Abstract
The conserved cp32 plasmid family of Borrelia burgdorferi was recently shown to be packaged into a bacteriophage particle (C. H. Eggers and D. S. Samuels, J. Bacteriol. 181:7308–7313, 1999). This plasmid encodes BlyA, a 7.4-kDa membrane-interactive protein, and BlyB, an accessory protein, which were previously proposed to comprise a hemolysis system. Our genetic and biochemical evidence suggests that this hypothesis is incorrect and that BlyA and BlyB function instead as a prophage-encoded holin or holin-like system for this newly described bacteriophage. An Escherichia coli mutant containing the blyAB locus that was defective for the normally cryptic host hemolysin SheA was found to be nonhemolytic, suggesting that induction of sheA by blyAB expression was responsible for the hemolytic activity observed previously. Analysis of the structural features of BlyA indicated greater structural similarity to bacteriophage-encoded holins than to hemolysins. Consistent with holin characteristics, subcellular localization studies with E. coli and B. burgdorferi indicated that BlyA is solely membrane associated and that BlyB is a soluble protein. Furthermore, BlyA exhibited a holin-like function by promoting the endolysin-dependent lysis of an induced lambda lysogen that was defective in the holin gene. Finally, induction of the cp32 prophage in B. burgdorferi dramatically stimulated blyAB expression. Our results provide the first evidence of a prophage-encoded holin within Borrelia.
The spirochete Borrelia burgdorferi is the causative agent of Lyme disease, the most prevalent arthropod-borne disease in the United States and one that is of increasing importance worldwide (9). If untreated, patients with Lyme disease develop an array of symptoms, often culminating in debilitating arthritis and neurologic disease (38). Clinical and animal model studies reveal the presence of an immune response to a variety of spirochetal antigens following infection and colonization (6, 40). However, the immune response is ineffective at eradication of the organism and may also play a role in the disease process in certain cases (2, 20). Down-regulation of antigen synthesis and antigenic variation have been suggested to be important factors in the potentiation of immune evasion (30, 43, 44, 49).
Considerable effort has been made to elucidate the molecular biology of B. burgdorferi (4, 34). Central to this effort has been the identification of protein targets for the development of antibodies and vaccines that can be used to diagnose and potentially prevent Lyme disease. Efforts are also being made to develop new and more powerful recombinant DNA techniques as tools for the genetic manipulation of B. burgdorferi. All of the B. burgdorferi genospecies reported to date contain an ∼1-Mbp linear chromosome and multiple linear and circular plasmids, the latter of which can account for up to one-third of the organism's coding capacity (11, 18). Plasmid-encoded genes are believed to play an important role in virulence, since prolonged in vitro cultivation of B. burgdorferi and loss of plasmids result in a concomitant loss of infectivity (36, 46). A large variety of antigens, many of which are plasmid-encoded membrane lipoproteins, have been described to date (for references, see references 11 and 23). However, little is known about the precise function of most of these proteins. Specific roles in the establishment or maintenance of infection have been suggested for certain proteins (19, 22, 35, 49). One of the major outer surface lipoproteins, OspA, has become the target for vaccine trials recently (37, 39).
We previously reported the isolation and preliminary characterization of the small membrane-interactive BlyA protein of B. burgdorferi strain B31, which, together with BlyB, promoted hemolytic activity in an Escherichia coli strain carrying this locus (21). In B. burgdorferi B31, the blyAB locus is located in a four-gene operon on the cp32 family of conserved circular plasmids and the lp56 linear plasmid (11, 12, 33, 42). The Borrelia species causing relapsing fever have also been shown to contain cp32 plasmids carrying the blyAB operon (41). cp32 has recently been shown to be the φBB-1 prophage, and linearized cp32 molecules are packaged into a bacteriophage particle upon induction with 1-methyl-3-nitro-nitrosoguanidine (MNNG) (16, 17). The results presented here indicate that the blyAB locus is likely to encode a bacteriophage holin or holin-like system. Holins, a component of the lysis mechanism for all known tailed phages, are small proteins that form stable, nonspecific pores in the membrane, allowing endolysin access to the peptidoglycan (1, 47, 48). In phage λ, gene S encodes the holin responsible for release of endolysin, encoded by gene R, into the periplasm (47, 48). This report is about the first identification and characterization of a nonstructural gene product involved in bacteriophage function from a bacteriophage of spirochetes.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. burgdorferi strains CA-11.2A (26) and B31 (ATCC 35210) were used. E. coli K-12 strains MM294 (27), MC4100 (10), and CFP201, containing the sheA::Tn5-2.1 allele (14), have already been described. MM294 sheA::Tn5-2.1 and MC4100 sheA::Tn5-2.1 (λcI857 Sam7) were constructed from CFP201 by P1 transduction. MC4100 (λcI857 Sam7), MC4100 (λcI857 ΔSR), and pUCS105R−, a pUC19 derivative containing the lambda S gene under the control of the lambda p′R promoter, were obtained from Ing-Nang Wang and Ry Young (Texas A&M University). pCD1 is a pUC19 derivative containing the blyA gene under the control of the lambda p′R promoter with the normal S gene ribosome-binding site. It was constructed utilizing a Seamless cloning kit in accordance with the manufacturer's (Stratagene) instructions as follows. Primers 5′-GGCTCTTCATCAACGTAAGGCGTTCCTCGATATGC-3′ and 5′-AACTCTTCAGTCTTACCCCCAATAAGGGGATTTGC-3′ were used to PCR amplify pUCS105R− exclusive of the lambda S gene, and primers 5′-CCCTCTTCCGACATGGATACTATTAAATTAACAGAACTTC-3′ and 5′-CCCTCTTCCTGATTAATCTCTTTTTTTAATGTGATTTTTGCC-3′ were used to PCR amplify the coding sequence of blyA from pTG3. The products were then cleaved with Eam1104I, and the resulting DNA fragments were ligated together to give rise to pCD1, which was verified by DNA sequence analysis. pCID552 containing the transcriptional regulatory gene mprA has been described previously (15). pUC18-derivative plasmids pDP1 and pTG3, which contain the blyAB locus of B. burgdorferi B31, as well as pDAK, in which this locus is deleted, have been described previously (21). EP18 is an MM294(pTG3) derivative containing the blyA-L10F allele (21).
Media and reagents.
B. burgdorferi was routinely cultivated in Barbour-Stoenner-Kelly complete medium (3) (Sigma) at 34°C with a 5% CO2 atmosphere. LB and LB plates supplemented with appropriate antibiotics were made as described by Miller (28). Nutrient blood agar plates containing 5% sheep erythrocytes were purchased commercially (Remel) and spread with antibiotics as needed. DNA restriction and modification enzymes were purchased from New England Biolabs. DNA primers used for PCR, mutagenesis, and DNA sequencing were purchased commercially (Integrated DNA Technologies). Plasmid DNA was prepared utilizing the Qiagen system. All other reagents were laboratory grade or better and were purchased from Sigma or a comparable supplier.
Antisera.
Antipeptide antibody directed against the C terminus of BlyA has been described previously (21). For development of an antipeptide antibody directed against the C terminus of BlyB, a 20-mer peptide (DLKFNQEGKPIYKERTNNAK) was synthesized commercially (TANA Biologicals) and conjugated to keyhole limpet hemocyanin. Two New Zealand White rabbits were injected with 1 mg of conjugate suspended in complete Freund's adjuvant and boosted five times over 3-week intervals with a comparable dose of peptide suspended in incomplete Freund's adjuvant. The antibody was affinity purified on a Sepharose column containing the immobilized peptide.
BlyA and BlyB analysis in B. burgdorferi.
Cultures of B. burgdorferi B31 and CA-11.2A (200 ml) were grown to log phase (>107 cells ml−1; A600, ≥0.05) and then split into two equal aliquots. One aliquot was treated with 10 μg of MNNG ml−1 as described previously (17), and the untreated control was treated similarly but without chemical induction. After an appropriate recovery time (∼60 h), the cells were sedimented at 8,000 × g for 10 min at 4°C, washed in 1 ml of dPBS++ (138 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 0.1 g of CaCl2 liter−1, 0.213 g of MgCl2 · 6 H2O liter−1), and resedimented at 14,000 × g for 5 min at 4°C in a microcentrifuge. The cell pellet was resuspended in 1 ml of dPBS++, and the cell density (A600) was determined. The cells were sedimented at 14,000 × g for 5 min at 4°C and resuspended in an amount of water equal to the A600 value multiplied by 200 μl. An equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading dye (125 mM Tris-HCl [pH 8.0], 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.0025% bromophenol blue) was added to each sample, which was heated at 100°C for 5 min. Aliquots were resolved by SDS-PAGE on duplicate 17.5% polyacrylamide gels. After electrophoresis, one gel was stained with Coomassie brilliant blue and destained while the other gel was blotted onto Immobilon-P. The membranes were probed with BlyA, BlyB, or OspC antiserum diluted 1:5,000.
RNA analysis.
Cultures of MNNG-treated and untreated B. burgdorferi B31 and CA-11.2A (100 ml) were prepared as described above. RNA extraction was done using the Trizol reagent (Sigma) as described by the manufacturer. The RNA pellet was resuspended in 50 to 100 μl of diethyl pyrocarbonate-treated water, and the A260 was measured and multiplied by a factor of 40 μg ml−1 and the dilution factor to determine the RNA concentration. RNA was resolved on a 1.2% agarose gel and transferred to Immobilon-Ny+ (Millipore) as described previously (25). The probes for Northern hybridization were generated using the Prime-it II labeling kit (Stratagene) in accordance with the manufacturer's instructions. Probes were created using PCR (25 cycles of 92°C for 30 s, 50°C for 30 s, and 72°C for 3 min) with primers 5′-CAGAACTTCTTATCAAT-3′ and 5′-GCCATTACCATTGCC-3′ (for blyA) or 5′-CCAAAGATAATGTTG-3′ and 5′-GATCTATGTTTGTATC-3′ (for blyB). Northern hybridization was performed as described previously (7).
BlyA and BlyB localization.
A culture of log-phase MNNG-treated B. burgdorferi CA-11.2A (100 ml) was prepared and sedimented to collect cells as described above. The cell pellet was washed in 2 ml of ice-cold TBSP (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg each of leupeptin, pepstatin, and aprotinin ml−1) and resedimented at 14,000 × g for 10 min at 4°C. The pellet was resuspended in 2 ml of TBSP and then sonicated on ice (eight cycles of 30 s at a setting of 3.5 with 1-min recovery intervals). Cell lysis was evaluated by dark-field microscopy. Unlysed cells were removed by sedimentation of the crude extract at 6,000 × g for 10 min at 4°C. Cell extracts were sedimented at 100,000 × g (53,000 rpm) for 3 h at 4°C in a TLA100.2 rotor (Beckman), and the supernatant (S100) and pellet (P100) fractions were recovered. P100 was resuspended in a volume of TBSP equivalent to that of the S100 fraction. Samples were analyzed by SDS-PAGE and Western blotting as described above.
E. coli cultures (500 ml) were grown in LB supplemented with ampicillin at 100 μg ml−1, when appropriate, for 15 h at 37°C. Cultures were chilled on ice, and cells were sedimented at 15,000 × g for 10 min at 4°C. The cell pellet was resuspended in 10 ml of ice-cold TBSP, and cells were disrupted by three passages through a prechilled French pressure cell (Aminco) at 16,000 lb/in2. Unbroken cells were removed by sedimentation at 3,000 × g for 10 min at 4°C. A 5-ml volume of cell extract was sedimented at 100,000 × g for 3 h at 4°C to give rise to supernatant (S100) and membrane pellet (P100) fractions. P100 was resuspended in an equivalent volume of TBSP. For SDS-PAGE analysis, samples were mixed with an equal volume of loading buffer (100 mM Tris-HCl [pH 6.8], 200 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) and heated at 100°C for 5 min and proteins were resolved by SDS–15% PAGE (24). Gels were subjected to Western blotting (8), and the BlyA and BlyB proteins were visualized utilizing a 1:5,000 dilution of the appropriate antibody and an ECL kit as described by the manufacturer (Amersham).
RESULTS
blyAB is nonhemolytic in an E. coli sheA mutant.
Guina and Oliver previously hypothesized that BlyA is a hemolysin and that BlyB is required in some manner for BlyA synthesis, stability, or activity (21). These conclusions were based on experiments performed with E. coli that contained the cloned blyAB locus. However, additional genetic characterization of this system led us to reevaluate this hypothesis. In particular, we noted that blyAB expression occurred specifically during stationary-phase growth, at which point the viability of the E. coli host declined precipitously (13). Furthermore, the hemolytic activity against sheep erythrocytes and the cytotoxic activity against the host strain could be uncoupled genetically in certain blyAB mutants. These observations led us to consider the possibility that other activities may be induced by blyAB expression rather than resulting directly from the products of these genes. We speculated that BlyA might not be a hemolysin but rather that it could induce expression of a normally cryptic E. coli hemolysin. In order to test this hypothesis, we obtained a recently described E. coli mutant that is defective for the cryptic hemolysin SheA (14). While sheA is normally silent in most laboratory E. coli K-12 strains, it can be derepressed under certain circumstances, such as by overproduction of particular transcriptional regulators, such as MprA (14, 15, 45). The appropriate plasmid-containing sheA+ and sheA::Tn5 isogenic strains were constructed and tested for hemolytic activity. The result indicated clearly that hemolytic activity requires both an intact blyAB locus and a wild-type sheA gene, since the blyAB-containing, sheA-defective host was nonhemolytic (Table 1). Furthermore, the hemolytic phenotype of MM294(pTG3) on blood agar plates was visually similar to that of MM294(pCID552), which overproduced the transcriptional regulator MprA. These results suggest that SheA is the hemolysin in this system and that blyAB expression serves to directly or indirectly (see Discussion) induce sheA expression. These data also point out the utility of using the sheA mutant as a better host for the isolation of heterologous hemolysin determinants.
TABLE 1.
sheA function is required for hemolytic activitya
| Strain | Relevant plasmid gene(s) | Presence of hemolytic activityb |
|---|---|---|
| MM294(pTG3) | blyAB | + |
| MM294(pDAK) | − | |
| MM294 sheA::Tn5(pTG3) | blyAB | − |
| MM294 sheA::Tn5(pDAK) | − | |
| MM294(pCID552) | mprA | + |
Strains were streaked on 5% blood agar plates supplemented with ampicillin (100 μg ml−1) and incubated at 37°C for 20 h, and then hemolysis was scored.
+, presence of hemolytic activity; −, absence of hemolytic activity.
BlyA structurally resembles holins.
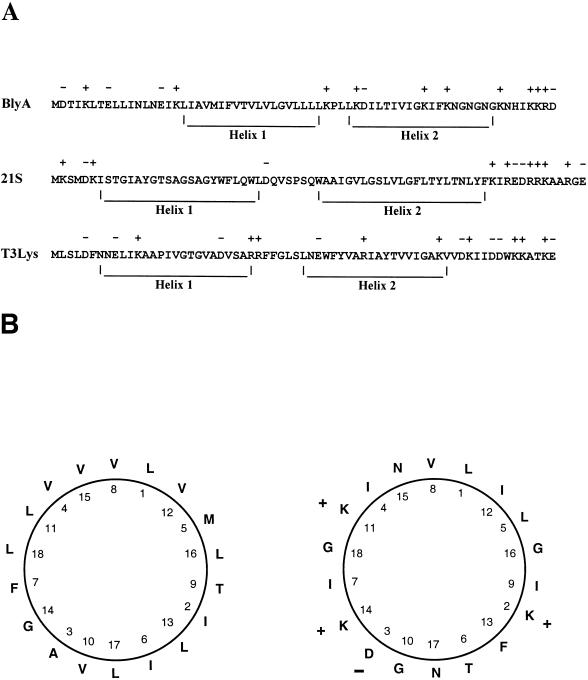
We noted previously that the BlyA protein is structurally similar to pore-forming toxins such as melittin and Staphylococcus aureus delta-hemolysin, since it contains a predicted hydrophobic α-helical region followed by a positively charged C terminus (21). The predicted α-helical region, however, contains more residues than are necessary to form a single transmembrane helix, as well as a proline, a residue known to induce turns and disrupt helices, at its center (31, 32). Taken together, these observations suggest that BlyA actually contains two α-helical regions interrupted by a proline residue. This structural model more closely resembles another class of channel-forming proteins called holins (48). A comparison of BlyA to two group II phage-encoded holins is shown in Fig. 1A. The first predicted helix is hydrophobic, while the second one is amphipathic (Fig. 1B). In an oligomerized state, the charged surface of multiple BlyA amphipathic helices could line the aqueous channel of the holin pore. Holins are produced by virtually all bacteriophage as a highly conserved mechanism to facilitate the release of endolysin and cause cell lysis. Consistent with our hypothesis that BlyA is a prophage-encoded holin is the recent report that the cp32 plasmids that encode BlyA are prophages (16, 17).
FIG. 1.
Predictive analysis of BlyA structure and comparison to holins. (A) Predicted helices within BlyA and holins from bacteriophages 21 and T3 are shown. A + or − indicates a positively or negatively charged amino acid residue, respectively. (B) Helical wheel diagram of helix 1 (left) and helix 2 (right) of BlyA. Charged residues are indicated as in panel A.
BlyA possesses holin activity.
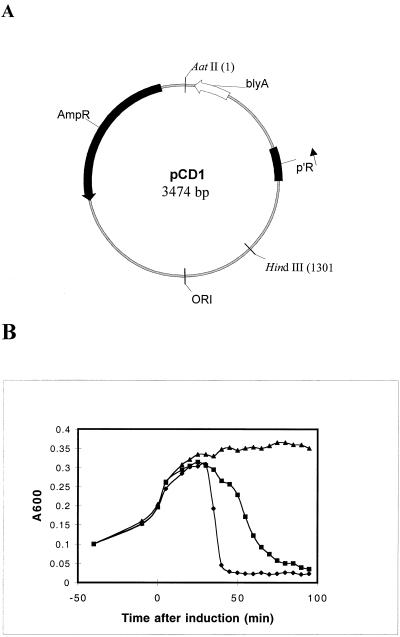
In order to test whether BlyA may possess holin activity, we utilized a genetic system developed by Wang and Young that incorporates the phage λ S gene, which encodes a holin (I.-N. Wang and R. Young, personal communication). This system involves complementation of a thermoinducible lambda lysogen that is defective for lambda holin (λcI857 Sam7) with a plasmid containing the test gene of interest under the control of the lambda p′R promoter. blyA was cloned into the appropriate plasmid vector under lambda p′R promoter control while maintaining the S gene ribosome-binding site (Fig. 2A). Thermoinduction of the blyA-containing lysogen MC4100 sheA::Tn5 (λcI857 Sam7)(pCD1) resulted in a similar onset and extent of cell lysis compared to the control lysogen containing lambda holin, MC4100 (λcI857 Sam7)(pUCS105R−), although the rate of lysis was somewhat slower than that promoted by lambda holin (Fig. 2B). The fact that the blyA-dependent lysis occurred in a sheA-defective lysogen indicated that the SheA hemolysin played no role in the observed result. Furthermore, an isogenic blyA-containing lysogen that lacked both the lambda holin and endolysin genes, MC4100 (λcI857 ΔSR)(pCD1), failed to undergo lysis in this assay, indicating that cell lysis was specific for the release and action of lambda endolysin and was not simply due to BlyA overproduction and toxicity. This result suggests that BlyA has holin-like activity and can transport λ endolysin through the E. coli membrane, although a less specific mechanism that results in loss of membrane integrity allowing release of endolysin and cell lysis to occur cannot be excluded by our experiments.
FIG. 2.
Analysis of BlyA for holin-like activity. (A) Plasmid map of pCD1. p′R, blyA, AmpR, and ORI indicate the major rightward promoter of lambda, the blyA gene, the β-lactamase gene, and the plasmid replicative origin, respectively. (B) Strains were grown in LB supplemented with ampicillin (100 μg ml−1) at 30°C for 70 min, after which the cell density of all cultures was adjusted to an A600 of 0.1. At an A600 of 0.2, the cultures were shifted to 42°C (0 min) for 15 min and then placed at 37°C for monitoring of cell lysis. Similar results were obtained in two different trials. Symbols: ⧫, MC4100 (λcI857 Sam7)(pUCS105R−); ■, MC4100 sheA::Tn5 (λcI857 Sam7)(pCD1); ▴, MC4100 (λcI857 ΔSR)(pCD1).
blyAB up-regulation upon phage induction.
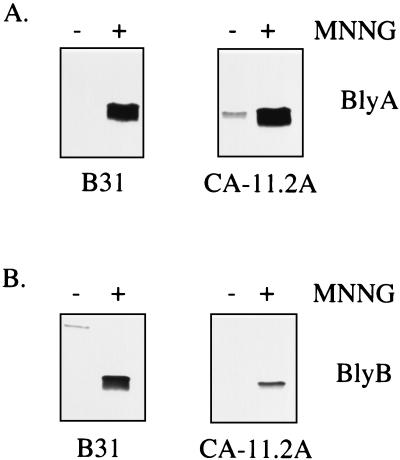
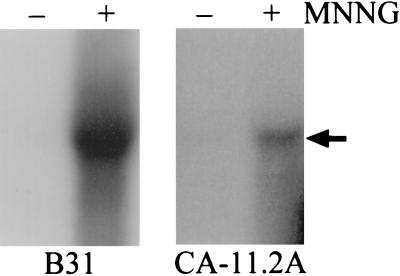
A previous attempt to visualize the BlyA protein from in vitro-grown cultures of B. burgdorferi by Western analysis was unsuccessful, although a minor amount of blyAB mRNA was detectable under these conditions (21, 33). Our hypothesis that BlyA may be a prophage-encoded holin was further tested by assaying blyAB expression upon prophage induction with MNNG (17). As expected for a holin, a marked increase in the level of the BlyA and BlyB proteins was observed in the MNNG-treated cultures while the basal level of these two proteins was low or undetectable in the untreated control cultures (Fig. 3). B. burgdorferi strain CA-11.2A constitutively produces low levels of the BlyA protein (Fig. 3A), consistent with its constitutively producing low levels of φBB-1 phage (17). The level of OspC, an outer surface protein encoded on a different plasmid (25), did not increase in either strain after MNNG treatment (data not shown). The increased level of the BlyA and BlyB proteins was correlated with the level of φBB-1 phage in the culture supernatant as assayed by the presence of phage DNA (data not shown). Furthermore, the blyAB transcript level was assayed upon phage induction. This analysis revealed a substantial increase in the level of blyAB mRNA after MNNG treatment (Fig. 4). Again, the extent of this increase was correlated with the level of φBB-1 DNA (linearized cp32) in the culture supernatant (data not shown).
FIG. 3.
Analysis of BlyA and BlyB protein levels in MNNG-induced B. burgdorferi. Cell extracts were prepared from B. burgdorferi B31 and CA-11.2A that were treated with MNNG (+) or left untreated (−) as described in Materials and Methods. Five microliters of each fraction was analyzed for BlyA (A) or BlyB (B) protein content by SDS-PAGE and Western blotting.
FIG. 4.
Analysis of blyAB mRNA in MNNG-induced B. burgdorferi. RNA was prepared from B. burgdorferi B31 (15 μg) and CA-11.2A (10 μg) that were treated with MNNG (+) or left untreated (−) and analyzed by Northern hybridization with a blyB probe as described in Materials and Methods. The arrow indicates the position of the ∼1.3-kb blyAB transcript. A similar result was obtained with a blyA probe.
Subcellular localization of BlyA and BlyB.
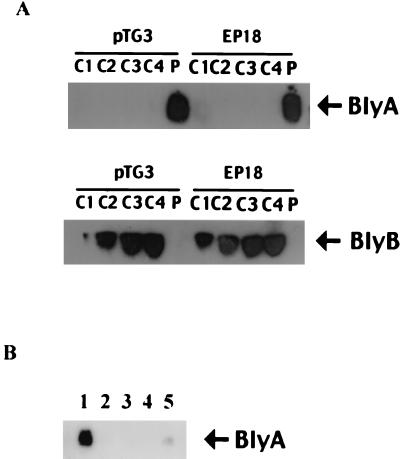
One reason that BlyA was originally suggested to be a hemolysin was based on its partitioning between soluble (∼25%) and membrane (∼75%) fractions in E. coli (21). By contrast, holins are solely membrane associated (48). In order to reinvestigate this issue, fractionation studies were performed with both B. burgdorferi and E. coli. In both organisms, BlyA was found to be entirely membrane associated (i.e., contained solely within the P100 fraction) while BlyB was found to be a soluble protein (i.e., contained within the S100 fraction) (Fig. 5 and 6). To reconcile our results with those published previously, after sedimentation of the E. coli extract, we divided the S100 into four fractions (from top to bottom), as well as a small amount of S100 that was closest to the pellet. Only the latter fraction contained a small quantity of BlyA protein, suggesting that the prior result was due to contamination of the soluble fraction by small membrane fragments (Fig. 6A and data not shown). A similar result was obtained with EP18, which contains the blyA-L10F allele and was previously suggested to produce mostly soluble BlyA protein (21). It is worthy of note that the BlyB protein was found to be enriched in the bottom S100 fractions despite the fact that most other soluble proteins were equally abundant in all four S100 fractions (Fig. 6A and data not shown). This result suggests that the small BlyB monomer (10 kDa) is likely to oligomerize or associate with a larger protein in E. coli. Finally, we tested whether our results were due to aggregation of the BlyA protein in E. coli rather than authentic membrane association. For this purpose, BlyA localization was repeated using floatation sedimentation to separate membranes from proteins based on their different densities. P100 was loaded at the bottom of a solution of metrizamide and sedimented to form a density gradient that allows floatation of membranes to the top of the gradient while proteins remain at the bottom. The BlyA protein was present in the top fraction along with membranes, suggesting an authentic association with the membranes (Fig. 6B).
FIG. 5.
Subcellular localization of BlyA and BlyB proteins in B. burgdorferi. A cell extract of MNNG-treated B. burgdorferi CA-11.2A was prepared as described in Materials and Methods. The cell extract was sedimented at 100,000 × g for 3 h at 4°C to obtain supernatant (S100) and pellet (P100) fractions. The pellet was resuspended in a volume of TBSP buffer equivalent to that of the supernatant. Five microliters of each fraction was analyzed for BlyA (left) or BlyB (right) protein content by SDS-PAGE and Western blotting.
FIG. 6.
Subcellular localization of BlyA and BlyB proteins in E. coli. (A) A cell extract of E. coli MM294(pTG3) or EP18 was prepared as described in Materials and Methods. Cell extracts were sedimented at 100,000 × g for 3 h at 4°C. The supernatant was divided into four fractions (C1, C2, C3, and C4), from the top to the bottom, while the pellet (P) was resuspended in a volume of TBSP buffer equivalent to that of the supernatant. Twenty (BlyA) or forty (BlyB) microliters of each fraction was analyzed for BlyA and BlyB protein content by SDS-PAGE and Western blotting. (B) Floatation sedimentation analysis of P100 from MM294(pTG3). P100 was loaded onto the bottom of a solution of metrizamide and subjected to floatation sedimentation analysis as described by Mitchell and Oliver (29). The gradient was divided into five fractions (1 to 5), from the top to the bottom. The positions of proteins BlyA and BlyB are shown.
DISCUSSION
Previously, the blyAB locus was isolated from B. burgdorferi B31 based on the hemolytic activity of an E. coli strain containing the cloned genes (21). From genetic and biochemical analyses of the system, Guina and Oliver suggested that BlyA is a hemolysin and that BlyB is required in some manner for BlyA function. In the present study, we were able to show that the blyAB locus is nonhemolytic in an E. coli sheA mutant. This result suggests that the otherwise cryptic SheA hemolysin is responsible for the observed hemolytic phenotype and that blyAB expression only serves to derepress sheA expression. Since sheA induction normally requires production of a transcriptional regulator, BlyB may be directly responsible for the transcriptional up-regulation of sheA, particularly given its previously documented role in BlyA synthesis or stability (21). A recent report demonstrated that a global transcriptional regulator for anaerobic growth from Pasteurella haemolytica, FnrP, also leads to sheA induction and a hemolytic phenotype in E. coli (45). Given the complexity of growth phase-specific genetic circuitry and the fact that blyAB expression is growth phase dependent in E. coli (13), indirect models of sheA induction by blyAB expression need to be considered.
The physiological role of the BlyA protein in B. burgdorferi is more likely illustrated by the dramatic decrease in cell growth and viability observed in the E. coli system during stationary-phase growth (13). Indeed, sheA expression is not ordinarily toxic to E. coli (14). We propose that BlyA has a cytotoxic role as a bacteriophage holin or holin-like protein. Holins are a widely distributed class of small, channel-forming membrane proteins encoded by bacteriophage that oligomerize during the phage lytic cycle to allow release of endolysin, resulting in cell lysis. They comprise at least two membrane-spanning helical domains and a highly charged C terminus (48).
Several lines of evidence are consistent with this hypothesis. First, based on the uniform size of the B. burgdorferi chromosome and the conserved size and distribution of the cp32 plasmid family, Casjens et al. proposed that cp32 is a prophage (11, 12). This suggestion has received experimental support by the recent isolation of bacteriophage φBB-1, which contains linearized cp32 molecules, from the supernatant of B. burgdorferi strains that constitutively shed virus or can be induced to produce virus by treatment with MNNG (17). In this paper, we show that both expression of blyAB and synthesis of the BlyA and BlyB proteins were dramatically increased when B. burgdorferi was treated with MNNG; this increase correlated with φBB-1 phage production. Second, like other holins, BlyA was found to fractionate solely with the membrane. Third, the structural architecture of BlyA is similar to that of other holins in that it is likely to contain two membrane-spanning α helices and a charged C terminus. Fourth, Western blots have demonstrated a tendency of BlyA to oligomerize (13). Fifth, blyA is one of 28 genes in a putative phage late operon (or late regulon), and its location near the 3′ end is a position occupied by lysis genes in many other temperate phages (16). Finally, and most importantly, a lysis system in which BlyA was substituted for lambda holin resulted in a rapid-lysis phenotype characteristic of holins. Our results provide the first indication for the presence of a prophage-encoded holin within Borrelia. However contrary to known holins, we recently found that BlyA-induced lysis of E. coli was prevented by treatment of the heat-induced lambda lysogen with cyanide (C. Damman and D. Oliver, unpublished results), which ordinarily triggers holin oligomerization and premature cell lysis (48). Although we emphasize the caveat that the kinetics of cell lysis mediated by BlyA in E. coli was slower than that mediated by λ S protein and cyanide did not induce lysis, a heterologous system was utilized to demonstrate function by complementation and the cell lysis was dependent on the λ R protein. The transport of λ endolysin through a putative Borrelia pore in an E. coli membrane is rather remarkable. Additional studies employing sophisticated biochemical and biophysical approaches are now warranted to confirm and extend our work, particularly given the difficulty with genetic approaches to the study of gene function in B. burgdorferi and the cp32 plasmid family.
Three possible roles for BlyB, which are not necessarily mutually exclusive, can be envisioned on the basis of our work. BlyB could be a regulatory factor, an assembly factor, or an endolysin. The observation that BlyB is required for BlyA production, as well as sheA derepression, is consistent with a role as a regulatory factor, and indeed, several transcriptional regulators have been isolated based on sheA induction (14, 15, 45). The two-way genetic interaction between blyA and blyB noted previously (blyA mutations affect BlyB levels and vice versa) may be explained by some sort of protein-protein interaction, albeit transient in nature (13, 21). This view is consistent with the previous proposal that BlyB serves as a chaperone or assembly factor for BlyA (21). Finally, in addition to a role in BlyA synthesis or stabilization, BlyB could be an endolysin, since endolysin genes are often located adjacent to their companion holin genes (16, 48). While BlyB shows no homology to any known endolysin and had no lytic activity in E. coli, the phylogenetic distance between spirochetes and proteobacteria and the difference in peptidoglycan composition between these two groups of organisms make these observations inconclusive (5). Additional characterization of the blyAB system in the biology of B. burgdorferi and phage φBB-1 should clarify these points. However, taken together, the structural and functional data presented here suggest that the BlyA and BlyB proteins play an important role in the lysis of B. burgdorferi cells during the last stage of the φBB-1 lytic cycle.
ACKNOWLEDGMENTS
We thank Tina Guina for guidance throughout this project, Shannon Kelly-Francis for help with production of the BlyB antibody, Tom Schwan for the OspC antibody, Patti Rosa for CA-11.2A, Sherwood Casjens for useful discussions, Ing-Nang Wang and Ry Young for strains and advice on the holin assay, and Francisco J. del Castillo for providing the sheA mutant.
We acknowledge the generous support of Procter and Gamble (D.O.), the National Institutes of Health (AI41559), the Arthritis Foundation, and the National Science Foundation (MCB-9722408) (D.S.S.). C.H.E. was a recipient of a Predoctoral Honors Fellowship from The University of Montana.
REFERENCES
- 1.Ackerman H W. Tailed bacteriophages: the order Caudovirales. Adv Virus Res. 1998;51:135–201. doi: 10.1016/S0065-3527(08)60785-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akin E, McHugh G L, Flavell R A, Fikrig E, Steere A C. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun. 1999;67:173–181. doi: 10.1128/iai.67.1.173-181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck G, Benach J L, Habicht G S. Isolation, preliminary chemical characterization, and biological activity of Borrelia burgdorferi peptidoglycan. Biochem Biophys Res Commun. 1990;167:89–95. doi: 10.1016/0006-291x(90)91734-a. [DOI] [PubMed] [Google Scholar]
- 6.Brandt M E, Riley B S, Radolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown T. Analysis of RNA by Northern and slot blot hybridization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Siedman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. unit 4.9. New York, N.Y: John Wiley & Sons, Inc.; 1999. pp. 21–27. [Google Scholar]
- 8.Cabelli R J, Dolan K M, Qian L, Oliver D B. Characterization of membrane-associated and soluble states of SecA protein from wild-type and secA51(Ts) mutant strains of Escherichia coli. J Biol Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 9.Campbell G L, Fritz C L, Fish D, Nowakowski J, Nadelman R B, Wormser G P. Estimation of the incidence of Lyme disease. Am J Epidemiol. 1998;148:1018–1026. doi: 10.1093/oxfordjournals.aje.a009568. [DOI] [PubMed] [Google Scholar]
- 10.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 11.Casjens S, Palmer N, van Vugt R, Huang W M, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson R J, Haft D, Hickey E, Gwinn M, White O, Fraser C M. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 12.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damman C. M.S. thesis. Middletown, Conn: Wesleyan University; 1999. [Google Scholar]
- 14.del Castillo F J, Leal S C, Moreno F, del Castillo I. The Escherichia coli K12 sheA gene encodes a 34-kDa secreted haemolysin. Mol Microbiol. 1997;25:107–117. doi: 10.1046/j.1365-2958.1997.4391813.x. [DOI] [PubMed] [Google Scholar]
- 15.del Castillo I, Gomez J M, Moreno F. mprA, an Escherichia coli gene that reduces growth-phase dependent synthesis of microcins B17 and C7 and blocks osmoinduction of proU when cloned on a high-copy-number plasmid. J Bacteriol. 1990;172:437–445. doi: 10.1128/jb.172.1.437-445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggers C H, Casjens S, Hayes S F, Garon C F, Damman C J, Oliver D B, Samuels D S. Bacteriophages of spirochetes. J Mol Microbiol Biotechnol. 2000;2:365–373. [PubMed] [Google Scholar]
- 17.Eggers C H, Samuels D S. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J Bacteriol. 1999;181:7308–7313. doi: 10.1128/jb.181.23.7308-7313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, Van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs H, Wallich R, Simon M M, Kramer M D. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci USA. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 21.Guina T, Oliver D B. Cloning and analysis of a Borrelia burgdorferi membrane-interactive protein exhibiting haemolytic activity. Mol Microbiol. 1997;24:1201–1213. doi: 10.1046/j.1365-2958.1997.4291786.x. [DOI] [PubMed] [Google Scholar]
- 22.Guo B P, Brown E L, Dorward D W, Rosenberg L C, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 23.Kornacki J A, Oliver D B. Lyme disease-causing Borrelia species encode multiple lipoproteins homologous to peptide-binding proteins of ABC-type transporters. Infect Immun. 1998;66:4115–4122. doi: 10.1128/iai.66.9.4115-4122.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Marconi R T, Samuels D S, Garon C F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993;175:926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis N, Rosa P A. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993;61:2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meselson M, Yuan R. DNA restriction enzyme from E. coli. Nature. 1968;217:1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 29.Mitchell C, Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery R R, Malawista S E, Feen K J, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson I, Saaf A, Whitley P, Gafvelin G, Waller C, von Heijne G. Proline-induced disruption of a transmembrane α-helix in its natural environment. J Mol Biol. 1998;284:1165–1175. doi: 10.1006/jmbi.1998.2217. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson I, von Heijne G. Breaking the camel's back: proline-induced turns in a model transmembrane helix. J Mol Biol. 1998;284:1185–1189. doi: 10.1006/jmbi.1998.2219. [DOI] [PubMed] [Google Scholar]
- 33.Porcella S F, Popova T G, Akins D R, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa P A. Microbiology of Borrelia burgdorferi. Semin Neurol. 1997;17:5–10. doi: 10.1055/s-2008-1040906. [DOI] [PubMed] [Google Scholar]
- 35.Sadziene A, Barbour A G, Rosa P A, Thomas D D. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993;61:3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 38.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 39.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson B, Porcella S F, Oie K L, Fitzpatrick C A, Raffel S J, Lubke L, Schrumpf M E, Schwan T G. The relapsing fever spirochete Borrelia hermsii contains multiple, antigen-encoding plasmids that are homologous to the cp32 plasmids of Lyme disease spirochetes. Infect Immun. 2000;68:3900–3908. doi: 10.1128/iai.68.7.3900-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson B, Zückert W, Akins D. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species. J Mol Microbiol Biotech. 2000;2:411–422. [PubMed] [Google Scholar]
- 43.Suk K, Das S, Sun W, Jwang B, Barthold S, Flavell R, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung S Y, McDowell J V, Carlyon J A, Marconi R T. Mutation and recombination in the upstream homology box-flanked ospE-related genes of the Lyme disease spirochetes result in the development of new antigenic variants during infection. Infect Immun. 2000;68:1319–1327. doi: 10.1128/iai.68.3.1319-1327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhlich G A, McNamara P J, Iandolo J J, Mosier D A. Cloning and characterization of the gene encoding Pasteurella haemolytica FnrP, a silent regulator of the Escherichia coli silent hemolysin SheA. J Bacteriol. 1999;181:3845–3848. doi: 10.1128/jb.181.12.3845-3848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y, Kodner C, Coleman L, Johnson R C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young R, Blasi U. Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev. 1995;17:191–205. doi: 10.1111/j.1574-6976.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Hardham J, Barbour A, Norris S. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]