Abstract
Objectives
We aimed to explore the radiographic definitions of types of New Bone formation (NBF) by focusing on the terminology, description and location of the findings.
Methods
Three systematic literature reviews were conducted in parallel to identify the radiographic spinal NBF definitions for spondyloarthritis (SpA), Diffuse Idiopathic Skeletal Hyperostosis (DISH) and Osteorathritis (OA). Study characteristics and definitions were extracted independently by two reviewers. Definitions were analysed and collated based on whether they were unique, modified or established from previous research.
Results
We identified 33 studies that indicated a definition for the NBF in SpA, 10 for DISH and 7 for spinal OA. In SpA, the variations in syndesmophytes included the description as well as the subtypes and locations. The differentiation of syndesmophytes from osteophytes were included in 12 articles, based on the origin and the angle of the NBF and associated findings. The definitions of DISH varied in the number of vertebrae, level and laterality. For OA, five articles indicated that osteophytes arose from the anterior or lateral aspects of the vertebral bodies, and two studies required a size cut-off.
Discussion
Our ultimate aim is to create formal NBF definitions for SpA, DISH and OA guided by an atlas, through a Delphi exercise with international experts. The improved ability to differentiate these conditions radiographically will not only allow the clinicians to accurately approach patients but also will help the researchers to better classify patient phenotypes and focus on accurate radiographic outcomes.
Keywords: new bone formation, spine, radiography
Key messages.
New bone formation can be seen in spondyloarthritis, osteoarthritis and DISH, with different underlying pathogenic mechanisms.
Literature shows the heterogeneity in the definition of new bone formation on spine radiographs.
The terminology of new bone formation in different diseases needs to be established.
Introduction
New bone formation (NBF) and destruction cycles provide normal bone turnover in a balanced and continuous manner. Osteoblasts play the main role in synthesis of new bone to maintain bone homeostasis. NBF occurs as a result of tissue repair mechanisms mediated by inflammatory, non-inflammatory and biomechanical forces [1].
Despite increasing research in this area, the cellular and molecular processes of NBF in humans are poorly understood. Bone morphogenic protein and wingless-type-like signalling pathways found to have a critical role in NBF in animal studies in spondyloarthritis (SpA) [2]. Furthermore, the complex bone remodelling mechanism of osteoblastic NBF with the interaction of cellular proliferation, differentiation, maturation, migration and cell death has been described in SpA. However, these osteoblastic changes are not specific to SpA, and a similar remodelling process can be seen in the degenerative changes in the spine leading to spinal damage Osteorathritis (OA) and Diffuse Idiopathic Skeletal Hyperostosis (DISH) [3, 4].
Regardless of the underlying mechanism, processes of NBF can sometimes occur in the same patient [5, 6]. Therefore, it can be challenging to differentiate NBFs in SpA, OA and DISH by conventional radiography. Moreover, these conditions can frequently coexist, which has been demonstrated to be related to a poorer outcome, and the management can be challenging [5, 7]. For example, marginal syndesmophytes can be mistaken for the early osteophytes, and bridging osteophytes mimic bridging para-marginal syndesmophytes [8, 9]. The ability to distinguish these entities through radiographic imaging holds clinical significance due to the diverse prognoses and differences in the treatment approaches associated with each. Differentiating the aetiology of NBF is also very important for the research on the pathogenesis of these diseases. This may be problematic in clinical trials that use radiographic progression on the spine as an outcome and has the potential to result in high rate of measurement error.
We believe an important element in establishing a comprehensive definition of radiographic spinal NBF is to review the radiographic definitions that are currently utilized in the literature. Our ultimate aim is to have a consensus on the definitions of NBF in order to differentiate NBF types from each other. We conducted three parallel systematic literature reviews (SLR), aiming to explore the definitions of various types of NBF in DISH, OA and SpA and combined results in this article. We will use the results to inform an international Delphi through an atlas to establish criteria to identify and differentiate NBF in each of these disease processes, which will be followed by prospective research to validate our efforts.
Methods
Study selection and search strategy
Three separate SLRs were performed by using a predefined PICO (population, intervention, comparator and outcome) strategy. MEDLINE, EMBASE and Cochrane Central Register databases were reviewed for publications between January 1980 and November 2023 by an experienced librarian (RS) at The University of Ottawa. Our research questions were identified as follows: ‘What are spine NBF radiography findings in patients with 1) DISH, 2) spine OA, 3) PsA, 4) AS, 5) SpA?’
Search strategies have been developed separately for these research questions. The following terms have been used for the literature search; ‘PsA’ OR ‘AS’ OR ‘Spondyloarthritis’ OR ‘Spine osteoarthritis’ OR ‘DISH’ AND ‘Radiography’ AND ‘Spine’ as MESH terms and text terms. Review process was done after merging the results of three diseases under the SpA topic.
The protocols have been registered to the International Prospective Register of Systematic Reviews database (Registration numbers for DISH: CRD42020197545, for OA: CRD42020197584, for SpA: CRD42020197760). Details of search strategies are given in Supplementary Tables S1–S3, available at Rheumatology Advances in Practice online.
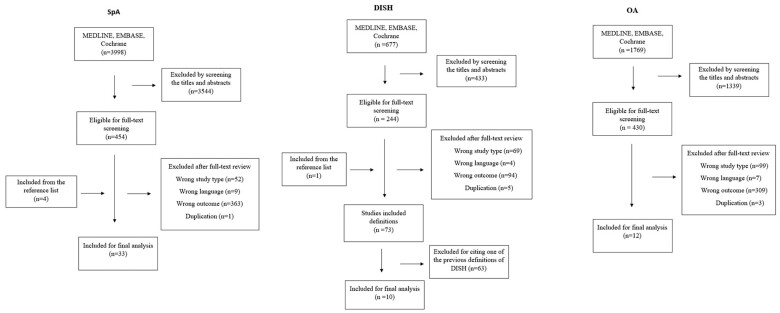
The titles and abstracts were independently screened by two reviewers (UGG&GA for SpA, NH&JD for DISH and OA). All abstracts with discrepancies were carried forward to a full-text review, to be as inclusive as possible, and the full texts were reviewed independently by the same investigators. Any disagreement at the stage of full text review was resolved by the third investigator (SZA). Articles that do not fulfil the inclusion criteria were identified, and the reason for exclusion was documented. Additionally, references of the included articles were manually searched. All screening processes for three SLRs were presented in a flow chart (Fig. 1). To be eligible for inclusion, studies had to meet the following criteria; either cross-sectional, case–control, cohort, observational (retrospective or prospective) study designs, literature reviews and case studies with more than 10 patients; studies including patients >18 years old with a diagnosis of SpA (AS, PsA, inflammatory bowel disease-associated arthritis, reactive arthritis), DISH or OA with the descriptions of axial plain radiographic NBF features and utilized this definition for diagnosis. Studies were excluded if they were in a language other than English, the wrong study type, with no displayed or inaccessible data, duplicate study population, the wrong outcome or modality (e.g. computed tomography or magnetic resonance imaging) or if only the abstract was available.
Figure 1.
Flow chart for study selection
Data extraction
After identifying of the articles to be included, data were extracted in parallel by two independent reviewers using a standardized sheet (UGG&GA for SpA, NH&JD for DISH and OA). Any discrepancies within the data extraction phase were resolved by discussion with a third reviewer (SZA). The descriptions of axial plain radiographic NBF features in patients with DISH, OA and DISH were the primary outcomes.
The location (cervical/thoracic/lumbar) and distribution of age groups of the defining disease types were the secondary outcome measures.
Results
The results of the SLRs are presented separately for SpA, DISH and OA:
SpA
Our literature search identified 32 studies (11 original studies and 21 review articles), which included a definition of NBFs on the spine radiography of SpA patients. The diagnostic subgroups, sample size, age distribution and spinal region in the original studies, as well as the definitions, were displayed in Table 1. For the review articles, definitions and disease subgroups were summarized in Supplementary Table S4, available at Rheumatology Advances in Practice online.
Table 1.
Original studies with NBF definitions in SpA
| Author/year | Country | SPA Type | Diagnosis criteria used | Study design | Sample size (n) | Gender (M/F) | Age | Anatomical site | Definition |
|---|---|---|---|---|---|---|---|---|---|
| Petcharat et al. (2021) [22] | Thailand | AS and PsA |
|
Cross-Sectional |
|
171/148 | 45.5 (12.2) | Cervical and lumbar spine | ‘Syndesmophyte was defined as bone growth originating from the vertebral endplate in the anterior one-quarter of the discovertebral space or from the anterior vertebral cortex’ |
| Gonzalez-Lopez et al. (2017) [10] | Mexico | AS | New York criteria | Prospective/case–control | 89 | 57/32 | 44.3 (11.4)a | Cervical/Lumbar | ‘Syndesmophytes were defined as bony protuberances associated with ossification of the spinal ligaments without involvement of the intervertebral discs’. |
| De Bruin et al. (2016) [35] | Netherlands | AxSpA | ASAS | Cross-sectional | 274 | 96/178 | 28.2 (9)a | All | ‘The differentiation between osteophytes and syndesmophytes is based on the site of origin and the angle between the bony spur and the vertebral endplate; syndesmophytes originate at the ligamentous insertion and have a more vertical configuration’. |
| Gamez-Nava et al. (2016) [11] | Mexico | AS | New York criteria | Case–control | 78 | 50/28 | Cervical/Lumbar | ‘Syndesmophytes were defined according to the presence of ossification of vertebral ligaments of > 5 mm in radiographs’. | |
| Baraliakos et al. (2014) [36] | Germany | AS | N/A | Cross-sectional | 73 | 63/10 | 40.5 (10.5)a | Cervical/Lumbar | ‘Assessment of syndesmophytes and differentiation from degenerative changes such as spondylophytes was made according to a recent proposal, where the former are considered by showing a growth parallel to the anterior vertebral side/anterior intervertebral ligament while the latter are considered by showing a growth parallel to the horizontal line’. |
| Haddad et al. (2013) [5] | Canada | PsA | CasparS | Prospective cohort | 78 | 57/21 | 62.9 (8.9)a | Cervical/Lumbar | ‘PsA-related changes (syndesmophytes) were considered if there was a growth angle of < 45° to the anterior vertebral side, while an angle > 45 was considered to be osteophytes’. |
| Baraliakos 2012 [37] | Germany | AS | mNew York criteria | Retrospective cohort | 146 | 81/65 | 54.2 (12.3)a | Cervical/Lumbar | ‘Measuring the horizontal angle of new bone formation on lateral spinal radiographs, AS-related changes (syndesmophytes) were assumed to typically show a growth angle of ≤ 45° to the anterior vertebral side, while a growth angle of > 45° was assumed to represent more DISH-related changes (spondylophytes)’. |
| Maejima et al. (2010) [23] | Japan | PsA | Caspar | Cross-sectional | 25 | 18/7 | N/A | All |
|
| Chandran et al. (2009) [24] | Canada | PsA | N/A | Retrospective cohort | 297 | 169/128 | 42.5a | All |
|
| Baraliakos 2007 [38] | Germany | AS | mNew York criteria | Prospective | 116 | N/A | 38.4a | All | ‘Bony changes with an angle ≤45° to the anterior vertebral side were defined as syndesmophytes, in contrast with changes with an angle of >45°, which were defined as ambiguous syndesmophytes’. |
| Helliwell et al. (1998) [25] | UK |
|
|
|
AS: 46a IBD = 48 PsA = 46 ReA = 43 | All |
|
||
| Hanly et al. (1988) [26] | Canada | PsA | NA | Cross-sectional | 52 | 30/22 |
|
All |
|
Mean (s.d.).
Median.
SpA: Spondyloarthritis; AxSpA: Axial Spondyloarthritis; ASAS: Assessment of Spondylo Arthritis International Society; ReA: Reactive arthritis; N/A: Not applicable.
Definition of syndesmophytes
Our literature search was able to identify only one description of syndesmophytes, that was used in 13 studies with only minor modifications [10–22]. According to that, a syndesmophyte was defined as ‘bony overgrowth (protuberances/projections) along the anterior longitudinal ligament or ossification within the outer fibres/layers of the annulus fibrosus’. In the majority of these articles, syndesmophytes’ shape was specified as thin and the orientation of the growth as vertical. Six of the 12 articles mentioned that syndesmophytes may also connect the angles of adjacent vertebral bodies or connect two vertebral bodies across the disc space, leading to bridging phenomena, although this was not a mandatory feature of the definition [12–15, 19, 21].
Definition of subtypes of syndesmophytes
Marginal syndesmophytes: Seven articles used a specific terminology of ‘marginal syndesmophytes’ [14, 23–28]. Six of these articles defined the marginal syndesmophytes as ‘vertebral ossifications/calcification/bony outgrowth arose from the edge of the vertebral body vertically and extend from the corner of one vertebra to the next’. Mattar et al. [14] defined the marginal syndesmophytes as ‘horizontal projections at the level of the vertebral end-plate, with its cortex and medulla continuous with those of the parent bone’. Also, three of seven articles additionally described them as ‘being thin’ [23, 27, 28].
Non-marginal (para-marginal) syndesmophytes: Within the included studies, eight articles had a definition for non-marginal (para-marginal) syndesmophytes [23, 26–32]. Three out of eight articles described the growing pattern of these NBFs as syndesmophytes arising from beyond/away from the edge/margin of the vertebral body [23, 26, 31]. Five articles stated that these bony growths are curvilinear. Also, the following features for the shape of para-marginal syndesmophytes were mentioned in the articles: asymmetrical, thick, bulky, fluffy and chunky. While three articles described these ossifications as being parallel to the vertebral bodies or intervertebral discs [29, 31, 32], Eshed et al. [27] defined them as horizontally oriented syndesmophytes.
Other NBF definitions in SpA
Paravertebral ossification: Four articles included the definition of paravertebral ossification. These ossifications were defined as being close to the vertebra; however, with a gap between the margins of the ossification and the vertebra [23, 28]. Also, Klecker et al. [31] described it as ‘coarse asymmetrical bony bridging, and relative sparing of the apophyseal joints’.
Squaring: Squaring of vertebral borders was described as a result of erosive changes at the corners of the vertebrae and straightening of the anterior curve of the vertebra by NBF. This lesion is defined as a typical feature of AS and is best visualized in the lumbar spine [12, 33, 34].
Finer ossification : It was separately defined only by Porter et al. as ‘more closely related to the disc margins and fusing with the rim of the vertebral body’.
Locations of the NBF in SpA
Among 11 original articles, five investigated the syndesmophytes on the cervical and lumbar spine, while six articles included the thoracic spine as well (Table 1). For para-syndesmophytes, three articles specifically indicated that the lower thoracic and upper lumbar spine or thoracolumbar junction were more commonly involved than the cervical and lower lumber spine [31, 32]. However, Sudol-Szopinska et al. [29] mentioned that cervical involvement may be typical for the para-marginal syndesmophytes in PsA.
For the other lesions, in two articles, squaring of vertebra was mentioned as they can be best visualized in the lumbar spine due to the concavity of the lumbar spine compared with the cervical and thoracic spine [33, 34].
Differentiation of the inflammatory lesions from degenerative changes
A differentiation between syndesmophytes and degenerative changes was made in 12 articles [5, 12, 13, 18, 21, 26, 35–40]. According to those articles, syndesmophytes originated at the ligamentous insertion and the growth was parallel to the anterior vertebral side/anterior intervertebral ligament, whereas osteophytes originated from the cartilaginous endplate, with a horizontal growth and was associated with disc space narrowing.
In parallel to this explanation, in five articles, an angle of 45° was used to differentiate, with SpA-related changes having an angle of ≤45° to the anterior vertebral side and an angle of > 45° being representative of degenerative changes [5, 12, 37, 38, 40].
Distinguishing spinal PsA findings from other SpA subtypes
Non-marginal syndesmophytes were indicated to be more typical for PsA [27, 29]. The main differences for NBF in PsA compared with other SpA entities were larger, asymmetric distribution with skipped vertebral bodies levels, unilaterality and separation from the lateral aspect of the vertebral bodies of syndesmophytes [27, 29, 40]. Also, in Reijnierse et al.’s [12] study, ossification in AS was specified as in the outer layers of the annulus fibrosus itself, which results as intervertebral bridging, while PsA-related ossification was indicated as paraspinal and separated from the vertebral bodies and discs.
DISH
Our literature search identified several dichotomous variations in the radiographic definition of spinal DISH, that could be grouped into 10 definitions in total (Table 2).
Table 2.
Dichotomous criteria for the radiographic diagnosis of spinal DISH
| Original Author | Description |
|---|---|
| Haddad et al. 2013 [5] | ‘…flowing bony bridges on the right aspect of at least four contiguous thoracic vertebrae seen on anteroposterior view and also confirmed to be flowing on the lateral thoracic spine radiograph, irrespective of the presence of radiographic sacroiliitis on the last available radiographic assessment’. |
| Denko et al. 2002 [47] | ‘Patients with DISH met the following criteria… All DISH patients were 45 years or older with symptoms of pain in the spine and characteristic radiological changes in the involved areas consisting of widened intervertebral disk space and exuberant osteophytosis’ |
| Guo et al. 1997 [46] | ‘…flowing ossification of at least four contiguous vertebral bodies’ |
| Marcelli et al. 1995 [45] |
|
| Rogers et al. 1987 [48] | ‘…the presence of massive vertical osteophytes on the right anterolateral surface of the bodies of the thoracic spine… The vertebrae may be ankylosed but disc spaces are normal and the facet joints… are almost always normal… there must also be extraspinal manifestations of new bone growth in ligaments, in tendinous insertions or in cartilage’. |
| Arlet and Mazières, 1985 [41] |
|
| Brigode et al. 1982 [42] | To be included in the vertebral ankylosing hyperostosis series, patient had to have ‘at least two complete intervertebral bridges and a typical bone case along one vertebral body’ |
| Resnick and Niwayama, 1976 [4] |
|
| Julkunen et al. 1975 [44] | ‘…prominent and complete bony bridge connecting two vertebrae in two or more different sites in the dorsal spine’ |
| Forestier and Lagier, 1971 [43] |
|
DISH: Diffuse idiopathic skeletal hyperostosis.
Definitions of DISH
Number of vertebrae: Within the 10 dichotomous definitions outlined, five definitions [41–45] required the involvement of at least three contiguous vertebrae (or two intervertebral bridges), and three definitions [4, 5, 46] required four contiguous vertebrae to classify as changes as consistent with DISH. Two studies [47, 48] did not require any specific number of contiguous vertebrae as part of their definition of DISH.
Level of NBF: Five out of 10 definitions [5, 41, 43, 44, 48] mandated that lesions of NBF consistent with DISH were found on the thoracic spine, and one definition [45] required the presence of changes in the thoracic or lumbar spine. None of the definitions specified criteria for cervical spine changes. There were four definitions [4, 42, 46, 47] that did not specify a spinal level of involvement to make the diagnosis of DISH.
Laterality: Two out of 10 definitions required the ossifications to be present on the right side [5, 48].
Description of NBF in DISH
Several descriptions of the bony lesions of DISH were found in the identified dichotomous definitions. The most common description specified changes as flowing or bridging ossifications/calcifications, found in seven of the definitions [4, 5, 37, 41, 43, 46]. Other descriptions included were exuberant osteophytosis [47], flame-shaped anterolateral bony bridges [45] and massive vertical osteophytes [48], all used in one definition each.
Differentiation of the DISH NBF from osteophytes
Most of the definitions differentiated DISH-related changes from OA by requiring a normal/relatively normal disc space. Preservation of disc height was mandated in five definitions [4, 41, 43, 47, 48], one of which specifically included ‘widening intervertebral disc space’ [47]. None of the studies included differentiation of NBFs of DISH and OA. One study described the NBF in DISH as osteophytes.
Differentiation of the DISH NBF from syndesmophytes
Three definitions [4, 41, 43] required the absence of sacroiliitis in order to make a definitive diagnosis of DISH [49, 50], with each of these definitions also including the absence of ankylosis in the facet joint and one in the apophyseal joint [43]. Outside of the 10 dichotomous definitions identified by our search, one study modified the classically accepted Resnick criteria to help differentiate the NBF of DISH and SpA based on the angle of new bone growth from the vertebrae, which was then used in another study [7, 37]. In both studies, a growth angle of >45° from a vertebral body was felt to be in keeping with DISH-related changes, whereas bony growth of ≤45° was felt to be in keeping inflammatory changes, either from PsA [7] or AS [37].
OA
There were seven studies identified that provided a definition for the identification of osteophytes in the context of spinal OA (Table 3).
Table 3.
Definitions of osteophytes identified in the literature
| Author (year) | Country | Study design | Age (mean) | Sample size (n) | Anatomical site | Definition | |
|---|---|---|---|---|---|---|---|
| Okpala (2018) [52] | Nigeria | Retrospective | 44.7 |
|
Lumbar | ‘…bony overgrowths especially at the anterior, lateral and less commonly, posterior aspects of the superior and inferior margins of vertebral bodies’ | |
| Middleto and Fish (2009) [51] | USA | Review | N/A | N/A | Lumbar | ‘…bony outgrowths arising primarily along the anterior and lateral perimeters of the vertebral end-plate apophyses. These hypertrophic changes are believed to develop at sites of stress to the annular ligament and most commonly occur at thoracic T9–10 and lumbar L3 levels, vacuum phenomenon and vertebral body reactive change’. | |
| Gallucci et al. (2007) [55] | Italy | Review | N/A | N/A | Cervical, Thoracic, Lumbar |
|
|
| Pitkanen et al. (2002) [54] | Finland | Retrospective | 43 | 215 | Lumbar | ‘Traction spur defined as horizontally directed and arising at the site of attachment of the outermost annular fibres about 2 mm away from the distal border of the anterior and lateral surfaces’ | |
| Jaovisidha et al. (2000) [66] | Thailand | Retrospective | 58.8 | 100 | Lumbar | ‘Osteophyte defined as a prominent bone proliferation along the anterior and lateral aspect of vertebral body. | |
| Pfirrmann and Resnick (2001) [53] | USA | Retrospective | 68.2 | 100 | Thoracic, Lumbar |
|
|
| Katevuo et al. (1985) [67] | Finland | Prospective | 46.2 | 311 | Cervical, Thoracic | ‘Spondylosis was recorded if there were changes in more than two vertebrae and if there was osteophytes longer than 2 mm’. | |
N/A: Not applicable; USA: United States of America.
Radiographic definitions for spinal osteophytes
Location of NBF: A total of five articles indicated that osteophytes arose from the anterior or lateral aspects of the vertebral bodies, with only two of these studies specifying that osteophytosis could also occur at other locations, including the posterior, superior and inferior margins [51, 52].
Size of NBF: Two studies required a size cut-off in their definitions of osteophytes in the context of OA. Pfirrmann et al. [53] classified large osteophytes as those with an ‘anteroposterior diameter greater than 3 mm’. Another study required the presence of osteophytes longer than 2 mm to define spondylosis [54].
Description of NBF: Of the seven studies that included definitions for osteophytes, five described osteophytes as a form of outgrowth or spur arising from the bone [51, 52, 54, 55]. One study simply defined osteophyte as ‘prominent bony proliferation’ [53].
Osteophyte subtypes: Two articles defined different subtypes of osteophytes [53, 55]. Both studies outlined criteria for traction osteophytes, and one of the articles also defined claw osteophytes. These definitions were based on the shape and direction of growth of the osteophyte itself. Traction osteophytes were noted to grow horizontally in both aforementioned studies.
Radiographic definitions for spondylosis
Eight studies were identified that outlined specific criteria for the diagnosis of spondylosis (Supplementary Table S5, available at Rheumatology Advances in Practice online). All studies defining spondylosis included the presence of osteophytes in their criteria for diagnosing spondylosis. Osteophytes were often an absolute criterion for the diagnosis of spondylosis, but not in every case. In all but one study, disc space narrowing was also included in the criteria for the definition of spondylosis, though it was not necessary to make the diagnosis in any study. Three articles included facet joint sclerosis in their descriptions of spondylosis.
Discussion
Our results showed heterogeneity and variations in defining NBF in these three diseases. For SpA, our SLR revealed the inconsistencies in the literature for the definitions of syndesmophytes in terms of the shape, location and growing patterns of syndesmophyte subtypes. Also, this study identified that there are many variations of definitions of spinal DISH in the literature, which may result in different outcomes. Osteophyte formation was the only feature consistently included in the definition of spinal OA. Otherwise, joint space narrowing was frequently part of the diagnostic criteria, but the inclusion of other features was variable. These results are important to generate knowledge on how the NBFs are defined in the literature and create a standardized approach in this field.
The pathophysiological mechanism of the NBF leading to ankylosis in SpA is still unclear. The slow progression of the process requires a long-term follow-up, making it difficult to understand the natural course. In addition, the spine is not accessible for the purpose of the biopsies. Previously, it has been suggested that inflammation is the initial lesion, followed by the replacement of the subchondral bone marrow by fibrosis as a repair mechanism [56]. On the other hand, it has also been shown that syndesmophytes can grow from the areas without inflammation [36, 57]. According to this hypothesis, there might be similar underlying mechanisms in inflammatory and degenerative diseases, such as mechanical and genetic factors. For example, in DISH, where the metabolic conditions have been identified as the underlying factors, the extra spinal NBF can be seen in similar locations as SpA, such as ligaments, tendons and entheses. Therefore, it may become even more complicated to differentiate SpA from DISH [58–60]. On the other hand, DISH and SpA can also occur concomitantly, with some observation that this co-occurrence may portend worse clinical outcomes [7]. A small study comparing patients with DISH and AS demonstrated that there was a preponderance of horizontal enthesophytes in the former versus vertical enthesophytes in AS [61]. In two studies, authors attempting to differentiate spinal DISH from inflammatory arthritis used the angle of new bone growth in the spine to differentiate these two processes [7, 37]. To our knowledge, this is the only attempt in the literature on plain radiographs to differentiate the two types of NBF based on the angle, which is based on the expert opinion and has not been validated. Research on the bony changes of DISH utilizing computed tomography scans has also suggested an osteophyte angle of larger than 90° in relation to the vertebral bodies to differentiate DISH from bridging degenerative osteophytes, which has not been defined or tested in plain radiographs [62]. It will be important for any future definitions of DISH to take concurrent cases of seronegative spondyloarthropathies and DISH into consideration rather than excluding inflammatory arthritis entirely as is traditional.
From a diagnostic perspective, a clear and comprehensive definition of OA will enable clinicians to differentiate other concurrent skeletal disease processes of NBF. Overlapping features of OA and inflammatory arthropathies have also been reported [63]. For instance, enthesophytes, more classically connected to inflammatory processes, have been associated with OA in patients where SpA has been excluded [63, 64]. Erosive OA, an uncommon presentation of OA, can be especially difficult to discern from other inflammatory arthropathies, particularly PsA of the hand [65]. Especially with the ageing population, it may be complicated during the disease course to differentiate OA changes from SpA progression.
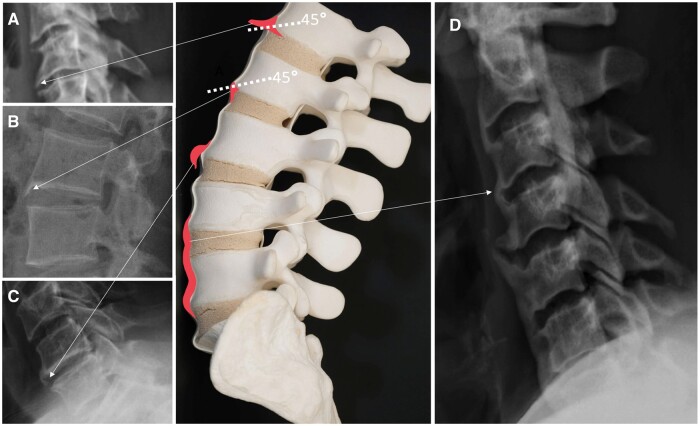
It is important to emphasize that the radiographic features of spinal NBF are not meant to be diagnostic for any disease as a stand-alone modality but rather be complimentary to the clinical features as well as other radiographic features- such as the sacroiliac joint findings. One key element to be able to differentiate the various NBF types lies under the recognition of which anatomical structures are getting ossified: Syndesmophytes are the ossification process of the annulus fibrosus, whereas para-syndesmophytes involve the soft tissues around the vertebral corners. The ‘flowing ossification’ in DISH mainly includes the ossification of the anterior longitudinal ligament, leading to a more widespread process exceeding the vertebral corners. An illustration of a variety of NBF types is provided in Fig. 2 with corresponding examples of the radiographs.
Figure 2.
Illustration of some of the new bone formation (NBF) types and representative radiographs. (A) Osteophyte: Horizontal bony outgrowth, with an angle of >45° to the hypothetical line that crosses the vertebral corner/ (B) Marginal syndesmophytes: vertebral ossifications/calcification/bony outgrowth arising from the edge of the vertebral body vertically, having an angle of ≤45° to the hypothetical line that crosses the vertebral corner. (C) Non-marginal (Para-marginal) syndesmophytes: asymmetrical, thick and bulky ossifications/calcification/bony outgrowth arising from away from the edge of the vertebral body. (D) DISH: Flowing ossifications and/or calcifications of the anterior longitudinal ligament.
The major limitation of this study is having only focused on plain radiographic definitions, excluding definitions identified by computed tomography and magnetic resonance imaging. While more advanced imaging techniques can certainly be important in differentiating different types of NBF, plain films balance both cost-effectiveness and radiation exposure for patients followed over time.
We propose that the description of NBF in DISH, SpA and OA needs to include a detailed description of the anatomical location, highlight the differences in different levels of the spine and include how to differentiate DISH from syndesmophytes seen in the context of PsA and other axial SpA well as the ‘tractions spurs’ or osteophytes in OA. The detailed findings from the literature will allow us to propose the definitions and conduct a Delphi exercise with a group of international experts to be able to create formal NBF definitions for disease groups.
The improved ability to differentiate these conditions radiographically will not only allow the clinicians to accurately approach their patients but also will help the researchers to better classify patient phenotypes and focus on accurate radiographic outcomes.
Supplementary Material
Acknowledment
Dr. Ozun Bayindir Tsechelidis and Dr. Ricardo Sabido-Sauri contributed to this manuscript for review and data extraction at the stage of revision.
Contributor Information
Ummugulsum Gazel, Division of Rheumatology, Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada.
Gizem Ayan, Division of Rheumatology, Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada.
Nicole Hryciw, Department of Medicine, The Ottawa Hospital, University of Ottawa, Ottawa, ON, Canada.
Jean-Philippe Delorme, Department of Radiology, The Ottawa Hospital, University of Ottawa, Ottawa, ON, Canada.
Elliot Hepworth, Division of Rheumatology, Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada.
Marcos Sampaio, Department of Radiology, The Ottawa Hospital, University of Ottawa, Ottawa, ON, Canada; Division of Radiology, Department of Medical Imaging, The Ottawa Hospital, Ottawa, ON, Canada.
Zaid Jibri, Department of Radiology, The Ottawa Hospital, University of Ottawa, Ottawa, ON, Canada; Division of Radiology, Department of Medical Imaging, The Ottawa Hospital, Ottawa, ON, Canada.
Jacob Karsh, Division of Rheumatology, Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada; Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Sibel Zehra Aydin, Division of Rheumatology, Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada; Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Supplementary material
Supplementary material is available at Rheumatology Advances in Practice online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosure statement: S.Z.A. received honoraria from Abbvie, Celgene, UCB, Novartis, Jannsen, Pfizer and Sanofi. The other authors have disclosed no conflicts of interest.
References
- 1. de Vlam K, Lories RJ, Luyten FP.. Mechanisms of pathologic new bone formation. Curr Rheumatol Rep 2006;8:332–7. [DOI] [PubMed] [Google Scholar]
- 2. Lories RJ, Luyten FP, de Vlam K.. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther 2009;11:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appel H, Maier R, Loddenkemper C. et al. Immunohistochemical analysis of osteoblasts in zygapophyseal joints of patients with ankylosing spondylitis reveal repair mechanisms similar to osteoarthritis. J Rheumatol 2010;37:823–8. [DOI] [PubMed] [Google Scholar]
- 4. Resnick D, Niwayama G.. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976;119:559–68. [DOI] [PubMed] [Google Scholar]
- 5. Haddad A, Thavaneswaran A, Toloza S, Chandran V, Gladman DD.. Diffuse idiopathic skeletal hyperostosis in psoriatic arthritis. J Rheumatol 2013;40:1367–73. [DOI] [PubMed] [Google Scholar]
- 6. Kuperus JS, Waalwijk JF, Regan EA. et al. Simultaneous occurrence of ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis: a systematic review. Rheumatology (Oxford) 2018;57:2120–8. [DOI] [PubMed] [Google Scholar]
- 7. Pappone N, Di Minno MND, Iervolino S. et al. The impact of concomitant diffuse idiopathic skeletal hyperostosis on the achievement of minimal disease activity in subjects with psoriatic arthritis. Rheumatol Int 2015;35:2041–6. [DOI] [PubMed] [Google Scholar]
- 8. Mathiessen A, Conaghan PG.. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riley MJ, Ansell BM, Bywaters EG.. Radiological manifestations of ankylosing spondylitis according to age at onset. Ann Rheum Dis 1971;30:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Lopez L, Fajardo-Robledo NS, Miriam Saldaña-Cruz A. et al. Association of adipokines, interleukin-6, and tumor necrosis factor-alpha concentrations with clinical characteristics and presence of spinal syndesmophytes in patients with ankylosing spondylitis: a cross-sectional study. J Int Med Res 2017;45:1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gamez-Nava JI, de la Cerda-Trujillo LF, Vazquez-Villegas ML et al Association between bone turnover markers, clinical variables, spinal syndesmophytes and bone mineral density in Mexican patients with ankylosing spondylitis. Scand J Rheumatol 2016;45:480–90. [DOI] [PubMed] [Google Scholar]
- 12. Reijnierse M. Radiographic/MR imaging correlation of paravertebral ossifications in ligaments and bony vertebral outgrowths: anatomy, early detection, and clinical impact. Magn Reson Imaging Clin N Am 2019;27:641–59. [DOI] [PubMed] [Google Scholar]
- 13. Braun J, Baraliakos X, Regel A, Kiltz U.. Assessment of spinal pain. Best Pract Res Clin Rheumatol 2014;28:875–87. [DOI] [PubMed] [Google Scholar]
- 14. Mattar M, Salonen D, Inman RD.. Imaging of spondyloarthropathies. Rheum Dis Clin North Am 2013;39:645–67. [DOI] [PubMed] [Google Scholar]
- 15. Olivieri I, D'Angelo S, Palazzi C, Padula A.. Spondyloarthritis and diffuse idiopathic skeletal hyperostosis: two different diseases that continue to intersect. J Rheumatol 2013;40:1251–3. [DOI] [PubMed] [Google Scholar]
- 16. Ostergaard M. Can imaging be used for inflammatory arthritis screening? Semin Musculoskelet Radiol 2012;16:401–9. [DOI] [PubMed] [Google Scholar]
- 17.Braum L, Hermann KGA. Utility of imaging in the diagnosis and assessment of axial spondyloarthritis. Int J Adv Rheumatol 2010;8:127–35. [Google Scholar]
- 18. Olivieri I, D'Angelo S, Palazzi C. et al. Diffuse idiopathic skeletal hyperostosis: differentiation from ankylosing spondylitis. Curr Rheumatol Rep 2009;11:321–8. [DOI] [PubMed] [Google Scholar]
- 19. Resnick D. Inflammatory disorders of the vertebral column: seronegative spondyloarthropathies, adult-onset rheumatoid arthritis, and juvenile chronic arthritis. Clin Imaging 1989;13:253–68. [DOI] [PubMed] [Google Scholar]
- 20. Kerr R, Resnick D.. Radiology of the seronegative spondyloarthropathies. Clin Rheum Dis 1985;11:113–46. [PubMed] [Google Scholar]
- 21. Baraliakos X. Imaging in axial spondyloarthritis. Isr Med Assoc J 2017;19:712–8. [PubMed] [Google Scholar]
- 22. Petcharat C, Srinonprasert V, Chiowchanwisawakit P.. Association between syndesmophyte and metabolic syndrome in patients with psoriatic arthritis or ankylosing spondylitis: a cross-sectional study. BMC Musculoskelet Disord 2021;22:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maejima H, Taniguchi T, Watarai A, Aki R, Katsuoka K.. Analysis of clinical, radiological and laboratory variables in psoriatic arthritis with 25 Japanese patients. J Dermatol 2010;37:647–56. [DOI] [PubMed] [Google Scholar]
- 24. Chandran V, Barrett J, Schentag CT, Farewell VT, Gladman DD.. Axial psoriatic arthritis: update on a longterm prospective study. J Rheumatol 2009;36:2744–50. [DOI] [PubMed] [Google Scholar]
- 25. Helliwell PS, Hickling P, Wright V.. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis 1998;57:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanly JG, Russell ML, Gladman DD.. Psoriatic spondyloarthropathy: a long term prospective study. Ann Rheum Dis 1988;47:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eshed I, Hermann KA, Zejden A, Sudol-Szopinska I.. Imaging to differentiate the various forms of seronegative arthritis. Semin Musculoskelet Radiol 2018;22:189–96. [DOI] [PubMed] [Google Scholar]
- 28. Taylor WJ, Porter GG, Helliwell PS.. Operational definitions and observer reliability of the plain radiographic features of psoriatic arthritis. J Rheumatol 2003;30:2645–58. [PubMed] [Google Scholar]
- 29. Sudoł-Szopińska I, Matuszewska G, Kwiatkowska B, Pracoń G.. Diagnostic imaging of psoriatic arthritis. Part I: etiopathogenesis, classifications and radiographic features. J Ultrason 2016;16:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spadaro A, Lubrano E.. Psoriatic arthritis: imaging techniques. Reumatismo 2012;64:99–106. [DOI] [PubMed] [Google Scholar]
- 31. Klecker RJ, Weissman BN.. Imaging features of psoriatic arthritis and Reiter's syndrome. Semin Musculoskelet Radiol 2003;7:115–26. [DOI] [PubMed] [Google Scholar]
- 32. Porter GG. Psoriatic arthritis. Plain radiology and other imaging techniques. Baillieres Clin Rheumatol 1994;8:465–82. [DOI] [PubMed] [Google Scholar]
- 33. Bazzocchi A, Aparisi Gomez MP, Guglielmi G.. Conventional radiology in spondyloarthritis. Radiol Clin North Am 2017;55:943–66. [DOI] [PubMed] [Google Scholar]
- 34. Ory PA. Radiography in the assessment of musculoskeletal conditions. Best Pract Res Clin Rheumatol 2003;17:495–512. [DOI] [PubMed] [Google Scholar]
- 35. de Bruin F, ter Horst S, Bloem HL. et al. Prevalence of degenerative changes of the spine on magnetic resonance images and radiographs in patients aged 16-45 years with chronic back pain of short duration in the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2016;55:56–65. [DOI] [PubMed] [Google Scholar]
- 36. Baraliakos X, Heldmann F, Callhoff J. et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis 2014;73:1819–25. [DOI] [PubMed] [Google Scholar]
- 37. Baraliakos X, Listing J, Buschmann J, von der Recke A, Braun J.. A comparison of new bone formation in patients with ankylosing spondylitis and patients with diffuse idiopathic skeletal hyperostosis: a retrospective cohort study over six years. Arthritis Rheum 2012;64:1127–33. [DOI] [PubMed] [Google Scholar]
- 38. Baraliakos X, Listing J, Rudwaleit M. et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007;66:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schueller-Weidekamm C, Mascarenhas VV, Sudol-Szopinska I et al Imaging and interpretation of axial spondylarthritis: the radiologist's perspective—consensus of the Arthritis Subcommittee of the ESSR. Semin Musculoskelet Radiol 2014;18:265–79. [DOI] [PubMed] [Google Scholar]
- 40. Lubrano E, Marchesoni A, Olivieri I. et al. The radiological assessment of axial involvement in psoriatic arthritis. J Rheumatol Suppl 2012;89:54–6. [DOI] [PubMed] [Google Scholar]
- 41. Arlet J, Mazieres B.. [Hyperostotic disease]. Rev Med Interne 1985;6:553–64. [DOI] [PubMed] [Google Scholar]
- 42. Brigode M, Francois RJ, Dory MA.. Radiological study of the sacroiliac joints in vertebral ankylosing hyperostosis. Ann Rheum Dis 1982;41:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forestier J, Lagier R.. Ankylosing hyperostosis of the spine. Clin Orthop Relat Res 1971;74:65???83–83. [PubMed] [Google Scholar]
- 44. Julkunen H, Heinonen OP, Knekt P, Maatela J.. The epidemiology of hyperostosis of the spine together with its symptoms and related mortality in a general population. Scand J Rheumatol 1975;4:23–7. [PubMed] [Google Scholar]
- 45. Marcelli C, Yates AJ, Barjon MC. et al. Pagetic vertebral ankylosis and diffuse idiopathic skeletal hyperostosis. Spine (Phila Pa 1976) 1995;20:454–9. [DOI] [PubMed] [Google Scholar]
- 46. Guo B, Jaovisidha S, Sartoris DJ. et al. Correlation between ossification of the stylohyoid ligament and osteophytes of the cervical spine. J Rheumatol 1997;24:1575–81. [PubMed] [Google Scholar]
- 47. Denko CW, Boja B, Malemud CJ.. Growth hormone and insulin-like growth factor-I in symptomatic and asymptomatic patients with diffuse idiopathic skeletal hyperostosis (DISH). Front Biosci 2002;7:a37–43. [DOI] [PubMed] [Google Scholar]
- 48. Rogers J, Waldron T, Dieppe P, Watt I.. Arthropathies in palaeopathology: the basis of classification according to most probable cause. J Archaeol Sci 1987;14:179–93. [Google Scholar]
- 49. Forestier J, Rotes-Querol J.. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis 1950;9:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Resnick D, Shaul SR, Robins JM.. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier's disease with extraspinal manifestations. Radiology 1975;115:513–24. [DOI] [PubMed] [Google Scholar]
- 51. Middleton K, Fish DE.. Lumbar spondylosis: clinical presentation and treatment approaches. Curr Rev Musculoskelet Med 2009;2:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Okpala FO. Comparison of Four Radiographic Angular Measures of Lumbar Lordosis. J Neurosci Rural Pract 2018;9:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pfirrmann CW, Resnick D.. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology 2001;219:368–74. [DOI] [PubMed] [Google Scholar]
- 54. Pitkanen MT, Manninen HI, Lindgren KA. et al. Segmental lumbar spine instability at flexion-extension radiography can be predicted by conventional radiography. Clin Radiol 2002;57:632–9. [DOI] [PubMed] [Google Scholar]
- 55. Gallucci M, Limbucci N, Paonessa A, Splendiani A.. Degenerative disease of the spine. Neuroimaging Clin N Am 2007;17:87–103. [DOI] [PubMed] [Google Scholar]
- 56. Sieper J, Appel H, Braun J, Rudwaleit M.. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum 2008;58:649–56. [DOI] [PubMed] [Google Scholar]
- 57. Maksymowych WP, Morency N, Conner-Spady B, Lambert RG.. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 2013;72:23–8. [DOI] [PubMed] [Google Scholar]
- 58. Mader R, Verlaan JJ, Buskila D.. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol 2013;9:741–50. [DOI] [PubMed] [Google Scholar]
- 59. Ramos-Remus C, Gomez-Vargas A, LeClercq S, Russell AS.. Radiologic features of DISH may mimic ankylosing spondylitis. Clin Exp Rheumatol 1993;11:603–8. [PubMed] [Google Scholar]
- 60. Mader R, Sarzi-Puttini P, Atzeni F. et al. Extraspinal manifestations of diffuse idiopathic skeletal hyperostosis. Rheumatology (Oxford) 2009;48:1478–81. [DOI] [PubMed] [Google Scholar]
- 61. Maertens M, Mielants H, Verstraete K, Veys EM.. Evaluation of the involvement of axial entheses and sacroiliac joints in relation to diagnosis: comparison among diffuse idiopathic skeletal hyperostostis (DISH), osteoarthrosis and ankylosing spondylitis. Clin Rheumatol 1992;11:551–7. [DOI] [PubMed] [Google Scholar]
- 62. Oudkerk SF, de Jong PA, Attrach M. et al. Diagnosis of diffuse idiopathic skeletal hyperostosis with chest computed tomography: inter-observer agreement. Eur Radiol 2017;27:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McGonagle D, Hermann KG, Tan AL.. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology (Oxford) 2015;54:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Menz HB, Marshall M, Thomas MJ. et al. Associations Between Calcaneal Enthesophytes and Osteoarthritis of the Hands and Feet. Arthritis Care Res (Hoboken) 2020;72:1343–8. [DOI] [PubMed] [Google Scholar]
- 65. Punzi L, Ramonda R, Sfriso P.. Erosive osteoarthritis. Best Pract Res Clin Rheumatol 2004;18:739–58. [DOI] [PubMed] [Google Scholar]
- 66. Jaovisidha S, Techatipakorn S, Apiyasawat P. et al. Degenerative disk disease at lumbosacral junction: plain film findings and related MRI abnormalities. J Med Assoc Thai 2000;83:865–71. [PubMed] [Google Scholar]
- 67. Katevuo K, Aitasalo K, Lehtinen R, Pietila J.. Skeletal changes in dentists and farmers in Finland. Community Dent Oral Epidemiol 1985;13:23–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.