 to 10 times more than it does base substitutions. This difference may result from the specific target sequences employed or may reflect the intrinsic instability of nucleotide runs. In general, frameshift mutations, particularly in runs of G (33), are more frequent than base substitutions in the Cupples-Miller plasmids (5). Even though the dnaE1338 mutant is functionally mismatch repair deficient for base substitution mutations, there was a sevenfold increase in mutation rate for frameshifts when comparing dnaE1338 mutS to dnaE1338 (Table 6) There was a decrease in the base substitution rate resulting from the introduction of mutS into dnaE173 but an almost twofold increase in the mutation rate for frameshifts. Introduction of a dnaE1336 or a dnaE1337 mutation into a mutS background makes a 6- to 12-fold difference in the rate of base substitution mutations but only a 2- to 2.5-fold increase in the rate of frameshifts. Introduction of a dnaE173 mutation into a genetically mutS strain results in a sixfold increase in the rate of frameshifts but decreases the number of base substitutions (Table 6).
Interaction of dnaE and dinB.
The experiments above indicate that mutations in dnaE can increase the frequency of frameshift mutations. However, recent observations implicate another gene product, the dinB polymerase (pol IV), in the production of frameshifts (20, 49, 50; S. R. Kim, K. Matsui, M. Yamada, P. Gruz, and T. Nohmi, Proc. Am. Soc. Microbiol. Conf. DNA Repair Mutagenesis, 1999, abstr. p. 94). We therefore decided to determine the effect of inactivating dinB on mutation. Most of our previous studies utilized the Cupples-Miller CC107 F′ factor, which reverts by the addition of a G to a run of six G's, and this F′ factor contains a copy of the dinB gene. dinB is located at 5.4 min on the E. coli chromosome, and lac is at 7.8 min (2). Presumably the same event inserted both lac and dinB genes into the episome. Our strains therefore carry two copies of dinB, one on the chromosome and one on the F′ factor. We obtained F′CC107 with the dinB gene deleted from P. Foster, and we constructed a strain with dinB deleted from F′CSH143 (see Materials and Methods). Both ΔdinB plasmids were introduced into a strain with its chromosome carrying ΔdinB. The deletion (or rather substitution of the Kanr cassette for dinB), as confirmed by PCR analysis and sequencing (data not shown), runs from a position 158 bases 3′ to the dinB start codon to a position 94 bases 3′ to the termination codon of the dinB gene (20).
Deletion of dinB results in a statistically significant lowering of both frameshift and base substitution mutation rates for the wild type (dnaE+ mutS+) and dnaE1336 and dnaE1336 mutS strains but not for the dnaE+ mutS strain (Table 7). These rates were calculated from the median of at least 28 independent frequency determinations for the mutS and dnaE mutS strains and at least 18 determinations for the wild-type and dnaE strains. Deletion of dinB from the BS40 dnaE1336 mutS double mutant resulted in a fourfold decrease in F′ factor mutation rates for both frameshifts and base substitutions and a two- to threefold decrease for the chromosomal Rifr mutations.
TABLE 7.
Effect of deletion of the dinB gene from both chromosome and episome on mutation ratesa
| Strain | Mutation rate (106)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Lac−→Lac+
|
Rifs→Rifr
|
|||||||
| Frameshift
|
Base substitution
|
F′CC107
|
F′CSH143
|
|||||
| dinB+ | ΔdinB | dinB+ | ΔdinB | dinB+ | ΔdinB | dinB+ | ΔdinB | |
| BS40 | 0.048 | 0.03 | 0.01 | 0.004 | 0.007 | 0.002 | 0.004 | 0.002 |
| BS40dnaE1336 | 0.34 | 0.13 | 0.247 | 0.14 | 0.076 | 0.03 | 0.076 | 0.045 |
| BS40mutS | 34.4 | 32.4 | 0.685 | 0.51 | 0.187 | 0.178 | 0.197 | 0.128 |
| BS40dnaE1336mutS | 61.0 | 14.3 | 6.68 | 1.48 | 1.28 | 0.641 | 2.00 | 0.576 |
Values for lac mutations in dinB+ strains use the same data as given in Table 6. The data were examined statistically as described in Materials and Methods. The probability of a difference in the mutation frequency with and without dinB was highly significant (P < 0.001) for all comparisons except BS40mutS/F′CC107 (P = 0.6835) and BS40mutS/F′CSH143 (P = 0.0958). The probabilities for BS40/F′CC107 (P = 0.0055) and BS40dnaE1336/F′CSH143 (P = 0.0109) were higher but still significant.
DISCUSSION
Changes producing the mutator effect are in dnaE, but the increased number of mutations observed is not necessarily due to an increased number of polymerization errors made by the product of the mutant dnaE gene.
The dnaE mutants studied fall into two classes, based on their reaction to rich and minimal media. dnaE1336 and dnaE1337 mutants give a modest increase in mutation rate over the wild type when grown on minimal or complex medium. The second group, dnaE1338 and dnaE173 mutants (22), also give a modest increase in mutation rate over the wild type when grown on minimal medium but a very large increase when grown on complex medium. A possible explanation for our data is that the two mutants in this second class each interact with the proofreading subunit (the ɛ subunit) in a way which does not promote efficient proofreading. Maki et al. (22) considered this hypothesis as a result of finding decreased capacity for proofreading in pol III core derived from dnaE173 when coupled to DNA synthesis. The dnaE1338 and dnaE173 mutant strains are functionally mismatch repair deficient on rich but not on minimal medium (Table 5). This behavior is similar to that of certain dnaQ mutants described by Schaaper and Radman (35). Why there should be a difference between rich and minimal medium is not clear. Schaaper (34) indicates that simple explanations due to different growth rates on the two media are not sufficient. We suppose that amino acid position 655 (dnaE1338) is important for both catalysis (measured by complementation ability) and interaction with the ɛ subunit (measured by the mutator effect). We do not know whether the mutants carrying mutations at codon 655 which do not complement at 40°C have any polymerase activity at 30°C because we have no way of selecting chromosomal mutants of an inactive replicative polymerase. We do know that the mutator activity of the plasmid is independent of its ability to complement (Table 3). A hypothesis that accounts for the finding that the noncomplementing Asn, Gly, and Tyr substitutions are stronger mutators than the complementing Ala is that an inactive polymerase produced by the plasmid-borne dnaE ties up the proofreader so that errors made by the chromosomal dnaE (or some other polymerase) are not corrected. This hypothesis also accounts for the dominant effect of a polymerase with a Tyr at codon 655 introduced on a plasmid into a wild-type chromosomal background. Mutator activity, even in a polymerase molecule, need not require that the errors in replication be made by the mutated molecule. The hypothesis that proofreading is deficient in the dnaE173 and dnaE1338 mutants also implies that proofreading as well as mismatch repair plays a role in the correction of slippage errors in the six-guanine-long stretch in the CC107 plasmid, since introduction of either allele into a mutS strain results in a four- to sixfold increase in the mutation rate (Table 6), a result which is understandable if both of these dnaE alleles are functionally deficient in proofreading. A role for exonuclease in the prevention of frameshifts in repetitive runs has been suggested for Saccharomyces cerevisiae (45).
Frameshifts in a run of G's and base substitutions are handled differently by E. coli.
The introduction of the dnaE1336 mutation into a mutS strain results in a 12-fold increase in the rate of base substitutions but only a 2-fold increase in the rate of frameshifts. The introduction of a genetic mutS deficiency into either dnaE173 or dnaE1338 increases the rate of frameshifts two- or sevenfold but either decreases or hardly increases base substitution mutations (Table 6). It might be supposed that the mismatch repair system has a greater affinity for frameshifts, but this hypothesis does not satisfactorily account for the finding (Table 6) that mismatch repair is saturated in dnaE173 and dnaE1338 strains for base substitutions but not for frameshifts. The difference could be accounted for by supposing that the mismatch repair proteins recognize incipient frameshifts during replication and degrade the strand with an extrahelical region, since the bacterial mismatch repair system can act without the mutH product when confronted with a free end (53). This speculation supposes that mismatch repair in E. coli also acts during replication, as appears to be the case in eukaryotes, in which the mismatch repair apparatus is part of a large replication complex (16, 36, 47, 51). However, the suggestion implies that the mutH product should not be required for the repair of frameshifts in the long CC107 repeats; it has already been shown (5) that the frequency of +1G frameshifts is particularly high in a mutH strain, and we have confirmed this result. Either mutH is required for the operation of the complex in vivo, even when dealing with free ends, or some other explanation is needed to account for the difference between base substitution and frameshift mutation.
Replicative polymerase may not be responsible for all spontaneous point mutations.
Although dinB is induced as part of the SOS response, its lexA binding site is of relatively low efficiency (8), and the gene is likely to be transcribed to some extent even in the absence of SOS induction. Deletion of dinB significantly decreases the mutation rates for both frameshift (Kim et al., abstr.) and base substitution mutations. This decrease is seen in a wild-type and in a dnaE1336 mutant strain but is particularly striking in a double mutant with both a partially disabled replicative DNA polymerase and a mismatch repair deficiency. Although reports on dinB have stressed its effect on frameshift mutation (20), the effect of dinB deletion is identical for base substitutions (Table 7). However, the difference in handling the two types of mutation remains: ΔdinB dnaE1336 mutS strains have a lower mutation rate than ΔdinB dnaE+ mutS strains for frameshifts but a higher rate for base substitutions.
The data suggest that mutation occurs as a result of the interaction of at least four elements: the replicative polymerase, the dinB polymerase (pol IV), the proofreading exonuclease, and the mismatch repair system. The simplest explanation of the data (Table 7) is that an impaired replicative polymerase makes it possible for pol IV to compete for the growing end of the DNA chain when replication is stalled. The decrease in frameshift mutation seen in the comparison of dnaE+ mutS ΔdinB and dnaE1336 mutS ΔdinB strains may be the result of an increased chance for the proofreading function to eliminate frameshifts that might otherwise be fixed if an active DinB polymerase were present. It will be recalled that most frameshifts in runs are not corrected by the dnaQ proofreader (40), but given the inefficiency of the dnaE1336 polymerase, there might be time for the extrahelical “bulge” to migrate to the growing point, where the exonuclease is effective. This effect would not be seen with base substitution mutations, which occur and are corrected at the growing point (40). The observation that there is no statistically significant effect of deletion of dinB in a mutS strain carrying a wild-type replicative polymerase could simply mean that pol IV does not compete effectively with the wild-type polymerase. This conclusion requires that we suppose the statistically significant effects on both frameshift and base substitution mutation of deleting dinB from a wild-type strain (BS40, Table 7) are in some way artifacts due to the peculiarities of the experimental system. There is a more interesting speculation. The demonstration that dinB deletion in a mutS strain carrying a wild-type replicative polymerase has no effect can be restated as meaning that the mismatch repair proteins are required for pol IV to gain access to the 3′ OH end of the growing DNA strand in the presence of wild-type but not mutant replicative polymerase. This could mean that pol IV action comes during the processes of traditional mismatch repair, but it could also mean that the mismatch repair proteins play a role during replication. For example, the MutS proteins might form a sliding clamp on the DNA which follows the replicative polymerase, as has been suggested by Gradia et al. (13), and help dissociate the replication complex when it is stalled, permitting access by pol IV.
What is the source of the errors that appear as spontaneous mutations in normal cells? It has been carefully demonstrated that mutations, mostly single base frameshifts due to slippage, can be produced by a complete DNA replication apparatus reconstituted in vitro (11). The experiments described in this paper imply that 75% of the mutations made by a dnaE mutant present in a mismatch repair-defective strain are actually due to the action of the dinB polymerase, pol IV. Our experiments, taken at face value, also indicate that between 40 and 60% of the errors made by a wild-type or a dnaE1336 mutant are due to pol IV. A substantial fraction of spontaneous mutation is therefore likely to be due to the operation of the auxiliary polymerases. This hypothesis has some interesting consequences for human biology. If spontaneous mutations are not necessarily due to the operation of the replicative polymerases, then, for example, one might reduce the incidence of mutation in tumor progression by inhibiting these auxiliary polymerases without necessarily inhibiting normal replication.
ACKNOWLEDGMENTS
Financial support for this project was provided by a grant from the National Cancer Institute (CA32436).
We thank Charles McHenry for providing the cloned dnaE plasmid pDDS7-11, K. Murphy for plasmid pTP223, Roger Woodgate for providing the sequence of dnaE74, the E. coli Genetic Stock Center for the mutS and recA strains, Pat Foster for providing the F′ ΔdinB derivatives, and T. Nohmi for the chromosomal dinB deletion. Phoebe Rice called our attention to the Multalin program. Jennifer Chou, N. Romaniv, and K. Rumilla, undergraduate students at the University of Chicago, contributed to the mutant isolations. We thank Malti Lavassa and Edith Turkington for technical assistance in the early part of this study. Ted Karrison (University of Chicago Cancer Center) provided the statistical analysis. We thank Douglas Bishop for his comments on an earlier version of the manuscript and Mary Berlyn and an unknown reviewer for advice on the terminology of dnaE alleles.
REFERENCES
- 1.Beard W A, Minnick D T, Wade C L, Prasad R, Won R L, Kumar A, Kunkel T A, Wilson S H. Role of the “helix clamp” in HIV-1 reverse transcriptase catalytic cycling as revealed by alanine-scanning mutagenesis. J Biol Chem. 1996;271:12213–12220. doi: 10.1074/jbc.271.21.12213. [DOI] [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett G, 3rd, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Brotcorne-Lannoye A, Maenhaut-Michel G, Radman M. Involvement of DNA polymerase III in UV-induced mutagenesis of bacteriophage lambda. Mol Gen Genet. 1985;199:64–69. doi: 10.1007/BF00327511. [DOI] [PubMed] [Google Scholar]
- 4.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cupples C G, Cabrera M, Cruz C, Miller J H. A set of lacZ mutations in Escherichia coli that allow rapid detection of specific frameshift mutations. Genetics. 1990;125:275–280. doi: 10.1093/genetics/125.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake J W. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci USA. 1991;88:7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake J W, Charlesworth B, Charlesworth D, Crow J F. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez De Henestrosa A R, Ogi T, Aoyagi S, Chafin D, Hayes J J, Ohmori H, Woodgate R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol Microbiol. 2000;35:1560–1572. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 9.Fijalkowska I J, Schaaper R M. Antimutator mutations in the alpha subunit of Escherichia coli DNA polymerase III: identification of the responsible mutations and alignment with other DNA polymerases. Genetics. 1993;134:1039–1044. doi: 10.1093/genetics/134.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fijalkowska I J, Schaaper R M. Effects of Escherichia coli dnaE antimutator alleles in a proofreading-deficient mutD5 strain. J Bacteriol. 1995;177:5979–5986. doi: 10.1128/jb.177.20.5979-5986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii S, Akiyama M, Aoki K, Sugaya Y, Higuchi K, Hiraoka M, Miki Y, Saitoh N, Yoshiyama K, Ihara K, Seki M, Ohtsubo E, Maki H. DNA replication errors produced by the replicative apparatus of Escherichia coli. J Mol Biol. 1999;289:835–850. doi: 10.1006/jmbi.1999.2802. [DOI] [PubMed] [Google Scholar]
- 12.Gerlach V L, Aravind L, Gotway G, Schultz R A, Koonin E V, Friedberg E C. Human and mouse homologs of Escherichia coli dinB (DNA polymerase IV), members of the umuC/dinB superfamily. Proc Natl Acad Sci USA. 1999;96:11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gradia S, Acharya S, Fishel R. The role of mismatched nucleotides in activating the hMSH2-hMSH6 molecular switch. J Biol Chem. 2000;275:3922–3930. doi: 10.1074/jbc.275.6.3922. [DOI] [PubMed] [Google Scholar]
- 14.Harris R S, Feng G, Ross K J, Sidhu R, Thulin C, Longerich S, Szigety S K, Winkler M E, Rosenberg S M. Mismatch repair protein MutL becomes limiting during stationary-phase mutation. Genes Dev. 1997;11:2426–2437. doi: 10.1101/gad.11.18.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James A A, Morrison P T, Kolodner R. Genetic recombination of bacterial plasmid DNA: analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982;160:411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R E, Kovvali G K, Guzder S N, Amin N S, Holm C, Habraken Y, Sung P, Prakash L, Prakash S. Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem. 1996;271:27987–27990. doi: 10.1074/jbc.271.45.27987. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R E, Washington M T, Prakash S, Prakash L. Bridging the gap: a family of novel DNA polymerases that replicate faulty DNA. Proc Natl Acad Sci USA. 1999;96:12224–12226. doi: 10.1073/pnas.96.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonczyk P, Nowicka A, Fijalkowska I J, Schaaper R M, Ciesla Z. In vivo protein interactions within the Escherichia coli DNA polymerase III core. J Bacteriol. 1998;180:1563–1566. doi: 10.1128/jb.180.6.1563-1566.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelman Z, O'Donnell M. DNA polymerase III holoenzyme: structure and function of a chromosomal replicating machine. Annu Rev Biochem. 1995;64:171–200. doi: 10.1146/annurev.bi.64.070195.001131. [DOI] [PubMed] [Google Scholar]
- 20.Kim S R, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damaged DNA. Proc Natl Acad Sci USA. 1997;94:13792–13797. doi: 10.1073/pnas.94.25.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindahl T, Wood R D. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 22.Maki H, Mo J Y, Sekiguchi M. A strong mutator effect caused by an amino acid change in the alpha subunit of DNA polymerase III of Escherichia coli. J Biol Chem. 1991;266:5055–5061. [PubMed] [Google Scholar]
- 23.Maloy S R, Dunn W D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981;145:1110–1112. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 25.Minnick D T, Astatke M, Joyce C M, Kunkel T A. A thumb subdomain mutant of the large fragment of Escherichia coli DNA polymerase I with reduced DNA binding affinity, processivity, and frameshift fidelity. J Biol Chem. 1996;271:24954–24961. doi: 10.1074/jbc.271.40.24954. [DOI] [PubMed] [Google Scholar]
- 26.Mo J Y, Maki H, Sekiguchi M. Mutational specificity of the dnaE173 mutator associated with a defect in the catalytic subunit of DNA polymerase III of Escherichia coli. J Mol Biol. 1991;222:925–936. doi: 10.1016/0022-2836(91)90586-u. [DOI] [PubMed] [Google Scholar]
- 27.Murphy K C. Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J Bacteriol. 1998;180:2063–2071. doi: 10.1128/jb.180.8.2063-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham P T, Olson M W, McHenry C S, Schaaper R M. The base substitution and frameshift fidelity of Escherichia coli DNA polymerase III holoenzyme in vitro. J Biol Chem. 1998;273:23575–23584. doi: 10.1074/jbc.273.36.23575. [DOI] [PubMed] [Google Scholar]
- 29.Poteete A R, Fenton A C. Lambda red-dependent growth and recombination of phage P22. Virology. 1984;134:161–167. doi: 10.1016/0042-6822(84)90281-2. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard A E, McHenry C S. Identification of the acidic residues in the active site of DNA polymerase III. J Mol Biol. 1999;285:1067–1080. doi: 10.1006/jmbi.1998.2352. [DOI] [PubMed] [Google Scholar]
- 31.Reznikoff W S. The lactose operon-controlling elements: a complex paradigm. Mol Microbiol. 1992;6:2419–2422. doi: 10.1111/j.1365-2958.1992.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosche W A, Foster P L. Determining mutation rates in bacterial populations. Methods. 2000;20:4–17. doi: 10.1006/meth.1999.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagher D, Hsu A, Strauss B. Stabilization of the intermediate in frameshift mutation. Mutat Res. 1999;423:73–77. doi: 10.1016/s0027-5107(98)00227-9. [DOI] [PubMed] [Google Scholar]
- 34.Schaaper R M. Mechanisms of mutagenesis in the Escherichia coli mutator mutD5: role of DNA mismatch repair. Proc Natl Acad Sci USA. 1988;85:8126–8130. doi: 10.1073/pnas.85.21.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaaper R M, Radman M. The extreme mutator effect of Escherichia coli mutD5 results from saturation of mismatch repair by excessive DNA replication errors. EMBO J. 1989;8:3511–3516. doi: 10.1002/j.1460-2075.1989.tb08516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekowski J W, Han S, Hickey R J, Malkas L H. The human cell DNA replication machinery contains DNA mismatch repair proteins that are structurally altered in cancer. Proc Am Assoc Cancer Res. 2000;41:715. [Google Scholar]
- 37.Sevastopoulos C, Glaser D. Mutator action by Escherichia coli strains carrying dnaE mutations. Proc Natl Acad Sci USA. 1977;74:3947–3950. doi: 10.1073/pnas.74.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwood L. Human physiology. 2nd ed. Minneapolis, Minn: West Publishing Co.; 1993. [Google Scholar]
- 39.Siegel E C, Wain S L, Meltzer S F, Binion M L, Steinberg J L. Mutator mutations in Escherichia coli induced by the insertion of phage mu and the transposable resistance elements Tn5 and Tn10. Mutat Res. 1982;93:25–33. doi: 10.1016/0027-5107(82)90122-1. [DOI] [PubMed] [Google Scholar]
- 40.Strauss B S, Sagher D, Acharya S. Role of proofreading and mismatch repair in maintaining the stability of nucleotide repeats in DNA. Nucleic Acids Res. 1997;25:806–813. doi: 10.1093/nar/25.4.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taft-Benz S A, Schaaper R M. The C-terminal domain of dnaQ contains the polymerase binding site. J Bacteriol. 1999;181:2963–2965. doi: 10.1128/jb.181.9.2963-2965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang M, Shen X, Frank E G, O'Donnell M, Woodgate R, Goodman M F. UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenaillon O, Toupance B, Le Nagard H, Taddei F, Godelle B. Mutators, population size, adaptive landscape and the adaptation of asexual populations of bacteria. Genetics. 1999;152:485–493. doi: 10.1093/genetics/152.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasiewicz H G, McHenry C S. Sequence analysis of the Escherichia coli dnaE gene. J Bacteriol. 1987;169:5735–5744. doi: 10.1128/jb.169.12.5735-5744.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran H T, Gordenin D A, Resnick M A. The 3′→5′ exonucleases of DNA polymerases delta and epsilon and the 5′→3′ exonuclease Exo1 have major roles in postreplication mutation avoidance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:2000–2007. doi: 10.1128/mcb.19.3.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran H T, Keen J D, Kricker M, Resnick M A, Gordenin D A. Hypermutability of homonucleotide runs in mismatch repair and DNA polymerase proofreading yeast mutants. Mol Cell Biol. 1997;17:2859–2865. doi: 10.1128/mcb.17.5.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umar A, Buermeyer A B, Simon J A, Thomas D C, Clark A B, Liskay R M, Kunkel T A. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 48.Vulic M, Lenski R E, Radman M. Mutation, recombination, and incipient speciation of bacteria in the laboratory. Proc Natl Acad Sci USA. 1999;96:7348–7351. doi: 10.1073/pnas.96.13.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner J, Gruz P, Kim S R, Yamada M, Matsui K, Fuchs R P, Nohmi T. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- 50.Wagner J, Nohmi T. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J Bacteriol. 2000;182:4587–4595. doi: 10.1128/jb.182.16.4587-4595.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Cortez D, Yazdi P, Neff N, Elledge S J, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 52.Wertman K F, Wyman A R, Botstein D. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene. 1986;49:253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi M, Dao V, Modrich P. MutS and MutL activate DNA helicase II in a mismatch-dependent manner. J Biol Chem. 1998;273:9197–9201. doi: 10.1074/jbc.273.15.9197. [DOI] [PubMed] [Google Scholar]