Patients are frequently given the opportunity to participate in making decisions about their care. To assist them in making their decisions data that are as accurate and as complete as appropriate will need to be quickly available. These data will be needed not only by patients but also by their doctors. A life table constructed from regularly published statistics on national morbidity and mortality can be used to display the likelihood of developing or dying of a disease (a cumulative probability) at any given age. Such a life table may be a valuable tool in helping patients to make decisions about their care. In this paper we discuss the use of life tables to present to patients the absolute and relative risks of breast cancer and their use in comparing the risks from breast cancer with those from other life threatening conditions.
Summary points
A life table constructed from national statistics on morbidity and mortality can be a valuable resource in helping patients to make decisions about their care
Coordinating absolute and relative risks in a life table offers patients and doctors the opportunity to make more informed judgments
Life table analysis allows patients and doctors to estimate the cumulative probability of dying of breast cancer before a patient reaches a given age
The risk of developing or dying of breast cancer should be seen in the context of other life threatening conditions
For women who smoke the cumulative probability of dying of lung cancer matches that of dying of breast cancer when women reach their early 50s; this probability doubles by age 65 and triples by age 75
Cumulative incidence
Recently, notices in London’s underground warned that women have a 1 in 12 risk of developing breast cancer. Women in America have been warned that their risk is 1 in 8; consequently, “fear of breast cancer is so pervasive among US women that it is causing them to ignore far more serious health threats.”1 For women who manage to escape other, greater health risks and survive to age 75 or 80, risks of 1 in 12 and 1 in 8 are approximately correct. (The greater risk in America may reflect the earlier introduction of mass screening, the fact that women are screened at younger ages, and the overdiagnosis of tumours of low grade malignancy or of ductal carcinoma in situ—conditions that might never become clinically detectable or life threatening.2,3)
More relevant to the concerns of younger women is the risk of developing breast cancer earlier in their lives. A life table analysis of the cumulative incidence of breast cancer in England and Wales shows that the risk for women under age 35 is 1 in 625. This rises to 1 in 56 by age 50, to 1 in 18 by age 65, and to 1 in 13 by age 75.4 This type of age specific data should offset disproportionate fear of the disease among women but it falls short of meeting the information needs of individuals. Life table estimates based on the entire population are averages; the risk for an individual varies depending on the presence or absence of predisposing risk factors such as a family history of the disease, age at menarche, age at first pregnancy, age at menopause, whether oral contraceptives have been used, and the amount of alcohol consumed. In postmenopausal women risk factors include the use of hormone replacement therapy and obesity in women not receiving hormone replacement therapy.
Coordinating absolute and relative risks
The quantitative significance of risk factors has been poorly presented to both the public and the medical profession. In 1996, many women were concerned after publication of a review of the association between breast cancer and hormonal contraceptives which stated that the risk of breast cancer was increased by 20% among women who used oral contraceptives.5 If the risk had been presented as a cumulative increase in absolute risk from 16 to 18.7 per 10 000 women for a woman aged between 30 and 35, which was how it was presented in the review (table 15), then different assessments of personal risk might have occurred. This increase in absolute risk represents one additional case of breast cancer occurring annually (excess cumulative incidence) among 3700 women taking oral contraceptives; this would increase to one additional case occurring among 2100 women as a woman became five years older. For a woman aged 30 to 35 this could be compared to a 40% increase in the risk of developing breast cancer if she consumed the equivalent of “24 g (1 oz) of absolute alcohol daily (about two drinks daily).”6 This risk would convert to an excess cumulative incidence of one additional case of breast cancer occurring among about 1500 women.
Table 1.
Estimated number of cases of breast cancer occurring in Europe or North America in women who never used combined oral contraceptives and in women who used them from age 25 to 29.5 Used with permission from Elsevier Science
| Age at diagnosis | Breast cancers diagnosed in women who never used combined oral contraceptives (n=10 000)
|
Breast cancers diagnosed in women who used combined oral contraceptives from age 25 to 29 (n=10 000)
|
|||||
|---|---|---|---|---|---|---|---|
| 5 year incidence* | Cumulative incidence | Relative risk | 5 year incidence | Cumulative incidence | Excess cumulative incidence (SD) | ||
| Using estimates of relative risk for all users: | |||||||
| 20-24 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0 | |
| 25-29 | 3.5 | 4 | 1.24 | 4.3 | 4.8 | 0.8 (0.1) | |
| 30-34 | 12 | 16 | 1.15 | 13.9 | 18.7 | 2.7 (0.5) | |
| 35-39 | 28 | 44 | 1.07 | 30.0 | 48.7 | 4.7 (1.0) | |
| 40-44 | 56 | 100 | 0.98 | 55.1 | 103.7 | 3.7 (2.0) | |
| 45-49 | 80 | 180 | 1.01 | 80.8 | 184.5 | 4.5 (3.6) | |
| Using estimates of relative risk for users with total duration of use of oral contraceptives >1 year: | |||||||
| 20-24 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0 | |
| 25-29 | 3.5 | 4 | 1.22 | 4.3 | 4.8 | 0.8 (0.1) | |
| 30-34 | 12 | 16 | 1.15 | 13.8 | 18.6 | 2.6 (0.5) | |
| 35-39 | 28 | 44 | 1.06 | 29.6 | 48.2 | 4.2 (1.0) | |
| 40-44 | 56 | 100 | 0.96 | 53.8 | 102.0 | 2.0 (2.0) | |
| 45-49 | 80 | 180 | 0.98 | 78.5 | 180.5 | 0.5 (3.9) | |
Annual incidences per 100 000 never users were assumed to be 160 at ages 45-49 and 0.007(age−17) at ages 20-44; these rates are intermediate between rates in the United Kingdom and the United States in the mid-1980s.
The benefits and risks of treatment are perceived differently by health professionals7,8 and the public depending on whether they are presented as absolute or relative improvements. Members of a health authority were presented with the results of a large Swedish trial in which a relative risk reduction of 34% was reported and asked if they would vote to fund mammographic screening.8,9 The majority said that they would favour funding the screening. When the same results were disguised as a different trial and presented as an absolute risk reduction of 0.06%, the majority indicated that they would vote against funding screening. When presented with 1592 patients as the number needed to treat to save one life, half of those surveyed indicated that they would be in favour of screening. Presenting these data in a life table similar to table 1 would have offered a better opportunity for the members of the health authority to make a more informed judgment.
Cumulative mortality
A woman probably wants to know her risk of developing breast cancer at her age and with her risk factors; she may have a better appreciation of the risk if the numbers can be seen as absolute as well as relative risks. But this is not enough. A woman will also want to know her prognosis and, in particular, the probability that she will live or die. She might also want to know how such a probability compares with that of dying of other life threatening conditions.
Analysing death rates may lead to some surprises for doctors and their patients. The first surprise is that there is an increasingly large discrepancy between the incidence of and mortality from breast cancer in America; since 1940 mortality has remained constant while incidence has nearly doubled.2 This discrepancy can only partially be accounted for by the growing success of treatment. Thus, the woman concerned about the possibility of developing breast cancer in the future should know that the likelihood of subsequently dying of breast cancer is relatively small and is becoming smaller each year. A life table analysis based on 1995 mortality statistics for England and Wales10 allows us to estimate the cumulative probability of a patient dying of breast cancer before she reaches a given age.
Mortality from breast cancer among women in different age groups was derived from data from the Office for National Statistics (table 2).10 The data were adjusted for the number of women surviving in each age group. The number of women dying from all causes in each age group was calculated from the resulting life table, which was based on mortality in the female population. The cumulative number of deaths from breast cancer is found by adding the number of deaths from the disease occurring at each age interval. The per cent probability of dying from breast cancer before the end of each age interval is found by dividing the number in the cumulative risk column by 1000. In England and Wales before the age of 50 only one woman out of 136 dies of breast cancer. By the age of 60 this is one out of 65, by the age of 70 it is one out of 39, and by the age of 80 only one woman out of 26 dies of breast cancer (table 2).
Table 2.
Cumulative probability of death from breast cancer in England and Wales in 1995 per 100 000 women. Mortality was adjusted for the number of women surviving in each age group. Data derived from Office for National Statistics10
| Age interval | No of women dying of breast cancer in each age interval/100 000 women | Cumulative No of deaths from breast cancer occurring by the end of each age interval/100 000 women | Probability of dying of breast cancer by end of each age interval |
|---|---|---|---|
| 25-34 | 34.6 | 34.6 | 1/2873 |
| 35-44 | 176.3 | 210.9 | 1/474 |
| 45-54 | 522.5 | 733.4 | 1/136 |
| 55-64 | 794.7 | 1528.1 | 1/65 |
| 65-74 | 1060.4 | 2588.0 | 1/39 |
| 75-84 | 1189.5 | 3778.0 | 1/26 |
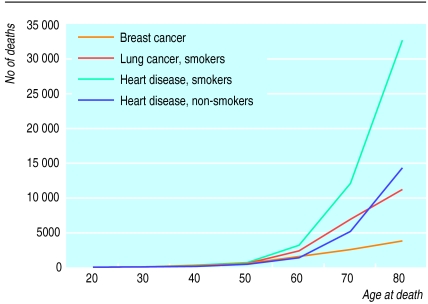
The risk of dying of breast cancer should be seen in the context of other risks to a woman’s life. The risk of dying of heart disease has been underestimated by women and its threat is perceived to be low.11 Women may believe that deaths from cardiac causes primarily affect men and elderly women whereas, at least in smokers, the number of deaths occurring in women as a result of cardiac causes exceeds the number of deaths from breast cancer at all ages (figure).11 Fearing death from breast cancer more than death from heart disease reflects not simply an ignorance of probabilities. “Heart disease, which offers at least the possibility of sudden death, may not frighten people quite as much [as cancer]: for many, for perhaps irrational reasons, it is preferred. That certainly is the conclusion from responses to the question ‘How would you like to die?’”1
Death from heart disease may be preferred by some to death from breast cancer; death from lung cancer, certainly as unpleasant as that from breast cancer, offers a more apposite comparison. For women who smoke the cumulative probability of dying of lung cancer matches that of dying of breast cancer when women reach their early 50s; this probability doubles by age 65 and triples by age 75 (figure). Although there has been a modest fall in the number of women who smoke (mainly among older women), there is little evidence that the fear of developing lung cancer matches the fear of developing breast cancer. Ironically, lung cancer has a cure rate of <5% and can be almost entirely prevented by avoiding tobacco but, on average, 70% of patients treated for breast cancer can expect to survive for 10 years. In contrast to lung cancer there is comparatively little that can be done to prevent breast cancer.
Conclusion
The statistic that 1 in 12 women will develop breast cancer is thus correct only for women who have escaped a number of equally serious but more likely threats to life at an earlier age. For most women the lifetime risk of dying of breast cancer is only 1 in 26; the other 25 women will die of something else. Life table analyses show that the incidence of breast cancer and mortality from the disease are much lower among younger women and these risks should be understood in the context of other serious threats to life.
Figure.
Cumulative number of deaths in 1995 from breast cancer, lung cancer in smokers, heart disease in smokers, and heart disease in non-smokers per 100 000 women in England and Wales. A risk ratio of 12.5:1 for lung cancer and 2.3:1 for heart disease for smokers v non-smokers was assumed12
References
- 1.Assessing the odds [editorial] Lancet. 1997;350:1563. [PubMed] [Google Scholar]
- 2.Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer. New Engl J Med. 1992;327:319–328. doi: 10.1056/NEJM199207303270505. [DOI] [PubMed] [Google Scholar]
- 3.Feuer EJ, Wun L-M. How much of the recent rise in breast cancer incidence can be explained by increases in mammography utilization? A dynamic population model approach. Am J Epidemiol. 1992;136:1423–1436. doi: 10.1093/oxfordjournals.aje.a116463. [DOI] [PubMed] [Google Scholar]
- 4.Office for National Statistics. 1991 cancer statistics registrations. London: Stationery Office; 1997. (Series MB1, No 24.) [Google Scholar]
- 5.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: further results. Contraception. 1996;54(suppl 3):1–106S. doi: 10.1016/s0010-7824(15)30002-0. [DOI] [PubMed] [Google Scholar]
- 6.Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 1988;260:652–656. [PubMed] [Google Scholar]
- 7.Fahey T, Griffiths S, Peters TJ. Evidence based purchasing: understanding results of clinical trials and systematic reviews. BMJ. 1995;311:1056–1060. doi: 10.1136/bmj.311.7012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McColl A, Smith H, White P, Field J. General practitioners’ perceptions of the route to evidence based medicine: a questionnaire survey. BMJ. 1998;316:361–365. doi: 10.1136/bmj.316.7128.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabar L, Fagerberg CJG, Gad A, Baldetorp L, Holmberg LH, Grontoft O, et al. Reduction in mortality from breast cancer after mass screening with mammography: randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;i:829–832. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- 10.Office for National Statistics. 1995 mortality statistics: cause. London: Stationery Office; 1997. (Series DH2, No 22.) [Google Scholar]
- 11.Pilote L, Hlatky MA. Attitudes of women toward hormone therapy and prevention of heart disease. Am Heart J. 1995;129:1237–1238. doi: 10.1016/0002-8703(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 12.Peto R, Lopez AD, Boreham J, Thun M, Heath C. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]