Abstract
Hypertrophic cardiomyopathy is an important cause of heart failure and arrhythmias, including sudden death, with a major impact on the healthcare system. Genetic causes and different phenotypes are now increasingly being identified for this condition. In addition, specific medications, such as myosin inhibitors, have been recently shown as potentially able to modify its symptoms, hemodynamic abnormalities and clinical course. Our article aims to provide a comprehensive outline of the epidemiology, diagnosis and treatment of hypertrophic cardiomyopathy in the current era.
Keywords: hypertrophic cardiomyopathy, management, update
Introduction
Hypertrophic cardiomyopathy (HCM) is one of the most common inherited heart diseases.1–4 Modern research on HCM began in the 1960s,5–8 and after >60 years of development, great progress has been made so far in the etiology, diagnosis, treatment, and management of HCM.9–12 The aim of this review is to summarize available data on the epidemiology, diagnosis and treatment of HCM in the current era.
Definition and epidemiology
The hallmark of HCM is increased ventricular wall thickness mainly caused by mutations in sarcomere proteins and is transmitted with an autosomal dominant inheritance pattern in the absence of another cardiac, systemic, or metabolic disease capable of producing that magnitude of hypertrophy.
HCM is a global disease, and early epidemiological surveys based on echocardiography showed that the prevalence of HCM in general adults ranged from 0.16% to 0.23%,13–16 with an average of 0.20% (1/500). Later, it was found that the incidence of pathogenic variants in the sarcomeric gene was higher in the general population than previously expected;17 advances in genetic testing technology have identified more patients carrying pathogenic variants in sarcomeric genes in the absence of clinical manifestations of left ventricular hypertrophy (LVH), known as ‘genotype positive phenotype negative’ individuals18; and clinical application of modern cardiac imaging techniques such as cardiac magnetic resonance (CMR) has detected a number of HCM phenotypes that are commonly missed or underdiagnosed using echocardiography.19,20 Therefore, the contemporary prevalence of HCM was estimated to be higher than that in previous survey results and could reach 1/200 (0.5%).21,22
Etiology
Pathogenic genes
HCM is mostly a monogenic inherited heart disease, and approximately 60% of HCM patients have pathogenic genetic variants mainly encoding myocardial sarcomere proteins, including thick myofilaments, intermediate filaments and thin myofilaments.1–4,9–11 Since Geisterfer-Lowrance et al.23 first identified the beta–myosin heavy chain 7 (MYH7) gene mutation as causative in a family with HCM in 1990, over 1500 variants associated with the pathogenesis of HCM have been identified to date in at least 8 genes encoding myocardial sarcomere protein (known as ‘core pathogenic gene’ of HCM) (Table 1),10 among which, MYBPC3 gene and MYH7 gene are the two most common pathogenic genes, which account for approximately 70% of patients with HCM positive for genetic variations.
Table 1.
Common pathogenic genetic variants in hypertrophic cardiomyopathy
| Pathogenic genes | Gene mapping | Genes encoded | Detection frequency (%) | Inheritance model |
| Thick myofilament protein | ||||
| MYH7 | 14q11.2 | Myosin heavy chain 7 | 20–30 | AD |
| MYL2 | 12q24.11 | Myosin light chain 2 | 2–4 | AD |
| MYL3 | 3q21.31 | Myosin light chain 3 | 1–2 | AD, minimal AR |
| Intermediate filament protein | ||||
| MYBPC3 | 11p11.2 | Cardiac myosin binding protein C | 30–40 | AD, minimal AR |
| Thin myofilament protein | ||||
| TNNT2 | 1q32.1 | Cardiac troponin T2 | 5–10 | AD |
| TNNI3 | 19q13.42 | Cardiac troponin I3 | 4–8 | AD |
| TPM1 | 15q22.2 | alpha tropomyosin | <1 | AD |
| ACTC1 | 15q14 | alpha actin 1 | <1 | AD |
Note: AD, autosomal dominant; AR, autosomal recessive.
Genetic pattern
Most genetic patterns of HCM caused by genetic variants are autosomal dominant. However, due to incomplete penetrance and age-dependent expression of pathogenic genetic variants, carriers of pathogenic genetic variants do not necessarily develop a clinical phenotype.10
Complex genetic variants
Some patients with HCM may carry multiple variants of a single gene (compound variation) or heterozygous variants of at least two identical or different genes, collectively known as the complex genetic variant phenomenon. Studies have found that patients carrying complex genetic variants have earlier onset, more severe clinical phenotype, and worse prognosis.24
Gene-elusive cases
Current studies have found that pathogenic genetic variants are not detectable in up to 40% of HCM patients, mainly seen in some sporadic cases (called ‘nonfamilial HCM’) or small families, usually with late onset and relatively mild clinical phenotype,25 suggesting that different mechanisms may be involved in the pathogenesis of HCM.
Pathophysiological mechanisms
HCM has complex pathophysiological mechanisms, mainly including left ventricular outflow tract obstruction (LVOTO), mitral regurgitation (MR) and diastolic dysfunction with a major role also for myocardial ischemia and autonomic dysfunction.1–4 At the individual level, one mechanism may be predominant or complex interactions between multiple mechanisms may be involved.
Left ventricular outflow tract obstruction
LVOTO is defined as an instantaneous peak left ventricular outflow tract (LVOT) pressure gradient at ≥30 mmHg (1 mmHg = 0.133 kPa) detected by Doppler echocardiography. Of note, LVOTO is dynamic and sensitive to changes in ventricular preload, afterload and myocardial contractility.26 Importantly, for HCM patients without LVOTO at rest, provocation test is required to assess latent LVOTO, which can be performed by doing a Valsalva maneuver, or repeated bedside transition from a supine to standing position or from a squatting to standing position, or upright treadmill or semi-supine bicycle test, etc.27,28 Due to the lack of specificity, use of dobutamine for determination of provocative LVOTO is not recommended in current guidelines.1–4 Notably, studies have shown that LVOTO significantly increased the risk of adverse events such as sudden cardiac death (SCD), progression to moderate to severe heart failure (HF), stroke and death in HCM patients.29–31 A resting or provoked LVOT pressure gradient ≥50 mmHg is generally considered to be the threshold for septal reduction therapy (SRT) in patients with drug-refractory symptoms.
Mitral regurgitation
There are two main causes of MR: secondary to systolic anterior motion (SAM) phenomenon caused by LVOTO; primary or intrinsic mitral apparatus abnormalities, such as excessively elongated mitral valve leaflets, abnormal papillary muscle insertion, and anteriorly displaced papillary muscles.32
Diastolic dysfunction
The mechanism mainly includes two aspects: myocardial ischemia, hypoxia, energy metabolism disorders, resulting in abnormal diastolic intracellular calcium re-uptake of myocardium and impaired myocardial active relaxation; ventricular hypertrophy, myocardial fibrosis and changes in ventricular geometry resulting in reduced ventricular wall compliance (increased stiffness) and restrictive ventricular filling.1–4
Myocardial ischemia
Its causes may include the following: cardiomyocyte hypertrophy, resulting in an imbalance of myocardial oxygen supply and demand; intramural coronary vessels are apparently narrowed by medial hypertrophy, and vascular distribution density in the ventricular wall reduced;33 coronary microvascular dysfunction, resulting in reduced coronary flow reserve; and coronary myocardial bridging and coronary atherosclerotic lesions in some cases.
Autonomic dysfunction
It is mainly manifested by abnormal heart rate recovery,34 inappropriate vasodilatation and abnormal blood pressure response to exercise,35 and is related to the prognosis of HCM.3,4
Clinical evaluation
Clinical evaluation of HCM patients includes family history, symptoms and signs, etc. For patients with suspected HCM, a comprehensive physical examination, detailed personal history, and family history of at least three generations should be performed at the initial evaluation.
Family history
Family history is very important for the diagnosis and prognosis of patients with HCM. Relevant family history includes diagnosis of HCM in family members or occurrence of SCD in the first-degree relative aged ≤50 years, HF, heart transplantation, and history of implantable cardioverter defibrillator (ICD) therapy.36
Symptoms
The clinical manifestations of HCM vary greatly, ranging from completely asymptomatic patients to exercise-related symptoms, such as dyspnea, chest pain, palpitation and syncope, and to SCD as the first manifestation. An increasing proportion of clinically asymptomatic patients with HCM are incidentally diagnosed following physical examination, electrocardiography and/or echocardiography as part of the diagnostic workup for other diseases or as a result of cascade family screening in relatives of patients with a pathogenic gene mutation.
Approximately 15–25% of HCM patients have experienced at least one episode of syncope or presyncope.37 The occurrence of unexplained syncope within 6 months prior to the initial evaluation has been shown to be an independent predictor of SCD in patients with HCM, and the risk is nearly five times greater than that in patients without syncope – a relationship that exists in different age groups.38
Signs
Signs in patients with obstructive HCM are mainly related to LVOTO. Rough ejection systolic murmur can be heard in the third to fourth intercostal space located on the left sternal border, without conduction to the neck, and murmur can be enhanced by enhancing myocardial contractility or reducing cardiac preload and afterload; conversely, murmur can be attenuated by weakening myocardial contractility or increasing cardiac preload and afterload. This kind of murmur needs to be differentiated from MR as well as murmur of aortic stenosis (AS).
Auxiliary examination
Patients with HCM need to complete relevant auxiliary examinations during initial evaluation and regular follow-up, including the following aspects.39
Electrocardiogram
The mainly includes standard 12-lead ECG and 24–48 h ambulatory ECG.
1. Standard 12-lead ECG: This can provide information about QRS complex voltage reflecting left ventricular hypertrophy, repolarization abnormalities and arrhythmia.40–42 The ECG recording of HCM patients demonstrates abnormalities in up to 94% of cases at the first visit, including left ventricular high voltage, pathological Q wave and ST–T changes.43 In patients with suspected HCM, a normal ECG greatly reduces the possibility of an underlying HCM. Standard 12-lead ECG can be used for the initial evaluation, regular follow-up and screening of family members of patients with HCM.
2. 24–48 h ambulatory ECG: Different types of arrhythmia can be seen, including supraventricular and ventricular arrhythmia.44 Among them, the prevalence of non-sustained ventricular tachycardia (NSVT, defined as ≥3 consecutive ventricular beats with heart rate ≥100 beats/min and duration <30 s) is 20%–30%,45 which is independently associated with an increased risk of SCD in young patients aged <35 years; and faster rate (>200 beats/min), longer (>7 beats), and repetitive runs of NSVT are more highly predictive.46
Echocardiography
Echocardiography is currently the first-choice examination method for clinical diagnosis, disease monitoring, treatment selection and therapeutic effect evaluation in HCM patients. At present, there are a variety of new ultrasound techniques applied in clinical practice, such as tissue Doppler imaging, myocardial contrast echocardiography, speckle tracking echocardiography for myocardial strain or strain rate imaging, and their applications mainly include the following aspects.39,47–49
1. Measure ventricular wall thickness to assess LVH: It is recommended to measure the maximal ventricular wall thickness of all ventricular segments in short-axis view from the base to the apex at the end of diastole, avoiding overestimation of ventricular wall thickness in long-axis view with M-mode ultrasound.
2. Measure LVOT pressure gradient to assess LVOTO: Peak LVOT pressure gradient at rest is initially measured to assess resting LVOTO and provocation test is recommended to assess latent LVOTO if the resting LVOT pressure gradient is <50 mmHg.
Cardiac biomarkers
At present, detection of cardiac biomarkers mainly refers to natriuretic peptide and cardiac troponin. Natriuretic peptide detection is used to assess cardiac load or cardiac function, and cardiac troponin detection is used to assess myocardial injury, both of which are helpful for risk stratification and prognosis judgment of HCM patients.50–53 Thus, it is recommended to perform routine detection of cardiac biomarker parameters during the initial evaluation and regular follow-up of HCM patients.
Cardiac magnetic resonance
Cardiac magnetic resonance (CMR) is currently the most accurate examination method for the diagnosis of HCM and the current noninvasive imaging method of first choice for the assessment of myocardial fibrosis.39,54 Clinical applications of CMR in patients with HCM mainly include:
1. Measure ventricular wall thickness and cardiac chamber size: For special sites where hypertrophy occurs such as the anterolateral wall of the left ventricle, posterior septum, right ventricle and apex or apical ventricular aneurysm and thrombosis, CMR is of greater diagnostic value than TTE, which is helpful for the accurate classification of HCM.
2. Assess myocardial fibrosis: Focal replacement fibrosis (myocardial scar) can be detectable by late gadolinium enhancement (LGE), which is helpful for risk stratification and prognosis of HCM.55,56 The presence of extensive LGE, such as ≥15% left ventricular mass, has been shown to increase the risk of SCD and is associated with poor prognosis in HCM.55
Genetic testing
Genetic testing is of great value in the diagnosis and differential diagnosis of HCM, family screening and prenatal and postnatal care,57,58 and is also of certain value in the prognostic evaluation and risk stratification of HCM.59,60 For patients with HCM, it is recommended to perform genetic testing so as to clarify the genetic basis and help identify individuals at high risk of developing HCM in the family.
Nowadays, genetic testing is mainly performed using next-generation sequencing technology, which is characterized by high throughput, rapid detection and low cost.61
As for genetic testing in HCM, it is usually preferred to sequence ‘target genes’ or ‘gene panels’ containing at least eight ‘core pathogenic genes’ (MYH7, MYBPC3, MYL2, MYL3, TNNT2, TNNI3, TPM1 and ACTC1) that are clearly associated with the pathogenesis of HCM. If the above genetic testing fails to identify pathogenic gene mutation, gene panel sequencing for cardiomyopathy, or whole exome sequencing or whole genome sequencing may be considered.62 For detection results of genetic variants, only those with pathogenic (P) and likely pathogenic (LP) are clinically significant.63
Of note, the pathogenicity of genetic variants depends on the current number or level of evidence, and the pathogenicity classification of genetic variants may change over time. Thus, for HCM patients with genetic testing, it is recommended to periodically reevaluate the pathogenicity of genetic variants to determine whether reclassification is required.64
Exercise tests
Cardiopulmonary exercise test (CPET) can be objectively evaluated in HCM patients to have a quantitative assessment of exercise tolerance or degree of functional limitation which is associated with poor prognosis in HCM patients.65 For patients with end-stage (ES) HCM (ES–HCM), CPET can assist in the evaluation of indications for heart transplantation or mechanical circulatory support.
Endomyocardial myocardial biopsy
Endocardial myocardial biopsy is an invasive examination that is mainly used when clinical evaluation suggests infiltrative, storage or inflammatory diseases (HCM ‘phenocopies’) that cannot be definitely diagnosed by routine noninvasive imaging, or for patients with HCM who have lower response to routine treatment and thus require further clarification of the cause.66
Diagnostic criteria and algorithm
Ventricular wall thickening is a requisite criterion for the diagnosis of HCM. Different cardiac imaging methods such as echocardiography, CMR or cardiac CT can be used.
1. Diagnostic criteria for HCM in adults (≥18 years of age): A maximal end-diastolic ventricular wall thickness of ≥ 15 mm in one or more left ventricular myocardial segments as measured by any cardiac imaging technique. Moreover, maximal thickness of left ventricular wall of ≥ 30 mm is called extreme LVH.67 For family members other than the proband in familial HCM or individuals with positive genetic testing (carriers of HCM pathogenic genetic variants), maximal end-diastolic ventricular wall thickness of ≥ 13 mm can also be diagnosed with HCM.
2. Diagnostic criteria for HCM in children (<18 years of age): Different diagnostic cut-off values are used depending on the children's age, body surface area, and pretest probability of diagnosing HCM: for asymptomatic children with no family history of HCM, HCM can be diagnosed when the body surface area adjusted z-score is > 2.5 standard deviations above the mean. For children with a definite family history of HCM or a positive genetic test, a threshold of a z-score of >2.0 standard deviations above the mean value may be appropriate.
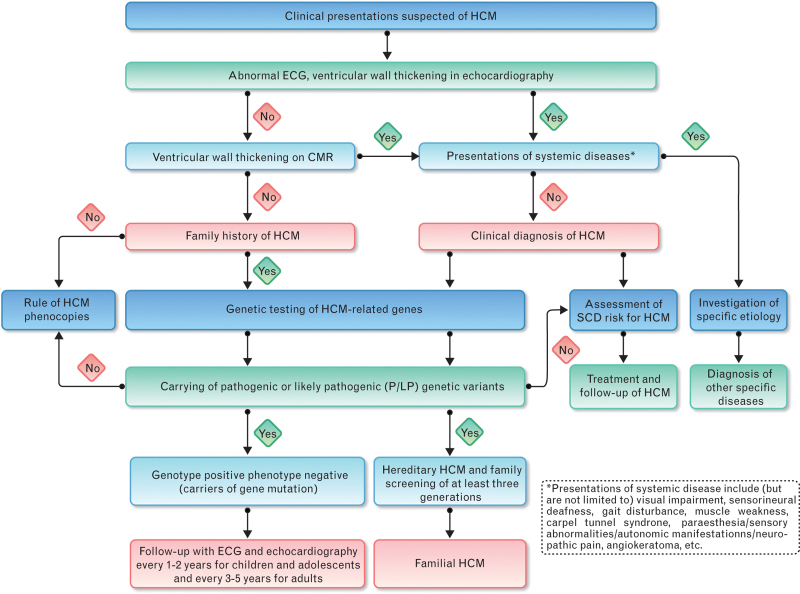
The diagnostic algorithms of HCM patients are detailed in Fig. 1.
Fig. 1.
Diagnostic algorithm for hypertrophic cardiomyopathy (CMR, cardiac magnetic resonance; ECG, electrocardiography; HCM, hypertrophic cardiomyopathy; SCD, sudden cardiac death).
Genetic evaluation and family screening
Genetic evaluation
Genetic evaluation usually includes two parts: genetic counseling and genetic testing. Genetic evaluation first involves the first patient diagnosed with HCM in the family (called a ‘proband’ or ‘index case’).
1. Genetic counseling: Generally, genetic counseling includes pretest genetic counseling and posttest genetic counseling.1–4 The aims of the former are to carry out family history survey and pedigree mapping, and to explain in detail to patients and relatives the benefits and potential hazards of genetic testing for detection of inherited heart diseases. Furthermore, the latter is recommended to provide a professional interpretation of genetic testing results based on the patient's phenotype to assess the value of risk stratification for individuals and family members, and to inform patients and relatives that the genetic testing results need to be re-interpreted regularly based on progress in the pathogenicity classification of genetic variants.68
2. Genetic testing: See ‘Auxiliary examination, genetic testing’ for details.
Family screening
Family screening should include two parts: clinical evaluation and genetic testing. Clinical evaluation includes symptom evaluation and auxiliary examination. Symptom evaluation mainly refers to evaluation of exercise-related dyspnea, chest pain, palpitation or syncope. Auxiliary examination includes at least 12-lead ECG and echocardiography.
For first-degree child relatives from families with positive HCM genotype, it is recommended that clinical evaluation and genetic testing should be initiated when HCM is diagnosed in other family members, and ECG and echocardiography should be regularly performed every 1–2 years thereafter; for other types of first-degree child relatives, it is recommended that clinical evaluation should be initiated at any time (but not later than puberty) after HCM is diagnosed in family members, and then ECG and echocardiography should be regularly performed every 2–3 years thereafter,3 with clinical evaluation to be continued at least to middle age (50 years old).3
The corresponding family screening strategy are shown as follows.69,70
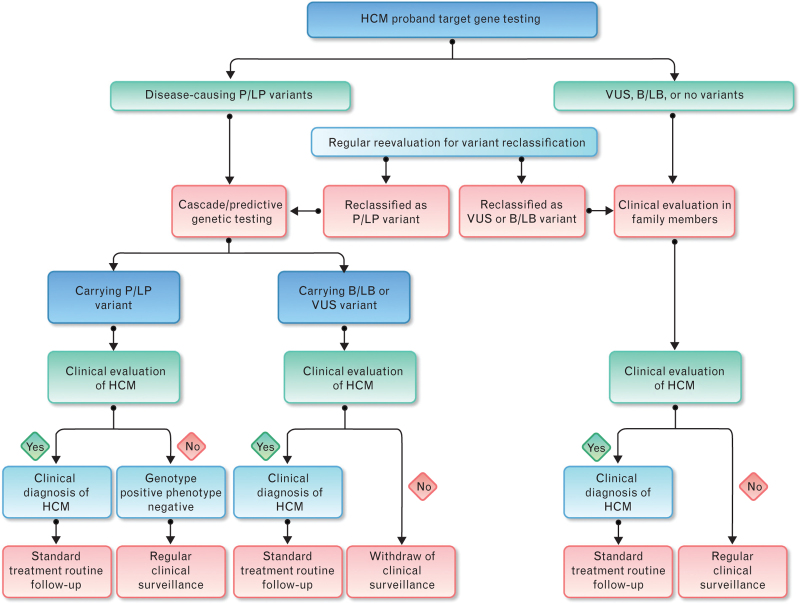
1. For families with a genetic variant of P/LP type detected in the proband, it is recommended to carry out cascade/predictive family screening. If the first-degree relative carries the same pathogenic genetic variant as the proband and has HCM-related clinical manifestations, HCM is definitely diagnosed, and standardized treatment and routine follow-up should be performed. If the first-degree relative carries the same pathogenic genetic variant as the proband but does not have evidence of LVH and is asymptomatic, a diagnosis of ‘genotype positive phenotype negative’ (G+P−) individual is made, and it is recommended to perform long-term regular follow-up to monitor the disease progression. If no pathogenic genetic variant is detected in relatives and with no HCM-related clinical manifestation on initial evaluation, further clinical monitoring can be removed (Fig. 2).
Fig. 2.
Flow chart for genetic testing and family screening in hypertrophic cardiomyopathy (B/LB, benign/likely benign; HCM, hypertrophic cardiomyopathy; P/LP, pathogenic/likely pathogenic; VUS, variant of unknown significance).
2. For families without a genetic variant of P/LP type detected in the proband: If the proband has not yet undergone genetic testing, or the test result is negative, or the result is VUS or B/LB variant, it is recommended that relatives should undergo clinical evaluation. If HCM-related clinical manifestations are found, HCM is diagnosed clinically, and standardized treatment and routine follow-up should be performed. If no HCM-related clinical manifestations are revealed in the initial evaluation, perform regular clinical evaluation (Fig. 2).
Genotype positive phenotype negative (G+P−) individuals
1. Definition: (G+P−) individuals refer to individuals carrying HCM-related P/LP genetic variants (G+) but have no evidence of LVH on cardiac imaging (P−) and are clinically asymptomatic.10
2. Subclinical structural and/or functional abnormalities: Despite no evidence of LVH on cardiac imaging, some (G+P−) individuals may have ECG abnormalities and may have subtle cardiac imaging abnormalities.71,72 The clinical significance of these subclinical structural and/or functional changes remains unclear.
3. Phenotypic transformation: Some (G+P−) individuals can increase ventricular wall thickness during follow-up, meeting the diagnostic criteria for HCM and have phenotypic transformation,73 with an incidence ranging from 6% to 30% and varied time from positive genotype to positive phenotype.
4. Prognosis: Generally, (G+P−) individuals have a better prognosis as long as they undergo no phenotypic transformation, and most of them have no clinical symptoms. However, some cases can undergo phenotypic transformation, develop clinical symptoms and even SCD.
Clinical classification and staging
Clinical classification
HCM can have different clinical classifications according to different criteria.
1. According to hemodynamics: Obstructive HCM: This can be further divided into LVOTO, mid-ventricular obstruction and left ventricular apical obstruction according to the site of ventricular wall hypertrophy. Generally, obstructive HCM refers to LVOTO, that is, with instantaneous peak LVOT pressure gradient of ≥30 mmHg; it can be divided into resting obstructive (with LVOTO present at rest) and latent obstructive (with LVOTO after provocation but absent at rest). Nonobstructive HCM: This is defined as a peak LVOT pressure gradient at <30 mmHg both at rest and with provocation. Clinically, resting obstructive, latent obstructive, and nonobstructive HCM account for approximately one-third, respectively.74
2. According to hypertrophy site: Septal hypertrophy: This is the most common clinical phenotype, mainly involving the basal interventricular septum, characterized by ASH. The diagnostic criterion is the ratio of end-diastole interventricular septum to left ventricular posterior wall thickness ≥1.3−1.5. Apical hypertrophy: This is also known as apical hypertrophic cardiomyopathy (ApHCM), which refers to ventricular hypertrophy mainly involving the apex below the papillary muscle of the left ventricle.75 ApHCM is a relatively rare subtype of HCM which is relatively more prevalent in Asian populations, with a higher prevalence in males, and progresses relatively slowly. The diagnostic criteria are maximal left ventricular end-diastolic ventricular wall thickness at the apex ≥15 mm, and the ratio of the maximal apical to posterior wall thickness ≥1.5. It can be subclassified into two forms: ‘pure’, with isolated apical hypertrophy; and ‘mixed’, with both apical and septal hypertrophy but with the apex thickest.75 The typical feature on ECG is giant negative T wave with voltage often >1.0 mV, which can be seen in leads I, aVL and V3–6, with increased R wave and depressed ST segment in corresponding leads, especially in lead V4. Echocardiography and CMR imaging are the most valuable imaging test methods, which show ‘black spade’ pattern-like changes in the left ventricular cavity. Mid-ventricular hypertrophy: This is also known as mid-ventricular obstructive hypertrophic cardiomyopathy (MVOHCM), which refers to myocardial hypertrophy at the level of the papillary muscle in the middle of the left ventricle and in the middle of the interventricular septum, with an end-systolic pressure gradient between the apex and the base of the left ventricle.76 Diagnostic criteria include: maximal end-diastolic mid-ventricular wall thickening with wall thickness of ≥ 15 mm, or ventricular wall thickness of ≥13 mm with definite family history; and mid-ventricular end-systolic peak pressure gradient of ≥30 mmHg.
3. According to genetic characteristics: Familial/inherited HCM: The disease is caused by pathogenic genetic variants and its diagnostic criteria includes at least two members of three generations of relatives with HCM in addition to proband or presence of same genetic variants as the proband. Sporadic HCM: There is no familial aggregation of the disease. It is not caused by genetic variants, or variants carried by patients are ‘de novo’ variants.
Clinical staging
In 2012, Olivotto et al.77 proposed a clinical staging for HCM patients based on objective evidence of clinical and disease progression, dividing HCM into four stages: stage I (nonhypertrophic/preclinical), stage II (classic phenotype), stage III (adverse remodeling) and stage IV (overt dysfunction/end-stage).
Special phenotypes
1. ES–HCM: ES–HCM was defined as HCM with severe left ventricular systolic dysfunction and the main diagnostic criterion is left ventricular ejection fraction (LVEF) of < 50% by echocardiography.78 Based on the previous reports, prevalence of ES–HCM in HCM ranged from 2.4% to 15.7%,78–83 and about 0.5–1.5% of patients with HCM progress to ES–HCM every year.78–81 Previous studies showed that patients with ES–HCM suffered from a severe clinical phenotype and poor prognosis, with an annual mortality rate of 11.0%,78 with HF and SCD as the main causes of death. However, a recent report by Rowin et al.83 showed that the annual mortality rate of ES–HCM patients decreased significantly to 1.9% on current optimal anti-HF treatment, which was significantly lower than the previously reported annual mortality rate (8.0%), but still higher than the annual mortality rate for HCM patients with preserved LVEF (0.2%). These results suggest that current treatment strategies, including ICD and heart transplantation, significantly improve the prognosis of ES–HCM patients.
2. HCM with restrictive phenotype (RP–HCM): According to the study conducted by Kubo et al.,84 RP–HCM echocardiographic diagnostic criteria include the following aspects: significant enlargement of both atria, mitral inflow E/A ratio of ≥ 2 and mitral deceleration time of ≤ 150 ms; absent or only mild LVH; ventricular cavity reduced or of normal size; LVEF normal or mildly decreased. Prevalence of RP–HCM in HCM patients ranged from 1.5% to 5.9% in different studies, suggesting that RP–HCM is relatively uncommon.84,85 Studies have shown that MYH7, TNTT3 and MYL2 gene variants are more common in patients with RP–HCM.84,86 Compared with patients with classic HCM, patients with RP–HCM demonstrate severe clinical phenotype, poor prognosis, and high mortality from HF.84,86
Complications
Atrial fibrillation
Atrial fibrillation (AF) is one of the most common arrhythmias in patients with HCM, which is present in 18–20% of patients87–89 with an annual incidence of approximately 3%.88 Advanced age, left atrial enlargement, and New York Heart Association (NYHA) functional classes III and IV are the main risk factors for AF in HCM patients. AF affects the quality of life and increases the risk of stroke and peripheral embolism with a prevalence and annual incidence of 27% and 4%, respectively.88 On current effective treatment, AF-related mortality has been reduced, showing no significant difference from HCM patients without AF. However, AF remains an important cause of thrombotic events in HCM patients.89
Heart failure
Patients with HCM may present with symptoms of dyspnea, mainly caused by abnormal left ventricular diastolic function, manifested as heart failure with preserved ejection fraction (HFpEF, LVEF ≥ 50%). A few patients progress to ES–HCM, which manifests as heart failure with reduced ejection fraction (HFrEF, LVEF ≤ 40%) and heart failure with mildly reduced ejection fraction (HFmrEF, LVEF 41–49%).90
Left ventricular apical aneurysm
Left ventricular apical aneurysm (LVAA) is a relatively rare complication of HCM with an incidence of between 1% and 5%,91 and is more common in special types of HCM such as ApHCM and MVOHCM.92,93 LVAA is one of the risk factors for SCD in patients with HCM and is closely related to the poor prognosis of HCM.94
Intracardiac thrombosis
Intracardiac thrombosis can be divided into atrial thrombosis and ventricular thrombosis by location. Atrial thrombosis, including atrial appendage thrombosis, is primarily associated with AF. HCM patients with AF have a high risk of stroke.82 Left ventricular thrombosis is relatively rare in patients with HCM,95 and is more common in special types of HCM such as ApHCM and MVOHCM, especially in patients with LVAA.
Differential diagnosis
There are a variety of physiological and pathological factors that can lead to ventricular wall thickening, known as ‘phenocopies’,96,97 which need to be differentiated from HCM (Table 2).
Table 2.
The differential diagnosis of HCM and other ‘phenocopies’
| Disease | Etiology | Clinical manifestation | Auxiliary examinations | ||||||||
| Pathogenic gene | Inheritance pattern | Cardiac | Extra-cardiac | Electrocardiography | Echocardiography | Cardiac magnetic resonance | Endomyocardial biopsy | Others | Treatment | ||
| Hypertrophic cardiomyopathy | MYBPC3, MYH7, TNNT2, TNNI3, MLY2, MYL3, TPM1, ACTC1, etc. | AD | Asymptomatic, or exercise-related symptoms, such as dyspnea, chest pain, palpitation and syncope, SCD, cardiac murmur | No | LV high voltage, T-wave inversion | Asymmetric septal hypertrophy ≥ 15 mm, normal or higher systolic function, varied diastolic dysfunction, reduced LV GLS | LV wall thickness, focal septal LGE, increased native T1 value and ECV | Cardiomyocyte hypertrophy and disarray, interstitial fibrosis, intramural coronary artery abnormalities | No | Beta blocker, cardiac myosin inhibitor, nondihydropyridine CCB, septal reduction therapy, ICD | |
| Athlete's heart | No | No | Asymptomatic | No | Normal | Mild symmetrical LVH (usually ≤ 15 mm), normal systolic or diastolic function | Normal | Normal | No | Withdrawal from exercises | |
| Aortic stenosis | No | No | Dyspnea, chest pain, palpitation and syncope, SCD, cardiac murmur | No | LV high voltage | Mild symmetrical LVH (usually ≤ 15 mm), aortic valve lobe thickened and valve orifice area decreased | Nonspecific | Nonspecific | No | Transcatheter aortic valve replacement, surgical aortic valve replacement | |
| Cardiac amyloidosis | AL | No | No | HF (dyspnea, edema, etc.), arrhythmia, myocardial ischemia, hypotension, syncope, angina | Polyneuropathy, nephropathy, autonomic dysfunction, macroglossia, periorbital purpura, liver involvement | Low QRS voltage and decreased voltage/mass ratio, pseudo-infarction, conduction disturbance | Symmetrical pseudo-hypertrophy involving LV, right ventricle, atrial septum, valves, with speckle sign, mildly systolic dysfunction, severe diastolic dysfunction, decreased LV GLS with apical sparing, pericardial effusion | LV wall thickness, diffuse LGE with subendocardial and transmural patterns, increased native T1 value and ECV | Amyloid deposition, stained with Congo red, ‘apple green’ birefringence on polarized light microscope, interstitial fibrosis, 8–12 nm unbranched structures observed on electron microscopy, amyloid typing using immunohistochemical analysis/mass spectrometry | Serum free light chain (FLC) assay: abnormal κ/λ ratio; serum (SPIE), and urine (UPIE) protein electrophoresis with immunofixation: clonal immunoglobulin and/or clonal light chain | Antiplasma cell therapy, including protein proteasome inhibitor, daratumumab, immunomodulatory drug, autologous stem cell transplantation or cardiac transplantation |
| ATTRv | TTR (common genotypes: Val30Met, Val122Ile, Thr60Ala) | AD | Polyneuropathy, orthostatic hypotension, vitreous opacities, gastrointestinal problems | 99 mTc-pyrophosphate (PYP): grade 2 or 3 myocardial uptake of radiotracer | Genetic silencer, TTR tetramer stabilizer, TTR disruption/reabsorption, liver transplantation or cardiac transplantation | ||||||
| ATTRwt | No | No | Carpal tunnel syndrome, lumbar spinal stenosis, ruptures biceps tendon | ||||||||
| Fabry disease | GLA | XR | HF, arrhythmia, myocardial ischemia | Angiokeratoma, hypohidrosis, cornea verticillata, gastrointestinal symptoms, neuropathic pain, renal damage, early-onset cerebral infarction, hearing impairment, etc. | Short PR interval without preexcitation, bradycardia | Concentrically LVH with normal systolic function, aortic root dilation, reduced longitudinal strain in the basal inferolateral segment as well as loss of the base-to-apex circumferential strain gradient | Hypertrophy of papillary muscles, mid-layer posterolateral LGE, lower native T1 value | No | No | Enzyme replacement therapy, agalsidase α (Replagal) or agalsidase β (Fabrazyme) | |
| Danon disease | LAMP2 | XD | Severe symmetrical LVH (usually > 30mm) or DCM, inherited preexcitation syndrome | Proximal skeletal myopathy and mental retardation | Preexcitation syndrome | Severe symmetrical LVH or DCM, decreased LV GLS with apical sparing | LGE in the anterior, lateral and/or posterior wall of the subendocardium, myocardium or transmural, usually not involved in the middle interventricular septum | Electron microscopy shows intracytoplasmic vacuoles containing autophagic material and glycogen in both skeletal muscle and endomyocardial tissue biopsy | No | No | |
| Pompe disease | GAA | AD | Increased myocardial wall thickness | Skeletal myopathy often presents with progressive muscle weakness | Short PR interval without preexcitation | LV hypertrophy | LV hypertrophy | Electron microscopy of a muscle biopsy indicates vacuolar myopathy with glycogen accumulation in lysosomes and free glycogen in the cytoplasm | No | No | |
| PRKAG2 cardiac syndrome | PRKAG2 | AD | Symmetrical LVH (> 15 mm), inherited preexcitation syndrome, conduct disorder, and supraventricular tachycardia | No | Preexcitation syndrome | LV hypertrophy | LV hypertrophy | No | No | No | |
| Friedreich's ataxia | FXN | AR | Increased myocardial wall thickness | No | No | No | No | No | No | No | |
| RASopathies | PTPN11, BRAF, etc. | AD | Left ventricular hypertrophy, pulmonary valve stenosis | Cardio-facio-cutaneous syndrome, retardation of growth | Electrocardiographic conduction defect | LV hypertrophy | LV hypertrophy | Intracytoplasmic vacuoles with osmiophilic ‘medullary corpuscles’ | No | No | |
AD, autosomal dominant; AR, autosomal recessive; CCB, calcium channel blocker; DCM, dilated cardiomyopathy; ECV, extracellular volume; GLS, global longitudinal strain; HF, heart failure; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; LVH, left ventricular hypertrophy; XD, X-linked dominant; XR, X-linked recessive.
Myocardial hypertrophy caused by regular exercise (athlete heart)
Long-term regular exercise can lead to adaptive changes in the heart, manifested as mild symmetrical hypertrophy of the left ventricle (usually ≤15 mm), with normal diastolic function, good cardiopulmonary exercise function, no family history of cardiomyopathy, and negative genetic testing. The degree of myocardial hypertrophy can be alleviated or subsides after 3 months of withdrawal from exercises.
Hypertension
Generally, patients with long-term uncontrolled blood pressure may have mild symmetrical hypertrophy (≤15 mm), ultrasonic hypoecho for hypertrophic myocardium, and possibly enlarged left ventricular cavity in decompensated stage. ECG may indicate left ventricular high voltage, and genetic testing is generally negative. LVH can be alleviated or subsides after 6–12 months of strict blood pressure control.
Aortic stenosis
AS can cause increased cardiac afterload, resulting in compensatory hypertrophy of the myocardium, generally mild symmetrical hypertrophy (≤15 mm), with different location, transmission and influencing factors of murmur. Echocardiography indicates aortic valve thickening, limited systolic opening and reduced valve orifice area and cardiac catheterization reveals systolic pressure gradient between the left ventricle and aorta help to differentiate from HCM.
Cardiac amyloidosis
Cardiac amyloidosis (CA) can have extra-cardiac and cardiac manifestations including symmetrical ventricular wall thickening with low or normal voltage on ECG and, thus with decreased ratio of QRS voltage to wall thickness.98 Echocardiography99 and CMR100 help to discriminate CA from HCM. Blood and urine free light chain test and immunofixation electrophoresis, pyrophosphate radionuclide bone scan and transthyretin gene detection are used to assist in the diagnosis of CA101; pathological examination results of affected organs and/or endocardial biopsies are the gold standard for the diagnosis and classification of CA.102
Congenital metabolic disorders
1. Fabry disease:103 This is caused by mutations in the alpha-galactosidase A (GLA) gene with a X-linked recessive inheritance pattern. Cardiac involvement is characterized by concentric myocardial hypertrophy, aortic and mitral valve thickening with mild to moderate regurgitation, with ECG often displaying left ventricular high voltage and conduction system involvement and also showing short PR interval without preexcitation. LGE in the mid-posterior lateral wall is seen on CMR. Alpha-galactosidase A enzyme activity detection and genetic testing are helpful in confirming the diagnosis.
2. Glycogen storage disease: This is often accompanied by multiple organ involvement, mainly including the following: Danon disease:104 This is a X-link dominant disorder, caused by mutations in the LAMP2 coding gene located at Xq24. It is characterized by a typical triad phenotype: cardiac hypertrophy with inherited preexcitation, muscle weakness and mental retardation. Typical pathological features are intracytoplasmic vacuoles in skeletal muscle cells, with LAMP-2 insufficiency suggested by immunohistochemical staining. It can be differentiated based on genetic testing and characteristic pathological changes. RKAG2 cardiac syndrome:105 This is caused by mutations in the PRKAG2 gene with autosomal dominant inheritance. Cardiac involvement is characterized by myocardial hypertrophy, inherited preexcitation, conduction system disorders and supraventricular arrhythmia tetralogy, with few or rare other systemic manifestations. Genetic testing is helpful to confirm the diagnosis.
Neuromuscular disorders
Friedreich's ataxia:106 This is caused by mutations in the FXN gene encoding the soluble mitochondrial protein frataxin, with an autosomal recessive inheritance pattern, and cardiac involvement is mainly characterized by cardiac hypertrophy. Genetic testing is helpful to confirm the diagnosis.
Malformation syndromes
These include Noonan syndrome,107 LEOPARD syndrome,108 etc., collectively referred to as RASopathies.109 Noonan syndrome and LEOPARD syndrome are caused by mutations in the PTPN11 gene encoding the nonreceptor protein tyrosine phosphatase type 11 and in proto-oncogenes c-Raf or RAF1 and BRAF gene, with an autosomal dominant inheritance pattern. Genetic testing is helpful in diagnosis.
Mitochondrial diseases
Primary mitochondrial diseases are caused by mutations in nuclear DNA or mitochondrial DNA, resulting in energy metabolism disorders and symptoms of multisystem involvement, including brain, skeletal muscle and myocardial manifestations with high demand for aerobic metabolism. Cardiac involvement mainly presents with cardiac hypertrophy. Genetic testing helps to achieve a diagnosis.110
Treatment
The treatment goals of HCM include relieving clinical symptoms, improving cardiac function, delaying disease progression, and reducing disease death.
1. For patients with symptomatic obstructive HCM, medical treatment or invasive treatment can be used to improve symptoms.
2. For patients with symptomatic nonobstructive HCM, treatment is mainly aimed at comorbidities.
3. For patients with asymptomatic HCM, it is required to perform regular clinical evaluation. Even in the presence of LVOTO, SRT is not recommended.
4. All patients with HCM should routinely undergo risk assessment and risk stratification for SCD for corresponding prevention and treatment.
Drug therapy for patients with symptomatic obstructive hypertrophic cardiomyopathy
At present, the types of drug treatment for patients with symptomatic obstructive HCM mainly include the following:1–4
Beta blockers: As the earliest drugs studied for the treatment of patients with HCM, currently, beta blockers are used as first-line treatment.1–4 For patients with symptomatic obstructive HCM, it is recommended to use beta blockers without vasodilator effects, including propranolol, metoprolol, and bisoprolol, starting with a small dose and titrated gradually to a therapeutically effective (for symptomatic relief) or maximally tolerated dose (usually defined as a resting heart rate of 55–60 beats/min).111–113
2. Cardiac myosin inhibitors: Mavacamten is a selective allosteric inhibitor of cardiac myosin that inhibits the excessive formation of myosin-actin cross-bridges by selectively reducing the ATPase activity of cardiac myosin heavy chain.114 Results from the EXPLORER-HCM study115 demonstrated that treatment with mavacamten can significantly reduce resting and provoked LVOT pressure gradients. Moreover, mavacamten can improve exercise capacity, cardiac function and health status, and decrease N-terminal-pro B type natriuretic peptide (NT-proBNP) and cardiac troponin I (cTnI) levels.116,117 VALOR-HCM study118 showed that 16 weeks of treatment with mavacamten significantly reduced the proportion of patients who met the criteria for SRT. In the following active-controlled period, mavacamten treatment showed sustained reduction in the proportion proceeding to SRT through 32 weeks.119 The exploratory diastolic function sub-study demonstrated that treatment with mavacamten is associated with improvement in the average E/e’ ratio and left atrial volume index, independently of changes in the LVOT gradient pressure and improvement in MR.120 The MAVERICK-HCM study121 showed that treatment with mavacamten in nonobstructive HCM can reduce levels of NT-proBNP and cTnI. Mavacamten was approved by the US Food and Drug Administration (FDA) in April 2022 and is the first and only cardiac myosin inhibitor approved for the treatment of adult patients with symptomatic NYHA functional class II to III obstructive HCM to improve functional capacity and symptoms. In the 2023 European Society of Cardiology (ESC) Guidelines on the management of cardiomyopathy, cardiac myosin ATPase inhibitors have been recommended for the treatment of symptomatic patients with obstructive HCM (resting or provocable LVOT ≥ 50 mmHg) to relieve symptoms as second-line therapy when beta blockers, calcium antagonists, and/or disopyramide are ineffective or poorly tolerated with class of recommendation (COR) IIa, level of evidence (LOE) A.122 Mavacamten should be uptitrated to the maximum tolerated dose (max 15 mg/day) under careful echocardiographic monitoring for decline in LVEF. Of note, cardiac myosin ATPase inhibitors should not be used with disopyramide.
Aficamten (CK-27) is a novel, selective, small molecule inhibitor of cardiac myosin.123,124 Results from REDWOOD-HCM study125 demonstrated that aficamten significantly reduced LVOT gradient pressure and NT-proBNP levels compared with placebo. Currently, both the FDA and the Center for Drug Evaluation of the National Medical Products Administration have granted ‘breakthrough therapy’ designation to aficamten for the treatment of symptomatic obstructive HCM.
Of note, initiation of cardiac myosin inhibitors in patients with LVEF of < 55% is not recommended and should be interrupted if LVEF is <50% at any visit or if the patient experiences HF symptoms or worsening clinical status.
3. Nondihydropyridine calcium channel blockers (CCBs): These have negative inotropic and negative frequency effects and thus can alleviate LVOTO, improve diastolic ventricular filling, and improve patient symptoms. For patients with symptomatic obstructive HCM who are refractory to, intolerant of, or have contraindications to beta blocker therapy, it is recommended to use nondihydropyridine CCBs,1–4 including verapamil or diltiazem. However, the use of nondihydropyridine CCBs is not recommended in patients with signs of severe dyspnea or HF at rest, hypotension or cardiogenic shock, sick sinus syndrome, or second- or third-degree atrioventricular block (unless a pacemaker is implanted), and markedly elevated resting LVOT pressure gradients (> 80–100 mmHg).
4. Disopyramide: This belongs to Class Ia antiarrhythmic drugs, has a potent negative inotropic effect, can inhibit myocardial contractility, alleviate SAM phenomenon and the degree of MR, and can decrease the LVOT pressure gradient.126,127 A recent study explored the electrophysiological profile of disopyramide and provided evidence for the efficient reduction of outflow gradients but also the limited prolongation of the QT interval and the absence of arrhythmic side effects.128 For patients who still have persistent severe symptoms related to LVOTO after use of beta blockers or nondihydropyridine CCBs, it is recommended to use disopyramide in combination with beta blockers or nondihydropyridine CCBs and gradually titrate to the maximal tolerated dose.
5. Cibenzoline: This also belongs to Class Ia antiarrhythmic drugs, which can decrease the LVOT pressure gradient and alleviate LVOTO, and can also improve left ventricular diastolic function.129 For patients who remain symptomatic after beta blockers or nondihydropyridine CCBs, it is recommended to use cibenzoline in combination with beta blockers or nondihydropyridine CCBs and gradually titrate to the maximal tolerated dose.
Invasive therapy for patients with symptomatic obstructive hypertrophic cardiomyopathy
Invasive treatment measures include surgical septal myectomy (SSM), septal myocardial ablation (SMA), and dual-chamber pacemaker implantation, and the first two modalities can reduce the thickness of the interventricular septum, and are thus collectively referred to as SRT.
1. General indications for SRT include: Clinical criteria: Presence of severe dyspnea or chest pain symptoms (NYHA functional class III or IV) after optimal drug treatment or other symptoms related to exercise (such as recurrent syncope or presyncope) is related to LVOTO and affects the patient's daily activities and quality of life. Hemodynamic criteria: Peak LVOT pressure gradient of ≥ 50 mmHg at rest or with provocation is associated with septal hypertrophy or mitral SAM phenomenon. Anatomical criteria: It is at the discretion of the operator to determine whether the septal thickness is sufficient in order to perform the operation safely and effectively. SRT is not recommended for asymptomatic obstructive HCM patients with normal exercise tolerance or if symptoms can be controlled with optimized medical therapy.
2. Specific indications for SSM include1–4,130: In patients who meet indications for SRT and are able to undergo cardiac surgery, it is recommended to perform SSM131–135; in patients with related heart diseases requiring surgical treatment, it is also recommended to perform SSM134,136; in patients who failed SMA treatment (residual obstruction) or have postoperative recurrence, it is recommended to perform SSM137,138; those meeting indications for SRT and with presence of moderate to severe MR not caused by the SAM phenomenon of mitral valve alone after detailed evaluation, mitral valve repair (preferred) or replacement should be considered.139 Mitral valve replacement solely for the relief of LVOTO is not recommended.
Main surgical procedures include: Thoracotomy and transaortic extended septal myectomy or modified Morrow operation is the main surgical procedure,130,140,141 which was first reported by Morrow et al. in 1961.7 Currently, a transapical approach myectomy can be performed for ApHCM,142 and a combined transaortic and transapical approach to myectomy can be performed for MVOHCM.143 SSM can rapidly and significantly alleviate LVOTO and resolve MR, which allows the long-term survival rate for patients to be close to that of the general population. Studies in Western countries showed that the 1-, 5-, and 10-year survival rates for patients with symptomatic obstructive HCM after SSM were 98%, 96%, and 83%, respectively, which were similar to those of the general population, but were significantly higher than those of patients who failed to receive SSM (1-, 5-, and 10-year survival rates were 90%, 79%, and 61%).131 Similarly, study results in China showed that the 1-year and 5-year cumulative survival rates for patients with symptomatic obstructive HCM after SSM were 99% and 97%, respectively.134 Currently, the surgery-related mortality rate for SSM alone is < 1%, while the mortality rate for SSM combined with mitral valve surgery is 3–5%.135 Other common early postoperative complications include complete atrioventricular block (AVB), left bundle branch block (LBBB), atrial fibrillation, ventricular septal perforation and aortic valve insufficiency.
3. Specific indications for percutaneous transluminal septal myocardial ablation (PTSMA) include patients who meet indications for SRT but cannot undergo surgery due to advanced age or severe comorbidities, patients at higher risk or with contraindications to surgery, or patients unwilling to undergo surgery, for whom it is recommended to perform PTSMA.1–4,144 For patients with SSM treatment failure (residual obstruction) or postoperative recurrence of PTSMA, PTSMA can be considered again.145 Alcohol septal ablation (ASA) is mainly used currently which is the classic PTSMA using anhydrous alcohol (96–99% ethanol) for chemo-ablation and blocking the septal branch artery, resulting in regional myocardial necrosis, thereby eliminating interventricular septal hypertrophy and reducing LVOTO. PTSMA can significantly reduce the peak LVOT pressure gradient, alleviate LVOTO and resolve MR, thereby improving patient symptoms and cardiac function, and improving exercise tolerance and quality of life. A decrease in the peak LVOT pressure gradient by ≥ 50% or a peak LVOT pressure gradient of < 30 mmHg is usually considered to be a marker of successful PTSMA operation. PTSMA can also significantly improve the long-term survival rates for patients with symptomatic obstructive HCM, making them close to those for the general population matched for age and gender.146,147 The incidence of major adverse cardiac events after ASA is < 2%. Complications mainly include AVB (< 10% requiring implantation of permanent cardiac pacemaker), right bundle branch block (RBBB) (affecting supply of blood to first septal branch artery), myocardial infarction and myocardial scarring-induced arrhythmia, etc.
4. SSM vs. ASA: Both SSM and ASA are effective treatments for obstructive HCM, with no significant differences in alleviating LVOTO, improving symptoms and quality of life, and short- and long-term mortality in experienced centers.148 However, ASA is associated with a higher incidence of postoperative residual obstruction, which may require reintervention, and a higher risk of AVB, requiring permanent pacemaker implantation,149,150 especially among patients with preexisting conduction system disease. Although limited data are available, ASA may be less effective in patients with very severe hypertrophy (≥30 mm).151 In an recent analysis of 3859 patients with obstructive HCM, ASA was associated with an increase of 68% in the 10-year all-cause mortality risk compared with SSM after adjustment for age, sex, and comorbidities.152 In clinical practice, individualized selection needs to be made after comprehensive evaluation according to factors such as patient age, site and degree of obstruction, concomitant diseases and patient wishes.
5. Other SMA procedures include: Percutaneous intramyocardial septal radiofrequency ablation (PIMSRA, also known as the Liwen procedure):153,154 This may be considered as an alternative treatment for SSM or ASA at experienced centers, and its long-term efficacy deserves further investigation. Endocardial radiofrequency ablation of septal hypertrophy (ERASH):155,156 ERASH has a high incidence of complications, and the long-term outcome requires further observation.
6. Dual-chamber pacemaker implantation: Nowadays, there are few applications of dual-chamber pacemakers in the treatment of patients with obstructive HCM for symptom control since the long-term efficacy is uncertain. For patients with SSM or ASA who are at high risk of heart conduction block after operation, dual-chamber pacemaker implantation may be considered.4
Treatment of hypertrophic cardiomyopathy with heart failure
Heart failure with preserved ejection fraction
1. The goal of medical treatment is to control the heart rate to reduce left ventricular end-diastolic pressure and improve left ventricular filling. Beta blockers and nondihydropyridine CCBs are recommended as first-line treatment. Small doses of diuretics (loop diuretics or thiazide diuretics) should be considered with caution in patients with exertional dyspnea or signs of fluid retention despite other treatment.
2. End-stage HCM: Drug therapy: Guideline-directed medical therapy (GDMT) is recommended based on the updated guidelines on HF,157,158 and discontinuation of negative inotropic drugs, such as nondihydropyridine CCBs, disopyramide, and cibenzoline, should be considered. ICD: Patients with ES–HCM have a higher risk of SCD, for whom ICD implantation should be considered.
3. Advanced HF: Heart transplantation: For patients with HCM who have serious symptoms (NYHA functional classes III or IV) or recurrent fatal arrhythmia after GDMT (regardless of LVEF level), it is recommended to perform heart transplantation evaluation according to the latest waiting list criteria for heart transplantation.159 Long-term prognosis for patients with HCM after heart transplantation is similar to those without HCM. Ventricular assist device (VAD): For some HCM patients with end-stage HF, VAD implantation should be considered as a transition before heart transplantation to reduce deaths while waiting for heart transplantation.
Treatment of arrhythmias
1. Treatment of HCM with AF: Anticoagulation therapy: AF significantly increases the risk of stroke and peripheral embolism in HCM patients,83 independently of the CHA2DS2–VASc score.160 Thus, it is recommended that HCM patients with AF should be given anticoagulant therapy, regardless of the CHA2DS2–VASc score, as long as there is no contraindication. Direct-acting oral anticoagulants (DOACs) are recommended as first-line treatment, and oral vitamin K antagonists (warfarin) as second-line treatment.161 Heart rate control strategy: For HCM patients with AF who choose a heart rate control strategy, beta blockers or nondihydropyridine CCBs are recommended according to the patient's propensity and comorbidities. If the ventricular rate is difficult to control with medication, or intolerable adverse events occur, atrioventricular node ablation may be considered.4 Rhythm control strategy: Main treatments include antiarrhythmic drugs (AADs), direct current cardioversion, catheter ablation and surgical maze procedure. Conversion of sinus rhythm with AADs (e.g. amiodarone) should be considered in HCM with AF;3,4 maintenance of sinus rhythm with amiodarone or AADs (e.g. disopyramide, sotalol, etc.) should be considered.4 If accompanied by hemodynamic instability or severe symptoms of angina pectoris or HF, direct current cardioversion should be considered to restore sinus rhythm.2–4 Catheter ablation should be considered in patients with AF that is difficult to control with medical therapy, or in patients with HCM who are intolerant of (or contraindicated to) or refuse medical therapy.162 The surgical maze procedure is an effective treatment for AF, and concurrent surgical maze surgery should be considered for HCM patients undergoing SSM.163
2. Ventricular arrhythmias: Medication: As first-line choice, a beta blocker is recommended.1–4 If symptomatic ventricular arrhythmia persists despite beta blockers, or recurrent discharges occur after ICD implantation, it is recommended to be combined with AADs, including amiodarone, or sotalol, dofetilide, mexiletine, etc.164 Nondrug therapy: Implantation of an ICD is recommended for the prevention of SCD due to fatal cardiac arrhythmias. After ICD implantation, it is necessary to optimize drug therapy and pacemaker programming to reduce the risk of appropriate or inappropriate discharges. Catheter ablation should be considered in case of recurrent episodes of symptomatic, sustained, monomorphic ventricular tachycardia (VT) of focal origin. Heart transplantation: For patients with HCM who have recurrent fatal arrhythmia, it is recommended to perform heart transplantation evaluation according to the latest waiting list criteria for heart transplantation.
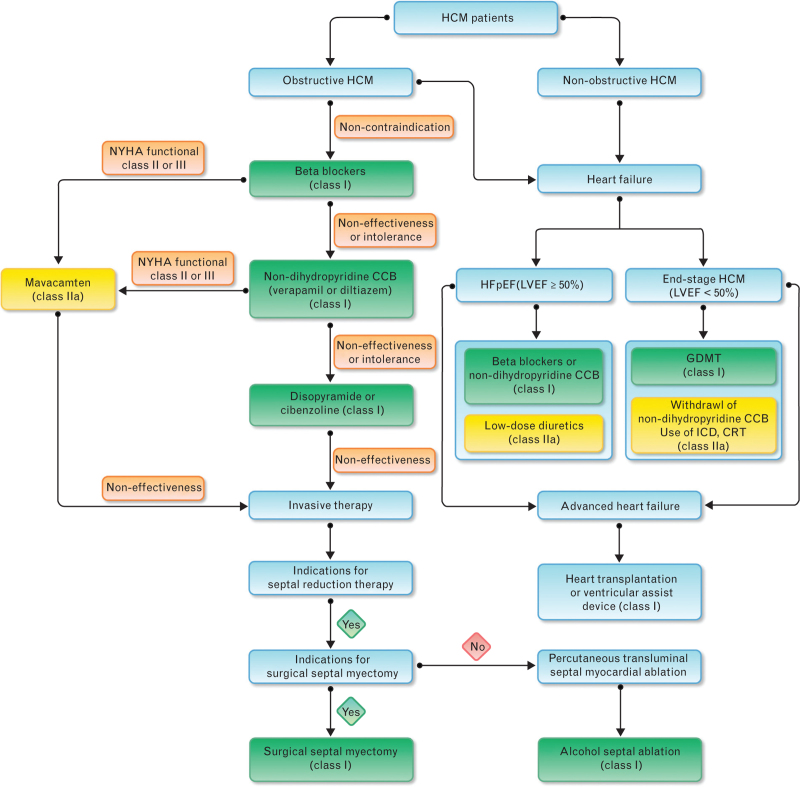
The flow chart for treatment of patients with HCM is shown in Fig. 3.
Fig. 3.
Flow chart for treatment of patients with hypertrophic cardiomyopathy (CCB, calcicum channel blocker; CRT, cardiac resynchronization therapy; GDMT, guideline-directed medical therapy; HCM, hypertrophic cardiomyopathy; HFpEF, heart failure with preserved ejection fraction; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association).
Risk assessment, prevention, and treatment of sudden cardiac death
SCD is the most common cause of death and one of the most serious complications in patients with HCM. Therefore, it is recommended that all HCM patients should be assessed for SCD risk at the time of initial diagnosis, and then every 1–2 years or when the clinical condition changes, and preventive and therapeutic measures should be decided depending on the risk of SCD. Currently, ICD is the most effective measure to prevent and treat SCD.
Risk factors for sudden cardiac death in adult patients with hypertrophic cardiomyopathy
Risk factors for SCD in adult patients with HCM include: Previous documented cardiac arrest or hemodynamic instability due to sustained VT (duration > 30 s); family history of early-onset SCD associated with HCM, including SCD, cardiac arrest, or sustained arrhythmia in at least one first-degree relative aged ≤50 years, definitely or probably caused by HCM; recent (within 6 months) unexplained syncope, which is considered to be possibly caused by arrhythmia (judged by history unlikely to be of vasovagal etiology); extreme left ventricular hypertrophy (maximal left ventricular wall thickness ≥ 30 mm); LVAA; ES–HCM; NSVT on ambulatory ECG; extensive myocardial fibrosis (≥15% left ventricular mass) on CMR.1–4 Of note, studies on LVAA in patients with HCM were retrospective and the absolute number of events was too small to assess the independent predictive value of LVAA in HCM. Thus, individualized ICD decisions should be based using well established risk factors and not solely on the presence of an LVAA.122
Prediction models for sudden cardiac death in adult hypertrophic cardiomyopathy patients
At present, the most widely used prediction model for the 5-year risk of SCD in adult HCM patients is the HCM Risk–SCD model,165 which is recommended by many guidelines or consensus for the risk prediction of SCD in adult HCM patients. This prediction model defines the risk to be high if the 5-year SCD risk is ≥ 6%, moderate if the 5-year SCD risk is ≥ 4% but <6%, and low if the 5-year SCD risk is < 4%. However, the model is only suitable for adult HCM patients aged ≥16 years with no previous history of cardiac arrest or sustained arrhythmia, and this model cannot be used in athletes or in individuals with metabolic/infiltrative diseases; this model did not use exercise-induced LVOT gradients; there is also the possibility of underestimation of risk for HCM patients with maximal ventricular wall thickness of ≥ 35 mm; and the model did not include new SCD risk factors, such as ES–HCM, LVAA, and LGE on CMR. In addition, this model has not been validated after SSM.166
Enhanced ACC/AHA strategy for prevention of SCD in HCM patients. The strategy includes seven major risk factors for SCD in adult HCM patients, including family history of early-onset SCD, recent unexplained syncope, LVH (especially maximal left ventricular wall thickness of ≥ 30 mm), NSVT, LVEF <50%, LVAA, and myocardial fibrosis with diffuse and extensive distribution detected by CMR.167 Of note, LVAA should be considered along with other validated tools for stratifying the risk of arrhythmic death among patients with HCM. According to the above risk factors, study participants are divided into high-risk population and low-risk population.
Risk factors and prediction models for sudden cardiac death in children (< 18 years of age) with hypertrophic cardiomyopathy patients
1. Risk factors: Main risk factors related to SCD in children with HCM included1–4: a family history of early-onset SCD associated with HCM; a personal history of unexplained syncope; extreme left ventricular hypertrophy (maximal wall thickness ≥ 30 mm or z-value > 6); and NSVT on ambulatory ECG monitoring. There are few studies in children with risk factors such as LVAA, ES–HCM, and extensive myocardial fibrosis detected by CMR, and thus, the predictive value of these markers is uncertain.
2. Prediction model: Currently, there are two prediction models for SCD in pediatric patients with HCM: the HCM Risk–kids prediction model proposed by European scholars168 and the PRIMACY prediction model proposed by American scholars.169 However, the predictive value of both prediction models needs more external validation.
Prevention of sudden cardiac death
For HCM patients (including adult and pediatric patients) who have previous documented cardiac arrest or hemodynamic instability due to sustained VT, it is recommended to perform ICD implantation for secondary prevention.
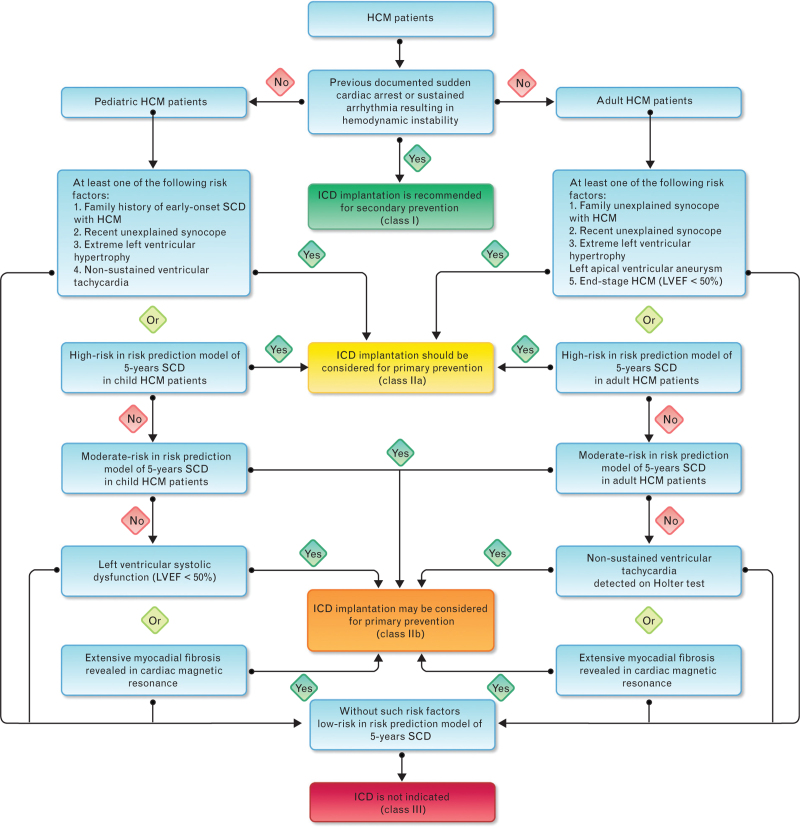
1. For adult HCM patients with at least one risk factor or assessed at high risk (≥ 6%) using the SCD risk prediction model, ICD implantation should be considered for primary prevention. In the absence of such risk factors, ICD implantation for primary prevention may also be considered if NSVT is found on ambulatory ECG, extensive myocardial fibrosis is indicated on CMR, or moderate risk (≥4%, <6%) is indicated by the SCD risk prediction model. For adult HCM patients without the above risk factors, ICD implantation is not recommended (Fig. 4).
Fig. 4.
Flow chart for selection of ICD implantation population in patients with hypertrophic cardiomyopathy (HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; SCD, sudden cardiac death).
2. For pediatric HCM patients with at least one of the risk factors or assessed as being at high risk using the SCD risk prediction model, ICD implantation should be considered for primary prevention. In the absence of these risk factors, ICD implantation for primary prevention may also be considered if the LVEF is < 50%, extensive myocardial fibrosis is indicated on CMR, or moderate risk is indicated using the SCD risk prediction model. For HCM patients without the above risk factors, ICD implantation is not recommended (Fig. 4).
Both for adult and pediatric HCM patients, the choice between these options is recommended by a shared decision-making approach after a comprehensive shared discussion with patients and their relatives on the benefits and risks of each option.170
Management of special population
Hypertrophic cardiomyopathy and pregnancy
1. Prepregnancy and prenatal assessment and counseling: Pregnancy increases plasma volume and cardiac output, decreases systemic vascular resistance, and hypercoagulable state, which may increase the risk of HCM in pregnancy, especially in patients with LVOTO and abnormal left ventricular diastolic function showing poor tolerance to volume load. Therefore, women HCM should undergo comprehensive prepregnancy and prenatal risk assessment and counseling.171 Echocardiography is the first-line choice to assess cardiac function, MR and LVOTO. Cardiac events occur mainly in women with symptoms before pregnancy or previous cardiac events; thus, active measures should be taken to improve symptoms before pregnancy for these women.
2. Management during pregnancy: Pregnancy monitoring and assessment: For female patients with HCM, pregnancy monitoring and assessment focus on symptoms, cardiac function, LVOTO as well as arrhythmias. Drug treatment during pregnancy: For medication during pregnancy, it is required to take into account the needs of pregnant women and possible effects of drugs on the fetus. ① Beta blockers: Use of most beta blockers (including metoprolol, bisoprolol, labetalol, propranolol) are safe during pregnancy, but atenolol with potential fetal risk is not recommended. For the use of beta blockers during pregnancy, close monitoring of fetal growth and fetal heart rate is required. ② Nondihydropyridine CCBs and disopyramide: Avoid use during pregnancy. ③ AADs: Most AADs have potential teratogenic effects and are contraindicated during pregnancy. Amiodarone has the risks of causing fetal thyrotoxicosis, growth retardation and bradycardia, and should be avoided during pregnancy. ④ Anticoagulation therapy: For female HCM patients with AF or other anticoagulation indications, low-molecular-weight heparin can be used for anticoagulation in the first trimester (the first 3 months) and after gestational week 36, or low-dose warfarin (<5 mg/day) can be used for anticoagulation in the second and third trimesters. At present, the safety data on the use of DOACs during pregnancy are limited, so their use is not recommended.
Hypertrophic cardiomyopathy and exercises
Because patients with HCM, especially those with obstructive HCM, are at risk for exercise-induced SCD, HCM patients need to be assessed for potential SCD risk before participating in competitive sports, and a decision should be made after weighing up the pros and cons.172 Patients with clinically diagnosed HCM are not recommended to participate in moderate- or vigorous-intensity competitive sports, stimulating recreational activities, and vigorous-intensity physical activity, especially for those with obstructive HCM.1–4,170 For (G+P−) individuals, participation in physical activity is usually not restricted because of low SCD risk, unless family history suggests high SCD risk. However, the type of exercise needs to be considered and decided jointly with the patient and family, and the safety of participating in physical activity is assessed every 1–2 years.3,172
Natural course and prognosis
HCM is a highly heterogeneous cardiac disease that is likely to develop from infants through to the elderly. At present, the annual mortality rate for adult patients with HCM is 0.5–1.0%, and the main causes of death include SCD, HF and stroke caused by atrial fibrillation.1–4,10,173 Among them, the main cause of death is SCD in younger patients, and HCM-related HF and stroke in older patients. Most patients with HCM require close follow-up.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Yuhui Zhang, Marianna Adamo and Changhong Zou contributed equally to this article.
References
- 1.Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Heart Failure and Cardiomyopathy. Chinese guidelines on the management of hypertrophic cardiomyopathy 2017. Chin J Heart Fail Cardiomyopathy 2017; 1:65–86. [Google Scholar]
- 3.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020; 76:3022–3055. [DOI] [PubMed] [Google Scholar]
- 4.Kitaoka H, Tsutsui H, Kubo T, et al. Japanese Circulation Society Joint Working Group. JCS/JHFS 2018 guideline on the diagnosis and treatment of cardiomyopathies. Circ J 2021; 85:1590–1689. [DOI] [PubMed] [Google Scholar]
- 5.Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J 1958; 20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brent LB, Aburano A, Fisher DL, et al. Familial muscular subaortic stenosis: an unrecognized form of ‘idiopathic heart diseases,’ with clinical and autopsy observation. Circulation 1960; 21:167–180. [DOI] [PubMed] [Google Scholar]
- 7.Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy. Ann Surg 1961; 154:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunwald E, Aygen MM. Idiopathic myocardial hypertrophy without congestive heart failure or obstruction to blood flow: clinical, hemodynamic and angiocardiographic studies in fourteen patients. Am J Med 1963; 35:7–19. [DOI] [PubMed] [Google Scholar]
- 9.Liew AC, Vassiliou VS, Cooper R, et al. Hypertrophic cardiomyopathy: past, present and future. J Clin Med 2017; 6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017; 121:749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2022; 79:372–389. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Desai MY, Nishimura RA, et al. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2022; 79:390–414. [DOI] [PubMed] [Google Scholar]
- 13.Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study: coronary artery risk development in (young) adults. Circulation 1995; 92:785–789. [DOI] [PubMed] [Google Scholar]
- 14.Maron BJ, Mathenge R, Casey SA, et al. Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J Am Coll Cardiol 1999; 33:1590–1595. [DOI] [PubMed] [Google Scholar]
- 15.Maron BJ, Spirito P, Roman MJ, et al. Prevalence of hypertrophic cardiomyopathy in a population-based sample of American Indians aged 51-77 years (the strong heart study). Am J Cardiol 2004; 93:1510–1514. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y, Song L, Wang Z, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8 080 adults. Am J Med 2004; 116:14–18. [DOI] [PubMed] [Google Scholar]
- 17.Bick AG, Flannick J, Ito K, et al. Burden of rare sarcomere gene variants in the Framingham and Jackson Heart Study cohorts. Am J Hum Genet 2012; 91:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maron BJ, Semsarian C. Emergence of gene mutation carriers and the expanding disease spectrum of hypertrophic cardiomyopathy. Eur Heart J 2010; 31:1551–1553. [DOI] [PubMed] [Google Scholar]
- 19.Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009; 54:220–228. [DOI] [PubMed] [Google Scholar]
- 20.Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2012; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015; 65:1249–1254. [DOI] [PubMed] [Google Scholar]
- 22.Maron BJ, Rowin EJ, Maron MS. Global burden of hypertrophic cardiomyopathy. JACC Heart Fail 2018; 6:376–378. [DOI] [PubMed] [Google Scholar]
- 23.Geisterfer-Lowrance AA, Kass S, Tanigawa G, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 1990; 62:999–1006. [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, Maron MS, Semsarian C. Double or compound sarcomere mutations in hypertrophic cardiomyopathy: a potential link to sudden death in the absence of conventional risk factors. Heart Rhythm 2012; 9:57–63. [DOI] [PubMed] [Google Scholar]
- 25.Ingles J, Burns C, Bagnall RD, et al. Nonfamilial hypertrophic cardiomyopathy: prevalence, natural history, and clinical implications. Circ Cardiovasc Genet 2017; 10:e001620. [DOI] [PubMed] [Google Scholar]
- 26.Geske JB, Sorajja P, Ommen SR, et al. Variability of left ventricular outflow tract gradient during cardiac catheterization in patients with hypertrophic cardiomyopathy. JACC Cardiovasc Interv 2011; 4:704–709. [DOI] [PubMed] [Google Scholar]
- 27.Ayoub C, Geske JB, Larsen CM, et al. Comparison of valsalva maneuver, amyl nitrite, and exercise echocardiography to demonstrate latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am J Cardiol 2017; 120:2265–2271. [DOI] [PubMed] [Google Scholar]
- 28.Reant P, Dufour M, Peyrou J, et al. Upright treadmill vs. semi-supine bicycle exercise echocardiography to provoke obstruction in symptomatic hypertrophic cardiomyopathy: a pilot study. Eur Heart J Cardiovasc Imaging 2018; 19:31–38. [DOI] [PubMed] [Google Scholar]
- 29.Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003; 348:295–303. [DOI] [PubMed] [Google Scholar]
- 30.Elliott PM, Gimeno JR, Tomé MT, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J 2006; 27:1933–1941. [DOI] [PubMed] [Google Scholar]
- 31.Avegliano G, Politi MT, Costabel JP, et al. Differences in the extent of fibrosis in obstructive and nonobstructive hypertrophic cardiomyopathy. J Cardiovasc Med (Hagerstown) 2019; 20:389–396. [DOI] [PubMed] [Google Scholar]
- 32.Sherrid MV, Balaram S, Kim B, et al. The mitral valve in obstructive hypertrophic cardiomyopathy: a test in context. J Am Coll Cardiol 2016; 67:1846–1858. [DOI] [PubMed] [Google Scholar]
- 33.Raphael CE, Cooper R, Parker KH, et al. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: insights from wave intensity analysis and magnetic resonance. J Am Coll Cardiol 2016; 68:1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciampi Q, Olivotto I, Peteiro J, et al. Prognostic value of reduced heart rate reserve during exercise in hypertrophic cardiomyopathy. J Clin Med 2021; 10:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivotto I, Maron BJ, Montereggi A, et al. Prognostic valueof systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999; 33:2044–2051. [DOI] [PubMed] [Google Scholar]
- 36.Ranthe MF, Carstensen L, Øyen N, et al. Risk of cardiomyopathy in younger persons with a family history of death from cardiomyopathy: a nationwide family study in a cohort of 3.9 million persons. Circulation 2015; 132:1013–1019. [DOI] [PubMed] [Google Scholar]
- 37.Williams L, Frenneaux M. Syncope in hypertrophic cardiomyopathy: mechanisms and consequences for treatment. Europace 2007; 9:817–822. [DOI] [PubMed] [Google Scholar]
- 38.Spirito P, Autore C, Rapezzi C, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation 2009; 119:1703–1710. [DOI] [PubMed] [Google Scholar]
- 39.Nagueh SF, Phelan D, Abraham T, et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: an update from the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2022; 35:533–569. [DOI] [PubMed] [Google Scholar]
- 40.Finocchiaro G, Sheikh N, Biagini E, et al. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm 2020; 17:142–151. [DOI] [PubMed] [Google Scholar]
- 41.Monzo L, Martino A, Lanzillo C, et al. Electrocardiographic voltage criteria in patients with hypertrophic cardiomyopathy. J Cardiovasc Med (Hagerstown) 2020; 21:696–703. [DOI] [PubMed] [Google Scholar]
- 42.Tiron C, Ramos R, Iglesies J, et al. The Peguero-Lo Presti ECG criteria improve diagnostic accuracy of left ventricular hypertrophy in hypertrophic cardiomyopathy patients. J Cardiovasc Med (Hagerstown) 2021; 22:946–947. [DOI] [PubMed] [Google Scholar]
- 43.McLeod CJ, Ackerman MJ, Nishimura RA, et al. Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram. J Am Coll Cardiol 2009; 54:229–233. [DOI] [PubMed] [Google Scholar]
- 44.Adabag AS, Casey SA, Kuskowski MA, et al. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 45:697–704. [DOI] [PubMed] [Google Scholar]
- 45.Monserrat L, Elliott PM, Gimeno JR, et al. Nonsustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003; 42:873–879. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Lian Z, Rowin EJ, et al. Prognostic implications of nonsustained ventricular tachycardia in high-risk patients with hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol 2017; 10:e004604. [DOI] [PubMed] [Google Scholar]
- 47.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28:1–39. [DOI] [PubMed] [Google Scholar]
- 48.Echocardiography Group of Chinese Society of Echocardiography of Chinese Medical Association. Guideline on the echocardiographic measurements in Chinese adults. Chinese J Ultrasonogr 2016; 25:645–666. [Google Scholar]
- 49.Haland TF, Edvardsen T. The role of echocardiography in management of hypertrophic cardiomyopathy. J Echocardiogr 2020; 18:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geske JB, McKie PM, Ommen SR, et al. B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol 2013; 61:2456–2460. [DOI] [PubMed] [Google Scholar]
- 51.Coats CJ, Gallagher MJ, Foley M, et al. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J 2013; 34:2529–2537. [DOI] [PubMed] [Google Scholar]
- 52.Zhang C, Liu R, Yuan J, et al. Significance and determinants of cardiac troponin I in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 2015; 116:1744–1751. [DOI] [PubMed] [Google Scholar]
- 53.Gommans DHF, Cramer GE, Fouraux MA, et al. Usefulness of high-sensitivity cardiac troponin T to predict long-term outcome in patients with hypertrophic cardiomyopathy. Am J Cardiol 2021; 152:120–124. [DOI] [PubMed] [Google Scholar]
- 54.Chinese Society of Cardiology of Chinese Medical Association, Chinese College of Cardiovascular Physician of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Cardiology. Chinese expert consensus on clinical application of magnetic resonance imaging in cardiomyopathy. Chin J Cardiol 2015; 43:673–681. [Google Scholar]
- 55.Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014; 130:484–495. [DOI] [PubMed] [Google Scholar]
- 56.Mentias A, Raeisi-Giglou P, Smedira NG, et al. Late gadolinum enhancement in patients with hypertrophic cardiomypathy and preserved systolic function. J Am Coll Cardiol 2018; 72:857–870. [DOI] [PubMed] [Google Scholar]
- 57.Wilde AAM, Semsarian C, Márquez MF, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the State of Genetic Testing for Cardiac Diseases. Heart Rhythm 2022; 19:e1–e60. [DOI] [PubMed] [Google Scholar]
- 58.Ahluwalia M, Ho CY. Cardiovascular genetics: the role of genetic testing in diagnosis and management of patients with hypertrophic cardiomyopathy. Heart 2021; 107:183–189. [DOI] [PubMed] [Google Scholar]
- 59.Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018; 138:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathew J, Zahavich L, Lafreniere-Roula M, et al. Utility of genetics for risk stratification in pediatric hypertrophic cardiomyopathy. Clin Genet 2018; 93:310–319. [DOI] [PubMed] [Google Scholar]
- 61.Mogensen J, van Tintelen JP, Fokstuen S, et al. The current role of next-generation DNA sequencing in routine care of patients with hereditary cardiovascular conditions: a viewpoint paper of the European Society of Cardiology working group on myocardial and pericardial diseases and members of the European Society of Human Genetics. Eur Heart J 2015; 36:1367–1370. [DOI] [PubMed] [Google Scholar]
- 62.Spracklen TF, Keavney B, Laing N, et al. Modern genomic techniques in the identification of genetic causes of cardiomyopathy. Heart 2022; 108:1843–1850. [DOI] [PubMed] [Google Scholar]
- 63.Richards S, Aziz N, Bale S, et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deignan JL, Chung WK, Kearney HM, et al. ACMG Laboratory Quality Assurance Committee. Points toconsider in the reevaluation and reanalysis of genomic test results: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2019; 21:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bayonas-Ruiz A, Muñoz-Franco FM, Ferrer V, et al. Cardiopulmonary exercise test in patients with hypertrophic cardiomyopathy: a systematic review and meta-analysis. J Clin Med 2021; 10:2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seferović PM, Tsutsui H, McNamara DM, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position Statement on endomyocardial biopsy. Eur J Heart Fail 2021; 23:854–871. [DOI] [PubMed] [Google Scholar]
- 67.Guo X, Fan C, Wang H, et al. The prevalence and long-term outcomes of extreme right versus extreme left ventricular hypertrophic cardiomyopathy. Cardiology 2016; 133:35–43. [DOI] [PubMed] [Google Scholar]
- 68.Bombard Y, Brothers KB, Fitzgerald-Butt S, et al. The responsibility to recontact research participants after reinterpretation of genetic and genomic research results. Am J Hum Genet 2019; 104:578–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Velzen HG, Schinkel AFL, Baart SJ, et al. Outcomes of contemporary family screening in hypertrophic cardiomyopathy. Circ Genom Precis Med 2018; 11:e001896. [DOI] [PubMed] [Google Scholar]
- 70.Lorenzini M, Norrish G, Field E, et al. Penetrance of hypertrophic cardiomyopathy in sarcomere protein mutation carriers. J Am Coll Cardiol 2020; 76:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maron BJ, Yeates L, Semsarian C. Clinical challenges of genotype positive (+)-phenotype negative (-) family members in hypertrophic cardiomyopathy. Am J Cardiol 2011; 107:604–608. [DOI] [PubMed] [Google Scholar]
- 72.Williams LK, Misurka J, Ho CY, et al. Multilayer myocardial mechanics in genotype-positive left ventricular hypertrophy-negative patients with hypertrophic cardiomyopathy. Am J Cardiol 2018; 122:1754–1760. [DOI] [PubMed] [Google Scholar]
- 73.Maurizi N, Michels M, Rowin EJ, et al. Clinical course and significance of hypertrophic cardiomyopathy without left ventricular hypertrophy. Circulation 2019; 139:830–833. [DOI] [PubMed] [Google Scholar]
- 74.Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006; 114:2232–2239. [DOI] [PubMed] [Google Scholar]
- 75.Hughes RK, Knott KD, Malcolmson J, et al. Apical hypertrophic cardiomyopathy: the variant less known. J Am Heart Assoc 2020; 9:e015294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Efthimiadis GK, Pagourelias ED, Parcharidou D, et al. Clinical characteristics and natural history of hypertrophic cardiomyopathy with midventricular obstruction. Circ J 2013; 77:2366–2374. [DOI] [PubMed] [Google Scholar]
- 77.Olivotto I, Cecchi F, Poggesi C, et al. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail 2012; 5:535–546. [DOI] [PubMed] [Google Scholar]
- 78.Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006; 114:216–225. [DOI] [PubMed] [Google Scholar]
- 79.Biagini E, Coccolo F, Ferlito M, et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol 2005; 46:1543–1550. [DOI] [PubMed] [Google Scholar]
- 80.Thaman R, Gimeno JR, Murphy RT, et al. Prevalence and clinical significance of systolic impairment in hypertrophic cardiomyopathy. Heart 2005; 91:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawarai H, Kajimoto K, Minami Y, et al. Risk of sudden death in end-stage hypertrophic cardiomyopathy. J Card Fail 2011; 17:459–464. [DOI] [PubMed] [Google Scholar]
- 82.Xiao Y, Yang KQ, Yang YK, et al. Clinical characteristics and prognosis of end-stage hypertrophic cardiomyopathy. Chin Med J (Engl) 2015; 128:1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowin EJ, Maron BJ, Carrick RT, et al. Outcomes in patients with hypertrophic cardiomyopathy and left ventricular systolic dysfunction. J Am Coll Cardiol 2020; 75:3033–3043. [DOI] [PubMed] [Google Scholar]
- 84.Kubo T, Gimeno JR, Bahl A, et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol 2007; 49:2419–2426. [DOI] [PubMed] [Google Scholar]
- 85.Biagini E, Spirito P, Rocchi G, et al. Prognostic implication of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol 2009; 104:1727–1731. [DOI] [PubMed] [Google Scholar]
- 86.De Bortoli M, Vio R, Basso C, et al. Novel missense variant in MYL2 gene associated with hypertrophic cardiomyopathy showing high incidence of restrictive physiology. Circ Genom Precis Med 2020; 13:e002824. [DOI] [PubMed] [Google Scholar]
- 87.Siontis KC, Geske JB, Ong K, et al. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014; 3:e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guttmann OP, Rahman MS, O′Mahony C, et al. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart 2014; 100:465–472. [DOI] [PubMed] [Google Scholar]
- 89.Rowin EJ, Hausvater A, Link MS, et al. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation 2017; 136:2420–2436. doi:10.1161/CIRCULATIONAHA.117.029267. [DOI] [PubMed] [Google Scholar]
- 90.Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definitioand classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021; 23:352–380. [DOI] [PubMed] [Google Scholar]
- 91.Rowin EJ, Maron BJ, Haas TS, et al. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 2017; 69:761–773. [DOI] [PubMed] [Google Scholar]
- 92.Yang K, Song YY, Chen XY, et al. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur Heart J Cardiovasc Imaging 2020; 21:1341–1350. [DOI] [PubMed] [Google Scholar]
- 93.Cui L, Tse G, Zhao Z, et al. Mid-ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm: an important subtype of arrhythmogenic cardiomyopathy. Ann Noninvasive Electrocardiol 2019; 24:e12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee DZJ, Montazeri M, Bataiosu R, et al. Clinical characteristics and prognostic importance of left ventricular apical aneurysms in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2022; 15:1696–1711. [DOI] [PubMed] [Google Scholar]
- 95.Hamada M. Left ventricular thrombus in hypertrophic cardiomyopathy. Intern Med 2019; 58:465–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lodi E, Lucio D, Federica F, et al. Hypertrophic-phenotype cardiopathy: the great simulator. J Cardiovasc Med (Hagerstown) 2022; 23:748–751. [DOI] [PubMed] [Google Scholar]
- 97.Autore C, Bariani R, Bauce B, et al. From the phenotype to precision medicine: an update on the cardiomyopathies diagnostic workflow. J Cardiovasc Med (Hagerstown) 2023; 24: (Suppl 2): e178–e186. [DOI] [PubMed] [Google Scholar]
- 98.Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res 2023; 118:3517–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merlo M, Pagura L, Porcari A, et al. Unmasking the prevalence of amyloid cardiomyopathy in the real world: results from Phase 2 of the AC-TIVE study, an Italian nationwide survey. Eur J Heart Fail 2022; 24:1377–1386. [DOI] [PubMed] [Google Scholar]
- 100.Fontana M, Ćorović A, Scully P, et al. Myocardial amyloidosis: the exemplar interstitial disease. JACC Cardiovasc Imaging 2019; 12 (11 Pt 2):2345–2356. [DOI] [PubMed] [Google Scholar]
- 101.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 102.Joury A, Gupta T, Krim SR. Cardiac amyloidosis: presentations, diagnostic work-up and collaborative approach for comprehensive clinical management. Curr Prol Cardiol 2021; 46:100910. [DOI] [PubMed] [Google Scholar]
- 103.Expert consensus for diagnosis and treatment of Fabry disease in China (2021). Chin J Intern Med 2021; 60:321–330. [DOI] [PubMed] [Google Scholar]
- 104.Lotan D, Salazar-Mendiguchía J, Mogensen J, et al. Clinical profile of cardiac involvement in Danon disease: a multicenter European Registry. Circ Genom Precis Med 2020; 13:e003117. [DOI] [PubMed] [Google Scholar]
- 105.Porto AG, Brun F, Severini GM, et al. Clinical spectrum of PRKAG2 syndrome. Circ Arrhythm Electrophysiol 2016; 9:e003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cook A, Giunti P. Friedreich's ataxia: clinical features, pathogenesis and management. Br Med Bull 2017; 124:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roberts AE, Allanson JE, Tartaglia M, et al. Noonan syndrome. Lancet 2013; 381:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martínez-Quintana E, Rodríguez-González F. LEOPARD syndrome: clinical features and gene mutations. Mol Syndromol 2012; 3:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aoki Y, Niihori T, Inoue S, et al. Recent advances in RASopathies. J Hum Genet 2016; 61:33–39. [DOI] [PubMed] [Google Scholar]
- 110.Ng YS, Bindoff LA, Gorman GS, et al. Mitochondrial disease in adults: recent advances and future promise. Lancet Neurol 2021; 20:573–584. [DOI] [PubMed] [Google Scholar]
- 111.Dybro AM, Rasmussen TB, Nielsen RR, et al. Randomized trial of metoprolol in patients with obstructive hypertropic cardiomyopathy. J Am Coll Cardiol 2021; 78:2505–2517. [DOI] [PubMed] [Google Scholar]
- 112.Dybro AM, Rasmussen TB, Nielsen RR, et al. Effects of metoprolol on exercise hemodynamics in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2022; 79:1565–1575. [DOI] [PubMed] [Google Scholar]
- 113.Monda E, Lioncino M, Palmiero G, et al. Bisoprolol for treatment of symptomatic patients with obstructive hypertrophic cardiomyopathy: the BASIC (bisoprolol AS therapy in hypertrophic cardiomyopathy) study. Int J Cardiol 2022; 354:22–28. [DOI] [PubMed] [Google Scholar]
- 114.Zampieri M, Argirò A, Marchi A, et al. Mavacamten, a novel therapeutic strategy for obstructive hypertrophic cardiomyopathy. Curr Cardiol Rep 2021; 23:79. [DOI] [PubMed] [Google Scholar]
- 115.Olivotto I, Oreziak A, Barriales-Villa R, et al. EXPLORER-HCM study investigators. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020; 396:759–769. [DOI] [PubMed] [Google Scholar]
- 116.Spertus JA, Fine JT, Elliott P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021; 397:2467–2475. [DOI] [PubMed] [Google Scholar]
- 117.Hegde SM, Lester SJ, Solomon SD, et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertensive cardiomyopathy. J Am Coll Cardiol 2021; 78:2518–2532. [DOI] [PubMed] [Google Scholar]
- 118.Desai MY, Owens A, Geske JB, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol 2022; 80:95–108. [DOI] [PubMed] [Google Scholar]
- 119.Desai MY, Owens AT, Geske JB, et al. Dose-blinded myosin inhibition in patients with obstructive HCM referred for septal reduction therapy: outcomes through 32-weeks. Circulation 2023; 147:850–863. [DOI] [PubMed] [Google Scholar]
- 120.Cremer PC, Geske JB, Owens A, et al. Myosin inhibition and left ventricular diastolic function in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: insights from the VALOR-HCM Study. Circ Cardiovasc Imaging 2022; 15:e014986. [DOI] [PubMed] [Google Scholar]
- 121.Ho CY, Mealiffe ME, Bach RG, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2020; 75:2649–2660. [DOI] [PubMed] [Google Scholar]
- 122.Arbelo E, Protonotarios A, Gimeno JR, et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J 2023; ehad194.online ahead of print. [Google Scholar]
- 123.Chuang C, Collibee S, Ashcraft L, et al. Discovery of Aficamten (CK-274), a next generation cardiac myosin inhibitor for the treatment of hypertrophic cardiomyopathy. J Med Chem 2021; 64:14142–14152. [DOI] [PubMed] [Google Scholar]
- 124.Masri A, Olivotto I. Cardiac myosin inhibitors as a novel treatment option for obstructive hypertrophic cardiomyopathy: addressing the core of the matter. J Am Heart Assoc 2022; 11:e024656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maron MS, Masri A, Choudhury L, et al. REDWOOD-HCM Steering Committee and Investigators. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2023; 81:34–45. [DOI] [PubMed] [Google Scholar]
- 126.Sherrid MV, Shetty A, Winson G, et al. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with (-blockade or verapamil. Circ Heart Fail 2013; 6:694–702. [DOI] [PubMed] [Google Scholar]
- 127.Adler A, Fourey D, Weissler-Snir A, et al. Safety of outpatient initiation of disopyramide for obstructive hypertrophic cardiomyopathy patients. J Am Heart Assoc 2017; 6:e005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coppini R, Ferrantini C, Pioner JM, et al. Electrophysiological and contractile effects of disopyramide in patients with obstructive hypertrophic cardiomyopathy: a translational study. JACC Basic Transl Sci 2019; 4:795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hamada M, Ikeda S, Ohshima K, et al. Impact of chronic use of cibenzoline on left ventricular pressure gradient and left ventricular remodeling in patients with hypertrophic obstructive cardiomyopathy. J Cardiol 2016; 67:279–286. [DOI] [PubMed] [Google Scholar]
- 130.Maron BJ, Dearani JA, Smedira NG, et al. Ventricular septal myectomy for obstructive hypertrophic cardiomyopathy (analysis spanning 60 years of practice): AJC Expert Panel. Am J Cardiol 2022; 180:124–139. [DOI] [PubMed] [Google Scholar]
- 131.Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 46:470–476. [DOI] [PubMed] [Google Scholar]
- 132.Wang S, Luo M, Sun H, et al. A retrospective clinical study of transaortic extended septal myectomy for obstructive hypertrophic cardiomyopathy in China. Eur J Cardiothorac Surg 2013; 43:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maron BJ, Dearani JA, Ommen SR, et al. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol 2015; 66:1307–1308. [DOI] [PubMed] [Google Scholar]
- 134.Wang S, Cui H, Yu Q, et al. Excision of anomalous muscle bundles as an important addition to extended septal myectomy for treatment of left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg 2016; 152:461–468. [DOI] [PubMed] [Google Scholar]
- 135.Maron MS, Rastegar H, Dolan N, et al. Outcomes over follow-up ≥ 10 years after surgical myectomy for symptomatic obstructive hypertrophic cardiomyopathy. Am J Cardiol 2022; 163:91–97. [DOI] [PubMed] [Google Scholar]
- 136.Holst KA, Hanson KT, Ommen SR, et al. Septal myectomy in hypertrophic cardiomyopathy: national outcomes of concomitant mitral surgery. Mayo Clin Proc 2019; 94:66–73. [DOI] [PubMed] [Google Scholar]
- 137.Yang Q, Zhu C, Cui H, et al. Surgical septal myectomy outcome for obstructive hypertrophic cardiomyopathy after alcohol septal ablation. J Thorac Dis 2021; 13:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rudenko KV, Lazoryshynets VV, Nevmerzhytska LO, et al. Septal myectomy with mitral valve surgery in patients after alcohol septal ablation. Interact Cardiovasc Thorac Surg 2022; 34:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lio A, D’Ovidio M, Chirichilli I, et al. Extended septal myectomy for obstructive hypertrophic cardiomyopathy and its impact on mitral valve function. J Cardiovasc Med (Hagerstown) 2024; 25:210–217. [DOI] [PubMed] [Google Scholar]
- 140.Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal Ablation. Circ Res 2017; 121:771–783. [DOI] [PubMed] [Google Scholar]
- 141.Nguyen A, Schaff HV. Surgical myectomy: subaortic, midventricular, and apical. Cardiol Clin 2019; 37:95–104. [DOI] [PubMed] [Google Scholar]
- 142.Schaff HV, Brown ML, Dearani JA, et al. Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2010; 139:634–640. [DOI] [PubMed] [Google Scholar]
- 143.Hang D, Schaff HV, Ommen SR, et al. Combined transaortic and transapical approach to septal myectomy in patients with complex hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2018; 155:2096–2102. [DOI] [PubMed] [Google Scholar]
- 144.China Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology, Group of Septal Ablation Treatment of Hypertrophic Obstructive Cardiomyopathy. Chinese expert consensus for septal myocardial ablation of hypertrophic obstructive cardiomyopathy. Chin J Cardiol 2011; 39:886–891. [PubMed] [Google Scholar]
- 145.Veselka J, Faber L, Liebregts M, et al. Long-term outcome of repeated septal reduction therapy after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: insight from the Euro-ASA registry. Arch Med Sci 2020; 16:1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J 2016; 37:1517–1523. [DOI] [PubMed] [Google Scholar]
- 147.Batzner A, Pfeiffer B, Neugebauer A, et al. Survival after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2018; 72:3087–3094. [DOI] [PubMed] [Google Scholar]
- 148.Nguyen A, Schaff HV, Hang D, et al. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a propensity score-matched cohort. J Thorac Cardiovasc Surg 2019; 157:306–315. [DOI] [PubMed] [Google Scholar]
- 149.Nguyen A, Schaff HV, Hang D, et al. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a propensity score-matched cohort. J Thorac Cardiovasc Surg 2019; 157:306–315. e3. [DOI] [PubMed] [Google Scholar]
- 150.Faber L, Welge D, Fassbender D, et al. Percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: managing the risk of procedure-related AV conduction disturbances. Int J Cardiol 2007; 119:163–167. [DOI] [PubMed] [Google Scholar]
- 151.Veselka J, Jensen M, Liebregts M, et al. Alcohol septal ablation in patients with severe septal hypertrophy. Heart 2020; 106:462–466. [DOI] [PubMed] [Google Scholar]
- 152.Cui H, Schaff HV, Wang S, et al. Survival following alcohol septal ablation or septal myectomy for patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2022; 79:1647–1655. [DOI] [PubMed] [Google Scholar]
- 153.Liu L, Li J, Zuo L, Zhang J, et al. Percutaneous intramyocardial septal radiofrequency ablation for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2018; 72:1898–1909. [DOI] [PubMed] [Google Scholar]
- 154.Zhou M, Ta S, Hahn RT, et al. Percutaneous intramyocardial septal radiofrequency ablation in patients with drug-refractory hypertrophic obstructive cardiomyopathy. JAMA Cardiol 2022; 7:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lawrenz T, Kuhn H. Endocardial radiofrequency ablation of septal hypertrophy: a new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy. Z Kardiol 2004; 93:493–499. [DOI] [PubMed] [Google Scholar]
- 156.Lawrenz T, Lawin D, Radke K, et al. Acute and chronic effects of endocardial radiofrequency ablation of septal hypertrophy in HOCM. J Cardiovasc Electrophysiol 2021; 32:2617–2624. [DOI] [PubMed] [Google Scholar]
- 157.McDonagh TA, Metra M, Adamo M, et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 158.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145:e876–e894. [DOI] [PubMed] [Google Scholar]
- 159.Mehra MR, Canter CE, Hannan MM, et al. International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016; 35:1–23. [DOI] [PubMed] [Google Scholar]
- 160.Guttmann OP, Pavlou M, O’Mahony C, et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur J Heart Fail 2015; 17:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jung H, Yang PS, Jang E, et al. Effectiveness and safety of nonvitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest 2019; 155:354–363. [DOI] [PubMed] [Google Scholar]
- 162.Providencia R, Elliott P, Patel K, et al. Catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Heart 2016; 102:1533–1543. [DOI] [PubMed] [Google Scholar]
- 163.Bogachev-Prokophiev AV, Afanasyev AV, Zheleznev SI, et al. Concomitant ablation for atrial fibrillation during septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Thorac Cardiovasc Surg 2018; 155:1536–1542. e2. [DOI] [PubMed] [Google Scholar]
- 164.Santangeli P, Muser D, Maeda S, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm 2016; 13:1552–1559. [DOI] [PubMed] [Google Scholar]
- 165.O’Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014; 35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 166.Buongiorno AL, Blandino A, Bianchi F, et al. Effectiveness of 2014 ESC HCM-Risk-SCD score in prediction of appropriate implantable-cardioverter-defibrillator shocks. J Cardiovasc Med (Hagerstown) 2023; 24:313–314. [DOI] [PubMed] [Google Scholar]
- 167.Maron MS, Rowin EJ, Wessler BS, et al. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol 2019; 4:644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Norrish G, Ding T, Field E, et al. Development a novel risk prediction model for sudden cardiac death in childhood hypertrophic cardiomyopathy (HCM Risk-Kids). JAMA Cardiol 2019; 4:918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miron A, Lafreniere-Roula M, Steve Fan CP, et al. A validated model for sudden cardiac death risk prediction in pediatric hypertrophic cardiomyopathy. Circulation 2020; 142:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maron BJ, Nishimura RA, Maron MS. Shared decision-making in HCM. Nat Rev Cardiol 2017; 14:125–126. [DOI] [PubMed] [Google Scholar]
- 171.Saberi S. Hypertrophic cardiomyopathy in pregnancy. Cardiol Clin 2021; 39:143–150. [DOI] [PubMed] [Google Scholar]
- 172.Pelliccia A, Sharma S, Gati S, Bäck M, et al. ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021; 42:17–96. [DOI] [PubMed] [Google Scholar]
- 173.Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018; 379:655–668. [DOI] [PubMed] [Google Scholar]