Abstract
The objective of this study was to establish a nausea-free ward model and evaluate the effect of an intervention procedure guided by this model on chemotherapy-induced nausea and vomiting (CINV) in cancer patients. A total of 105 chemotherapy patients from March to September 2022 before the establishment of nausea-free ward in the Chongqing Jiulongpo District People’s Hospital were selected as the control group as well as 105 chemotherapy patients from March to September 2023 after the establishment of nausea-free ward as the intervention group. The intervention group was managed by comprehensive standardized CINV management on the basis of the control group. Finally, the Chinese Society of Clinical Oncology grading tool for nausea and vomiting and the Functional Living Index-Emesis were used to evaluate the effect. Under the intervention of the nausea-free ward model, the intervention group exhibited significantly lower ratings of nausea and vomiting compared to the control group (all P-value <.05). The nausea score, vomiting score, and total score of the intervention group were significantly lower than the control group (all P-value <.05). Our study found CINV symptoms and quality of life can be significantly improved by the application of the nausea-free ward model. The nausea-free ward model is instructive in clinical practice and can guide clinical work as well as bring management experience to clinical workers.
Keywords: chemotherapy-induced nausea and vomiting, Functional Living Index-Emesis, multidisciplinary collaboration, nausea-free ward, retrospective
1. Introduction
Cancer was the leading cause of death in China, and because of its large population base, it also served as a major public health problem.[1] According to China’s cancer statistics, lung, liver, stomach, and esophagus cancers were the 4 main types of cancer in China.[1] For patients with advanced cancer, chemotherapy is one of the most important means to improve prognosis and clinical outcomes. With the continuous progress of medicine, targeted therapy, immunotherapy, etc were increasingly updated and popularized, but chemotherapy still had an unshakable position as a classical means of tumor treatment. However, the administration of chemotherapeutic agents often leads to chemotherapy-induced nausea and vomiting (CINV), which is the most prevalent adverse reaction associated with chemotherapy.[2] A previous cross-sectional study showed that nausea and vomiting were experienced by nearly half (48%) of the majority of patients (88%) who received chemotherapy.[3] CINV affects the food intake of the patients, which in turn leads to malnutrition during the treatment period.[4] Persistent dietary intake problems and malnutrition adversely affect treatment by reducing patient immunity.[5] In addition, CINV significantly decreasing the patient’s tolerance and compliance with chemotherapy, leading to a reduction or interruption of chemotherapy, and severely affecting the efficacy of antineoplastic drugs.[6] The use of antiemetic drugs as the most commonly used intervention, but there were still a majority of patients who could not get effective relief of nausea and vomiting symptoms.[7,8] Therefore, more effective interventions are needed to improve CINV in cancer patients.
There are many interventions for CINV, which can be generally categorized into pharmacologic and nonpharmacologic interventions. Many recent studies have shown that laughter yoga,[9] ginger,[10,11] acupuncture,[12] music therapy,[13] dietary interventions,[14] etc combined or noncombined antiemetics can alleviate CINV to varying degrees. It can be seen that CINV can be intervened by exercise, Chinese medicine, music, and diet in combination with antiemetic drugs. However, due to the lack of a systematic and comprehensive standardized intervention process and system, multidisciplinary cooperation is difficult to maintain well. Since the Chinese Society of Clinical Oncology (CSCO) initiated the CINV standardized management project, our research group has been exploring and establishing a nausea-free ward working model with the characteristics of whole process optimization and comprehensive intervention. Through multidisciplinary collaboration between healthcare professionals and communication between doctors and patients, individualized antiemetic programs for patients are formulated and comprehensive management is carried out to reduce the incidence of vomiting, improve the quality of life of patients, and allow patients to carry out the process of tumor chemotherapy with peace of mind and comfort.
2. Materials and methods
2.1. Study population
Patients who underwent chemotherapy for malignant tumors at Chongqing Jiulongpo District People’s Hospital from March 2022 to September 2023 were selected. The Ethics Committee of Chongqing Jiulongpo District People’s Hospital approved this study and the ethical number was 202402.
The detailed inclusion and exclusion criteria of this study population are as follows. The inclusion criteria were: patients who were clearly diagnosed with malignant tumors by histology or pathology; patients who underwent intermediate/highly emetogenic chemotherapy regimens. The exclusion criteria were: patients who age <18 years; patients who failed to complete the current chemotherapy due to death from disease progression; patients who interrupted the treatment due to inability to tolerate the chemotherapy; patients who had used antiemetic drugs in the 24 hours prior to chemotherapy or who had already experienced vomiting prior to chemotherapy; patients with neurological tumors or brain metastases, with intracranial hypertension that causes vomiting or affects the narrative of the condition and the those who were observed for adverse reactions. Overall, the study included patients who satisfied both the inclusion criteria and did not exhibit any of the exclusion criteria. A total of 210 participants were included in this study, 105 participants from March 2022 to September 2022 were selected as the control group and 105 participants from March 2023 to September 2023 were selected as the intervention group.
2.2. Study design and improvement measure
2.2.1. Chemotherapy related care before improvement
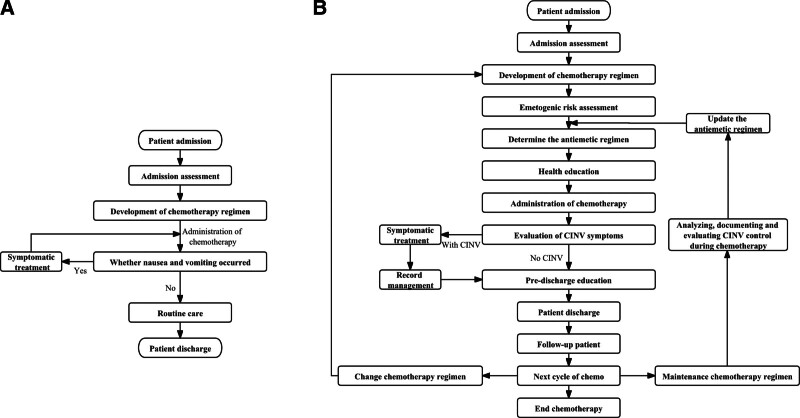
Before the establishment of the nausea-free ward model, the department adopted routine diagnosis and treatment procedures for chemotherapy patients. After admission, doctors and nurses collected general characteristics such as age, sex, place of residence (e.g., urban, rural), education (e.g., junior high school and below, high school or secondary school, college and above), marital status (e.g., unmarried, married, divorced, or widowed), smoking history (e.g., yes, no), drinking history (e.g., yes, no), and KarnofskyPerformance Status. Further, doctors formulated and implemented chemotherapy regiments according to patients’ specific malignant tumor types and stages, and paid close attention to patients’ vital signs and manifestations of nausea and vomiting during the perichemotherapy period. In addition, nausea and vomiting were further graded through the guidelines for the prevention and treatment of nausea and vomiting related to antitumor therapy of the CSCO. Specific details are shown in Supplementary additional file Table S1, http://links.lww.com/MD/M664. If the patient had symptoms of nausea and vomiting during chemotherapy, symptomatic treatment and empirical antiemetic drugs would be given, otherwise routine nursing would be carried out until the patient was discharged after this chemotherapy cycle. Finally, patients were followed up by nurses and questionnaire Functional Living Index-Emesis (FLIE) was completed.[15] A flow chart of the preimprovement management of chemotherapy patients is shown in Figure 1A.
Figure 1.
Flowchart for management of CINV symptoms in patients. (A) Before the nausea-free word was established; (B) After the nausea-free ward is established. CINV = chemotherapy-induced nausea and vomiting.
2.2.2. Chemotherapy related care based on the nausea-free ward model
On the basis of the control group, the intervention group was guided by the nausea-free ward model, with the purpose of reducing the incidence and severity of nausea and vomiting caused by chemotherapy, reducing the incidence of adverse reactions caused by chemotherapy and the need to reduce the dose, stop the drug, prolong the chemotherapy cycle and thus affect the curative effect.
2.2.2.1. Learn about CINV
Prior to the development of the nausea-free ward, the team members retrospectively analyzed the CINV control situation in the previous departments, and formulated training contents according to the shortcomings through on-site survey, medical record review, and questionnaires. The training content included: the pathophysiology of CINV, evaluation methods, guideline development, drug therapy and so on. Regular assessment was carried out to ensure that the nausea-free ward team had a solid grasp of the basic knowledge and the latest progress of CINV. The training of doctors and nurses is a tiered approach due to the different clinical focus. Physician training focused on chemotherapy drug toxicity, emetogenicity of chemotherapeutic drugs, and chemotherapy regimen development. Nurses were trained in the assessment of chemotherapeutic nausea and vomiting and the management of adverse reactions to chemotherapeutic drugs.
2.2.2.2. Establishment of CINV standardized treatment team
The management model of the nausea-free ward was a physician-nurse-patient model. The oncology department was the leading department, with the assistance of the pharmacy, nutrition, Chinese medicine and mental health departments. The team members include a department director, a medical team leader (master’s degree), a head nurse (bachelor’s degree), 3 doctors (1 master’s degree, 2 bachelor’s degree), and 3 nurses (bachelor’s degree). The department head coordinated staffing and developed detailed team collaboration mechanisms. Physicians were responsible for developing chemotherapy protocols, conducting risk assessments, developing standardized treatment procedures for CINV, and developing physician job responsibilities and training. Nurses were responsible for developing the standardized nursing process of CINV, formulating the job responsibilities and training of nurses, monitoring and giving feedback to patients throughout the chemotherapy process, and implementing the antiemetic plan formulated by doctors. The medical team leader followed up and evaluated the antiemetic effect through regular ward rounds and regular follow-up.
2.2.2.3. Finding defects
Panelists examined the current status of CINV in 74 patients who underwent chemotherapy between April 22, 2021 and May 10, 2021 through on-site survey and case reviews. Specifically, the 5 programs of CINV assessment, education, treatment, follow-up, and awareness were checked. Further, according to the 80/20 rule, the 3 items of no assessment of emetogenic risk, unstandardized diagnosis and treatment, and single choice of medication were considered to be the focus of improvement. Then, these 3 deficiencies were analyzed in all aspects from doctors, nurses, patients, materials, methods, and environment. Through these steps, 5 major defects were identified. Specifically: Inadequate awareness of CINV among healthcare professionals. No emetogenic risk assessment form. No continuity evaluation of antiemetic effect. Antiemetic regimen inconsistent with guidelines (i.e., empirical medication). Lack of medication and professional staff.
2.2.2.4. Formulating countermeasures to improve the defects
Countermeasure 1: Establish a comprehensive management system. The Medical Department took the lead in setting up a standardized management team for CINV, clarifying the functional responsibilities of relevant departments and personnel at all levels. A multidisciplinary management team for CINV was established, including oncology, pharmacy, nutrition, Chinese medicine and mental health. Establish a mechanism for pharmacists to provide regular clinical services and coordinate to ensure drug supply. Formulate training programs, study guidelines, and conduct regular training and assessment of CINV-related knowledge. Establish an assessment mechanism to strengthen quality supervision and control. In general, the formation of systems, responsibilities, assessment standards and standardized implementation.
Countermeasure 2: Establishment of model “ACEI-2C.” “A” means “Assess.” Patients were evaluated in 3 aspects: medical history (e.g., presence of brain metastases, uremia, hyperglycemia, intestinal obstruction, electrolyte disorders, etc), risk assessment of personal risk factors (e.g., female, history of vomiting in pregnancy, anxiety, age <50 years, history of opioid painkillers, no or little alcohol consumption, history of motion sickness, etc), and risk assessment of chemotherapy drug induced vomiting.[16] Each constructed a screening card to check patients individually. In addition, patients with 2 or more risk factors were identified as high-risk groups, high-risk groups with moderate-risk chemotherapy regimens were identified as high-risk, and high-risk groups with mild-risk chemotherapy regimens were identified as moderate-risk. Ultimately, chemotherapy patients were assessed and categorized as high risk, moderate risk, low risk, and slight risk.[16] “C” means “Cure.” Treatment standardization was improved through graded interventions, comprehensive therapy, and early intervention for adverse drug reactions. Graded management based on assessment results was reflected in the recommendation of no antiemetic drug medication for slight risk, single drug therapy for low risk, 2 or 3 drug combinations for moderate risk, and 3 or 4 drug combinations for high risk.[17] Refractory CINV was defined as failure of treatment with prophylactic and/or rescue antiemetic therapy in previous chemotherapy cycles and continued vomiting in subsequent chemotherapy cycles.[16–18] Comprehensive treatment referred to the use of multidisciplinary integrated treatment model for refractory CINV, which was manifested in the comprehensive diagnosis and treatment by physicians, full tracking by specialized nurses, guidance on the use of medication by pharmacists, dietary guidance by dietitians, psychological interventions by psychologists, and the application of traditional Chinese medicine treatments (such as aromatherapy,[19] relaxation therapy,[20] acupuncture,[12] acupressure,[21] etc). For the common adverse reactions of chemotherapy drugs, patients were guided to self-management at home through prophylactic drug preparation and education. In addition, in order to ensure the smooth development of the nausea-free ward, the management team formulated the assessment rules for the nausea-free ward, and regularly implemented nausea-free training every month to update the latest progress of the chemotherapy antiemetic program, and regularly carried out the assessment of nausea-free knowledge of the medical and nursing staff. According to the results of the assessment, the unqualified personnel would be strengthened to ensure the safety, efficiency and sustainability of the nausea-free ward. “E” means “Evaluate.” After the use of antiemetic drugs, it was necessary to evaluate all aspects of the patient’s CINV status and assist the patient in adjusting antiemetic drugs and nonpharmacological management measures (e.g., dietary habits, rationalization of work and rest schedules, etc). “I” means “Individuation.” Individualized treatment plans were developed for specific patients. The antiemetic regimen needed to be developed with full knowledge of the patient’s disease history and drug use. For example, the use of olanzapine increased the risk of prolongation of the Q-T interval on the electrocardiogram, so it should be used with caution in patients with underlying heart disease and arrhythmias. “2C” means “Whole Coursendividuation” and “Whole Cycle.” Whole coursendividuation referred to the use of a visual analogue scale of nausea, a CINV care record sheet, and a discharge self-management record sheet to establish a continuous dynamic evaluation model before, during and after chemotherapy. The whole process means that the doctor is responsible for the whole process of the patient and adjusts the relevant treatment in a timely manner. In the whole multi-cycle treatment process, do a good job in the whole process of “ACEI” guidance. Figure 1B shows the management flow chart after the establishment of the nausea-free ward.
Countermeasure 3: Establish an information interaction platform. Delayed CINV was defined as nausea and vomiting that occurred after 24 hours after the administration of antineoplastic drugs (chemotherapeutic agents).[16–18,22] For chemotherapy patients with malignant tumors, the cycle of in-hospital chemotherapy was generally 1 to 3 days, and the intermittent period of out-of-hospital chemotherapy was 21 days. According to the survey, a considerable portion of patients had experienced delayed CINV in the past. Therefore, the construction of the informatized doctor-patient interaction platform was conducive to tracking and follow-up of patients outside the hospital as well as interactive communication between doctors and patients, and further discharge guidance and reassessment of patients. This not only helped to improve the patient medical experience and ensure the sustainability of the follow-up process, but also facilitated physicians to guide the next cycle of chemotherapy.
2.3. Relevant assessment tools
2.3.1. Patient socio-demographic questionnaire
Based on prior experience and literature review, a socio-demographic questionnaire was created by the CINV treatment team to investigate the general condition of the patients. The patient’s name, gender, age, place of residence, marital status, educational level, smoking history, drinking history, and KarnofskyPerformance Status were included in the questionnaire. In addition, cancer type, stage, chemotherapy regimen, and antiemetic drug regimen were also recorded.
2.3.2. CSCO evaluation tool
According to the guidelines of CSCO prevention and treatment of nausea and vomiting caused by antitumor therapies, the nausea was categorized into 4 dimensions, grades 0, 1, 2, and 3, respectively. Vomiting was categorized into 5 dimensions, graded 0, 1, 2, 3, and 4, respectively. Supplementary additional file Table S1, http://links.lww.com/MD/M664 describes the specific grading requirements in detail.
2.3.3. FLIE questionnaire
The extent to which CINV affected the quality of life of patients was assessed by the FLIE questionnaire developed by Lindley et al.[23] The questionnaire had 2 sets of 18 questions dealing with nausea and vomiting. Each question was scored through Likert 7-level scoring method, with a score of 1 indicating no effect while a score of 7 indicating severe effect. Further, the patient’s nausea score, vomiting score and total score were calculated with higher scores indicating greater impact.
2.4. Statistical analysis
IBM SPSS 26.0 and R 4.2.1 were utilized to analyze the data in this study. Measurements that conformed to a normal distribution were expressed as mean ± standard deviation, otherwise they were expressed as median. Further, the independent samples t-test and nonparametric Mann-Whitney U-test were used for comparison between the 2 groups, respectively. Percentages were used to represent enumeration data and chi-square test was used to compare the 2 groups. The criterion for statistical significance was P < .05.
3. Results
3.1. Comparison of baseline data in the study population
The results in Table 1 shows that there were no significant differences in the baseline data between 2 groups (all P-value >.05). This indicated comparability between 2 groups.
Table 1.
Comparison of general demographic data between 2 groups.
| Variables | Categories | The control group (n = 105) | The intervention group (n = 105) | T/χ2 | P-value |
|---|---|---|---|---|---|
| Age | 65.18 ± 9.47 | 64.94 ± 11.84 | 0.161 | .872 | |
| Gender | Male | 74 | 75 | 0.023 | .879 |
| Female | 31 | 30 | |||
| Education | Junior high school and below | 43 | 41 | 0.974 | .615 |
| High school or secondary school | 55 | 53 | |||
| College and above | 7 | 11 | |||
| Place of residence | Urban | 77 | 72 | 0.578 | .447 |
| Rural | 28 | 33 | |||
| Marital status | Unmarried | 3 | 2 | 0.815 | .665 |
| Married | 92 | 96 | |||
| Divorced or widowed | 10 | 7 | |||
| Smoking history | No | 57 | 51 | 0.686 | .407 |
| Yes | 48 | 54 | |||
| Drinking history | No | 84 | 77 | 1.304 | .253 |
| Yes | 21 | 28 | |||
| The score of KPS | ≤70 | 13 | 15 | 1.165 | .685 |
| ≥80 | 92 | 90 |
KPS = Karnofsky performance status.
3.2. Comparison of grades of nausea and vomiting between 2 groups
After the establishment of the nausea-free ward, the intervention group exhibited significantly lower ratings of nausea and vomiting compared to the control group (all P-value <.05). The specific information is shown in Table 2.
Table 2.
Comparison of grades of nausea and vomiting between 2 groups.
| Variables | The control group (n = 105) | The intervention group (n = 105) | z | P-value |
|---|---|---|---|---|
| Nausea grade | 1.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | –5.370 | .000 |
| Vomiting grade | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | –3.879 | .000 |
3.3. Comparison of FLIE scores between 2 groups
As could be seen from the results shown in Table 3, after the establishment of the nausea-free ward, the nausea score, vomiting scores and total scores were significantly lower than those of the control group, and the difference were all statistically significant (all P-value <.05).
Table 3.
Comparison of FLIE scores between 2 groups.
| Variables | The control group (n = 105) | The intervention group (n = 105) | t | P-value |
|---|---|---|---|---|
| Nausea scores | 25.67 ± 13.82 | 20.64 ± 8.24 | 3.202 | .002 |
| Vomiting scores | 15.05 ± 11.33 | 9.53 ± 5.48 | 4.489 | .000 |
| Total scores | 40.71 ± 23.47 | 30.17 ± 12.27 | 4.080 | .000 |
FLIE = Functional Living Index-Emesis.
4. Discussion
4.1. Pathophysiology of CINV
The mechanisms of CINV development are mediated by a combination of therapeutic drugs, psycho-psychological factors, and advanced tumor complications, and involves signaling between a variety of neurotransmitters and receptors in the central nervous system and gastrointestinal tract.[24] Currently, neurotransmitters including dopamine, serotonin and substance P have been shown to contribute to the pathogenesis of CINV.[25] Specifically, CINV can occur through both central and peripheral pathways.[25,26] The central pathway is manifested in the stimulation of the vomiting center in the brain by chemotherapeutic agents, which in turn act on neurokinin-1 receptors in the central nervous system by releasing substance P via the vagus nerve.[25] The peripheral pathway is manifested in the stimulation of enterochromaffin cells by chemotherapeutic drugs to release serotonin to act on 5-hydroxytrypta-mine type 3 receptors, which ultimately transmits the stimulus to the brain.[24]
4.2. Nausea-free ward model significantly improves the degree of CINV in patients
The results of this study showed a significant reduction in the level of nausea and vomiting in the intervention group compared to the control group. Our group believes that this is related to the multidisciplinary management and guidance as well as the implementation of the “ACEI-2C” management system. There were many nonpharmacological management measures for CINV, which should be combined with the clinical situation and the patient’s wish, and follow the principle of individuation.[27] By applying the nausea-free ward model in this study, the results showed significant improvement in CINV in chemotherapy patients. This model emphasized multidisciplinary cooperation, continuous identification of deficiencies and improvement of patients’ CINV symptoms. Through dynamic monitoring, follow-up, and intervention, which not only improved patient satisfaction, but also served as a reference for clinicians in constructing intervention programs.
4.3. Nausea-free ward model significantly improves patients’ quality of life
Chemotherapy may enhance patients’ survival rates, yet it does not guarantee an optimal quality of life. As tumors are gradually entering the mode of chronic disease management, the degree of patients’ need for quality of life is increasing. Controlling the adverse effects of chemotherapy on patients, especially CINV,[28,29] has become the focus of research. The results of this study showed that the nausea score, vomiting score, and total score of the intervention group were significantly lower than the control group under the guidance of the nausea-free ward model. This may be related to the formation of a standardized management process for the whole process of chemotherapy in oncology patients, the multidisciplinary participation in assisting the management as well as the joint implementation of dietary, pharmacological, and psychological measures. In this study, although the patients’ FLIE scores improved significantly compared with the control group, the nausea scores remained high. This suggests, to some extent, that CINV is a symptom that accumulates in patients over time during chemotherapy, and it is difficult to achieve effective remission through standardized management and intervention over a short period of time. In addition, nausea can only be measured subjectively by the patient. Patients may also refer to taste disorders and other symptoms as nausea, which would cause bias.[24] Therefore, long-term assessment and follow-up are needed to focus on and manage patients’ CINV symptoms. Because of this, the initial purpose of the nausea-free ward model is that it can guide doctors to standardize the management of the patient’s entire cycle of chemotherapy, record and assess the previous symptoms of CINV, and provide a basis for better management of CINV when entering the next cycle of chemotherapy, which further proves the value of the nausea-free ward model in the application of chemotherapy for cancer patients.
4.4. Strengths and weaknesses of this study
The advantages of this study were as follows. Firstly, before the establishment of the nausea-free ward, through a large amount of data accumulation and personnel study, which provided us with a rich theoretical basis. Secondly, this study included the study population through a strict screening strategy, which increased the credibility of the findings to a certain extent. Thirdly, the “ACEI-2C” management model is the first time proposed in this study, which has important practical significance. In addition, this study had some limitations. As the model of the nausea-free ward has not been established for a long time, the sample size needs to be further expanded in the future. Secondly, there were fewer indicators for outcome evaluation, and more effective evaluation tools need to be included in the future. In the future, this study will further expand the sample size through a multi-center approach, and in addition, richer scales (e.g., depression-related scales, anxiety-related scales, etc) will be applied.
5. Conclusions
In this study, a standardized whole course management scheme for CINV symptoms in cancer chemotherapy patients was developed by establishing a model of a nausea-free ward and using it as a guide. The results of this study suggested that implementing the nausea-free ward model could enhance patients’ nausea and vomiting symptoms and improve their quality of life. Through comprehensive intervention of CINV, not only can bring many benefits to patients, but also provide clinical workers with management experience.
Acknowledgments
We appreciated Chongqing Jiulongpo District People’s Hospital for supporting this study. We also appreciated all participants in this study.
Author contributions
Conceptualization: Yingying Wang, Li Cao, Zhenglin Wang, Yip Yang.
Data curation: Yingying Wang, Mingyou Deng, Yong Huang, Li Liu, Yin Xiao, Lei Hu.
Formal analysis: Yingying Wang, Mingyou Deng, Yong Huang, Li Liu, Yin Xiao, Lei Hu.
Methodology: Yingying Wang, Mingyou Deng, Yip Yang.
Project administration: Yingying Wang, Mingyou Deng, Yip Yang.
Resources: Yingying Wang, Mingyou Deng, Yip Yang.
Validation: Yingying Wang, Mingyou Deng, Yong Huang, Li Liu, Yin Xiao, Lei Hu.
Visualization: Yingying Wang, Mingyou Deng, Yong Huang, Li Liu, Yin Xiao, Lei Hu.
Writing – original draft: Yingying Wang, Mingyou Deng, Yong Huang.
Writing – review & editing: Yingying Wang, Li Liu, Yin Xiao, Lei Hu, Yip Yang.
Supplementary Material
Abbreviations:
- CINV
- chemotherapy-induced nausea and vomiting
- CSCO
- Chinese Society of Clinical Oncology
- FLIE
- Functional Living Index-Emesis
All patients have signed an informed consent form.
This study was approved by the Ethics Review Committee of Chongqing Jiulongpo District People’s Hospital, Ethics No. 202402.
The author have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Wang Y, Deng M, Huang Y, Liu L, Xiao Y, Hu L, Cao L, Wang Z, Yang Y. Establishing a nausea-free ward model to reduce chemotherapy-induced nausea and vomiting: A retrospective study. Medicine 2024;103:22(e38357).
YW, MD, YH, LL, YX, and LH contributed equally to this work.
Contributor Information
Mingyou Deng, Email: 441682245@qq.com.
Yong Huang, Email: 2023150453@stu.cqmu.edu.cn.
Li Liu, Email: 1364819149@qq.com.
Yin Xiao, Email: 290532049@qq.com.
Lei Hu, Email: 357523521@qq.com.
Li Cao, Email: 1364819149@qq.com.
Zhenglin Wang, Email: wangzhenglinchqing@126.com.
Yiping Yang, Email: 1147235419@qq.com.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Razvi Y, Chan S, McFarlane T, et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer. 2019;27:87–95. [DOI] [PubMed] [Google Scholar]
- [3].Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. [DOI] [PubMed] [Google Scholar]
- [4].Marx W, Kiss N, McCarthy AL, McKavanagh D, Isenring L. Chemotherapy-induced nausea and vomiting: a narrative review to inform dietetics practice. J Acad Nutr Diet. 2016;116:819–27. [DOI] [PubMed] [Google Scholar]
- [5].Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aapro M. CINV: still troubling patients after all these years. Support Care Cancer. 2018;26(Suppl 1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Apfel CC, Korttila K, Abdalla M, et al.; IMPACT Investigators. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dranitsaris G, Molassiotis A, Clemons M, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28:1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Namazinia M, Mazlum SR, Mohajer S, Lim Abdullah K, Salehian M. A structured laughter yoga therapy program on patients with chemotherapy-induced nausea and vomiting: a randomized clinical trial. Asia Pac J Oncol Nurs. 2024;11:100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crichton M, Marshall S, Isenring E, et al. Effect of a standardized ginger root powder regimen on chemotherapy-induced nausea and vomiting: a multicenter, double-blind, placebo-controlled randomized trial. J Acad Nutr Diet. 2024;124:313–330.e6. [DOI] [PubMed] [Google Scholar]
- [11].Choi J, Lee J, Kim K, Choi HK, Lee SA, Lee HJ. Effects of ginger intake on chemotherapy-induced nausea and vomiting: a systematic review of randomized clinical trials. Nutrients. 2022;14:4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yan Y, López-Alcalde J, Zhang L, Siebenhüner AR, Witt CM, Barth J. Acupuncture for the prevention of chemotherapy-induced nausea and vomiting in cancer patients: a systematic review and meta-analysis. Cancer Med. 2023;12:12504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhong FP, Zhong J, Zhong MY. Effect of music therapy on chemotherapy-induced nausea and vomiting in gastrointestinal cancer: a systematic review and meta-analysis. World J Gastrointest Surg. 2023;15:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gala D, Wright HH, Zigori B, Marshall S, Crichton M. Dietary strategies for chemotherapy-induced nausea and vomiting: a systematic review. Clin Nutr. 2022;41:2147–55. [DOI] [PubMed] [Google Scholar]
- [15].Lindley CM, Hirsch JD, O’Neill CV, Transau MC, Gilbert CS, Osterhaus JT. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1:331–40. [DOI] [PubMed] [Google Scholar]
- [16].Berger MJ, Ettinger DS, Aston J, et al. NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Canc Netw. 2017;15:883–93. [DOI] [PubMed] [Google Scholar]
- [17].Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3240–61. [DOI] [PubMed] [Google Scholar]
- [18].Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43. [DOI] [PubMed] [Google Scholar]
- [19].Efe Ertürk N, Taşci S. The effects of peppermint oil on nausea, vomiting and retching in cancer patients undergoing chemotherapy: an open label quasi-randomized controlled pilot study. Complement Ther Med. 2021;56:102587. [DOI] [PubMed] [Google Scholar]
- [20].Moradi Y, Jafarizadeh H, Asghari R, Mirzamohammadi O, Alinejad V. Single and combined use of benson relaxation technique and oxygen therapy on chemotherapy-induced nausea and vomiting in gastric cancer patients. Explore (NY). 2023;19:587–93. [DOI] [PubMed] [Google Scholar]
- [21].Hu J, Shen Y, Zhang G, et al. Effect of acupoint therapies on chemotherapy-induced nausea and vomiting: a systematic review protocol. Medicine (Baltim). 2019;98:e17109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–8. [DOI] [PubMed] [Google Scholar]
- [23].Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003;11:522–7. [DOI] [PubMed] [Google Scholar]
- [24].Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356–67. [DOI] [PubMed] [Google Scholar]
- [25].Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–94. [DOI] [PubMed] [Google Scholar]
- [26].Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li K, Cai Y, Xie S, et al. Evidence summary for nonpharmacological management of chemotherapy-induced nausea and vomiting. Biomed Res Int. 2022;2022:4741193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol. 2016;99:13–36. [DOI] [PubMed] [Google Scholar]
- [29].Grassi L, Berardi MA, Ruffilli F, et al.; IOR-IRST Psycho-Oncology and UniFE Psychiatry Co-Authors. Role of psychosocial variables on chemotherapy-induced nausea and vomiting and health-related quality of life among cancer patients: a European study. Psychother Psychosom. 2015;84:339–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.