Abstract
Glucose transport kinetics and mRNA levels of different glucose transporters were determined in Saccharomyces cerevisiae strains expressing different sugar kinases. During exponential growth on glucose, a hxk2 null strain exhibited high-affinity hexose transport associated with an elevated transcription of the genes HXT2 and HXT7, encoding high-affinity transporters, and a diminished expression of the HXT1 and HXT3 genes, encoding low-affinity transporters. Deletion of HXT7 revealed that the high-affinity component is mostly due to HXT7; however, a previously unidentified very-high-affinity component (Km = 0.19 mM) appeared to be due to other factors. Expression of genes encoding hexokinases from Schizosaccharomyces pombe or Yarrowia lipolytica in a hxk1 hxk2 glk1 strain prevented derepression of the high-affinity transport system at high concentrations of glucose.
The yeast Saccharomyces cerevisiae utilizes a variety of carbon sources for growth, but glucose and related hexoses are used preferentially. Glucose elicits a variety of responses that ensure its preferential use, from modulation of enzyme activity to repression or induction of genes (for reviews, see references 4 and 9). A great number of proteins participate in the process (for a review, see reference 9) of glucose repression, including hexokinase II, which is encoded by the gene HXK2 (6, 7).
One of the activities regulated by glucose is sugar uptake. Glucose transport in S. cerevisiae is mediated by proteins encoded by several HXT genes (20; for reviews, see references 3 and 11). Glucose transport in yeast exhibits dual kinetics, with a high- and a low-affinity kinetic component (2) whose proportions depend on the culture conditions (5, 25). The kinetics observed are the result of the differential expression of the HXT genes, whose products have different affinities for glucose. HXT1 and HXT3 encode low-affinity transporters (Km = 50 to 100 mM), HXT2 and HXT4 encode intermediate-affinity transporters (Km ∼ 10 mM), and HXT6 and HXT7 encode high-affinity transporters (Km = 1 to 2 mM) (19). Glucose represses HXT genes encoding high- and intermediate-affinity transporters and induces HXT3 expression; these effects are relieved in hxk2 mutants (12, 14, 18, 26). In this study, we have analyzed in parallel the kinetics of hexose uptake and the transcription of hexose transporter genes in S. cerevisiae strains carrying deletions in the HXK2 gene and in strains expressing only HXK2 or genes encoding the hexokinases from Schizosaccharomyces pombe or Yarrowia lipolytica.
We deleted the HXK2 gene in the S. cerevisiae strain CEN.PK113-7D (MATa MAL2-8c SUC2) to create strain KY116 and in strain CEN.PK113-5D (MATa MAL2-8c SUC2 ura3-52) to create strain KY114, using the method of Wach et al. (24) and the primers AK53 (GTTGTAGGAATATAATTCTCCACACATAATAAGTACGCTAATTCGTACGCTGCAGGTCGAC) and AK54 (AAAAGGGCACCTTCTTGTTGTTCAAACTTAATTTACAAATTAAGTATCGATGAATTCGAGCTCG) (underlined nucleotides correspond to the DNA immediately 5′ and 3′ of the HXK2 open reading frame, respectively). The HXT7 gene was replaced in KY114 by URA3 to produce strain KY168 via amplification of the URA3 gene in plasmid pRS406 (22), using the primers JD3 (TATGCCAATACTTCACAATGTTCGAATCTATTCTTCATTTGCAGCGTATCACGAGGCCCTTTCGTC) and JD4 (ATGCACAAATTAGAGCGTGATCATGAATTAATAAAAGTGTTCGCAAAACGTTTACAATTTCCTGATGCGG) (underlined nucleotides correspond to DNA 5′ and 3′ of the HXT7 open reading frame, respectively). In both cases correct disruption was checked by using an analytical PCR. To construct strains expressing only one hexokinase, the following plasmids were introduced into strain THG1 (MATa leu2-1 ura3-52 hxk1::LEU2 hxk2::LEU2 glk1::LEU2) (15): pCEN/ScHXK2, carrying the S. cerevisiae HXK2 gene (17); pTP5, carrying the S. pombe Sphxk2+ gene (encoding hexokinase 2) (15); or pDB20/YlHXK1, carrying the Y. lipolytica YlHXK1 gene (16). The heterologous genes were under the control of the S. cerevisiae ADH1 promoter. Hexokinase activity was measured as described previously (10). Cells were grown in batch at 250 rpm and 30°C in a minimal medium containing 2% (wt/vol) glucose, 0.1 M potassium phthalate (pH 5.0), and amino acids (21) as required. For transport assays, cells were harvested by centrifugation at 4°C (5 min, 4,000 × g), washed twice in ice-cold 0.1 M potassium phosphate buffer (pH 6.5), resuspended in this buffer to a cell concentration of approximately 7.5 g of protein liter−1, and kept on ice until use. The zero trans-influx rate of hexoses was determined according to the method described by Walsh et al. (25), at 30°C in 0.1 M potassium phosphate buffer (pH 6.5). Kinetic parameters of glucose transport were derived from least-squares fitting of the data to one- or two-component Michaelis-Menten models using Enzfitter software.
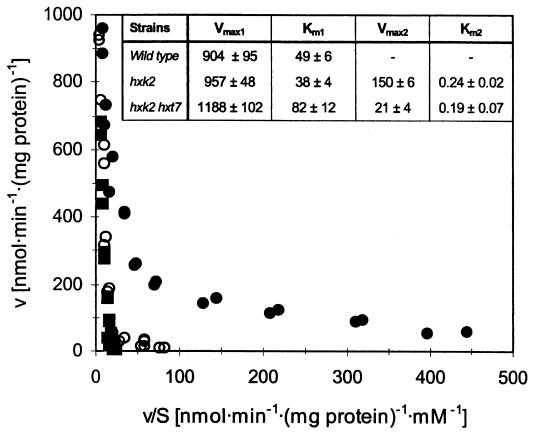
The kinetics of glucose uptake by S. cerevisiae cells depends on the stage of the culture; a low-affinity component is observed during exponential growth at high glucose concentrations, and a high-affinity component is observed when glucose is exhausted (1, 5, 25). During exponential growth in glucose, a wild-type strain and an isogenic hxk2 mutant displayed a major low-affinity component with similar Vmax and Km values (Fig. 1). However, the hxk2 strain showed, in addition, a very-high-affinity component with a Km of 0.24 mM and a Vmax that was about 16% of that of the low-affinity component (Fig. 1). No high-affinity component could be found in the wild type when the data were fitted to a two-component system. Fructose and mannose transport also showed an additional high-affinity component in cells of the hxk2 mutant harvested during exponential growth on glucose (results not shown). Similar kinetics of glucose transport were obtained with strains of a different genetic background: DFY1 (MATa lys1-1 leu2-1) and the isogenic hxk2 deletant DFY567 (MATa lys1-1 leu2-1 hxk2::LEU2).
FIG. 1.
Kinetics of glucose uptake in S. cerevisiae strains with successive deletions of the HXK2 and HXK7 genes. Zero trans-influx was determined for strain CEN.PK113-7D (wild type, ■), KY116 (hxk2Δ, ●), and KY168 (hxk2Δ hxk7Δ, ○) in cells from exponential cultures, as described in the text. S, extracellular glucose concentration; v, zero trans-influx rate of glucose. The kinetic parameters (shown in the inset) were calculated using the Enzfitter software. Vmax is expressed in nanomoles per minute per milligram of protein, and Km is in millimolar units.
When glucose was depleted, wild-type cells, as well as those of the hxk2 mutant, displayed only high-affinity glucose uptake (Km, around 2 mM). Also, no differences in the kinetics of fructose and mannose transport were found between the wild-type and hxk2 mutant strains under these conditions (Km for fructose, ca. 7 mM; Km for mannose, ca. 14 mM). Deletion of the HXT7 gene in the hxk2 mutant eliminated a substantial proportion of the high-affinity component of glucose uptake during exponential growth on glucose (Fig. 1). However, in the double mutant, a component with very high affinity for glucose remained (Km = 0.19 mM) but had a low activity (ca. 2% of that of the low-affinity component). This activity might be due to HXT8 to HXT17. However, we favor the idea that the very-high-affinity component is due to the high level of HXT2 expression observed in the hxk2 strain grown at high glucose concentrations. This possibility is consistent with previous observations that suggested that the kinetics of Hxt2 for glucose is modulated by the growth conditions; it exhibits intermediate affinity in cells grown at high glucose concentrations while it presents dual kinetics, with a high- and a low-affinity component, in cells grown at low glucose concentrations (19).
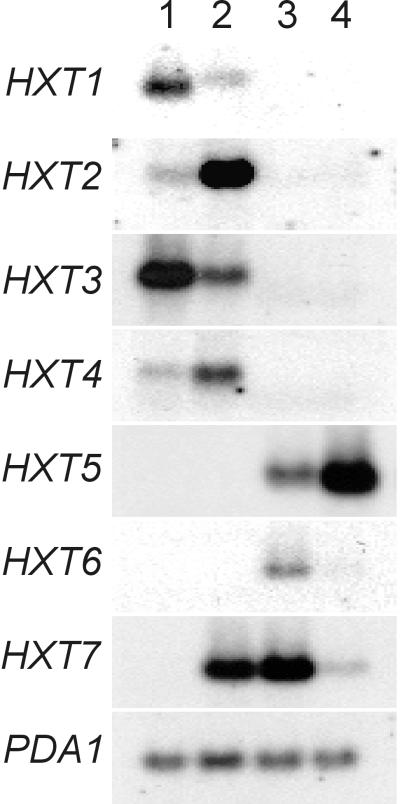
We determined the abundance of HXT transcripts at different stages of growth on glucose by blotting hybridization with oligonucleotides highly specific for each HXT gene, as described previously (5) (Fig. 2). The wild-type strain expressed predominantly HXT1 and HXT3 during exponential growth on glucose, a result consistent with the low-affinity glucose uptake displayed (Fig. 1) (19). At the diauxic shift, transcription of the high-affinity transporter gene HXT7, and to a lesser extent HXT6, was increased and almost no mRNA corresponding to the low-affinity transporter genes HXT1 and HXT3 was detected. Under these conditions HXT2 and HXT4 mRNAs were not detected and HXT5 mRNA was transcribed at a moderate level. In the hxk2 deletion strain, high levels of HXT2 and HXT7 mRNA and reduced levels of HXT1 and HXT3 mRNA were observed during exponential growth on glucose; no mRNA corresponding to HXT6 was detected. After glucose exhaustion, the levels of all HXT mRNAs, except that of HXT5, were quite low in the hxk2 mutant. A similar expression pattern was observed using the DFY1 and DFY567 strains (results not shown).
FIG. 2.
Expression of different HXT mRNAs in S. cerevisiae wild-type (CEN.PK113-7D) and hxk2Δ (KY116) strains at different stages of growth on glucose. Cells were harvested simultaneously for RNA analysis and for the transport assays shown in Fig. 1. Lane 1, wild type, exponential growth (optical density at 600 nm = 1); lane 2, hxk2Δ, exponential growth (optical density at 600 nm = 1); lane 3, wild type, glucose exhaustion; lane 4, hxk2Δ, glucose exhaustion. The PDA1 mRNA levels were used as controls for RNA loading (27).
The hexose uptake kinetics as well as the pattern of HXT transcription in glucose-grown cells shows that HXK2 influences the expression of the HXT genes. During growth at high glucose concentrations, the deletion of HXK2 strongly increases the high-affinity component of hexose transport and the expression of the HXT2 and HXT7 genes. This indicates that HXK2 represses the appearance of the high-affinity component. Mutations in either HXK1 or GLK1 do not alter the expression pattern (13, 23). To determine the specificity of this effect of HXK2, we used S. cerevisiae strains that expressed only one gene encoding hexokinases from other yeast species.
An S. cerevisiae strain expressing only S. cerevisiae HXK2 or the heterologous hexokinase genes from S. pombe or Y. lipolytica showed transport kinetics similar to that of a wild-type strain. During exponential growth on glucose, the strains displayed only low-affinity glucose transport. The best fit of the data was found by using a one-component system that yielded a mean (± standard deviation) Vmax of 419 ± 30 and a Km of 31 ± 3 for the strain expressing the S. pombe hxk2+ gene and a Vmax of 496 ± 44 and a Km of 23 ± 3 for the strain expressing the Y. lipolytica HXK1 gene (Vmax and Km are expressed in nanomoles per minute per milligram of protein and in millimolar units, respectively). These results indicate that the heterologous hexokinases can replace the S. cerevisiae protein in exerting glucose repression on high-affinity glucose uptake. The heterologous hexokinases are also active in invertase repression (16).
The presence in S. cerevisiae of a large family of glucose transporters that have different affinities for their substrates and whose expression is finely regulated remains an enigma. From a physiological point of view it appears reasonable that high-affinity transporters are expressed only at low external glucose concentrations. However, it is not immediately clear how expression of these transporters at high glucose concentrations could be detrimental to the cell.
Acknowledgments
We are grateful to M. van Gaalen for technical assistance. We are thankful to P. Kötter and D. Fraenkel for making available some of their yeast strains.
This work was supported in part by The Netherlands Foundation for Research (NWO), by the Association for Biotechnological Research Schools in The Netherlands (ABON), and by grant PB97-1213-CO2-01 from the Spanish CICYT. T.P. gratefully acknowledges the receipt of a FEBS short-term fellowship for research in Amsterdam and a Marie Curie Biotechnology program grant from the European Union (ERB-4001GT980575) for research in Delft.
REFERENCES
- 1.Bisson L F, Fraenkel D G. Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. J Bacteriol. 1984;159:1013–1017. doi: 10.1128/jb.159.3.1013-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisson L F, Fraenkel D G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1983;80:1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boles E, Hollenberg C P. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev. 1997;21:85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlson M. Glucose repression in yeast. Curr Opin Microbiol. 1999;2:202–207. doi: 10.1016/S1369-5274(99)80035-6. [DOI] [PubMed] [Google Scholar]
- 5.Diderich J A, Schepper M, van Hoek P, Luttik M A H, van Dijken J P, Pronk J T, Klaassen P, Boelens H F M, Teixeira de Mattos M J, van Dam K, Kruckeberg A L. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J Biol Chem. 1999;274:15350–15359. doi: 10.1074/jbc.274.22.15350. [DOI] [PubMed] [Google Scholar]
- 6.Entian K D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178:633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- 7.Entian K D, Zimmermann F K, Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphorylation. Mol Gen Genet. 1977;156:99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- 8.Gamo F J, Lafuente M J, Gancedo C. The mutation DGT1–1 decreases glucose transport and alleviates carbon catabolite repression in Saccharomyces cerevisiae. J Bacteriol. 1994;176:7423–7429. doi: 10.1128/jb.176.24.7423-7429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gancedo J M. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gancedo J M, Clifton D, Fraenkel D G. Yeast hexokinase mutants. J Biol Chem. 1977;252:4443–4444. [PubMed] [Google Scholar]
- 11.Kruckeberg A L. The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol. 1996;166:283–292. doi: 10.1007/s002030050385. [DOI] [PubMed] [Google Scholar]
- 12.Liang H, Gaber R F. A novel signal transduction pathway in Saccharomyces cerevisiae defined by Snf3-regulated expression of HXT6. Mol Biol Cell. 1996;7:1953–1966. doi: 10.1091/mbc.7.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClellan C J, Bisson L F. Glucose uptake in Saccharomyces cerevisiae grown under anaerobic conditions: effect of null mutations in the hexokinase and glucokinase structural genes. J Bacteriol. 1988;170:5396–5400. doi: 10.1128/jb.170.11.5396-5400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Özcan S, Schulte F, Freidel K, Weber A, Ciriacy M. Glucose uptake and metabolism in grr1/cat80 mutants of Saccharomyces cerevisiae. Eur J Biochem. 1994;224:605–611. doi: 10.1111/j.1432-1033.1994.00605.x. [DOI] [PubMed] [Google Scholar]
- 15.Petit T, Blázquez M A, Gancedo C. Schizosaccharomyces pombe possesses an unusual and a conventional hexokinase: biochemical and molecular characterization of both hexokinases. FEBS Lett. 1996;378:185–189. doi: 10.1016/0014-5793(95)01451-9. [DOI] [PubMed] [Google Scholar]
- 16.Petit T, Gancedo C. Molecular cloning and characterization of the gene HXK1 encoding the hexokinase from Yarrowia lipolytica. Yeast. 1999;15:1573–1584. doi: 10.1002/(SICI)1097-0061(199911)15:15<1573::AID-YEA478>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Petit T, Herrero P, Gancedo C. A mutation Ser213/Asn in the hexokinase 1 from Schizosaccharomyces pombe increases its affinity for glucose. Biochem Biophys Res Commun. 1998;251:714–719. doi: 10.1006/bbrc.1998.9538. [DOI] [PubMed] [Google Scholar]
- 18.Randez-Gil F, Sanz P, Entian K D, Prieto J A. Carbon source-dependent phosphorylation of hexokinase PII and its role in the glucose-signaling response in yeast. Mol Cell Biol. 1998;18:2940–2948. doi: 10.1128/mcb.18.5.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reifenberger E, Boles E, Ciriacy M. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997;245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 20.Reifenberger E, Freidel K, Ciriacy M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol. 1995;16:157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 21.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 22.Sikorski R J, Hieter P. A system of shuttle vectors and host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits H P. Mechanism and regulation of glucose transport in Saccharomyces cerevisiae. Ph.D. thesis. Amsterdam, The Netherlands: University of Amsterdam; 1996. [Google Scholar]
- 24.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 25.Walsh M C, Smits H P, Scholte M, van Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176:953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wendell D L, Bisson L F. Expression of high-affinity glucose transport protein Hxt2p of Saccharomyces cerevisiae is both repressed and induced by glucose and appears to be regulated posttranslationally. J Bacteriol. 1994;176:3730–3737. doi: 10.1128/jb.176.12.3730-3737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzel T J, Teunissen A W, Steensma H Y. PDA1 mRNA: a standard for quantitation of mRNA in Saccharomyces cerevisiae superior to ACT1 mRNA. Nucleic Acids Res. 1995;23:883–884. doi: 10.1093/nar/23.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye L, Kruckeberg A L, Berden J A, van Dam K. Growth and glucose repression are controlled by glucose transport in Saccharomyces cerevisiae cells containing only one glucose transporter. J Bacteriol. 1999;181:4673–4675. doi: 10.1128/jb.181.15.4673-4675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]