Abstract
Background
The omission of sentinel lymph node biopsy in low-risk elderly breast cancer patients has been introduced in several guidelines. Despite evidence to support its safety, this recommendation has not been implemented by many clinicians. We have examined two aspects of this recommendation that may explain why sentinel lymph node biopsy continues to be performed in most of these patients. Firstly, we quantified the proportion of patients diagnosed with axillary metastases postoperatively. Secondly, we examined adherence to antihormonal therapy in the same group of patients.
Methods
In this single-centre retrospective cohort study, the study population comprised 98 patients with breast cancer. Patients were aged ≥70 years and diagnosed with hormone receptor positive breast cancers less than 20 mm (T1). All patients underwent surgery and were subsequently prescribed five years of adjuvant antihormonal treatment.
Results
Axillary lymph node metastases, as confirmed by the postoperative histology report, were seen in 36.3%. Nonadherence was seen in 33.7% of the patients. Primary nonadherence, that is, patients that never collect their first or subsequent prescriptions at the pharmacy, comprised 11.2% of the total study population.
Conclusion
The high proportion of axillary metastases demonstrated suggests that clinical examination of the axilla alone is not sufficient in the preoperative assessment of the axilla. The less-than-optimal adherence rates show that adherence in these patients cannot be taken for granted. We suggest that these factors reflect some of the reluctance among clinicians to omit the sentinel lymph node procedure in these patients.
1. Introduction
Focusing on the challenge of overtreatment in breast cancer has led to the deescalation of previously well-established therapies. Overtreatment can be defined as treatment that does not convey a benefit to the patient but rather may cause harm. Moreover, unnecessary treatment leads to unnecessary costs for health care systems [1–3]. This pervasive problem has gained increasing attention in recent years. Minimising overtreatment is of particular importance in elderly breast cancer patients where comorbidities and frailty frequently occur [4]. The population is aging [5], and over 30% of new breast cancer diagnoses in the US occur in women ≥70 years [6].
The Choosing Wisely Campaign was established in the US in 2012 with a goal to reduce unnecessary medical testing and treatment by engaging clinicians and patients in conversations about the topic. Since its inception, many countries around the world have adapted and implemented the campaign [7, 8]. The Choosing Wisely initiative published the following recommendation in 2016: “Don't routinely use sentinel node biopsy in clinically node negative women ≥70 years of age with early stage hormone receptor positive, HER-2 negative invasive breast cancer.” This has later been reaffirmed in updated versions in 2019, 2020, and 2021 [9]. The same recommendation was made by the American Society of Clinical Oncology (ASCO) in 2021 [10]. Both recommendations state that these patients should be treated with adjuvant antihormonal treatment. These recommendations were made mainly based on the findings in the Cancer and Leukaemia Group B 9343 (CALBG 9343) clinical trial published in 2004 [11] with a follow-up study in 2013 [12]. These trials demonstrated similar survival in women who received radiotherapy and tamoxifen compared to those who received tamoxifen only following breast conserving surgery. Omitting SLNB, and therefore radiotherapy, has consequently been deemed safe by the Choosing Wisely Campaign and ASCO. Other studies have showed concordant results [13–16].
Although there is extensive evidence showing that omitting sentinel lymph node biopsy (SLNB) and radiotherapy in these patients is safe, the implementation of this recommendation remains to be embraced by most clinicians treating breast cancer [13, 17–20]. Retrospective data have shown that between 68% [21] and over 80% [22, 23] of patients eligible for omission of SLNB according to the abovementioned recommendations still undergo the procedure. The explanation for this is multifactorial showing that effective deescalation relies on more than evidence from clinical trials [24–26].
We propose that there are two prerequisites to this recommendation that may explain its unsuccessful implementation. Firstly, preoperative examination of the axilla must verify the absence of axillary metastases (cN0), and secondly, the patient is expected to adhere to adjuvant antihormonal treatment for five years after diagnosis. The main aims of the present study were to quantify the proportion of patients in whom axillary lymph node metastases are detected postoperatively and to examine adherence to antihormonal treatment in this specific group of patients. Furthermore, we examine how adherence and axillary lymph node status relate to survival for these patients.
2. Materials and Methods
The study population comprised all patients aged ≥70 years at the time of diagnosis who underwent surgery for estrogen receptor positive breast cancer at St. Olav's University Hospital in the period 01.01.2004 to 31.12.2013. Information regarding age, tumour characteristics, lymph node status, and treatment modalities were collected from the hospital medical records. Patients underwent breast conserving treatment (BCT) or mastectomy of the tumour with SLNB and/or axillary lymph node dissection (ALND). Tumours were confirmed to be estrogen receptor positive and less than 20 mm in diameter (T1 tumours) according to the postoperative pathology report. All patients were prescribed adjuvant antioestrogen treatment for five years. Patients with tumours that were of histological grade 1 and TI were excluded from the material if national guidelines operative at the time of diagnosis did not recommend antihormonal treatment. Also, patients with estrogen receptor low-positive tumours (≥1% < 10%) diagnosed prior to 2011 were excluded as these patients were not recommended antihormonal treatment at that time. Patients who received neoadjuvant treatment were excluded. All patients were followed for at least five years.
Preoperative evaluation of the axilla consisted of clinical examination and axillary ultrasound. All SLNB were performed using dual tracer with radioactive isotope and blue dye. Lymph node positivity was defined as any detected metastases within a lymph node. During the study period, frozen sections were performed on all lymph nodes removed during the SLNB procedure. An axillary dissection was performed if any metastases ≥2 mm were detected.
Detailed information regarding the prescribed antihormonal treatment was retrieved from the Norwegian Prescription Database. Adherence was determined by the medical possession ratio (MPR). MPR was measured as a fraction where the numerator was the total amount of tablets dispensed. The denominator was five years. Patients were defined as nonadherent if the MPR was <80%. For those who died during the five-year course of antihormonal treatment, the denominator was adjusted to the length of time they were alive after commencing the treatment [27].
Nonadherent patients were further subclassified into primary nonadherent (PNA) or secondary nonadherent (SNA). Primary nonadherence occurs when a patient is prescribed a treatment but fails to collect the first and subsequent prescriptions at the pharmacy. Secondary nonadherence is defined as failure to take the medication as prescribed after the first prescription has been collected, with a measured MPR of <80% [28–30].
Overall survival and BCSS were assessed using Kaplan–Meier curves. Statistical analyses were performed using SPSS version 28.
3. Results
A total of 98 patients (Figure 1), 97 females and one male, were included in the study. The median age at diagnosis was 77 (range 70–93). Of the total study population, 66.3% (65/98) were adherent, 11.2% (11/98) were PNA, and 22.4% (22/98) were SNA to the prescribed antihormonal treatment (Table 1). No axillary lymph node metastases (pN0) were found in 59.2% (58/98), 1–3 axillary metastases were found in 25.5% (25/98), and ≥4 in 8.2% (8/98) of the study population.
Figure 1.
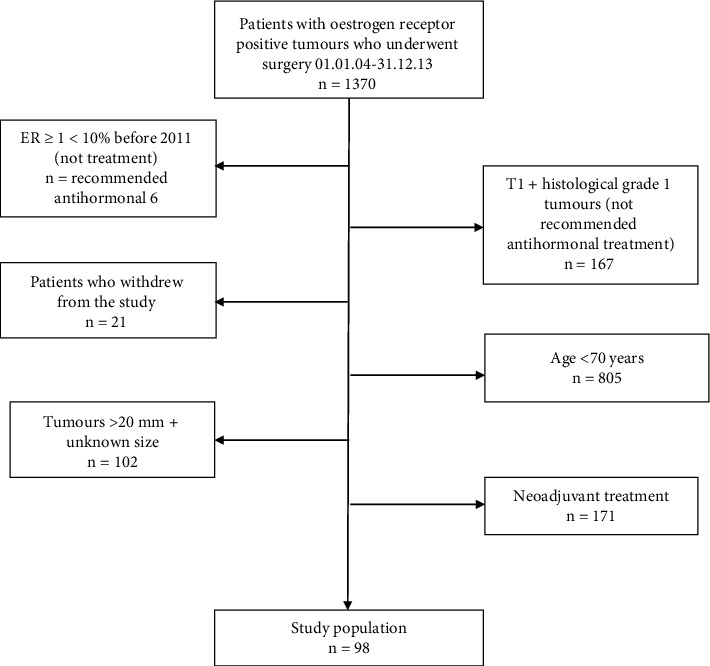
Flow chart of study population.
Table 1.
Patient characteristics.
| Total | Adherent | PNA | SNA | |
|---|---|---|---|---|
| N (%) | 98 | 65 (66.3) | 11 (11.2) | 22 (22.4) |
| Age at diagnosis | ||||
| 70–79 | 66 (67.3) | 52 (80) | 6 (54.5) | 8 (36.4) |
| ≥80 | 32 (32.7) | 13 (20) | 5 (45.5) | 14 (63.6) |
| T-stage | ||||
| pT1a | 1 (1) | 1 (1.5) | 0 | 0 |
| pT1b | 15 (15.3) | 8 (12.3) | 6 (54.5) | 1 (4.5) |
| pT1c | 82 (83.7) | 56 (86.2) | 5 (45.5) | 21 (95.5) |
| Histopathological type | ||||
| Ductal | 74 (75.5) | 50 (76.9) | 8 (72.7) | 16 (72.7) |
| Lobular | 13 (13.3) | 8 (12.3) | 2 (18.2) | 3 (13.6) |
| Other | 10 (10.2) | 7 (10.8) | 1 (9.1) | 2 (9.1) |
| Unknown | 1 (1) | 0 | 0 | 1 (4.5) |
| Histopathological grade | ||||
| Grade 1 | 14 (14.3) | 9 (13.8) | 1 (9.1) | 4 (18.2) |
| Grade 2 | 65 (66.3) | 42 (64.6) | 9 (81.8) | 14 (63.6) |
| Grade 3 | 17 (17.3) | 13 (20) | 1 (9.1) | 3 (13.6) |
| Unknown | 2 (2) | 1 (1.5) | 0 | 1 (4.5) |
| HER-2 status | ||||
| Negative | 66 (67.3) | 44 (67.7) | 8 (72.7) | 14 (63.6) |
| Positive | 12 (12.2) | 10 (15.4) | 1 (9.1) | 1 (4.5) |
| Unknown | 20 (20.4) | 11 (16.9) | 2 (18.2) | 7 (31.8) |
| Axillary lymph node metastases | ||||
| 0 | 58 (59.2) | 37 (56.9) | 9 (81.8) | 12 (54.5) |
| 1–3 | 25 (25.5) | 19 (29.2) | 1 (9.1) | 5 (22.7) |
| ≥4 | 8 (8.2) | 7 (10.8) | 0 | 1 (4.5) |
| Unknown | 7 (7.1) | 2 (3.1) | 1 (9.1) | 4 (18.2) |
| Radiotherapy | ||||
| No | 56 (57.1) | 36 (55.4) | 7 (63.6) | 13 (59.1) |
| Yes | 42 (42.9) | 29 (44.6) | 4 (36.4) | 9 (40.9) |
| Chemotherapy | ||||
| No | 94 (95.9) | 61 (93.8) | 11 (100) | 22 (100) |
| Yes | 4 (4.1) | 4 (6.2) | 0 | 0 |
At the end of the follow-up, 12.2% (12/98) of patients had died due to breast cancer, 30.6% (30/98) had died of causes other than breast cancer, and 57.1% (56/98) were alive (Table 2).
Table 2.
Axillary metastases and adherence according to patient status at the end of follow-up.
| Dead due to breast cancer | Dead due to other cause | Alive | |
|---|---|---|---|
| N (%) | 12 (12.2) | 30 (30.6) | 56 (57.1) |
| Axillary metastases | |||
| 0 | 5 (41.7) | 14 (46.7) | 39 (69.6) |
| 1–3 | 5 (41.7) | 7 (23.3) | 13 (23.2) |
| ≥4 | 1 (8.3) | 4 (13.3) | 3 (5.4) |
| Unknown | 1 (8.3) | 5 (16.7) | 1 (1.8) |
| Adherence status | |||
| PNA | 1 (8.3) | 2 (6.7) | 8 (14.3) |
| SNA | 1 (8.3) | 12 (40) | 9 (16.1) |
| Adherent | 10 (83.3) | 16 (53.3) | 39 (69.6) |
Figure 2 shows that the PNA patients tend to have better overall survival (OS) than the adherent patients (HR 0.61, 95% CI 0.18–2.02). SNA patients may be more likely to have worse OS compared to the adherent patients (HR 1.5, 95% CI 0.78–3.01). Figure 3 shows a worsening survival with an increasing number of axillary metastases. Overall survival was poorer for those with ≥4 metastases (HR 5.1, 95% CI 1.8–14.2) compared to patients with no axillary metastases.
Figure 2.
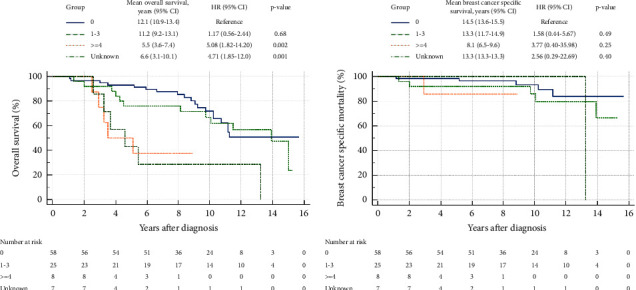
Overall survival (a) and breast cancer specific survival (b) according to the number of axillary lymph node metastases.
Figure 3.
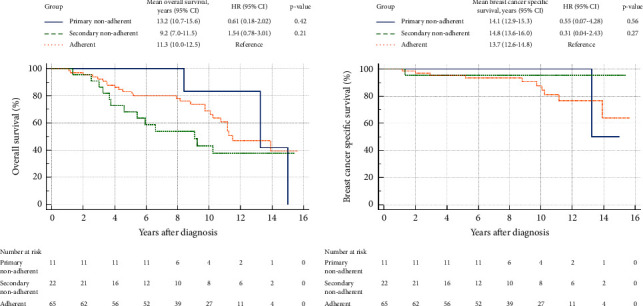
Overall survival (a) and breast cancer specific survival (b) according to adherence status.
4. Discussion
In this study, we found that about one-third of the patients had axillary lymph node metastases as documented on the postoperative pathology report. Nonadherence to the prescribed treatment was seen in approximately one-third of patients, and of these, one-third failed to collect their first and subsequent prescriptions.
In the CALBG 9343-trial [11], the majority of women had estrogen receptor positive T1 tumours, and all were ≥70 years of age. A total of 636 women were randomized to either breast conserving treatment with adjuvant radiotherapy and tamoxifen or breast conserving treatment and tamoxifen alone. In both arms, about one-third of the patients underwent axillary lymph node dissection (ALND). The study concluded that the two arms had similar survival and a similar risk of distant metastases. The only significant difference between the two groups was an increased risk of local or regional recurrences at five years for those who did not receive radiotherapy (4% risk for those not receiving radiotherapy and 1% risk for those receiving tamoxifen and radiotherapy). A long-term follow-up study of the CALBG 9343-trial published in 2013 [12] showed similar results. This study also demonstrated that the slightly increased risk of local recurrences in those treated without radiotherapy did not result in decreased survival.
Of the 91 patients in our study with known axillary status, 36.3% (33/91) had axillary metastases confirmed on the postoperative pathology report. According to the abovementioned guidelines, omitting SLNB is only done if the preoperative clinical examination of the axilla reveals no signs of metastases. It has been shown that the clinical evaluation of the axilla is inaccurate with regard to detecting axillary lymph node metastases [31, 32]. Similar to our results, a study of 5125 patients with negative preoperative clinical examinations of the axilla who underwent axillary dissection revealed that 34% had axillary metastatic disease according to the histopathological report [33]. A further study among women with no palpable axillary lymph nodes revealed that 32% had axillary metastases on histopathological examination [34].
Preoperative clinical data were unavailable to us. However, considering the rather low detection rates of axillary metastases by clinical examination alone, it is highly likely that metastases would have been missed. With the concomitant use of axillary ultrasound, a higher number of metastases would be detected. The use of both clinical examination and axillary ultrasound should therefore be encouraged [35, 36]. Some argue that axillary ultrasound should replace clinical examination of the axilla as they show that ultrasound has significantly higher sensitivity and accuracy than clinical examination alone [37]. However, ultrasound of the axilla is highly examinator dependent with a positive predictive value ranging from 77 to 83% and a negative predictive value between 52 and 67% [38, 39]. Furthermore, the necessity of axillary ultrasound in this setting can be questioned. The ACOSOG Z0011 study has changed the management of axillary metastases allowing for the omission of ALND in patients with one to two positive sentinel lymph nodes. Inclusion criteria in this study allowed patients with no palpable axillary lymphadenopathy eligible without the use of axillary ultrasound [40]. The SOUND trial randomized patients with T1 tumours and a negative axillary ultrasound to undergo SLNB or not. Of those who underwent SLNB, 13.7% had axillary metastases. However, there was no difference in distant disease-free survival or adjuvant treatment recommendations between the two groups [16]. Nevertheless, it must be acknowledged that in current clinical practice, axillary ultrasound is largely regarded to be standard-of-care in the preoperative evaluation [41].
As expected, we show that better survival is seen in patients with the fewest axillary lymph node metastases. Furthermore, studies have shown that a positive SLNB does affect adjuvant treatment decisions [42–44]. Radiation therapy in the CALBG 9343-trial was delivered as whole breast irradiation including axillary level I and II over 25 daily fractions. Due to the relatively low numbers of axillary metastases in these patients and the findings of similar survival for those who did, or did not, undergo axillary evaluation in the CALBG 9343-trials, the guidelines have deemed it safe to omit SLNB. Omitting SLNB means missing the opportunity to treat potential axillary metastases. However, the PRIME II study concluded that it is safe to omit radiotherapy in selected low-risk patients undergoing breast conserving surgery [14]. Omitting radiotherapy altogether might be difficult to accept for many clinicians, especially if the patient is in her early 70s with no or few comorbidities. Some argue that a more appropriate way to deescalate would be to give partial breast radiation to those with unknown axillary status [45]. However, others would be reluctant if axillary status is unknown for fear of missing undiagnosed axillary metastases [46].
Despite evidence that survival is not affected by the omission of SNLB, it might still be difficult to accept forgoing a well-established procedure. SLNB is considered to be safe and accurate and is associated with few complications [47, 48]. It is a relatively minor procedure performed at the same time as the primary breast surgery.
The present study shows that 33.7% (33/98) of the study population were nonadherent at five years of follow-up. Patients in the present study were subclassified into PNA or SNA. Although the numbers are low, over 10% of the patients were PNA. That is, they never initiated the antihormonal treatment they were prescribed. We suspect that these patients' lack of appreciation of the need for antihormonal treatment may be largely due to poor patient-doctor communication. If deescalating in surgery occurs by omitting the SLNB in elderly breast cancer patients, it is highly important that time is spent with the patient explaining the rationale for prescribing the antihormonal treatment in order to minimize both PNA and SNA. We observed that more than 20% of the patients were SNA. That is, they initiated the treatment, but for some reason, they failed to reach a MPR of ≥80%. The main reason for becoming nonadherent to antihormonal treatment is its side effects [49–51]. As many patients experience troublesome side effects affecting their quality of life, some of these patients choose to discontinue the treatment [52, 53]. Treating these side effects will help patients remain adherent to the treatment [52, 54]. We therefore suggest that in the case of deescalation by omitting SLNB, patients should be followed closely in order to uncover troublesome side effects or other reasons for nonadherence.
Various factors have been shown to affect adherence to antihormonal treatment. Advanced age and low-risk disease are known predictors of poor adherence to antihormonal treatment [49, 55, 56]. This puts the patients included in this recommendation at a higher risk of nonadherence compared to many other breast cancer patients. Omitting SLNB could potentially be a motivating factor for remaining adherent to the antihormonal treatment. This would perhaps give higher adherence rates than in the current study. Adherence behaviour is a complex matter, and causes of nonadherence are often multifactorial [57, 58]. It is therefore important to address the issue of adherence early, that is, even before the treatment is commenced.
A prerequisite of the abovementioned guidelines is five years of adherence to antihormonal treatment. However, previous literature has documented that adherence to adjuvant antihormonal treatment is poor [49, 52, 59–62]. Nonadherence rates have been described in the range of 10.8% [63] to 55% [54]. This wide range is probably due to the lack of a uniform definition of adherence and also varying ways of measuring it [64–66]. Based on these figures, it is likely that some of the patients included in the CALBG 9343-trial also were nonadherent. Despite this, survival rates were good. One could therefore argue that adherence to antihormonal therapy is not of importance in this population. However, it is our opinion that as we strive to improve patient care, adherence will continue to be of importance and even better survival rates could have been achieved with optimal adherence. It is therefore of importance to carefully select patients who will benefit from the treatment and avoid unnecessary side effects in those who will have a minimal effect of the treatment. Furthermore, the population is aging [5], and as we see a shift towards increased use of oral anticancer agents taken at home, the impact of poor adherence is likely to become even more important in the years to come [67, 68].
We have previously shown that PNA patients have better prognosis compared to both adherent and SNA patients [61]. Similarly, in the subgroup of patients in the present study, PNA patients tend to have better survival than both the adherent and SNA patients. This adds a contribution to the discussion regarding the possible overtreatment of low-risk patients as the PNA patients had the best survival.
Over the past decades, deescalation of treatments in breast cancer patients has gained increasing attention. As more treatment options become available and our knowledge expands, the need to tailor treatments to each individual patient has become an integral part of modern breast cancer management. Deescalation will spare the patient for potential morbidity associated with treatment and simultaneously reduce expenditure for health care systems [3, 24, 69]. However, deescalation should be carried out with caution and should be based on solid clinical research. Furthermore, implementation of deescalation guidelines should be monitored closely, especially when these guidelines do not seem to be widely accepted by clinicians.
From a financial point of view, health services would benefit from the omission of SLNB [70]. In an already pressurized health care system, the omission of SNLB would free up resources, shorten waiting lists, and allow for resources to be directed elsewhere [46].
In modern breast cancer management, treatment recommendations are increasingly based on tumour biology rather than nodal status [69]. Guidelines based on chronological age might not be the optimal way of stratifying patients. In the future, stratifying patients according to the biomarker profile and comorbidity are likely to become the preferred option in order to determine who is eligible for the omission of SLNB [71, 72].
One of the strengths of this study is the quantification of adherence rates in this specific group of patients and the subdivision of nonadherent patients into PNA and SNA, thus further examining the adherence behaviour of these patients. However, there are several limitations to this study. It is a small and retrospective study. Data regarding whether a patient underwent SLNB and axillary dissection or axillary dissection only were unfortunately not available to us. Patients with HER-2 positive tumours or with unknown HER-2 status were included in this study. These patients could have benefitted from SLNB as proven metastases would have affected adjuvant treatment decisions. Furthermore, patients with pT1 and histological grade 1 tumours were not recommended antihormonal treatment according to the national guidelines operative in the study period. These patients were therefore excluded from the material leaving the study population somewhat skewed towards larger tumours and higher histological grades. Furthermore, molecular subtyping was not available in this study population.
Some of the abovementioned patients with pT1 and histological grade 1 tumours did receive antihormonal treatment. These patients were therefore included in the study. We find this interesting as this group of patients was not treated according to the guidelines operative at the time of diagnosis. This confirms that the guidelines often are mere recommendations and that clinicians in selected cases choose to treat patients on an individual basis and according to the preferences of the patients. This shared decision-making will provide optimal care for each patient [73]. We believe that the individual assessment of each patient, especially with regard to comorbidity, is one of the main reasons for deviation from the guidelines.
5. Conclusion
Despite the small size of this study, it underpins much of the reluctance among clinicians to omit SLNB in elderly low-risk breast cancer patients. While reluctance is both understandable and necessary, the presence of axillary metastases in these low-risk breast cancer patients has not been shown to affect survival. In the future, we suggest that the selection of patients eligible for the omission of SLNB should be personalised based on the principles of shared decision-making including biomarker profiling and assessment of comorbidities.
Acknowledgments
This work was supported by The Dam Foundation (FO236264) and Central Norway Regional Health Authority (9051880).
Data Availability
Data that support the findings of this study are available from the Norwegian Prescription Database. Restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Patient data collected from the medical records in the hospital are not available due to patient confidentiality.
Ethical Approval
The study, including an outline of the statistical methods used, was approved by the Regional Committee for Medical and Health Research Ethics, Central Norway (561970), and the Regional Data Protection Office according to the Declaration of Helsinki.
Consent
Informed consent was obtained from all participants in the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
ID has performed the conceptualization of this article along with the data collection, data analysis, and interpretation of the data. Furthermore, she has drafted the article and revised it. AMB, HS, and MJE have contributed to the interpretation of the data, drafted the article, and provided final approval of the article. All authors have read and approved the final manuscript.
Supplementary Materials
STROBE checklist. STROBE statement: checklist of items that should be included in reports of cohort studies.
References
- 1.Brownlee S., Chalkidou K., Doust J., et al. Evidence for overuse of medical services around the world. The Lancet . 2017;390(10090):156–168. doi: 10.1016/s0140-6736(16)32585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ooi K. The pitfalls of overtreatment: why more care is not necessarily beneficial. Asian Bioethics Review . 2020;12(4):399–417. doi: 10.1007/s41649-020-00145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moynihan R., Doust J., Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ . 2012;344(4):p. e3502. doi: 10.1136/bmj.e3502. [DOI] [PubMed] [Google Scholar]
- 4.Santos Cruz R., Brito-Costa S., Santa Rosa B., Silvestre M. Overtreatment in elderly care: ethical considerations. Acta BioMedica . 2022;93(2) doi: 10.23750/abm.v93i2.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute on Aging. Why Population Aging Matters. A Global Perspective. 2007. https://www.nia.nih.gov/sites/default/files/2017-06/WPAM.pdf .
- 6.DeSantis C. E., Ma J., Gaudet M. M., et al. Breast cancer statistics, 2019. CA: A Cancer Journal for Clinicians . 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 7.Levinson W., Kallewaard M., Bhatia R. S., Wolfson D., Shortt S., Kerr E. A. Choosing Wisely’: a growing international campaign. BMJ Quality and Safety . 2015;24(2):167–174. doi: 10.1136/bmjqs-2014-003821. [DOI] [PubMed] [Google Scholar]
- 8.Grimshaw J. M., Patey A. M., Kirkham K. R., et al. De-implementing wisely: developing the evidence base to reduce low-value care. BMJ Quality and Safety . 2020;29(5):409–417. doi: 10.1136/bmjqs-2019-010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisely C. 2021. Society of Surgical Oncology, Don’t routinely use sentinel node biopsy in clinically node negative women ≥70 years of age with early stage hormone receptor positive HER2 negative invasive breast cancer. https://www.choosingwisely.org/clinician-lists/sso-sentinel-node-biopsy-in-node-negative-women-70-and-over/ [Google Scholar]
- 10.Brackstone M., Baldassarre F. G., Perera F. E., et al. Management of the axilla in early-stage breast cancer: ontario health (cancer care ontario) and ASCO guideline. Journal of Clinical Oncology . 2021;39(27):3056–3082. doi: 10.1200/jco.21.00934. [DOI] [PubMed] [Google Scholar]
- 11.Hughes K. S., Schnaper L. A., Berry D., et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. New England Journal of Medicine . 2004;351(10):971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 12.Hughes K. S., Schnaper L. A., Bellon J. R., et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. Journal of Clinical Oncology . 2013;31(19):2382–2387. doi: 10.1200/jco.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carleton N., Zou J., Fang Y., et al. Outcomes after sentinel lymph node biopsy and radiotherapy in older women with early-stage, estrogen receptor-positive breast cancer. JAMA Network Open . 2021;4:p. e216322. doi: 10.1001/jamanetworkopen.2021.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunkler I. H., Williams L. J., Jack W. J., Cameron D. A., Dixon J. M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. The Lancet Oncology . 2015;16(3):266–273. doi: 10.1016/s1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 15.Castelo M., Sutradhar R., Faught N., et al. The association between surgical axillary staging, adjuvant treatment use and survival in older women with early stage breast cancer: a population-based study. Annals of Surgical Oncology . 2023;30(7):3901–3912. doi: 10.1245/s10434-023-13274-0. [DOI] [PubMed] [Google Scholar]
- 16.Gentilini O. D., Botteri E., Sangalli C., et al. Sentinel lymph node biopsy vs No axillary surgery in patients with small breast cancer and negative results on ultrasonography of axillary lymph nodes: the SOUND randomized clinical trial. JAMA Oncology . 2023;9(11):1557–1564. doi: 10.1001/jamaoncol.2023.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu Q. D., Zhou M., Medeiros K. L., Peddi P., Wu X. C. Impact of CALGB 9343 trial and sociodemographic variation on patterns of adjuvant radiation therapy practice for elderly women (≥70 Years) with stage I, estrogen receptor-positive breast cancer: analysis of the national cancer data base. Anticancer Research . 2017;37(10):5585–5594. doi: 10.21873/anticanres.11992. [DOI] [PubMed] [Google Scholar]
- 18.Soulos P. R., Yu J. B., Roberts K. B., et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. Journal of Clinical Oncology . 2012;30(14):1601–1607. doi: 10.1200/jco.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidinger M., Maggi N., Dutilh G., et al. Use of sentinel lymph node biopsy in elderly patients with breast cancer – 10-year experience from a Swiss university hospital- 10-year experience from a Swiss university hospital. World Journal of Surgical Oncology . 2023;21(1):p. 176. doi: 10.1186/s12957-023-03062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T., Bredbeck B. C., Sinco B., et al. Variations in persistent use of low-value breast cancer surgery. JAMA Surg . 2021;156(4):353–362. doi: 10.1001/jamasurg.2020.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T., Baskin A., Miller J., et al. Trends in breast cancer treatment de-implementation in older patients with hormone receptor-positive breast cancer: a mixed methods study. Annals of Surgical Oncology . 2021;28(2):902–913. doi: 10.1245/s10434-020-08823-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minami C. A., Bryan A. F., Freedman R. A., et al. Assessment of oncologists’ perspectives on omission of sentinel lymph node biopsy in women 70 Years and older with early-stage hormone receptor-positive breast cancer. JAMA Network Open . 2022;5(8):p. e2228524. doi: 10.1001/jamanetworkopen.2022.28524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominici L. S., Sineshaw H. M., Jemal A., Lin C. C., King T. A., Freedman R. A. Patterns of axillary evaluation in older patients with breast cancer and associations with adjuvant therapy receipt. Breast Cancer Research and Treatment . 2018;167(2):555–566. doi: 10.1007/s10549-017-4528-6. [DOI] [PubMed] [Google Scholar]
- 24.Shubeck S. P., Morrow M., Dossett L. A. De-escalation in breast cancer surgery. NPJ Breast Cancer . 2022;8(1):p. 25. doi: 10.1038/s41523-022-00383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colla C. H., Mainor A. J. Choosing wisely campaign: valuable for providers who knew about it, but awareness remained constant, 2014-17. Health Affairs . 2017;36(11):2005–2011. doi: 10.1377/hlthaff.2017.0945. [DOI] [PubMed] [Google Scholar]
- 26.Smith M. E., Vitous C. A., Hughes T. M., Shubeck S. P., Jagsi R., Dossett L. A. Barriers and facilitators to de-implementation of the choosing Wisely® guidelines for low-value breast cancer surgery. Annals of Surgical Oncology . 2020;27(8):2653–2663. doi: 10.1245/s10434-020-08285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Research Square. Adjuvant antihormonal treatment in breast cancer. Understanding How Poor Adherence Affects Survival . 2023 [Google Scholar]
- 28.Cheen M. H. H., Tan Y. Z., Oh L. F., Wee H. L., Thumboo J. Prevalence of and factors associated with primary medication non-adherence in chronic disease: a systematic review and meta-analysis. International Journal of Clinical Practice . 2019;73(6):p. e13350. doi: 10.1111/ijcp.13350. [DOI] [PubMed] [Google Scholar]
- 29.Adams A. J., Stolpe S. F. Defining and measuring primary medication nonadherence: development of a quality measure. Journal of Managed Care and Specialty Pharmacy . 2016;22(5):516–523. doi: 10.18553/jmcp.2016.22.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyce G. F. Understanding primary nonadherence. Am. J. Pharm. Benefits . 2010;2:111–118. [PMC free article] [PubMed] [Google Scholar]
- 31.Lanng C., Hoffmann J., Galatius H., Engel U. Assessment of clinical palpation of the axilla as a criterion for performing the sentinel node procedure in breast cancer. European Journal of Surgical Oncology . 2007;33(3):281–284. doi: 10.1016/j.ejso.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Specht M. C., Fey J. V., Borgen P. I., Cody H. S. Is the clinically positive axilla in breast cancer really a contraindication to sentinel lymph node biopsy? Journal of the American College of Surgeons . 2005;200(1):10–14. doi: 10.1016/j.jamcollsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Voogd A. C., Coebergh J. W., Driel O. J. R. v., et al. The risk of nodal metastases in breast cancer patients with clinically negative lymph nodes: a population-based analysis. Breast Cancer Research and Treatment . 2000;62(1):63–69. doi: 10.1023/a:1006447825160. [DOI] [PubMed] [Google Scholar]
- 34.Majid S., Tengrup I., Manjer J. Clinical assessment of axillary lymph nodes and tumor size in breast cancer compared with histopathological examination: a population-based analysis of 2,537 women. World Journal of Surgery . 2013;37(1):67–71. doi: 10.1007/s00268-012-1788-5. [DOI] [PubMed] [Google Scholar]
- 35.Herrada J., Iyer R. B., Atkinson E. N., Sneige N., Buzdar A. U., Hortobagyi G. N. Relative value of physical examination, mammography, and breast sonography in evaluating the size of the primary tumor and regional lymph node metastases in women receiving neoadjuvant chemotherapy for locally advanced breast carcinoma. Clinical Cancer Research . 1997;3(9):1565–1569. [PubMed] [Google Scholar]
- 36.Balic M., Thomssen C., Würstlein R., Gnant M., Harbeck N. St. Gallen/vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care . 2019;14(2):103–110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., Li X., Fan Z., et al. Ultrasound as a replacement for physical examination in clinical staging of axillary lymph nodes in breast cancer patients. Thoracic Cancer . 2020;11(1):48–54. doi: 10.1111/1759-7714.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezvani A., Zahergivar A., Iranpour P., Akrami M., Kazemi S. Diagnostic accuracy of axillary ultrasonography compared with intra-operative pathological findings in patients with breast cancer. Asian Pacific Journal of Cancer Prevention . 2018;19(12):3615–3621. doi: 10.31557/apjcp.2018.19.12.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamaris S., Jamaluddin J., Islam T., et al. Is pre-operative axillary ultrasound alone sufficient to determine need for axillary dissection in early breast cancer patients? Medicine (Baltimore) . 2021;100(19):p. e25412. doi: 10.1097/md.0000000000025412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano A. E., Ballman K. V., McCall L., et al. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA . 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curigliano G., Burstein H. J., Gnant M., et al. Understanding breast cancer complexity to improve patient outcomes: the St gallen international consensus conference for the primary therapy of individuals with early breast cancer 2023. Annals of Oncology . 2023;34(11):970–986. doi: 10.1016/j.annonc.2023.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Chagpar A. B., Horowitz N., Sanft T., et al. Does lymph node status influence adjuvant therapy decision-making in women 70 years of age or older with clinically node negative hormone receptor positive breast cancer? The American Journal of Surgery . 2017;214(6):1082–1088. doi: 10.1016/j.amjsurg.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 43.Chagpar A. B., Hatzis C., Pusztai L., et al. Association of LN evaluation with survival in women aged 70 Years or older with clinically node-negative hormone receptor positive breast cancer. Annals of Surgical Oncology . 2017;24(10):3073–3081. doi: 10.1245/s10434-017-5936-x. [DOI] [PubMed] [Google Scholar]
- 44.Sun J., Mathias B. J., Sun W., et al. Is it wise to omit sentinel node biopsy in elderly patients with breast cancer? Annals of Surgical Oncology . 2021;28(1):320–329. doi: 10.1245/s10434-020-08759-1. [DOI] [PubMed] [Google Scholar]
- 45.Park K. U., Bazan J. G. Implications of omitting sentinel lymph node biopsy in patients older than 70 years. JAMA Surg . 2021;156(2):199–200. doi: 10.1001/jamasurg.2020.5008. [DOI] [PubMed] [Google Scholar]
- 46.McKevitt E., Nichol A. ASO author reflections: selective nodal staging and de-escalating adjuvant therapy. Annals of Surgical Oncology . 2021;28(11):5958–5959. doi: 10.1245/s10434-021-09937-5. [DOI] [PubMed] [Google Scholar]
- 47.Veronesi U., Paganelli G., Viale G., et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. New England Journal of Medicine . 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 48.Canavese G., Catturich A., Vecchio C., et al. Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: results of randomized trial. Annals of Oncology . 2009;20(6):1001–1007. doi: 10.1093/annonc/mdn746. [DOI] [PubMed] [Google Scholar]
- 49.Chirgwin J. H., Giobbie-Hurder A., Coates A. S., et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. Journal of Clinical Oncology . 2016;34(21):2452–2459. doi: 10.1200/jco.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells K. J., Pan T. M., Vázquez-Otero C., et al. Barriers and facilitators to endocrine therapy adherence among underserved hormone-receptor-positive breast cancer survivors: a qualitative study. Supportive Care in Cancer . 2016;24(10):4123–4130. doi: 10.1007/s00520-016-3229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paranjpe R., John G., Trivedi M., Abughosh S. Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Research and Treatment . 2019;174(2):297–305. doi: 10.1007/s10549-018-05073-z. [DOI] [PubMed] [Google Scholar]
- 52.Murphy C. C., Bartholomew L. K., Carpentier M. Y., Bluethmann S. M., Vernon S. W. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Research and Treatment . 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milata J. L., Otte J. L., Carpenter J. S. Oral endocrine therapy nonadherence, adverse effects, decisional support, and decisional needs in women with breast cancer. Cancer Nursing . 2018;41(1):E9–e18. doi: 10.1097/ncc.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atkins L., Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. European Journal of Cancer . 2006;42(14):2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Smaje A., Weston-Clark M., Raj R., Orlu M., Davis D., Rawle M. Factors associated with medication adherence in older patients: a systematic review. Aging Med (Milton) . 2018;1(3):254–266. doi: 10.1002/agm2.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Owusu C., Buist D. S., Field T. S., et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. Journal of Clinical Oncology . 2008;26(4):549–555. doi: 10.1200/jco.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 57.Gast A., Mathes T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Systematic Reviews . 2019;8(1):p. 112. doi: 10.1186/s13643-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yussof I., Mohd Tahir N. A., Hatah E., Mohamed Shah N. Factors influencing five-year adherence to adjuvant endocrine therapy in breast cancer patients: a systematic review. The Breast . 2022;62:22–35. doi: 10.1016/j.breast.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. European Journal of Cancer Care . 2012;21(1):10–19. doi: 10.1111/j.1365-2354.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 60.Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. The Lancet . 2011;378(9793):771–784. doi: 10.1016/s0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dragvoll I., Bofin A. M., Søiland H., Taraldsen G., Engstrøm M. J. Predictors of adherence and the role of primary non-adherence in antihormonal treatment of breast cancer. BMC Cancer . 2022;22(1):p. 1247. doi: 10.1186/s12885-022-10362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCowan C., Wang S., Thompson A. M., Makubate B., Petrie D. J. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. British Journal of Cancer . 2013;109(5):1172–1180. doi: 10.1038/bjc.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Güth U., Huang D. J., Schötzau A., et al. Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. British Journal of Cancer . 2008;99(3):428–433. doi: 10.1038/sj.bjc.6604525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cramer J. A., Roy A., Burrell A., et al. Medication compliance and persistence: terminology and definitions. Value in Health . 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 65.Güth U., Myrick M. E., Kilic N., Eppenberger-Castori S., Schmid S. M. Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Research and Treatment . 2012;131(2):491–499. doi: 10.1007/s10549-011-1801-y. [DOI] [PubMed] [Google Scholar]
- 66.Sattler E. L., Lee J. S., Perri M. Medication (Re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs and Aging . 2013;30(6):383–399. doi: 10.1007/s40266-013-0074-z. [DOI] [PubMed] [Google Scholar]
- 67.Given B. A., Spoelstra S. L., Grant M. The challenges of oral agents as antineoplastic treatments. Seminars in Oncology Nursing . 2011;27(2):93–103. doi: 10.1016/j.soncn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Partridge A. H., Avorn J., Wang P. S., Winer E. P. Adherence to therapy with oral antineoplastic agents. CancerSpectrum Knowledge Environment . 2002;94(9):652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 69.Pak L. M., Morrow M. Addressing the problem of overtreatment in breast cancer. Expert Review of Anticancer Therapy . 2022;22(5):535–548. doi: 10.1080/14737140.2022.2064277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McEvoy A. M., Poplack S., Nickel K., et al. Cost-effectiveness analyses demonstrate that observation is superior to sentinel lymph node biopsy for postmenopausal women with HR + breast cancer and negative axillary ultrasound. Breast Cancer Research and Treatment . 2020;183(2):251–262. doi: 10.1007/s10549-020-05768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davey M. G., Kerin E. P., McLaughlin R. P., et al. Evaluating the necessity for routine sentinel lymph node biopsy in postmenopausal patients being treated for clinically node negative breast cancer the era of RxPONDER. Clinical Breast Cancer . 2023;23(5):500–507. doi: 10.1016/j.clbc.2023.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Boughey J. C., Haffty B. G., Habermann E. B., Hoskin T. L., Goetz M. P. Has the time come to stop surgical staging of the axilla for all women age 70 Years or older with hormone receptor-positive breast cancer? Annals of Surgical Oncology . 2017;24(3):614–617. doi: 10.1245/s10434-016-5740-z. [DOI] [PubMed] [Google Scholar]
- 73.Montori V. M., Ruissen M. M., Hargraves I. G., Brito J. P., Kunneman M. Shared decision-making as a method of care. BMJ Evidence-Based Medicine . 2023;28(4):213–217. doi: 10.1136/bmjebm-2022-112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist. STROBE statement: checklist of items that should be included in reports of cohort studies.
Data Availability Statement
Data that support the findings of this study are available from the Norwegian Prescription Database. Restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Patient data collected from the medical records in the hospital are not available due to patient confidentiality.


