Abstract
C-REPEAT BINDING FACTORS (CBFs) are highly conserved plant transcription factors that promote cold tolerance. In Arabidopsis (Arabidopsis thaliana), three CBFs (CBF1 to CBF3) play a critical role in cold acclimation, and the expression of their corresponding genes is rapidly and transiently induced during this adaptive response. Cold induction of CBFs has been extensively studied and shown to be tightly controlled, yet the molecular mechanisms that restrict the expression of each CBF after their induction during cold acclimation are poorly understood. Here, we present genetic and molecular evidence that the decline in the induction of CBF3 during cold acclimation is epigenetically regulated through the Polycomb Repressive Complex (PRC) 2. We show that this complex promotes the deposition of the repressive mark H3K27me3 at the coding region of CBF3, silencing its expression. Our results indicate that the cold-inducible long noncoding RNA SVALKA is essential for this regulation by recruiting PRC2 to CBF3. These findings unveil a SVALKA-PRC2 regulatory module that ensures the precise timing of CBF3 induction during cold acclimation and the correct development of this adaptive response.
Introduction
Low temperatures adversely affect the growth and development of plants, and condition their geographic distribution and productivity. To cope with low temperatures and acquire freezing tolerance, plants from temperate regions have evolved a sophisticated adaptive response, termed cold acclimation, whereby they increase their freezing tolerance after being exposed to low nonfreezing temperatures (Thomashow 1999). In Arabidopsis (Arabidopsis thaliana), crucial to this response are the C-REPEAT BINDING FACTORS (CBFs), a small family of three tandemly clustered cold-inducible genes (CBF1 to CBF3) encoding transcription factors that activate the expression of different cold-regulated (COR) genes, known as the CBF regulon, to boost freezing tolerance (Gilmour et al. 1998; Jaglo-Ottosen et al. 1998; Liu et al. 1998; Medina et al. 1999; Novillo et al. 2007). The expression of CBFs during cold acclimation is characterized by a transient induction with a peak within 3 to 6 h of cold exposure and a subsequent rapid decline, after 8 to 12 h of exposition, to attain a relatively stable level (Gilmour et al. 1998; Liu et al. 1998; Medina et al. 1999). Despite extensive studies on the regulation of CBF cold induction, the molecular mechanisms underlying the posterior decline of expression remain unclear. Here, we show that the Arabidopsis cold-inducible long noncoding RNA (lncRNA) SVALKA, which is transcribed on the antisense strand between CBF3 and CBF1 (Kindgren et al. 2018), recruits the Polycomb Repressive Complex (PRC) 2 to the CBF3 gene for deposition of the H3K27me3 repressive mark, thus restraining its expression after its induction peak and ensuring the correct development of the cold acclimation process. Our study reveals that the expression of CBFs during cold acclimation is epigenetically regulated through a SVALKA-PRC2 module and this regulation is essential for this adaptive response.
Results and discussion
Under control conditions, CURLY LEAF (CLF), an essential subunit of the Arabidopsis PRC2 complex, has been associated to the genomic region harboring the CBFs (Xiao et al. 2017), and, consistently, the PRC2-dependent repressive mark H3K27me3 is substantially deposited at that region (Sequeira-Mendes et al. 2014). We then decided to explore the possibility that the PRC2 might be involved in repressing the expression of CBFs after their induction during cold acclimation. To test this possibility, we assessed the expression of CBFs in Arabidopsis clf28 swn7 mutants exposed 3 or 24 h to 4 °C, times at which they are highly induced or their induction has already drastically decreased in Col-0 wild-type (WT) plants, respectively (Supplementary Fig. S1). The clf28 swn7 mutants have a compromised PRC2 activity due to null mutations in CLF and SWN genes, which encode two of its core subunits, giving rise to small seedlings after germination that degenerate to a callus-embryo-like structure (Farrona et al. 2011). Reverse transcription quantitative PCR (RT-qPCR) assays with specific primers for each CBF (Fig. 1A, Supplementary Table S1) revealed that CBF1 and CBF3 transcripts accumulated at higher levels in mutant than in the WT plants in response to low temperature, this accumulation being much more significant after 24 h of cold exposure (Fig. 1B). The transcript levels of CBF2, however, were not affected in the mutants (Fig. 1B). These data indicated that PRC2 suppresses the expression of CBF1 and CBF3, mainly in the late phase of their induction by low temperature. The induction of CBF2 does not seem to be silenced by PRC2. Despite their redundant function in cold acclimation, the expression of CBF genes is positively and negatively governed through a plethora of common and specific regulators (Ding et al. 2020). Therefore, it is not surprising that PRC2 only silences CBF1 and CBF3. Understanding how CBF2 gene expression is repressed once it is induced under low temperature conditions requires additional experiments and will be the subject of future studies.
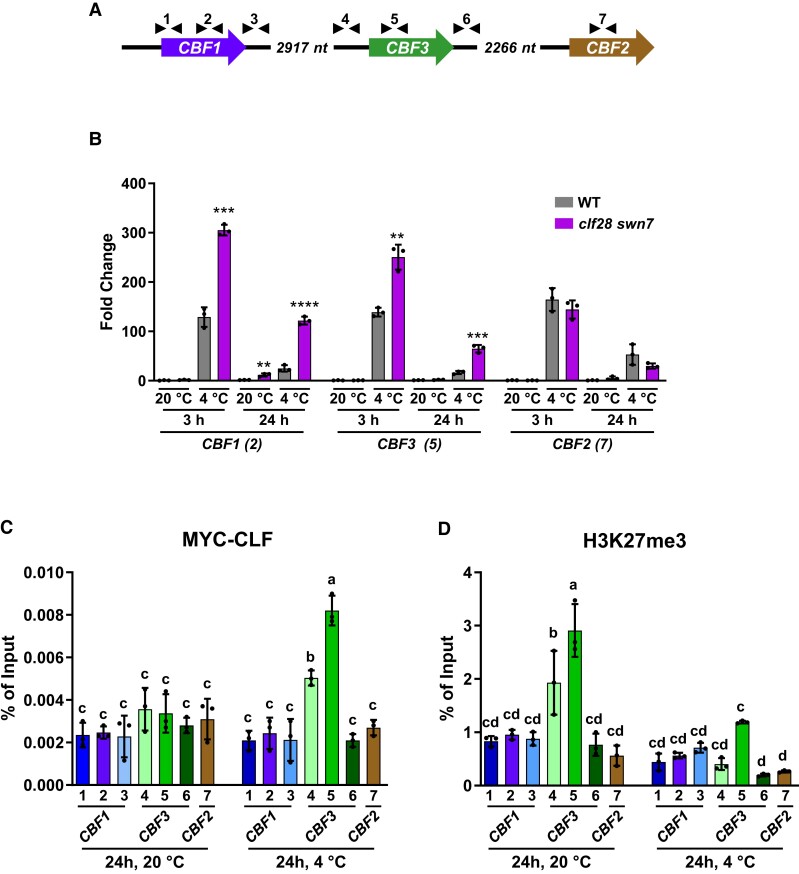
Figure 1.
The cold induction of CBF3 is repressed by the PRC2 complex. A) Schematic representation of the Arabidopsis genomic region containing the CBF genes. Arrowheads indicate the relative positions of primer sets used for expression analyses and ChIP experiments in this work. Each primer set is identified by a number. B) Expression analysis of CBF1, CBF3, and CBF2 in 2-wk-old Col-0 (WT) and clf28 swn7 plants maintained or exposed to 20 or 4 °C for 3 or 24 additional hours, respectively. In each condition (20 or 4 °C), transcript levels, determined by RT-qPCR, are represented as relative to the values of the corresponding WT. Asterisks indicate significant differences (**P < 0.01, ***P < 0.001, ****P < 0.0001) between clf28 swn7 and WT in each condition, as determined by two-sided t test. The numbers in parentheses indicate the primer sets used. C and D) ChIP experiments showing the levels of MYC-CLF (C) and H3K27me3 (D) on the chromatin of CBFs from 2-wk-old WT plants grown 24 additional hours at 20 or 4 °C. The relative amounts of DNA in the input and the immunoprecipitated samples were determined by qPCR with the CBF primer sets indicated below the bars in three biological replicates per sample. The levels of MYC-CLF and H3K27me3 were determined as the percentage of input DNAs recovered in the immunoprecipitates. Distinct letters indicate significant differences (P < 0.05) according to one-way ANOVA followed by Tukey's test. In B, C and D) data represent the mean of three independent experiments and error bars show the standard deviation.
From the results described above, we anticipated that, after 24 h of cold exposure, CLF would be substantially associated to CBF1 and CBF3 chromatin. This assumption was examined by determining the levels of CLF associated to CBFs by means of chromatin immunoprecipitation (ChIP) assays, using an anti-MYC antibody, chromatin isolated from the WT transgenic plants expressing a translational 35S:MYC:CLF fusion (Lodha et al. 2013) grown under control conditions or exposed to 4 °C for 24 h, and specific primers corresponding to different regions of the CBFs (Fig. 1A, Supplementary Table S1). Results showed that CLF was significantly associated to the CBF3 coding-region chromatin after 24 h of cold exposure. The levels of CLF associated to the chromatin upstream of the CBF3 coding region also resulted to be significantly high after 24 h at 4 °C, although to a lower extent than those detected in the chromatin of the coding region. We did not find a significant association of CLF to the chromatin downstream of the CBF3 coding region (Fig. 1C). Intriguingly, the levels of CLF associated with CBF1 chromatin were not significant in any of the regions analyzed neither in plants grown under control conditions nor exposed to low temperature (Fig. 1C). Consistent with the data described above (Fig. 1B), the CLF levels associated with CBF2 chromatin were not significant either (Fig. 1C). Then, we carried out ChIP assays with an anti-H3K27me3 antibody, chromatin from the WT plants grown under control conditions or subjected to 4 °C for 24 h, and the CBF-related primers used in the previous experiment (Fig. 1C, Supplementary Table S1). Under control conditions, coherently with the low expression of CBF3, the levels of the repressive histone mark H3K27me3 were highly significant in its chromatin corresponding to both promoter and coding regions (Fig. 1D). Although these regions showed very low levels of associated CLF (Fig. 1C), this is not surprising considering that around three quarters of the H3K27me3-marked genes have been proposed to not display CLF associated with their chromatin (Shu et al. 2019). These marks would have been deposited by a PRC2 containing a different catalytic subunit than CLF, such as SWN7 or MEA, or in a previous developmental stage (Costa and Dean 2019). Interestingly, after low temperature exposition, the levels of the H3K27me3 histone mark were significantly higher only in the CBF3 coding-region chromatin (Fig. 1D). Altogether, these results strongly suggested that the PRC2 complex represses the expression of CBF3 during cold acclimation, once attained its induction peak, through the deposition of the H3K27me3 mark at its coding region. The PRC2 complex would also suppress the expression of CBF1 during cold acclimation but independently of CLF, through another component of the PRC2 like SWN, or through an indirect pathway.
Now, the arising question was how the PRC2 complex is associated to the chromatin of CBF3 during cold acclimation. lncRNAs have recently emerged as potent regulators of gene expression and are recognized as important participants in PRC2 function (Margueron and Reinberg 2011). In Arabidopsis, COLDAIR, COLDWRAP, and AG-incRNA4 lncRNAs have been described to physically interact with the PRC2 subunit CLF, contributing to its localization to the corresponding target genes and their subsequent silencing through H3K27me3 deposition (Swiezewski et al. 2009; Kim and Sung 2017; Wu et al. 2018). A common feature of all these lncRNAs is that they are transcribed from the genomic locus of their target genes (Swiezewski et al. 2009; Kim and Sung 2017; Wu et al. 2018). Interestingly, as mentioned above, the Arabidopsis genome encodes a cold-induced lncRNA, SVALKA, that is transcribed on the antisense strand between CBF3 and CBF1. The transcription takes place from different transcription starting sites, originating short (α) and long (β) isoforms (Kindgren et al. 2018) (Supplementary Fig. S2A). Under control conditions, extensive transcription of the SVALKA β isoform throughout the CBF1 gene body generates a natural antisense transcript that negatively regulates CBF1 transcription (Zacharaki et al. 2023). In response to low temperature, the transcription of SVALKA isoforms is induced, reaching a stable maximum of induction after 8 to 12 h of treatment (Kindgren et al. 2018). It has been reported that a transcriptional read-through of the SVALKA β isoform limits the cold induction of CBF1 by means of a head-to-head RNA polymerase II (RNAPII) collision mechanism at the 3ʹ-end of this gene, negatively regulating cold acclimation (Kindgren et al. 2018). A function for the SVALKA isoforms, however, has not yet been described. Thus, we hypothesized that they could mediate the decline of CBF3 expression that takes place after its rapid induction during cold acclimation by recruiting PRC2 to CBF3. This possibility was assessed by studying the expression levels of CBF3 in a SVALKA T-DNA null mutant (svk-1) (Kindgren et al. 2018) exposed to 4 °C for different times. We did not find significant differences in CBF3 transcript levels between mutant and the WT plants after 3 or 8 h of cold exposure. However, CBF3 transcripts were significantly higher in svk-1 than in the WT plants after being exposed to 4 °C for 24 or 72 h (Fig. 2A). Very similar results were obtained when analyzing the SVALKA T-DNA mutant SVK OE, which overexpresses a partial and aberrant SVALKA transcript (Kindgren et al. 2018) (Fig. 2A). Our results, therefore, unveiled a role for SVALKA consisting in promoting the decline of CBF3 expression that takes place after its induction during cold acclimation. It is worth mentioning that, as displayed in Fig. 1B, clf28 swn7 mutations lead to increased expression of CBF3 at both 3 and 24 h after cold treatment, but disruption of SVALKA only affects CBF3 expression after 24 h of exposure at 4 °C (Fig. 2A). Considering that SVALKA is not expressed after 3 h of cold treatment (Kindgren et al. 2018), we can conclude that the effect of PRC2 on CBF3 expression in the early stage of cold acclimation is not mediated by SVALKA.
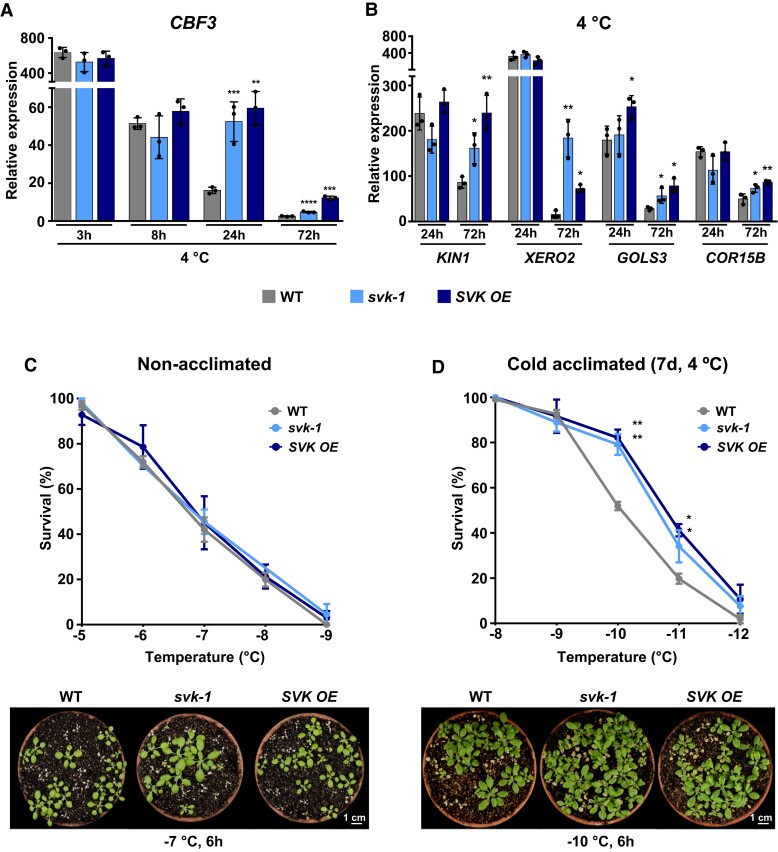
Figure 2.
SVALKA regulates the cold acclimation response by mediating the decline of CBF3 induction. A and B) Expression analysis of CBF3 (A) and CBF3 target genes KIN1, XERO2, GOLS2, and COR15B (B) in 2-wk-old WT, svk-1 and SVK OE plants exposed to 4 °C for the indicated additional hours (3, 8, 24, or 72 h). In each case, transcript levels, determined by RT-qPCR, are represented as relative to the values of the WT plants grown under control conditions. Primer set 5 in Fig. 1A was used for expression analyses of CBF3. C and D) Freezing tolerance of nonacclimated (C) and cold acclimated 7 d at 4 °C (D) 2-wk-old WT, svk-1 and SVK OE plants exposed to the indicated freezing temperatures for 6 h. Tolerance was estimated as the percentage of plants surviving each specific temperature after 1 wk of recovery under control conditions. Lower panels show the freezing tolerance of representative nonacclimated and cold acclimated plants. Images were digitally extracted for comparison. In all panels, asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) between svk-1 and SVK OE mutants and the corresponding WT plants, as determined by one-sided t test. Data represent the mean of three independent experiments and error bars show the standard deviation.
As shown above (Fig. 2A), svk-1 and SVK OE mutants had in common a delay in the decline of CBF3 induction during cold acclimation compared to the WT plants. Interestingly, however, they have been described to diverge in the induction levels of CBF1. Indeed, while the cold induction of CBF1 is increased in svk-1, it is significantly reduced in SVK OE (Kindgren et al. 2018). The SVK OE mutant, therefore, constitutes a very good material to evaluate the physiological significance of the role unveiled for the SVALKA lncRNA. First, we determine the mRNA levels corresponding to some CBF-activated COR genes such as KIN1, XERO2, GOLS3, and COR15B in SVK OE plants subjected to low temperature for 24 and 72 h. svk-1 mutants were also included in the analysis as a reference. Consistent with the high levels of CBF3 transcripts present in SVK OE and svk-1 plants after a long-term cold treatment (Fig. 2A), the expression of COR genes was significantly higher in both mutants than in the WT plants exposed 72 h to 4 °C (Fig. 2B). Then, we studied the effect of the late up-regulation of CBF3 and COR gene expression on the ability of SVK OE plants to tolerate freezing and cold acclimate. In this study, svk-1 mutants were again included for reference. Two-wk-old nonacclimated and cold acclimated (seven additional days at 4 °C) mutant plants were subjected to decreasing freezing temperatures and, after 2 wk of recovery under control conditions, scored for survival. Nonacclimated mutants presented a similar capacity to tolerate freezing as the WT, the LT50 (temperature that causes 50% lethality) values being in both cases around −6.8 °C (Fig. 2C). By contrast, cold acclimated mutants exhibited a significantly higher freezing tolerance than cold acclimated WT plants, the LT50 values in this case being −10.8 °C for both SVK OE and svk-1 mutants and −10.0 °C for the WT plants (Fig. 2D). As already mentioned, the expression of CBF1 is only slightly induced by low temperature in the SVK OE mutant (Kindgren et al. 2018). Therefore, our findings clearly indicated that SVALKA also contributes to the correct development of cold acclimation by mediating the decline of CBF3 induction during this adaptive response.
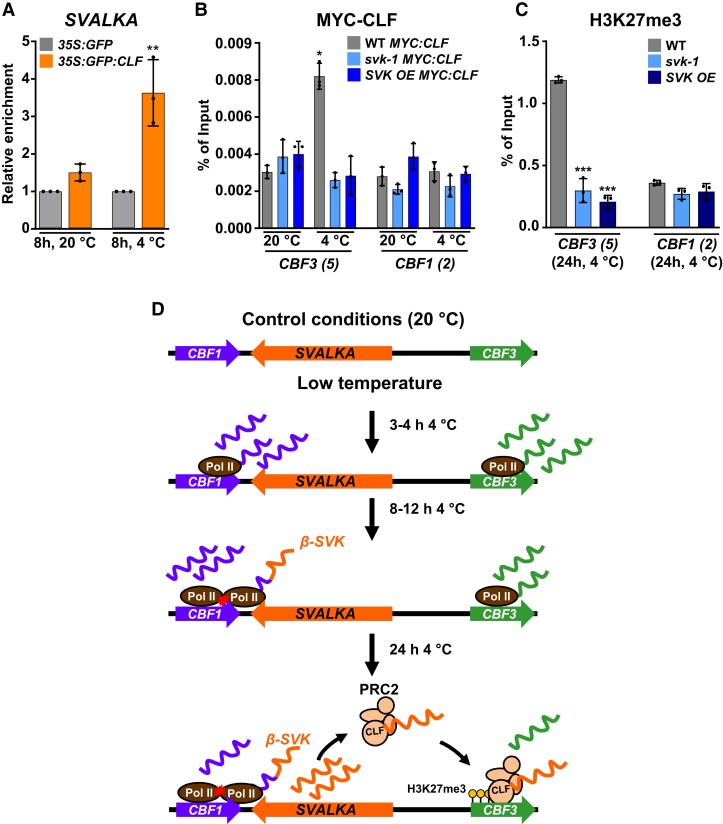
Next, we studied if SVALKA could contribute to suppress the induction of CBF3 during cold acclimation by targeting PRC2 to this gene. First, the possible interaction of SVALKA with CLF was analyzed by means of RNA immunoprecipitation (RIP) assays. We used null clf double mutant plants clf50 clf16 expressing a 35S:GFP:CLF fusion (Schubert et al. 2006) grown under control conditions or exposed to 4 °C for 8 h, a time at which the expression of SVALKA is highly induced (Kindgren et al. 2018), and an anti-GFP antibody. Plants WT expressing a 35S:GFP fusion were used as a control for specificity. The presence of SVALKA transcripts in the immunoprecipitates was determined and quantified by RT-qPCR with specific primers (Supplementary Fig. S2A, Supplementary Table S1) that, under our experimental conditions, solely amplify the spliced β isoform (Supplementary Fig. S2B). We only detected a significant enrichment of this isoform in the immunoprecipitated fraction corresponding to samples from plants expressing the 35S:GFP:CLF fusion exposed to 4 °C (Fig. 3A), indicating that, indeed, SVALKA physically interacts with CLF in response to low temperature. Then, we assessed whether the interaction of SVALKA with CLF was required for targeting this protein, and therefore PRC2, to the CBF3 coding region. ChIP experiments were performed with an anti-MYC antibody, chromatin isolated from svk-1, SVK OE and the WT plants expressing the 35S:MYC:CLF fusion grown under control conditions or exposed to 4 °C for 24 h, and the specific CBF3 primers corresponding to its coding region (Fig. 1A, Supplementary Table S1). The levels of MYC:CLF associated to the chromatin of CBF1 coding-region in WT, svk-1 and SVK OE plants under control and cold conditions were also quantified as internal controls in the experiments. The absence of a functional SVALKA in svk-1 and SVK OE plants did not affect the levels of CLF associated with the CBF3 chromatin in plants grown at 20 °C (Fig. 3B). After 24 h of cold exposure, however, the lack of a functional SVALKA led to significantly lower levels of associated CLF than in the WT plants, similar to those observed under control conditions (Fig. 3B). As expected, the levels of CLF associated with the CBF1 chromatin in svk-1 and SVK OE mutants were, in all cases, as in the WT plants, which, in turn, were similar to those associated to CBF3 chromatin in the WT plants grown under control conditions (Fig. 3B). These findings strongly supported that SVALKA interacts with CLF to promote PRC2 targeting to CBF3 coding region after its induction during cold acclimation, and anticipated that SVALKA should be necessary for the deposition of the repressive histone mark H3K27me3 by PRC2 at the coding region of CBF3. This possibility was evaluated by quantifying the levels of H3K27me3 at the CBF3 chromatin of WT, svk-1 and SVK OE plants exposed to 4 °C for 24 h. The levels of H3K27me3 at the chromatin of CBF1 were also quantified in these plants as an internal control. ChIP assays using an anti-H3K27me3 antibody and specific primers for CBF1 and CBF3 coding regions (Fig. 1A, Supplementary Table S1) revealed that, in fact, the levels of H3K27me3 at CBF3 were significantly lower in both svk-1 and SVK OE mutants than in the WT plants (Fig. 3C). Consistent with the results described above (Fig. 3B), the levels of H3K27me3 at CBF1 were similar in all genotypes (Fig. 3C). All in all, these data indicated that SVALKA physically interacts with CLF to target PRC2 to CBF3 coding region for deposition of H3K27me3 marks and repression of its induction during cold acclimation. As already proposed for different lncRNAs (Mattick et al. 2023), the specific recognition of the CBF3 coding region by PRC2 for deposition of the H3K27me3 mark could lay on the capacity of SVALKA to recognize and interact with a CBF3 specific nucleotide sequence, or with specific proteins associated to the CBF3 coding region chromatin.
Figure 3.
SVALKA interacts with CLF targeting PRC2 to CBF3 for H3K27me3 deposition. A) RIP assays performed with 2-wk-old clf50 clf16 plants expressing a 35S:GFP:CLF fusion maintained or exposed to 20 or 4 °C for eight additional hours, respectively, and an anti-GFP antibody. Two-wk-old Col-0 (WT) plants expressing a 35S:GFP fusion were used as interaction specificity controls. The relative amounts of SVALKA lncRNA (β isoform) in the input and the immunoprecipitated samples were determined by RT-qPCR, with three biological replicates per sample. The enrichment of SVALKA in the samples was determined as the percentage of input RNA recovered in the immunoprecipitates, and is represented relative to the enrichment in the corresponding 35S:GFP samples. B and C) ChIP experiments showing the levels of MYC-CLF on the CBF3 and CBF1 chromatin of 2-wk-old WT, svk-1 and SVK OE plants expressing the MYC:CLF fusion maintained at 20 °C or exposed 24 h to 4 °C (B), and the levels of H3K27me3 on the CBF3 and CBF1 chromatin of 2-wk-old WT, svk-1 and SVK OE plants exposed to 4 °C for 24 h (C). The relative amounts of DNA in the input and the immunoprecipitated samples were determined by qPCR, with the CBF primer sets indicated in parenthesis (see Fig. 1A), in three biological replicates per sample. The levels of MYC-CLF and H3k27me3 were determined as the percentage of input DNA recovered in the immunoprecipitates. D) Proposed model for SVALKA function in Arabidopsis cold acclimation. In (A) and (C), asterisks indicate significant differences (**P < 0.01, ***P < 0.001) between 35S:GFP:CLF and 35S:GFP, and svk-1 or SVK OE and WT in CBF3, respectively, as determined by one-sided t test. In (B), asterisk indicates significant differences (*P < 0.05) between svk-1 MYC:CLF or SVK OE MYC:CLF and WT MYC:CLF in CBF3 at 20 °C, as determined by one-way ANOVA followed by Tukey's test. Data represent the mean of three independent experiments and error bars show the standard deviation.
Based on the results described here, a model for SVALKA function in Arabidopsis cold acclimation is proposed in Fig. 3D. In response to low temperature, the expression of CBFs is rapidly induced, after 15 to 30 min, reaching the highest levels at 3 to 4 h of cold exposure. Around this time, a transcriptional read-through of SVALKA is activated, which would trigger head-to-head RNAPII collision over the CBF1 gene leading to a regulated limitation of CBF1 cold-induced levels. The transcription of SVALKA increases gradually during cold acclimation till attaining a maximum of induction after 8 to 12 h of exposure to low temperature. Then, the SVALKA β isoform would recruit the PRC2 complex to the coding region of CBF3 gene, promoting the deposition of the H3K27me3 repressive mark and, consequently, the decrease of its expression. As a result, after 24 h under low temperature conditions, the CBF3 expression would decay until reaching relatively stable low levels. Hence, SVALKA would serve as a regulatory hub for CBF expression in response to low temperature: through two distinct molecular mechanisms it differentially controls the induction levels of CBF1 and represses the expression of CBF3 after its peak of induction, respectively. The biological relevance of this dual functionality of SVALKA is evidenced by the fact that both mechanisms are essential to ensure the correct development of the cold acclimation process. Our findings unveil an epigenetic regulation of CBF expression in response to low temperature that is mediated by a SVALKA-PRC2 module, constitutes a particular layer of CBF regulation, and is required for an adequate cold acclimation. Identifying the molecular mechanisms that shape the transient expression patterns of CBF genes is a remarkable goal for future studies that should provide insights on how plants respond and adapt to adverse environments.
Materials and methods
Plant materials and growth conditions
Arabidopsis (A. thaliana) Col-0 ecotype, which was used in all experiments as the WT plant, and mutant lines SVK OE (SALK_007722) and svk-1 (GK-145A05) were obtained from the Nottingham Arabidopsis Stock Centre. The clf28swn7 double mutant and the clf50clf16 double mutant expressing the 35S:GFP:CLF fusion were kindly provided by José A. Jarillo and Manuel Piñeiro (Del Olmo et al. 2016). The WT plants expressing the 35S:MYC:CLF fusion (WT MYC:CLF) were obtained from Marja C. P. Timmermans (Lodha et al. 2013). SVK OE and svk-1 mutants containing the 35S:MYC:CLF fusion (SVK OE MYC:CLF) were generated by crossing the mutants with WT MYC:CLF plants. The binary vector pMDC45 was introduced in the WT plants via Agrobacterium tumefaciens (GV3101 strain) (Clough and Bent 1998), to generate the 35S:GFP plants. Arabidopsis seeds were surface-sterilized and grown under standard conditions [20 °C under long-day photoperiod (16 h of cool-white, fluorescent light, photon flux of 90 μmol m−2 s−1)] in pots containing a mixture of organic substrate and vermiculite (3/1, v/v) or in Petri dishes containing Murashige and Skoog medium supplemented with 1% (w/v) sucrose (germination media, GM) and solidified with 0.9% (w/v) plant agar.
Plant treatments and tolerance assays
Low temperature treatments for gene expression analyses and immunoprecipitation experiments were performed by transferring 2-wk-old plants growing in Petri dishes to a growth chamber set at 4 °C for different times under a long-day photoperiod (16 h of cool-white, fluorescent light, photon flux of 40 μmol m−2 s−1). In all cases, tissue samples were frozen in liquid nitrogen after treatment and stored at −80 °C until use. Freezing tolerance assays were carried out as previously described (Catala et al. 2011). All data reported about tolerances are expressed as standard deviations of the means of at least three independent experiments with 40 plants each.
Gene expression analysis
For gene expression analyses, total RNA was extracted using TRIzol (Thermo Fisher) according to the manufacturer's protocol. RNA samples were treated with DNase I (Roche) and quantified with a Nanodrop spectrophotometer (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized from each sample with the iScript cDNA Synthesis Kit (Bio-Rad) following the manufacturer's instructions, and was used as a template for RT-qPCR assays with the SsoFast EvaGreen Supermix (Bio-Rad) in an iQ2 thermal cycler machine (Bio-Rad) with the primers listed in Supplementary Table S1. In all cases, the relative expression values were calculated using the AT4G26410 gene as a reference (Czechowski et al. 2005), and the ΔΔCT method to determine fold changes (Livak and Schmittgen 2001). The identification of SVALKA splicing isoforms was carried out by RT-qPCR, using cDNA from 2-wk-old WT plants grown in Petri dishes exposed to 4 °C for eight additional hours and primers displayed in Supplementary Table S1. Genomic DNA from the same plants was isolated as described (Cenis 1992) and amplified by PCR with the same primers as a size reference. All reactions were carried out in triplicate using three independent RNA samples.
Nuclear RNA immunoprecipitation
Nuclear RIP was performed as described (Au et al. 2017) with some modifications. Two-wk-old 35S:GFP:CLF and 35S:GFP plants grown in Petri dishes under standard conditions were exposed to 20 °C or 4 °C for eight additional hours, cross-linked with 1% (v/v) formaldehyde and quenched with 0.125 M glycine. After grinding, 10 g of plant material were suspended in 40 mL Honda buffer (Supplementary Table S2). Nuclei were isolated by centrifugation (3,000 × g), and the resulting pellet washed four times with Nuclei Wash buffer (Supplementary Table S2), resuspended in Lysis buffer (Supplementary Table S2), and disrupted by sonication in a Sonifier 150D (Branson). One-third of each nuclei lysate was collected as input sample, and the remaining lysate diluted 10 times with Dilution buffer (Supplementary Table S2) and precleared with Pierce Control Agarose Resin (Thermo Scientific). Immunoprecipitation was performed by incubating the precleared nuclei lysate with 20 µL of GFP-Trap beads (Chromotek) o/n at 4 °C. After the incubation, GFP-beads were washed three times with Beads Wash buffer (Supplementary Table S2). RNA-protein complexes were eluted by incubation with 500 µL Elution buffer (Supplementary Table S2) during 1 h at 37 °C in the presence of 20 µg Proteinase K. Immunoprecipitated RNA was extracted using TRIzol (Thermo Fisher) according to the manufacturer's protocol. cDNA synthesis was performed using iScript Advanced cDNA Synthesis kit (Bio-Rad), and quantification of the SVALKA β RNA in the samples was performed by RT-qPCR as described above. In all cases, SVALKA β RNA levels obtained from the immunoprecipitated samples were relativized to the levels of SVALKA β RNA from the corresponding input samples. The primers used to amplify SVALKA β RNA are listed in Supplementary Table S1. All data reported are expressed as standard deviations of the means of at least three independent experiments.
Chromatin immunoprecipitation
ChIP experiments were carried out using 2-wk-old plants basically as previously described (Desvoyes et al. 2018). Ten grams of WT 35S:MYC:CLF, svk-1 35S:MYC:CLF and SVK OE 35S:MYC:CLF plants, or 2 g of WT, svk-1 and SVK OE plants, were cross-linked with 1% (v/v) formaldehyde by vacuum infiltration and quenched with 0.125 M glycine. After grinding, nuclei were isolated in Extraction buffer (Supplementary Table S2). Nuclei were pelleted by centrifugation (3,000 × g), resuspended in Lysis buffer (Supplementary Table S2), and disrupted by sonication in a Sonifier 150D (Branson) to obtain genomic fragments of 100 to 500 bp. One-tenth of each nuclei lysate was collected as input sample, and the remaining lysate was diluted 10 times using Dilution buffer (Supplementary Table S2). DNA/protein complexes were immunoprecipitated with anti-c-MYC monoclonal antibody (sc-40; Santa Cruz Biotechnology) or anti-H3K27me3 monoclonal antibody (ab6002, Abcam), using magnetic beads (Dynabeads Protein G, Invitrogen). De-cross-linking and DNA purification was carried out using Chelex 100 Resin (Bio Rad). Quantification of chromatin in each sample was determined by qPCR using the primers listed in Supplementary Table S1. In all cases, chromatin levels obtained in the immunoprecipitated samples were relativized to the levels of chromatin in the corresponding input samples. All data reported are expressed as standard deviations of the means of at least three independent experiments.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers indicated in Supplementary Table S3.
Supplementary Material
Acknowledgments
We thank all our colleagues who kindly provided us with the mutants and transgenic plants used in this work (see Materials and Methods section for details). Furthermore, we thank C. Carrasco for helpful discussions and comments.
Contributor Information
Diego Gómez-Martínez, Departamento de Biotecnología Microbiana y de Plantas, Centro de Investigaciones Biológicas Margarita Salas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain.
Javier Barrero-Gil, Departamento de Biotecnología Microbiana y de Plantas, Centro de Investigaciones Biológicas Margarita Salas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain.
Eduardo Tranque, Departamento de Biotecnología Microbiana y de Plantas, Centro de Investigaciones Biológicas Margarita Salas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain.
María Fernanda Ruiz, Departamento de Biotecnología Microbiana y de Plantas, Centro de Investigaciones Biológicas Margarita Salas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain.
Rafael Catalá, Departamento de Biotecnología Microbiana y de Plantas, Centro de Investigaciones Biológicas Margarita Salas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain.
Julio Salinas, Departamento de Biotecnología Microbiana y de Plantas, Centro de Investigaciones Biológicas Margarita Salas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain.
Author contributions
D.G., R.C., and J.S. conceived and designed the experiments; D.G., J.B., E.T., R.C., and M.F.R. performed the experiments; D.G., R.C., and J.S. analyzed the data; R.C. and J.S. wrote the paper.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1 . The induction of CBFs strongly declines after 24 h of cold exposure.
Supplementary Figure S2 . The β isoform is the SVALKA transcript analyzed in this study.
Supplementary Table S1 . Specific primers used in this study.
Supplementary Table S2 . Buffers used in this study.
Supplementary Table S3 . Full names of the genes mentioned in this study.
Funding
This research was supported by grants BIO2016-79187-R from MCIN/AEI/FEDER, UE, and PID2019-106987RB-I00 from MCIN/AEI/10.13039/501100011033 to J.S. D.G and E.T. were recipients of PhD FPI fellowships BES-2014-067840 and BES-2017-079952, respectively, from MCIN/AEI.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. All primers used in this work are described in Supplementary Table S1. Sequence data from the genes mentioned in this study can be found in the GenBank/EMBL data libraries under the accession numbers listed in Supplementary Table S3. The full names of these genes are also included in Supplementary Table S3.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Au PCK, Dennis ES, Wang M-B. Analysis of Argonaute 4-associated long non-coding RNA in Arabidopsis thaliana sheds novel insights into gene regulation through RNA-directed DNA methylation. Genes (Basel). 2017:8(8):198. 10.3390/genes8080198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala R, Medina J, Salinas J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc Natl Acad Sci USA. 2011:108(39):16475–16480. 10.1073/pnas.1107161108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenis JL. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992:20(9):2380. 10.1093/nar/20.9.2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998:16(6):735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Costa S, Dean C. Storing memories: the distinct phases of polycomb-mediated silencing of Arabidopsis FLC. Biochem Soc Trans. 2019:47(4):1187–1196. 10.1042/BST20190255 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK. Genome-wide identification and testing of superior reference genes for transcript normalization. Plant Physiol. 2005:139(1):5–17. 10.1104/pp.105.063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Olmo I, Lopez JA, Vazquez J, Raynaud C, Pineiro M, Jarillo JA. Arabidopsis DNA polymerase ɛ recruits components of polycomb repressor complex to mediate epigenetic gene silencing. Nucleic Acids Res. 2016:44(12):5597–5614. 10.1093/nar/gkw156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvoyes B, Vergara Z, Sequeira-Mendes J, Madeira S, Gutierrez C. A rapid and efficient ChIP protocol to profile chromatin binding proteins and epigenetic modifications in arabidopsis. Methods Mol. Biol. 2018:1675:71–82. 10.1007/978-1-4939-7318-7_5 [DOI] [PubMed] [Google Scholar]
- Ding Y, Shi Y, Yang S. Molecular regulation of plant responses to environmental temperatures. Mol Plant. 2020:13(4):544–564. 10.1016/j.molp.2020.02.004 [DOI] [PubMed] [Google Scholar]
- Farrona S, Thorpe FL, Engelhorn J, Adrian J, Dong X, Sarid-Krebs L, Goodrich J, Turck F. Tissue-specific expression of FLOWERING LOCUS T in Arabidopsis is maintained independently of polycomb group protein repression. Plant Cell. 2011:23(9):3204–3214. 10.1105/tpc.111.087809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998:16(4):433–442. 10.1046/j.1365-313x.1998.00310.x [DOI] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998:280(5360):104–106. 10.1126/science.280.5360.104 [DOI] [PubMed] [Google Scholar]
- Kim DH, Sung S. Vernalization-triggered intragenic chromatin loop formation by long noncoding RNAs. Dev Cell. 2017:40(3):302–312.e4. 10.1016/j.devcel.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindgren P, Ard R, Ivanov M, Marquardt S. Transcriptional read-through of the long non-coding RNA SVALKA governs plant cold acclimation. Nat Commun. 2018:9(1):4561. 10.1038/s41467-018-07010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998:10(8):1391–1406. 10.1105/tpc.10.8.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001:25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lodha M, Marco CF, Timmermans MCP. The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of polycomb-repressive complex2. Genes Dev. 2013:27(6):596–601. 10.1101/gad.211425.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011:469(7330):343–349. 10.1038/nature09784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME, Fitzgerald KA, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023:24(6):430–447. 10.1038/s41580-022-00566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Bargues M, Terol J, Pérez-Alonso M, Salinas J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol. 1999:119(2):463–470. 10.1104/pp.119.2.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA. 2007:104(52):21002–21007. 10.1073/pnas.0705639105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J. Silencing by plant polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 2006:25(19):4638–4649. 10.1038/sj.emboj.7601311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira-Mendes J, Aragüez I, Peiró R, Mendez-Giraldez R, Zhang X, Jacobsen SE, Bastolla U, Gutierrez C. The functional topography of the Arabidopsis genome is organized in a reduced number of linear motifs of chromatin states. Plant Cell. 2014:26(6):2351–2366. 10.1105/tpc.114.124578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J, Chen C, Thapa RK, Bian S, Nguyen V, Yu K, Yuan ZC, Liu J, Kohalmi SE, Li C, et al. Genome-wide occupancy of histone H3K27 methyltransferases CURLY LEAF and SWINGER in Arabidopsis seedlings. Plant Direct. 2019:3(1):e00100. 10.1002/pld3.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis polycomb target. Nature. 2009:462(7274):799–802. 10.1038/nature08618 [DOI] [PubMed] [Google Scholar]
- Thomashow MF. PLANT COLD ACCLIMATION: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999:50(1):571–599. 10.1146/annurev.arplant.50.1.571 [DOI] [PubMed] [Google Scholar]
- Wu HW, Deng S, Xu H, Mao HZ, Liu J, Niu QW, Wang H, Chua NH. A noncoding RNA transcribed from the AGAMOUS (AG) second intron binds to CURLY LEAF and represses AG expression in leaves. New Phytol. 2018:219(4):1480–1491. 10.1111/nph.15231 [DOI] [PubMed] [Google Scholar]
- Xiao J, Jin R, Yu X, Shen M, Wagner JD, Pai A, Song C, Zhuang M, Klasfeld S, He C, et al. Cis and trans determinants of epigenetic silencing by polycomb repressive complex 2 in Arabidopsis. Nat Genet. 2017:49(10):1546–1552. 10.1038/ng.3937 [DOI] [PubMed] [Google Scholar]
- Zacharaki V, Meena SK, Kindgren P. The non-coding RNA SVALKA locus produces a cis-natural antisense transcript that negatively regulates the expression of CBF1 and biomass production at normal temperatures. Plant Commun. 2023:4(4):100551. 10.1016/j.xplc.2023.100551 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request. All primers used in this work are described in Supplementary Table S1. Sequence data from the genes mentioned in this study can be found in the GenBank/EMBL data libraries under the accession numbers listed in Supplementary Table S3. The full names of these genes are also included in Supplementary Table S3.