Abstract
Fetal hyperthyroidism is a rare prenatal disease and can be life-threatening. The diagnosis is based on ultrasound in mothers with a history of Basedow–Graves' disease and elevation of thyrotropin receptor antibodies (TRAbs) levels. The treatment consists of antithyroid drugs. We present a mother with Basedow–Graves' disease, treated with radioactive iodine 16 years ago. She had an unplanned pregnancy at the age of 29 years, and an elevation of TRAbs (21 U/L) was found at the sixth week of pregnancy. At 22 weeks of gestation, fetal ultrasound displayed tachycardia, goiter, exophthalmos, and suspicion of craniosynostosis, hence methimazole was started. Concomitantly, suppressed maternal thyroid-stimulating hormone (TSH) was found. Her daughter was born at 33 + 6 weeks showing clinical and laboratory findings of hyperthyroidism. Consequently, treatment with methimazole was prescribed. Normal thyroid function was documented in the mother after giving birth. Clear explanation has not been found for the alteration of maternal TSH during pregnancy.
Keywords: fetal hyperthyroidism, Graves' disease, hyperthyroidism, fetal
Thyroid hormones are essential for fetal development during pregnancy, particularly for fetal neurogenesis. 1 At the 10th week of pregnancy, the synthesis of fetal thyroid hormones starts, prior to which the fetus depends exclusively on maternal hormones. 2 Around 20 weeks of gestation, fetal thyroid-stimulating hormone (TSH) receptors begin to respond to TSH and TSH receptor antibodies (thyrotropin receptor antibody [TRAb]). 3 TRAbs cross the fetoplacental barrier and act on fetal TSH receptors and fetuses of mothers who have a history of active Basedow–Graves' disease or treatment with thyroidectomy or radioiodine and currently being treated for hypothyroidism and who have high TRAb titers may develop hyperthyroidism after this period. 2 3 Fetal hyperthyroidism is a rare and transient prenatal condition; however, it can have lethal consequences for the fetus if it is not investigated and treated immediately. 4 Diagnosis is based on antenatal Doppler ultrasound from 20 weeks of pregnancy in at-risk mothers displaying signs of fetal thyroid dysfunction. The clinical and ultrasound manifestations are broad, ranging from tachycardia, goiter, growth retardation, and amniotic fluid volume alterations to more serious consequences such as microcephaly, craniosynostosis, hydrops fetalis, heart failure, premature birth, and stillbirth. 4 5 6 7 Once the diagnosis of fetal or maternal hyperthyroidism is made, the primary treatment is antithyroid drugs. 4
Case Report
A 29-year-old primigravid mother was diagnosed with Basedow–Graves' disease at the age of 13 years and was treated with radioiodine at 14 years of age, and developed posttreatment hypothyroidism. She showed progressive dysthyroid orbitopathy and underwent decompressive surgery at 19 years of age. Lately, she was referred to endocrinology in the context of her thyroid history and unintended pregnancy. Previously, she had been treated with levothyroxine 150 µg/d. Laboratory workup showed elevated TRAbs levels at the sixth week of pregnancy (21 U/L, normal value <0.1 U/L), with normal levels of thyroid function test during the first trimester of pregnancy ( Table 1 ). Repeated TRAbs levels at 18 weeks remained positive. At 22 weeks of gestation, a fetal ultrasound was performed, which showed suggestive signs of fetal hyperthyroidism, including tachycardia, goiter with increased central vascular flow, exophthalmos, and suspected craniosynostosis. Concomitantly, a mild suppression in maternal TSH level was detected. TRAb typing was requested and the presence of stimulating antibody (thyroid-stimulating immunoglobulin) was confirmed. Methimazole 10 mg was started which was later increased to 20 mg according to ultrasound findings ( Table 1 ). Interruption of pregnancy by cesarean section at 34 weeks due to progressive manifestations of fetal hyperthyroidism was decided; however, the pregnant woman went into labor at 33 + 6 weeks.
Table 1. Maternal–fetal evaluations since the beginning of the pregnancy.
| GA | 6 wk | 12 + 4 wk | 18 + 3 W | 22 + 4 wk | 24 + 3 wk | 27 + 4 wk | 30 + 2 wk | 33 wk | 33 + 6 wk | Normal reference ranges |
|---|---|---|---|---|---|---|---|---|---|---|
| TSH | 0.291 | 0.524 | 1.2 | 0.22 | 0.015 | 0.02 | 0.0011 | 0.3–4.2 µUI/mL | ||
| FT4 | 1.3 | 1.31 | 1.3 | 1.09 | 1.33 | 0.9 | 1.26 | 0.7–1.48 ng/dL | ||
| T4 | 10.3 | 9.81 | 10 | 11.4 | 12.9 | 11.2 | 10 | <16 wk: 5.5–11.5 µg/dL >16 wk: 8.25–15.25 µg/dL |
||
| TRAb s | 21.3 | 16.4 | 18.7 (TSI) | < 0.1 U/L | ||||||
| Treatment | LT4 150 µg | LT4 150 µg | LT4 150 µg | LT4 150 µg Methimazole 10 mg |
LT4 150 µg Methimazole 15 mg |
LT4 150 µg Thiamazole 20 mg |
LT4 150 µg Methimazole 20 mg |
Preterm labor | ||
| Fetal US | HR: 153 × min AF normal | Fetal tachycardia, goiter with central hypervascularization. Long bones >p90 | Sinus tachycardia, goiter improvement | Fetal tachycardia, goiter, exophthalmos | Similar goiter, normocardia, exophthalmos | Suspected craniosynostosis, HR: 160 × min | ||||
| Mother's physical examination | Exophthalmos, HR: 95 × min tremor (+) |
Abbreviations: AF, amniotic fluid; FT4, free thyroxine; GA, gestational age; HR, heart rate; LT4, levothyroxine; T4, thyroxine; TRAbs, thyrotropin receptor antibodies; TSH, thyroid-stimulating hormone; TSI, thyroid-stimulating immunoglobulin.
At birth, her daughter had a weight of 2,660 g (−0.47 standard deviation [SD]), height of 47.5 cm (1.2 SD), head circumference of 31.5 cm (−0.714 SD), and Apgar 8–9–9. Her physical examination revealed tachycardia, exophthalmos, bilateral palpebral retraction, and goiter. Treatment with methimazole 0.38 mg/kg/d and propranolol 1.5 mg/kg/d was immediately started. Thyroid function tests showed suppressed TSH, elevated free T4 (FT4) and T3. TRAbs levels were 9.187 IU/L. In the first hour of life, she presented respiratory distress syndrome, requiring continuous positive airway pressure for 15 hours, without hemodynamic instability. At 7 days of life, the dose of methimazole was increased to 0.68 mg/kg/d, progressing with slow normalization of thyroid tests. At 4 days of life, she presented hyperbilirubinemia with a cholestatic pattern, and ursodeoxycholic acid and vitamin E were prescribed. Her abdominal and brain ultrasound were normal. Thyroid ultrasound showed slightly heterogeneous hypoechoic diffuse goiter without focal lesions. Skull X-ray ruled out craniosynostosis. At 17 days of life due to persistent elevation of FT4, methimazole dose was increased (0.83 mg/kg/d). She was discharged at 22 days of life.
In her follow-up at the age of 2 months 5 days, the infant showed resolution of cholestasis, normalization of thyroid function with an adequate growth and weight gain; therefore, methimazole, ursodeoxycholic acid, and vitamin E were suspended, and levothyroxine was prescribed due to transient hypothyroidism secondary to methimazole ( Table 2 ). Ophthalmology evaluation reported regression of palpebral retraction with persistence of mild exophthalmos and her fundoscopic examination was normal. Nonetheless, dysphagia was suspected due to history of choking with feeding and saliva. She was evaluated by neurology and central hypotonia and an increased in her head circumference were noted ( Fig. 1 ). Magnetic resonance imaging displayed triventricular hydrocephalus and type 1 Chiari malformation and she underwent endoscopic ventricular cisternostomy at 12 months of age. She progressed with psychomotor developmental retardation; speech being predominantly affected. Brain stem evoked response audiometry was normal. At the age of 15 months, she underwent a new neurologic procedure owing to pseudomeningocele and a plasty of a cerebrospinal fluid noncommunicating fistula in the subgaleal space. She has been receiving speech therapy and has shown improvement in this area. Her growth has been adequate during follow-up ( Fig. 1 ).
Table 2. Clinical and laboratory follow-up of the newborn.
| Age | 0 | 4 d | 7 d | 10 d | 13 d | 17 d | 20 d | 22 d | 1 mo 5 d | 1 mo 17 d | 2 mo 5 d | 2 mo 11 d | 2 mo 25 d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | 36 + 3 | 38 + 5 | 3 d | 19 d | 25 d | 1 mo 9 d | |||||||
| Weight (g) | 2,660 | 2,130 | 2,090 | 2,205 | 2,270 | 2,345 | 2,390 | 2,380 | 3,100 | 3,510 | 4,040 | ||
| Height (cm) | 47.5 | 53.5 | 55 | ||||||||||
| TSH (mUI/mL) | 0.027 | <0.015 | 15 | <0.01 | <0.01 | 0.33 | 629 | <0.01 | |||||
| FT4 (ng/dL) | 5.59 | 6.37 | 5.07 | 3.63 | 3.22 | 3.48 | 3.03 | 2.27 | 1.0 | 0.7 | 0.4 | 0.54 | 1.7 |
| T3 (ng/m L) | 3.54 | 2.88 | 3.32 | 3.89 | 4.1 | 3.6 | 2.0 | 1.1 | 0.9 | 129 ng/dL | |||
| TRabs (UI/L) | 9.187 | 6.88 | 0.19 | ||||||||||
| Therapy | Methimazole 0.5–0.5 mg (0.38 mg/kg/d) Propranolol 2–2 mg (1.5 mg/kg/d) |
↓Propranolol 1–1 mg due to bradycardia, start of ursodiol and vitamin E | ↑Methimazole 1–0.5 mg (0.7 mg/kg/d) Propranolol 2–2 mg |
↑Methimazole 1–1 mg (0.83 mg/kg/d) Propranolol 2–2 (1.67 mg/kg/d) |
Propranolol suspension, Ursodiol 25 mg c/8 h (20 mg/kg/d) | ↓Methimazole 1–0.5 mg (0.48 mg/kg/d), ursodiol, vitamin E suspension | ↓Methimazole 0.5–0.5 mg (0.28 mg/kg/d) | Methimazole suspension | LVT 25 µg (6 µg/kg/d) | LVT suspension |
Abbreviations: FT4, free thyroxine; GA, gestational age; LVT, levothyroxine; T3, triiodothyronine; TRAbs, thyrotropin receptor antibodies; TSH, thyroid-stimulating hormone.
Note: References values: TRAbs (NR <1.5), LVT (0.78–2.19), T3 (0.97–1.7), and TSH (0.9–7.7).
Fig. 1.
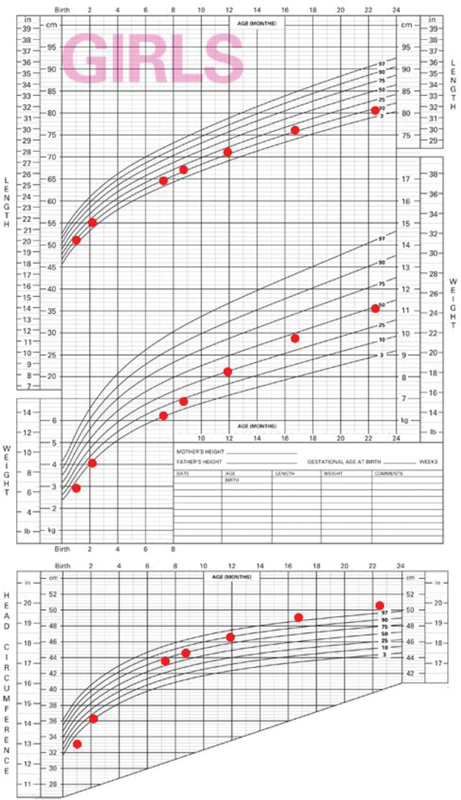
Height, weight, and head circumference growth charts.
Discussion
The most common management scenario in children of mothers with maternal hyperthyroidism due to Graves' disease is neonatal hyperthyroidism with several cases being described in the literature. Due to the severe consequences of fetal hyperthyroidism, 8 screening during pregnancy is mandatory in mothers with a history of active Graves' disease or those who have been treated with radioiodine and/or thyroidectomy. 4 Laboratory workup with TRAbs and thyroid function levels immediately after pregnancy diagnosis is suggested in the literature. 4 9 If TRAbs are negative, no further follow-up is required. Conversely, if TRAbs are positive, repeated levels should be requested at 18 to 22 weeks of pregnancy. If TRAbs remains positive at that time, new levels should be determined at 30 to 34 weeks of pregnancy. In addition, an obstetric ultrasound should be performed starting at 20 to 24 weeks of gestation every 4 to 6 weeks. 9
To the best of our knowledge, 15 cases of fetal hyperthyroidism have been reported in the literature ( Table 3 ). Six of those cases had a history of maternal radioiodine treatment for Graves' disease several years prior to pregnancy. This is consistent with data showing that high levels of TRAbs have been observed 5 years after treatment with I-131, in contrast to thyroidectomy. 10 In our case, TRABs remained positive after 16 years of radioiodine therapy.
Table 3. Diagnosis and management of previous cases of fetal hyperthyroidism reported in the literature.
| Case report | Cases | Prenatal maternal treatment | Maternal thyroid function and/or TRAbs/TSI | Gestational age at diagnosis (wk) | Diagnosis | US diagnosis | Treatment |
|---|---|---|---|---|---|---|---|
| Srisupundit et al (2008) 11 | 1 | Thyroidectomy | Euthyroidism | 28 | US + FBS | Tachycardia | PTU |
| Lembet et al (2004) 12 | 1 | PTU + propranolol | Hypothyroidism TRAb 46.7 UI/L |
26 | FBS | Fetal goiter, IUGR, and oligohydramnios | PTU |
| Spoke and Martin (2020) 8 | 1 | Radioiodine TT + desiccated thyroid extract and LVT | Hypothyroidism TRAb >40 UI/L |
17 | US | Fetal goiter, fetal tachycardia, and pericardial effusion | MMI at week 23. PTU since week 25 |
| Luton et al (1996) 13 | 2 | Partial thyroidectomy | TRAb 297 UI/L | 24 | FBS | Fetal goiter, pericardial effusion, and advance in bone maturation | PTU |
| Euthyroidism TRAb 469 UI/L |
25 | FBS | Fetal goiter, fetal tachycardia | PTU | |||
| Donnelly et al (2015) 14 | 1 | Thyroidectomy + LVT | Euthyroidism | 18 | US | Fetal goiter, tachycardia, bone maturation acceleration in proximal and distal tibial epiphysis, and dilated cardiomegaly | PTU |
| Kazakou et al (2018) 15 | 1 | TT + LVT | (–) | 24 + 3 | US + FBS | Fetal goiter, tachycardia, facial edema, pleural and pericardial effusion, IUGR, hydrops, oligohydramnios | MMI |
| Wallace et al (1995) 16 | 1 | Radioiodine TT >3 y |
Euthyroidism TSI > 500% | 32 | US | Tachycardia | PTU |
| Wenstrom et al (1990) 17 | 2 | Radioiodine TT | Hypothyroidism TSI >500% | 29 | US | Tachycardia, IURG, oligohydramnios | PTU |
| Hyperthyroidism TSI: 500% | 23 | FBS | (–) | MMI | |||
| Hadi and Strickland (1995) 18 | 1 | Radioiodine TT + PTU | Hyperthyroidism TSI: 470% | 30 | US + FBS | Fetal goiter, tachycardia | PTU |
| Porreco and Bloch (1990) 19 | 1 | Thyroidectomy | Euthyroidism TSI > 500% | 25 | FBS | Tachycardia | PTU |
| Vali et al (1993) 20 | 1 | Thyroidectomy | Hyperthyroidism TSI > 100% | 22 | US | Fetal goiter | Carbimazole |
| Belfar et al (1991) 21 | 1 | PTU + propranolol | Euthyroidism | 26 | US | Tachycardia | PTU |
| Pekonen et al (1994) 22 | 1 | Thyroidectomy | Euthyroidism | 31 | US | Thyroid cyst, tachycardia | Carbimazole |
| Hatjis (1993) 23 | 1 | Radioiodine TT | Hypothyroidism TSI: 770% | 26 | US | Fetal goiter, tachycardia, IUGR, oligohydramnios | PTU |
| Heckel et al (1997) 24 | 1 | (–) | Hyperthyroidism TSI > 500% | 28 | US + FBS | Fetal goiter, tachycardia | Carbimazole |
Abbreviations: FBS, fasting blood sugar; IUGR, intrauterine growth restriction; LVT, levothyroxine; MMI, methimazole; PTU, propylthiouracil; TRAbs, thyrotropin receptor antibodies; TSI, thyroid-stimulating immunoglobulin; TT, total thyroidectomy; US, ultrasound.
Regarding maternal thyroid function at the time of pregnancy diagnosis, our patient was receiving levothyroxine showing normal thyroid function, which was similar to another seven case reports ( Table 3 ).
Ultrasound and measurement of maternal TRAbs were used as diagnostic methods for fetal hyperthyroidism in concordance with 12 others reports. However, cordocentesis has also been described as a diagnostic tool following ultrasound suspicion of fetal hyperthyroidism in most of the cases. In our case, cordocentesis was not performed due to maternal and fetal risks, fetal death being the most feared. 25
In our patient, diagnosis was based on ultrasound findings of fetal tachycardia, goiter with central hyperemia, exophthalmos, and suspicion of craniosynostosis, whereas in the cases previously reported ( Table 3 ), goiter was described in 6 fetuses and fetal tachycardia in 13. Furthermore, other signs observed were advanced bone maturation, oligohydramnios, intrauterine growth restriction, pleural effusion, pericardial effusion, among others. 4 5 6 7
Once diagnosis of fetal hyperthyroidism is confirmed, mothers should receive low-dose antithyroid drugs: propylthiouracil during the first trimester of pregnancy, transitioning to methimazole or carbimazole later. Fetal hypothyroidism is the most risky complication of this management. 4 6 9
In our case, due to suppressed maternal TSH without having modified levothyroxine dose concomitant with manifestation of fetal hyperthyroidism on the ultrasound, methimazole was started at 22 weeks of gestation. Abnormal levels of TSH were normalized postpartum. TSH suppression is rare in a pregnant woman with a history of hypothyroidism secondary to radioactive iodine ablation. Additionally, an improvement in clinical and laboratory manifestations during pregnancy has been reported in Th1-predominant autoimmune diseases in pregnancy, such as Graves' disease, which make the hypothesis of reactivation of maternal hyperthyroidism as a cause of this TSH level abnormality unlikely. 26
On the other hand, human chorionic gonadotropin–induced maternal TSH receptor activation could not explain the suppression of TSH in our patient since this phenomenon began in the second trimester of pregnancy. 27
Other less common causes of TSH suppression in pregnancy are those related to autonomous secretion of thyroid hormones, destruction of thyroid follicles, and extrathyroidal sources of thyroid hormones. 28 These were also ruled out, considering the absence of a history of nodular goiter, viral or bacterial infection, anterior cervical pain, among others.
We hypothesized that the suppression of maternal TSH was due to a transplacental transfer of thyroid hormones from the fetus to the mother. This was suspected since TSH values began to fall concomitantly with the onset of ultrasound manifestations of fetal hyperthyroidism at the time when fetal thyroid hormone secretion begins, especially considering that TSH suppression was corrected postpartum.
Monocarboxylate transporter 8 could play a fundamental role in the transport of thyroid hormones of fetal origin toward the maternal circulation. This transporter is present in the placenta in both apical and basal membranes of maternal and fetal endothelial cells, in addition to the villous stroma and a potent and specific affinity for T3 has been described. 29
Another mechanism that may support the theory of bidirectional transport is reverse T3 (T3r), which is synthesized by the fetal thyroid and is transported from the fetus to the maternal circulation through the placenta, 30 showing 40 times greater affinity for placental nuclear binding sites than T4 and 63 times greater than T3. 31 However, it has been observed that T3r is functionally inactive and does not generate serum TSH modifications, 32 so if T3r played any role, it would be indirect and due to other mechanisms not yet established.
In summary, no clear explanation was found for the suppression of TSH during pregnancy in our patient. Further studies addressing transport or metabolism mechanisms of thyroid hormones are needed.
Conclusion
Health care providers must be alert to the eventual development of fetal hyperthyroidism in any mother with a history of GBD, even when she has received definitive treatment (surgery or radioiodine) several years before pregnancy, since the production of TRAbs can persist for a long time. Therefore, it is important that the entire care team for a pregnant woman inquire about maternal thyroid history and apply a protocol for monitoring TRAbs and ultrasound and, if necessary, treatment with antithyroid drugs.
Acknowledgment
We would like to acknowledge the motivation and participation of Dr. Laura Campos to make the publication of this case report possible.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Eng L, Lam L. Thyroid function during the fetal and neonatal periods. Neoreviews. 2020;21(01):e30–e36. doi: 10.1542/neo.21-1-e30. [DOI] [PubMed] [Google Scholar]
- 2.Polak M, Legac I, Vuillard E, Guibourdenche J, Castanet M, Luton D. Congenital hyperthyroidism: the fetus as a patient. Horm Res. 2006;65(05):235–242. doi: 10.1159/000092454. [DOI] [PubMed] [Google Scholar]
- 3.Léger J, Carel J C. Diagnosis and management of hyperthyroidism from prenatal life to adolescence. Best Pract Res Clin Endocrinol Metab. 2018;32(04):373–386. doi: 10.1016/j.beem.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Léger J. Management of fetal and neonatal Graves' disease. Horm Res Paediatr. 2017;87(01):1–6. doi: 10.1159/000453065. [DOI] [PubMed] [Google Scholar]
- 5.Luton D, Le Gac I, Vuillard E et al. Management of Graves' disease during pregnancy: the key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab. 2005;90(11):6093–6098. doi: 10.1210/jc.2004-2555. [DOI] [PubMed] [Google Scholar]
- 6.León M C. Hipertiroidismo en el embarazo. Recién nacido hijo de madre con Enfermedad de Graves Hyperthyroidism in pregnancy. Newborn of mother with Graves disease. Rev Española Endocrinol Pediatr. 2013;5(02):35–40. [Google Scholar]
- 7.Kurtoğlu S, Özdemir A. Fetal neonatal hyperthyroidism: diagnostic and therapeutic approachment. Turk Pediatri Ars. 2017;52(01):1–9. doi: 10.5152/TurkPediatriArs.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spoke C, Martin C. Maternal Graves disease and abnormal CYP2D6 genotype with fetal hyperthyroidism. AACE Clin Case Rep. 2020;6(04):e161–e164. doi: 10.4158/ACCR-2019-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander E K, Pearce E N, Brent G A et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(03):315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 10.Laurberg P, Wallin G, Tallstedt L, Abraham-Nordling M, Lundell G, Tørring O. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158(01):69–75. doi: 10.1530/EJE-07-0450. [DOI] [PubMed] [Google Scholar]
- 11.Srisupundit K, Sirichotiyakul S, Tongprasert F, Luewan S, Tongsong T. Fetal therapy in fetal thyrotoxicosis: a case report. Fetal Diagn Ther. 2008;23(02):114–116. doi: 10.1159/000111589. [DOI] [PubMed] [Google Scholar]
- 12.Lembet A, Eroglu D, Kinik S T, Gurakan B, Kuscu E. Non-invasive management of fetal goiter during maternal treatment of hyperthyroidism in Grave's disease. Fetal Diagn Ther. 2005;20(04):254–257. doi: 10.1159/000085080. [DOI] [PubMed] [Google Scholar]
- 13.Luton D, Fried D, Sibony O et al. Assessment of fetal thyroid function by colored Doppler echography. Fetal Diagn Ther. 1997;12(01):24–27. doi: 10.1159/000264419. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly M A, Wood C, Casey B, Hobbins J, Barbour L A. Early severe fetal Graves disease in a mother after thyroid ablation and thyroidectomy. Obstet Gynecol. 2015;125(05):1059–1062. doi: 10.1097/AOG.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 15.Kazakou P, Theodora M, Kanaka-Gantenbein C et al. Fetal hyperthyroidism associated with maternal thyroid autoantibodies: A case report. Case Rep Womens Health. 2018;20:e00081. doi: 10.1016/j.crwh.2018.e00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace C, Couch R, Ginsberg J. Fetal thyrotoxicosis: a case report and recommendations for prediction, diagnosis, and treatment. Thyroid. 1995;5(02):125–128. doi: 10.1089/thy.1995.5.125. [DOI] [PubMed] [Google Scholar]
- 17.Wenstrom K D, Weiner C P, Williamson R A, Grant S S.Prenatal diagnosis of fetal hyperthyroidism using funipuncture Obstet Gynecol 199076(3 Pt 2):513–517. [PubMed] [Google Scholar]
- 18.Hadi H A, Strickland D. Prenatal diagnosis and management of fetal goiter caused by maternal Grave's disease. Am J Perinatol. 1995;12(04):240–242. doi: 10.1055/s-2007-994462. [DOI] [PubMed] [Google Scholar]
- 19.Porreco R P, Bloch C A.Fetal blood sampling in the management of intrauterine thyrotoxicosis Obstet Gynecol 199076(3 Pt 2):509–512. [PubMed] [Google Scholar]
- 20.Vali A, Wiles P, Thomas N B, Ludlam A. Relapse of maternal thyrotoxicosis presenting as a second-trimester fetal goiter. Ultrasound Obstet Gynecol. 1993;3(06):429–431. doi: 10.1046/j.1469-0705.1993.03060429.x. [DOI] [PubMed] [Google Scholar]
- 21.Belfar H L, Foley T P, Jr, Hill L M, Kislak S. Sonographic findings in maternal hyperthyroidism. Fetal hyperthyroidism/fetal goiter. J Ultrasound Med. 1991;10(05):281–284. doi: 10.7863/jum.1991.10.5.281. [DOI] [PubMed] [Google Scholar]
- 22.Pekonen F, Teramo K, Mäkinen T, Ikonen E, Osterlund K, Lamberg B A. Prenatal diagnosis and treatment of fetal thyrotoxicosis. Am J Obstet Gynecol. 1984;150(07):893–894. doi: 10.1016/0002-9378(84)90473-3. [DOI] [PubMed] [Google Scholar]
- 23.Hatjis C G.Diagnosis and successful treatment of fetal goitrous hyperthyroidism caused by maternal Graves disease Obstet Gynecol 199381(5( Pt 2)):837–839. [PubMed] [Google Scholar]
- 24.Heckel S, Favre R, Schlienger J L, Soskin P.Diagnosis and successful in utero treatment of a fetal goitrous hyperthyroidism caused by maternal Graves' disease. A case report Fetal Diagn Ther 1997120154–58..11-18 [DOI] [PubMed] [Google Scholar]
- 25.Tongsong T, Wanapirak C, Kunavikatikul C, Sirirchotiyakul S, Piyamongkol W, Chanprapaph P. Fetal loss rate associated with cordocentesis at midgestation. Am J Obstet Gynecol. 2001;184(04):719–723. doi: 10.1067/mob.2001.111716. [DOI] [PubMed] [Google Scholar]
- 26.Somers E C. Pregnancy and autoimmune diseases. Best Pract Res Clin Obstet Gynaecol. 2020;64:3–10. doi: 10.1016/j.bpobgyn.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Kobaly K, Mandel S J. Hyperthyroidism and pregnancy. Endocrinol Metab Clin North Am. 2019;48(03):533–545. doi: 10.1016/j.ecl.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Cooper D S, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1(03):238–249. doi: 10.1016/S2213-8587(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 29.James S R, Franklyn J A, Kilby M D. Placental transport of thyroid hormone. Best Pract Res Clin Endocrinol Metab. 2007;21(02):253–264. doi: 10.1016/j.beem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Blackburn S. Maternal-fetal thyroid interactions. J Perinat Neonatal Nurs. 2009;23(04):312–313. doi: 10.1097/JPN.0b013e3181bfdc56. [DOI] [PubMed] [Google Scholar]
- 31.Banovac K, Ryan E A, O'Sullivan M J. Triiodothyronine (T3) nuclear binding sites in human placenta and decidua. Placenta. 1986;7(06):543–549. doi: 10.1016/s0143-4004(86)80140-0. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt R L, LoPresti J S, McDermott M T, Zick S M, Straseski J A. Does reverse triiodothyronine testing have clinical utility? An analysis of practice variation based on order data from a national reference laboratory. Thyroid. 2018;28(07):842–848. doi: 10.1089/thy.2017.0645. [DOI] [PubMed] [Google Scholar]


