Bladder cancer is the fifth most common malignancy in Europe and the fourth most common malignancy in the United States.1 About 75% of patients with bladder cancer are men. The most established risk factors for bladder cancer are cigarette smoking and occupational exposure to certain carcinogens.2 About 80% of bladder tumours are confined to the bladder mucosa, the so called superficial tumours, and 20% invade the muscle layer. The management and prognosis of the two types of cancer are completely different: superficial tumours are fairly benign, and invasive tumours are highly malignant.
Summary points
Exposure to industrial chemicals and cigarette smoke are risk factors for bladder cancer
Haematuria is the key symptom
In superficial bladder cancer, intravesical chemotherapy can prevent recurrences but not progression to invasive bladder cancer
Fifty per cent of patients with muscle invasive tumours will die within 5 years
Quality of life has been improved but not survival
Systemic chemotherapy for invasive bladder cancer has not proved to be beneficial in large series of patients
Methods
This article is based on the results of several clinical trials carried out during the past 20 years by the European Organisation for Research and Treatment of Cancer Genito-Urinary (EORTC-GU) Cancer Cooperative Group and the British Medical Research Council’s working party on superficial bladder cancer, and selected articles published in peer reviewed urological journals during the past two decades.
Aetiology
Bladder cancer is strongly linked to occupational and environmental exposure to chemicals. The development of the disease is associated with the excretion of carcinogenic metabolites in the urine.
In the early 1950s an investigation of bladder cancers in workers in British chemical industries showed that individuals exposed to benzidine and 2-naphtylamine had a 30 times greater risk of developing bladder cancer than the general population. The average latent period for the development of the disease was more than 15 years. Substantial evidence supports a relation between cigarette smoking and bladder cancer. Smokers have up to a fourfold higher incidence of bladder cancer than non-smokers.3 Studies from the United States, Europe, and Japan have shown that the association between smoking and bladder cancer is the same in both sexes. The risk is correlated with the number of cigarettes smoked, the length of time smoke is retained in the lungs, and the amount of smoke inhaled.4 Stopping smoking, even after many years, can be beneficial, as ex-cigarette smokers have a reduced incidence of bladder cancer compared with current smokers. One third of bladder cancer cases have been estimated to be related to cigarette smoking. No one specific carcinogen has been identified, but cigarette smoke contains many potential carcinogens.
Symptoms and diagnosis
Haematuria is the key symptom of both superficial and invasive bladder cancer. In most cases this is frank and painless.
Frank haematuria
Frank, visible haematuria is frequently associated with bladder cancer or tumours elsewhere in the urinary tract, and patients with haematuria require prompt referral to a urologist. Although occult haematuria is sometimes associated with tumours, it is far less common than with frank haematuria.
Although frank haematuria may occur with urinary tract infections, kidney and bladder stones, renal cell cancer, and many other renal diseases, its presence should always raise the possibility of bladder cancer. Bladder cancer may cause pain, urinary frequency, and bladder irritability, but these symptoms are more often seen in cases of cystitis or urolithiasis. One form of bladder cancer, carcinoma in situ, produces symptoms that may resemble urinary infection or prostatitis. Physical examination including digital rectal examination seldom leads to a diagnosis.
Classic diagnostic tools
A diagnosis of bladder cancer is classically made from analysing the urinary sediment, urine culture and cytology, intravenous urography, and cystoscopy. Intravenous urography can detect bladder cancer and urothelial tumours in the kidneys and ureters.
Most urologists now use a flexible cystoscope to examine the bladder. The procedure requires no anaesthesia and can be performed in a doctor’s surgery.
New diagnostic tools
Because cytology and the presence of microscopic haematuria are of low specificity and sensitivity, new diagnostic tools have been developed to detect bladder cancer. These sophisticated tests are carried out on voided urine, and they can detect the presence of antigens indicative of bladder cancer.5,6 Although these tests are more sensitive and more specific than cytology, they are insufficient for diagnosing or excluding bladder cancer. These tests should therefore be limited to enhancing or completing the diagnostic procedure.
Once a bladder tumour has been visualised it can be removed by transurethral resection. A histological examination of the tissue provides the diagnosis. As most (80%) bladder tumours are superficial, and therefore confined to the bladder mucosa, it is possible to remove all visible tumour tissue in most cses.
Staging procedure
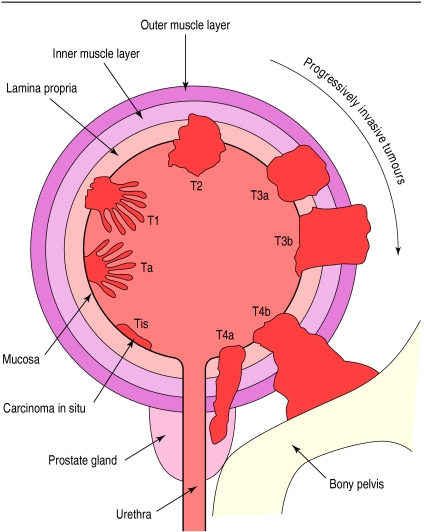
The TNM system is generally accepted for staging bladder tumours7: a tumour that is limited to the mucosa and lies flat is Tis (a carcinoma in situ); a tumour that is papillary and limited to the mucosa is pTa; and a tumour that penetrates the lamina propria but not the muscle layer is pT1 (fig 1). If the tumour invades muscle it may be staged from pT2 to pT4 according to the depth of infiltration of muscle tissue or the extent to which the surrounding tissue is affected. Tumours that invade the bladder muscle are highly malignant and have a strong potential to metastasise preferentially to regional lymph nodes, lungs, liver, and bone. Therefore computed tomography, chest x rays, magnetic resonance imaging (optional), and bone scanning (optional) are advised.
Figure 1.
Tumour staging in bladder cancer according to TNM system, 1997
Treatment of superficial tumours
Although it is possible to remove Ta, T1 tumours surgically, 50-70% of patients have a recurrence within 1-2 years. To prevent this, patients are treated adjuvantly with intravesical drugs. These drugs are instilled in the bladder as a watery solution, kept in the bladder for 1-2 hours, and then simply voided. Cytostatic agents such as thiotepa, adriamycin, mitomycin C, and epirubicin have been used, and during the past decade BCG has been one of the most effective drugs given intravesically.8
During the past two decades the EORTC-GU group and the British Medical Research Council’s working party have performed a series of randomised phase III studies investigating the prophylactic treatment of stage Ta, T1 bladder cancer after transurethral resection.
Many studies have shown the advantage of adjuvant treatment after transurethral resection in decreasing the recurrence rate of bladder tumours or in prolonging the disease free interval of patients.8 The studies failed, however, to show the superiority of one agent over another, probably with the exception of BCG. There is also no evidence that adjuvant prophylactic treatment is of long term benefit compared with transurethral resection alone in progression to muscle invasive disease and duration of survival.
Combined analysis and meta-analysis
The EORTC-GU group and the British Medical Research Council’s working party have recently performed a combined analysis of completed trials using meta-analysis techniques.9 The statistical power of the tests used to compare treatments is increased because data are available from six randomised trials, comprising 2535 patients.
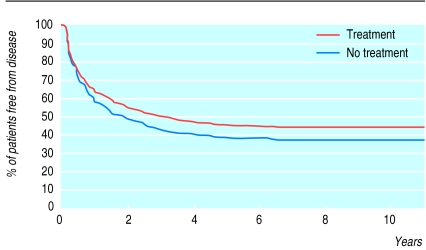
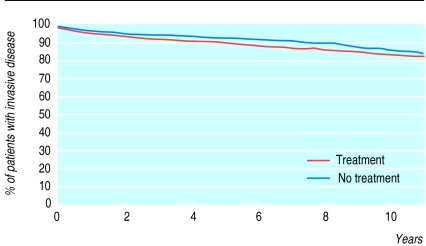
In all of these trials transurethral resection alone was compared with transurethral resection followed by intravesical chemotherapy using different drugs. There was a significant difference (P<0.01) in the disease free interval in favour of adjuvant treatment (fig 2). However, although statistically different, less than 10% of patients randomised to adjuvant treatment were disease free at follow up (fig 2). The time of progression to muscle invasive disease in the two treatment groups, and the overall duration of survival, was not significantly different (P>0.1) (fig 3). Urologists may need to assess the risks and benefits of intravesical chemotherapy to the patient, and its cost, before deciding on this treatment. Intravesical BCG, considered a form of immunotherapy, was not addressed in this combined analysis. In phase III trials investigating BCG there is insufficient follow up to allow a proper meta-analysis of survival as the end point.
Figure 2.
Kaplan-Meier curve of disease free interval of patients treated intravesically with or without adjuvant chemotherapy. Stratified log rank test, P<0.001
Figure 3.
Kaplan-Meier curve of progression to muscle invasive bladder cancer of patients treated intravesically with or without adjuvant chemotherapy. Stratified log rank test, P>0.1
Treatment of muscle invasive tumours
The role of transurethral resection in muscle invasive bladder cancer is limited to the diagnostic and staging procedure.
Invasive bladder cancer cannot be eradicated by transurethral resection alone or by intravesical instillations with cytostatic drugs or BCG. For 50 years, definitive treatment has consisted of radical surgery (cystectomy) or external beam radiotherapy. Improvements in surgical techniques and postoperative care have occurred. To avoid a urostoma it is now possible to create a new bladder for a patient from their own intestine.10 Improvements in radiotherapy have decreased the morbidity and increased the quality of life of patients with bladder cancer.11 Despite this, the survival of patients has not changed.12,13 Up to 50% of patients develop metastases and die within 5 years. Therefore many investigators have addressed the possible role of systemic chemotherapy as an addition to classic treatment.14,15 Chemotherapy given before cystectomy or definitive radiotherapy is termed neoadjuvant chemotherapy whereas that given after radical treatment is termed adjuvant chemotherapy.
Neoadjuvant chemotherapy
Although combination treatments with cisplatin, methotrexate, vinblastine, and adriamycin achieved complete remission in some patients, their impact on a large proportion of patients with bladder cancer remained unclear.
Randomised prospective phase III trials comprising 100-300 patients were not able to detect a difference between definitive treatment alone versus neoadjuvant chemotherapy followed by definitive treatment. This may lead to the conclusion that neoadjuvant chemotherapy does not improve the prognosis of patients or that the difference might be so small that it cannot be detected in a series with limited numbers of patients. On the basis of the expected proportion of response from previous studies, the EORTC-GU group and the British Medical Research Council working party embarked on an international phase III study of neoadjuvant cisplatin, methotrexate, and vinblastine, and definitive local treatment with radical cystectomy or radiotherapy.16 Other international oncology groups have also joined the study. A total of 975 patients with muscle invasive bladder tumours and without detectable metastases were randomised. Patients received either three cycles of cisplatin, methotrexate, and vinblastine or no chemotherapy before radical cystectomy or full dose external beam radiotherapy. The study aimed to detect an absolute increase of three year survival of at least 10% in the chemotherapy arm. After a median follow up of 22 months the overall survival for patients treated with the drug combination was 62%, and 60% for those not receiving such treatment (P=0.63).
As the follow up period is too limited, no definitive conclusion can be made yet. Apparently, however, if there is a difference in survival between the two groups it will be small. The results of this largest international series indicate that it is necessary to organise large prospective randomised studies to detect small differences, if any, between different treatments. Collaboration between trial organisations is therefore essential. Small series will not provide reliable conclusions, and patients might be harmed and efforts and funds wasted.
Adjuvant chemotherapy
The advantage of adjuvant chemotherapy after radical cystectomy or radiotherapy has not been proved.17 Studies have been hampered by small series of patients, patient selection, and high morbidity in elderly patients that have been exposed to extensive surgical procedures. In selected patients with minimal disease, adjuvant chemotherapy may improve survival18 but conclusive evidence and large series are lacking. New trials should be developed on the basis of current knowledge. Urologists, medical oncologists, and radiotherapists should collaborate in providing more extensive evidence rather than relying on the limited results of single studies.
Treatment considerations should include a cost benefit analysis. In most countries, three to four cycles of chemotherapy cost (at 1998 prices) the equivalent of about £3000, radiotherapy costs about £1500, and radical cystectomy including hospital stay costs about £6000. Such economic considerations may play an increasingly important role in the future treatment of bladder cancer.
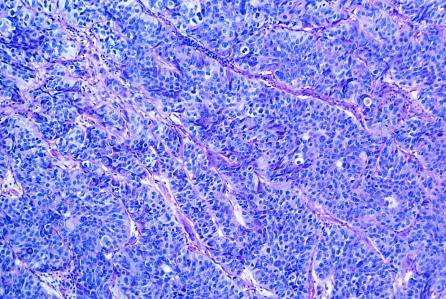
Figure.
Bladder cancer. Light micrograph of a section through a transitional cell carcinoma of the bladder. Malignant cells are poorly differentiated and contain large, dark staining nuclei
References
- 1.Jensen OM, Esteve J, Moller J, Renard H. Cancer in the European community and its member states. Eur J Cancer. 1990;26:1167–1177. doi: 10.1016/0277-5379(90)90278-2. [DOI] [PubMed] [Google Scholar]
- 2.Silverman D, Levin L, Hoover R. Occupational risks of bladder cancer in the United States in non white men. J Natl Cancer Inst. 1989;81:1480. doi: 10.1093/jnci/81.19.1472. [DOI] [PubMed] [Google Scholar]
- 3.Clavel J, Cordier S, Boccon-Gibod L, Hemon D. Tobacco and bladder cancer in males. Increased risk for inhalers and smokers of black tobacco. Int J Cancer. 1984;44:605–610. doi: 10.1002/ijc.2910440408. [DOI] [PubMed] [Google Scholar]
- 4.Augustine A, Hebert JR, Kabat GC, Wynder E. Bladder cancer in relation to cigarette smoking. Cancer Res. 1988;48:4405. [PubMed] [Google Scholar]
- 5.Sarosdy MF, de Vere White RW, Soloway M, Sheinfeld J, Hudson M, Schellhammer P, et al. Results of a multicenter trial using the BTA test to monitor for and diagnose recurrent bladder cancer. J Urol. 1995;154:379–384. doi: 10.1097/00005392-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Soloway MS, Briggman V, Carpinito G, Chodak G, Church P, Lamm D, et al. Use of a new tumour marker, urinary NMP 22 in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol. 1996;156:363–367. doi: 10.1097/00005392-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 7.International Union Against Cancer. TNM classification of malignant tumours, 5th ed. New York: Wiley-Liss; 1997. In: Sobin LH, Wittekind C, eds. [Google Scholar]
- 8.Herr HW, Loudone VP, Whitmore WF., Jr An overview of intravesical therapy for superficial tumours. J Urol. 1987;138:1363–1368. doi: 10.1016/s0022-5347(17)43644-5. [DOI] [PubMed] [Google Scholar]
- 9.Pawinsky A, Sylvester R, Kurth K-H, Bouffioux C, van der Meijden A, Parmar M, et al. for the members of the European Organisation for Research and Treatment of Cancer Genito-Urinary Cancer Cooperative Group and the Medical Research Council working party on superficial bladder cancer. A combined analysis of European Organisation for Research and Treatment of Cancer and Medical Research Council randomized clinical trials for the prophylactic treatment of stage Ta,T1 bladder cancer J Urol 19961561934–1941. [PubMed] [Google Scholar]
- 10.Hautman R, Miller K, Steiner U, Wenderoth U. The ileal neo bladder: 6 years of experience with more than 200 patients. J Urol. 1993;150:40–45. doi: 10.1016/s0022-5347(17)35392-2. [DOI] [PubMed] [Google Scholar]
- 11.Koiso K, Shipley W, Keuppens F, Baert L, Hall R, Hudson M, et al. The status of bladder preserving therapeutic strategies in the management of patients with muscle invasive bladder cancer. Int J Urol. 1995;2(suppl 2):49–57. doi: 10.1111/j.1442-2042.1995.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitmore WF., Jr Management of invasive bladder neoplasms. Semin Urol. 1983;1:4–10. [PubMed] [Google Scholar]
- 13.Gospodarowicz M, Quilty P, Scalliet P, Tsujii H, Fossa S, Horenblas S, et al. The place of radiotherapy as definitive treatment of bladder cancer. Int J Urol. 1995;2(suppl 2):41–48. doi: 10.1111/j.1442-2042.1995.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 14.Sternberg C, Yagoda A, Scher H, Watson R, Geller N, Herr H, et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium: Efficacy and patterns of response. Cancer. 1989;64:2448–2458. doi: 10.1002/1097-0142(19891215)64:12<2448::aid-cncr2820641209>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Tannock I. The current status of adjuvant chemotherapy for bladder cancer. Semin Urol. 1990;8:291–297. [PubMed] [Google Scholar]
- 16.Hall RR. Neo-adjuvant CMV chemotherapy and cystectomy or radiotherapy in muscle invasive bladder cancer. First analysis of Medical Research Council/European Organisation for Research and Treatment of Cancer intercontinental trial [abstract]. American Society of Clinical Oncology, 1996.
- 17.Sternberg C, Raghaven D, Ohi Y, Bajorin D, Herr H, Kato T, et al. Neo-adjuvant and adjuvant chemotherapy in advanced disease. What are the effects on survival and prognosis? Int J Urol. 1995;2(suppl 2):76–89. doi: 10.1111/j.1442-2042.1995.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 18.Skinner D, Daniels J, Russel C, Lieskovsky G, Boyd S, Nichols P, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer. A prospective comparative trial. J Urol. 1991;145:459–467. doi: 10.1016/s0022-5347(17)38368-4. [DOI] [PubMed] [Google Scholar]