Summary
Background
Despite increasing availability of rapid molecular tests for the diagnosis of tuberculosis in high-burden settings, many people with tuberculosis are undiagnosed. Reliance on sputum as the primary specimen for tuberculosis diagnostics contributes to this diagnostic gap. We evaluated the diagnostic accuracy and additive yield of a novel stool quantitative PCR (qPCR) assay for the diagnosis of tuberculosis in three countries in Africa with high tuberculosis burdens.
Methods
We undertook a prospective diagnostic accuracy study in Eswatini, Mozambique, and Tanzania from Sept 21, 2020, to Feb 2, 2023, to compare the diagnostic accuracy for tuberculosis of a novel stool qPCR test with the current diagnostic standard for Mycobacterium tuberculosis DNA detection from sputum and stool, Xpert-MTB/RIF Ultra (Xpert Ultra). Sputum, stool, and urine samples were provided by a cohort of participants, aged 10 years or older, diagnosed with tuberculosis. Participants with tuberculosis (cases) were enrolled within 72 h of treatment initiation for tuberculosis diagnosed clinically or following laboratory confirmation. Participants without tuberculosis (controls) consisted of household contacts of the cases who did not develop tuberculosis during a 6-month follow-up. The performance was compared with a robust composite microbiological reference standard (CMRS).
Findings
The cohort of adolescents and adults (n=408) included 268 participants with confirmed or clinical tuberculosis (cases), 147 (55%) of whom were living with HIV, and 140 participants (controls) without tuberculosis. The sensitivity of the novel stool qPCR was 93·7% (95% CI 87·4–97·4) compared with participants with detectable growth on M tuberculosis culture, and 88·1% (81·3–93·0) compared with sputum Xpert Ultra. The stool qPCR had an equivalent sensitivity as sputum Xpert Ultra (94·8%, 89·1–98·1) compared with culture. Compared with the CMRS, the sensitivity of the stool qPCR was higher than the current standard for tuberculosis diagnostics on stool, Xpert Ultra (80·4%, 73·4–86·2 vs 73·5%, 66·0–80·1; p=0·025 on paired comparison). The qPCR also identified 17–21% additional tuberculosis cases compared to sputum Xpert Ultra or sputum culture. In controls without tuberculosis, the specificity of the stool qPCR was 96·9% (92·2–99·1).
Interpretation
In this study, a novel qPCR for the diagnosis of tuberculosis from stool specimens had a higher accuracy in adolescents and adults than the current diagnostic PCR gold standard on stool, Xpert-MTB/RIF Ultra, and equivalent sensitivity to Xpert-MTB/RIF Ultra on sputum.
Funding
National Institutes of Health (NIH) Allergy and Infectious Diseases, and NIH Fogarty International Center.
Introduction
Over the past decade, tuberculosis has surpassed HIV as the highest cause of mortality from a single pathogen. WHO estimates that 10·6 million people were diagnosed with tuberculosis in 2021, and that 1·6 million people died of tuberculosis that year.1 A major contributor to tuberculosis deaths is a missed or delay in diagnosis.2 People living with HIV account for 12% of the global estimated tuberculosis burden, and this rate exceeds 50% in several countries in sub-Saharan Africa.1 Delays in tuberculosis diagnosis still drive morbidity and mortality, and current diagnostic methods remain insufficient to achieve WHO’s EndTB Strategy goals towards tuberculosis elimination.3
The diagnosis of pulmonary tuberculosis is difficult in specific populations such as children, adolescents, and people living with HIV. Non-specific clinical symptoms, the paucibacillary nature of sputum, and the challenge of collecting induced or expectorated sputum all contribute to the absence of diagnostic confirmation and the high rate of clinical diagnosis, especially in people living with HIV. In Eswatini, Tanzania, and Mozambique—the three countries with high HIV and tuberculosis burdens from where adults and adolescents participated in this study—bacteriological confirmation of tuberculosis occurs in only 67% of notified cases in Eswatini, 43% in Tanzania, and 38% in Mozambique.1 This gap highlights the urgent need for novel diagnostic strategies in these settings, despite the availability of a WHO-endorsed PCR diagnostic: GeneXpert MTB/RIF Ultra (Xpert Ultra).4
Testing of sputum specimens by Xpert Ultra is widely accepted as the initial diagnostic test for tuberculosis.5 It has a sensitivity of 90·9% (95% CI 86·2–94·7) and a specificity of 95·6% (93·0–97·4) in adults with presumptive tuberculosis when compared with sputum culture;6 in people living with HIV, sensitivity drops to 87·6% (75·4–94·1).7 Adults with minimally symptomatic tuberculosis and adults admitted to hosptial8 are often not able to provide an adequate sputum sample. Due to similar challenges with sputum collection in children, stool Xpert Ultra is now recommended by WHO as a first-line test in children with presumed tuberculosis.9–11 Although not yet recommended in adult guidelines, stool testing could represent an important new diagnostic strategy in this population, particularly in people with HIV for whom there is emerging evidence of additional yield with stool Xpert Ultra testing.12
Respiratory secretions are routinely emptied into the gastrointestinal tract. In 1933, this normal physiology was shown through mucociliary activity of the bronchial epithelium in the absence of cough.13 Mycobacterium tuberculosis bacilli can therefore be detected in stool following passage through the digestive tract.14 Collection of stool represents a simple, less invasive diagnostic strategy compared with sputum induction or bronchoscopy in people who cannot expectorate. Additionally, stool can easily be collected at the primary health facility or community level, thereby expanding the availability of diagnostic testing. Despite these advantages, evidence in children suggests that stool Xpert might have a lower sensitivity than sputum when compared with the reference standard of sputum culture.10,11 Fewer studies have investigated the diagnostic accuracy of stool Xpert Ultra in adolescents and adults. Current evidence indicates that use of a soil DNA extraction kit could improve DNA extraction from stool,14,15 thereby increasing the test sensitivity of this specimen type and making stool quantitative PCR (qPCR) an attractive diagnostic option for pulmonary tuberculosis.
Therefore, we evaluated the sensitivity of a soil DNA extraction kit combined with established in-house M tuberculosis stool qPCR for the detection of M tuberculosis compared with individual reference tests and a composite microbiological reference standard (CMRS) consisting of sputum culture, sputum Xpert Ultra, and urine lipoarabinomannan in people living with HIV. We assessed the specificity of the stool qPCR in a control population without tuberculosis. The study was designed to determine whether the stool qPCR had an equivalent sensitivity to sputum Xpert Ultra and to compare the diagnostic accuracy of the stool qPCR with stool Xpert Ultra.
Methods
Study design and population
This prospective diagnostic accuracy study was undertaken in three sub-Saharan African countries: Eswatini, Mozambique, and Tanzania, from Sept 21, 2020, to Feb 2, 2023. Sputum, stool, and urine samples were provided by a cohort of participants, aged 10 years orolder, diagnosed with tuberculosis. Participants with tuberculosis (cases) were enrolled within 72 h of treatment initiation for tuberculosis diagnosed clinically or following laboratory confirmation at seven study entry points, which included community-based, outpatient, and inpatient settings. Participants without tuberculosis (controls) consisted of household contacts of the cases; tuberculosis was excluded in controls on the basis of negative sputum Xpert Ultra, sputum culture, or urine lipoarabinomannan tests—if clinically indicated as decided by the study physician—or 6 months of longitudinal clinical evaluation to exclude the development of incident tuberculosis.
All participants provided written informed consent; for adolescents aged 10–17 years, written consent was provided by the legal guardian and assent by the participant before any study procedure. The study was conducted according to the principles of the Helsinki Declaration and approved by all required ethics bodies including Baylor Children’s Foundation-Eswatini Institutional Review Board, Eswatini Health and Human Research Review Board (EHHRRB037/2023), the National Bioethics Committee from Mozambique (CNBS), the Tanzania National Institute for Medical Research and the Mbeya Medical Research and Ethics Review Committee, and the Baylor College of Medicine Biomedical Research and Assurance Information Network (H-34210).
Laboratory procedures
Participants provided one respiratory specimen (minimum volume of 2 mL), a stool specimen (minimum volume of 5 g), and a urine specimen (minimum 2 mL) for diagnostic and study-specific procedures at enrolment. When participants were not able to provide enough sputum for both an Xpert Ultra and culture, the Xpert Ultra was prioritised in accordance with standard of care in the study settings. Specimens were also collected by home visits within 72 h of enrolment as needed. Participants with (cases) and without (controls) tuberculosis were clinically monitored for 6 months to ensure that baseline cohort classification was accurate. STARD (Standards for Reporting of Diagnostic Accuracy Studies) guidelines were used as reference for the study design and analysis.15
Study participants provided a spot sputum that was evaluated by Xpert Ultra (Cepheid, Sunnyvale, CA, USA) and liquid culture (BACTEC Mycobacteria Growth Indicator Tube [MGIT] system; Becton Dickinson, Franklin Lakes, NJ, USA). Xpert Ultra was used on sputum specimens in accordance with the manufacturer’s instructions; invalid results were repeated, and in case of a second error, were excluded. For completion of liquid culture, raw samples were decontaminated by the Kubica method,16 and 500 μL of the decontaminated pellet were inoculated into MGIT liquid medium and incubated in a BACTEC MGIT 960 mycobacterial detection instrument (Becton Dickinson, Franklin Lakes, NJ, USA). After 42 days without growth, samples were classified as negative. Reference tests were done without knowledge of index test results.
Stool samples were tested by Xpert Ultra, as a direct comparator, and the molecular assay under study (in-house stool M tuberculosis real-time qPCR). For completion of stool Xpert Ultra, fecal material was processed following the simple one-step stool processing method.12 For completion of stool real-time qPCR, DNA was extracted from 50 mg of stool using the MP FastDNA for Soil Kit (MP Biochemicals, Santa Anna, CA, USA) and the PCR targeted the IS6110 M tuberculosis gene as previously described14 (appendix p 11).
Urine samples in people living with HIV with advanced disease or tuberculosis symptoms were tested by lateral flow urine lipoarabinomannan (Alere, Waltham, MA, USA; and Abbott Laboratories, Abbott Park, IL, USA) following manufacturer instructions as a complementary comparator test, aligned with national guidelines.17 All diagnostic assays were completed in study-specific research laboratories in each country, with the exception of culture and sputum Xpert in Eswatini, which were completed in the National Reference Laboratory and in Ministry of Health clinical laboratories, respectively.
Statistical analysis
Recruitment was designed to determine whether the stool qPCR assay had equivalent diagnostic accuracy to sputum Xpert Ultra compared with a reference standard of sputum M tuberculosis culture. Assuming an equivalence margin of 10% for test sensitivity, 85% sensitivity for Xpert Ultra, and a 7% qPCR positive and Xpert Ultra negative rate, we determined that the study required 137 participants with tuberculosis (cases) to achieve 80% power using a two-sided equivalence test for correlated proportions comparing stool qPCR with the reference standard. Given an expected HIV prevalence of 60% among participants with tuberculosis, subgroup analysis was planned a priori among tuberculosis case participants living with HIV; accordingly, enrolment was continued to meet the subgroup target of 137 people living with HIV with tuberculosis.
Interpretation of qPCR results and colony forming unit cut point has been previously determined and qPCR was undertaken without knowledge of the reference test result.8 The sensitivity of the stool qPCR was calculated compared with a CMRS, which was positive if a participant had a positive sputum Xpert Ultra, sputum culture, or lipoarabinomannan in people living with HIV with advanced disease, but did not exclude participants who were missing a valid result for any of the assays. The clinical composite reference standard (CCRS) included all participants enrolled and treated for tuberculosis. The sensitivity of stool qPCR was also compared with a complete MRS, which was defined by the same criteria as the CMRS but limited to participants who had a valid result for all tests included in the reference standard, including sputum Xpert Ultra, sputum culture, and lipoarabinomannan, when indicated by national guidelines, in people living with HIV. We calculated the specificity of the stool qPCR in the control population without tuberculosis. We compared the sensitivity of stool qPCR and of sputum Xpert Ultra using two one-sided tests for proportions, with an equivalence margin of 10%.18 McNemar’s test was used to analyse the sensitivity of the stool qPCR and stool Xpert Ultra compared with the CMRS, CCRS, and complete MRS in cases, and to analyse the specificity in controls without tuberculosis. Subgroup analyses assessed the accuracy of the qPCR by HIV status and age. Friedman’s test was used to compare median cycle thresholds between sputum Xpert Ultra, stool Xpert Ultra, and the qPCR tests, followed by the Friedman effect size test to measure the correlation across tests.
To evaluate the additional diagnostic value of M tuberculosis stool qPCR, we enumerated the additional cases confirmed by M tuberculosis stool qPCR in participants with undetectable growth on sputum culture, undetectable M tuberculosis DNA on sputum or stool Xpert Ultra, and undetectable lipoarabinomannan from urine (in people living with HIV only). We also compared the additional diagnostic yield provided by stool qPCR to these standard diagnostics individually. All statistical analysis was done with R (version 4.1.2).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
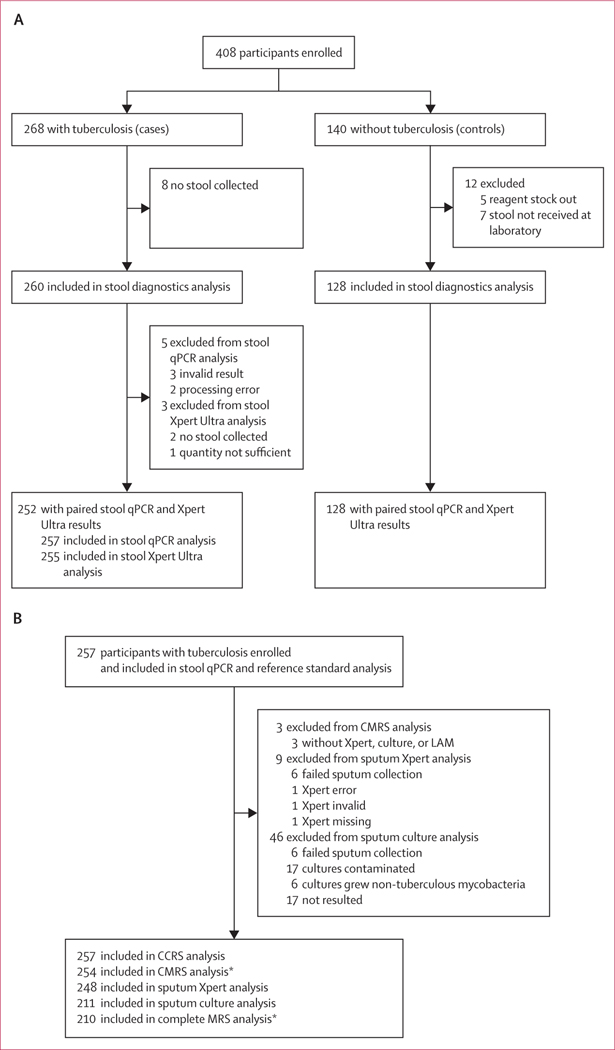
We recruited 408 participants, including 268 cases with tuberculosis and 140 controls without tuberculosis (figure 1). Among participants with tuberculosis and a valid stool qPCR result, 163 (63%) participants were confirmed by urine lipoarabinomannan, sputum Xpert Ultra, or sputum culture. The remaining 94 (37%) were diagnosed with tuberculosis on the basis of radiographic and clinical information. In participants with tuberculosis, 254 (95%) were included in the analysis evaluating the sensitivity of the stool qPCR compared with the CMRS, 257 (96%) in the analysis evaluating the sensitivity of the stool qPCR compared with the CCRS, and 210 (78%) in the analysis evaluating the sensitivity of the stool qPCR compared with complete MRS. Among participants without tuberculosis (controls), 128 (91%) were included in the analysis evaluating qPCR specificity.
Figure 1: Study profile.
STARD algorithm for inclusion of participants with tuberculosis and controls without signs and symptoms of tuberculosis who did not develop tuberculosis during a 6-month follow-up. (A) Participants in the stool diagnostics analysis. (B) Participants in the stool qPCR and reference standard analysis. qPCR=quantitative PCR. Xpert=Xpert Ultra assay. CMRS=composite microbiological reference standard. CCRS=clinical composite reference standard. Complete MRS=complete microbiological reference standard. LAM=lipoarabinomannan. *Participants living with HIV (n=147) who qualified by national guidelines (n=122) had a urine LAM test, which was included in these reference standards.
The study population was composed of 155 (38%) adolescents (aged 10–19 years) and 223 (55%) female participants; these groups were over-represented among controls compared with cases (table 1). Aligned with regional epidemiological risk factors, 147 (55%) participants with tuberculosis had HIV, compared with 11 (8%) control participants (table 1).
Table 1:
Characteristics of the study cohort
| All (n=408) | Cases (n=268) | Controls (n=140) | |
|---|---|---|---|
|
| |||
| Age, years | |||
| Median, IQR | 26·0 (15–42·4) | 32·9 (19·0–46·3) | 16 (12·5–28·6) |
| 10·19 | 155 (38%) | 71 (26%) | 84(60%) |
| ≥20 | 253 (62%) | 197 (74%) | 56 (40%) |
| Sex | |||
| Male | 185 (45%) | 135 (50%) | 50 (36%) |
| Female | 223 (55%) | 133 (50%) | 90 (64%) |
| BMI, kg/m2 | |||
| Median, IQR | 19·8 (17–23·2) | 19·8 (16·9–22·4) | 20·1 (17·0–25·0) |
| NA | 21 (5%) | 1 (<1%) | 20 (14%) |
| Enrolment site | |||
| Home | 111 (27%) | 41 (15%) | 70 (50%) |
| Inpatient | 27(7%) | 26 (10%) | 1 (1%) |
| Outpatient | 270 (66%) | 201 (75%) | 69 (49%) |
| HIV status | |||
| Positive | 158 (39%) | 147 (55%) | 11 (8%) |
| Negative | 245 (60%) | 119 (44%) | 126 (90%) |
| NA | 5 (1%) | 2 (1%) | 3 (2%) |
| BCG (scar or history) | |||
| Received | 372 (91%) | 249 (93%) | 123 (88%) |
| Not received | 22 (5%) | 9 (33%) | 13 (9%) |
| NA | 13 (3%) | 19 (3%) | 0 |
| TPT | |||
| Current | 3 (1%) | 2 (1%) | 1 (1%) |
| TPT last year | 10 (2%) | 9 (3%) | 1 (1%) |
| TPT >1 year ago | 12 (3%) | 11 (4%) | 1 (1%) |
| Never received | 383 (94%) | 246 (92%) | 137 (97%) |
| Site | |||
| Eswatini | 181 (44%) | 114 (43%) | 67 (48%) |
| Mozambique | 169 (41%) | 121 (45%) | 48 (34%) |
| Tanzania | 58 (14%) | 33 (12%) | 25 (17%) |
Data are n (%), unless otherwise indicated. TPT=tuberculosis preventive therapy. NA=not available.
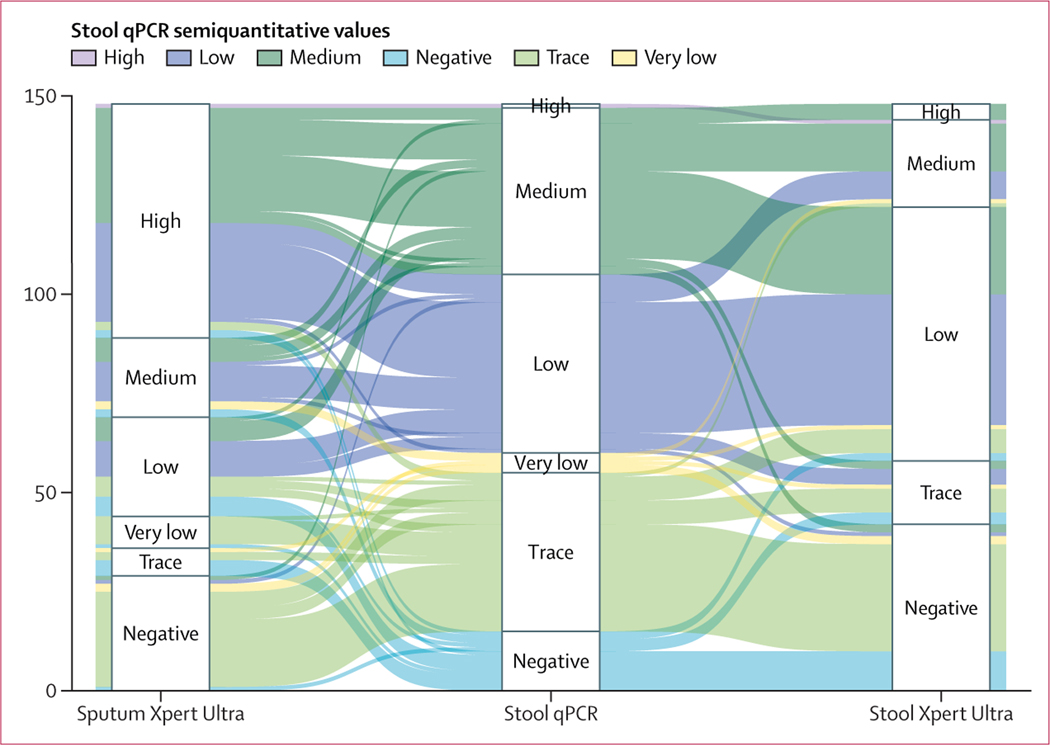
Stool and sputum were collected in 260 and 261 of 268 participants with tuberculosis, respectively. When both tests were performed, there was strong agreement between the results of the index test (stool qPCR) and recommended reference tests, ranging from 85·6% (95% CI 81·2–89·3) for sputum Xpert Ultra, 85·6% (80·6–89·7) for sputum culture, and 87·8% (84·1–90·9) for stool Xpert Ultra (appendix p 3). Median cycle threshold values between the stool Xpert Ultra (16·9, IQR 16·5–18·9), sputum Xpert Ultra (16·3, 16·1–16·6), and the stool qPCR differed (22·9, 19·9–26·5; p<0·0001) but were strongly correlated (W=0·82; figure 2).
Figure 2: Alluvial plot of semiquantitative values of qPCR, sputum Xpert Ultra, and stool Xpert Ultra.
Alluvial plot showing the relationship between semiquantitative measures of the cycle threshold recorded in stool by a novel qPCR and by Xpert Ultra. Included are 148 participants treated for tuberculosis who had a sputum Xpert Ultra, stool Xpert Ultra, and a stool qPCR undertaken and had a positive result for at least one test. qPCR=quantitative PCR.
Among the 268 participants with clinically or microbiologically diagnosed tuberculosis, sputum culture test results were positive in 117 of 242 (48%) tests undertaken, sputum Xpert Ultra in 142 of 260 (55%), stool Xpert Ultra in 125 of 260 (48%), and stool qPCR in 148 of 257 (58%). Compared with participants with detectable growth on M tuberculosis culture, stool qPCR had a sensitivity of 93·7% (95% CI 87·4–97·4) and confirmed tuberculosis in 29 (21%) additional participants who were negative by culture (appendix p 7). We compared the sensitivity of stool qPCR (93·7%, 95% CI 87·4–97·4) and sputum Xpert Ultra (94·8%, 89·1–98·1) against sputum culture using two one-sided-tests for proportions, and showed equivalence. Compared with sputum Xpert Ultra, stool qPCR had a sensitivity of 88·1% (95% CI 81·3–93·0), and was positive in 27 (17%) additional participants with tuberculosis who were negative by sputum Xpert Ultra (appendix p 7). Stool Xpert Ultra had a sensitivity of 92·7% (95% CI 86·0–96·8) compared with culture and 82·1% (74·5–88·2) compared with sputum Xpert Ultra. On paired comparison, there was no difference in sensitivity between the stool qPCR and stool Xpert Ultra (table 2) compared with sputum Xpert Ultra or sputum culture; however, compared with stool Xpert Ultra alone, stool qPCR was positive in an additional 31 (20%) of 154 participants with tuberculosis (appendix p 7).
Table 2:
Sensitivity of stool qPCR and stool Xpert Ultra compared with sputum culture and sputum Xpert Ultra among participants with tuberculosis, with paired comparisons limited to participants with stool qPCR and stool Xpert Ultra results
| Versus positive sputum culture (n=108) |
Versus sputum Xpert Ultra with MTB detected result (n=132) |
|||||||
|---|---|---|---|---|---|---|---|---|
| TP | FN | Sensitivity (%, 95% CI) | p value | TP | FN | Sensitivity (%, 95% CI) | p value | |
|
| ||||||||
| Stool qPCR | 102 | 6 | 94·4% (88·3–97·9) | p=0·77 | 116 | 16 | 87·9% (81·1–92·9) | p=0·11 |
| Stool Xpert | 100 | 8 | 92·6% (85·9–96·7) | .. | 108 | 24 | 81·8% (74·2–88·0) | .. |
TP=true positive. FN=false negative. qPCR=quantitative PCR. MTB=Mycobacterium tuberculosis.
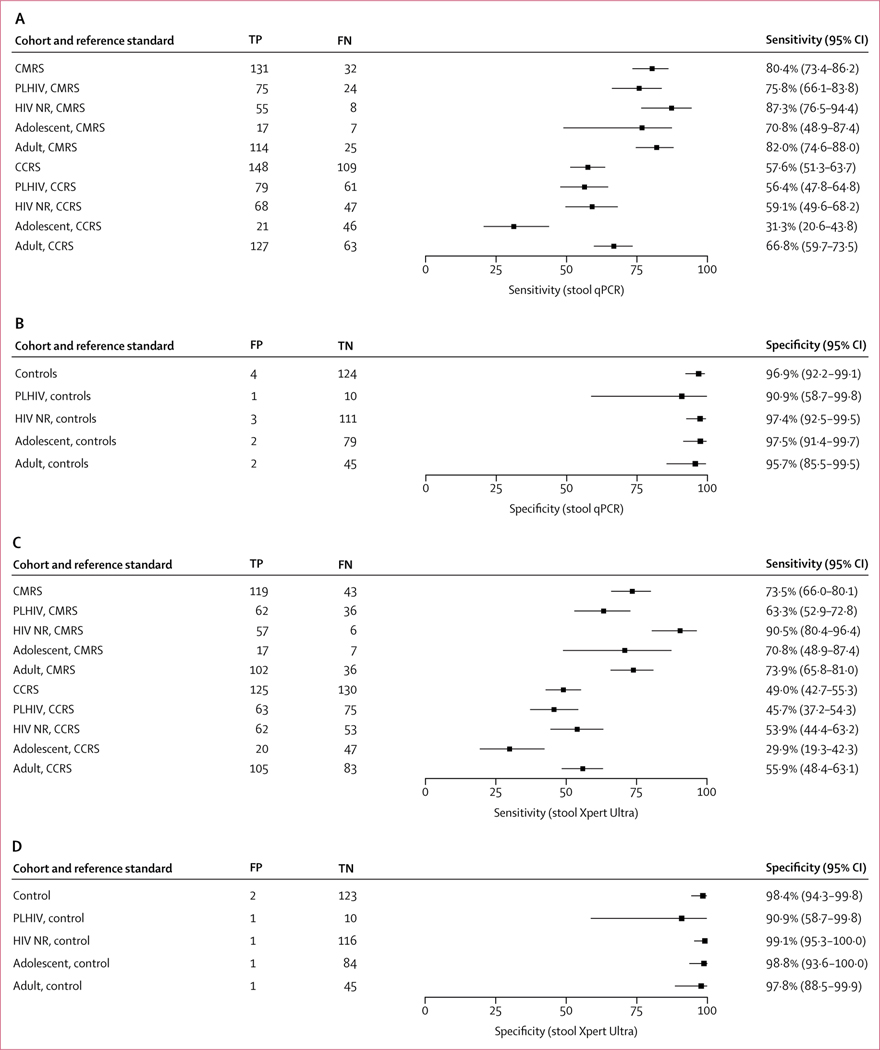
When compared with the CMRS that included a positive result by sputum Xpert Ultra, sputum culture, or urine lipoarabinomannan in people living with HIV, stool qPCR assay had a sensitivity of 80·4% (95% CI 73·4–86·2; figure 3A) and was the only positive test in 17 of 254 (7%) participants. Stool Xpert Ultra had a sensitivity of 73·5% (95% CI 66·0–80·1) compared with the CMRS (figure 3C). The sensitivity of the stool qPCR and stool Xpert Ultra was similar when compared to the complete MRS (appendix p 4). The sensitivity of the stool qPCR was superior to stool Xpert Ultra in a paired comparison with the CMRS (p=0·025; table 3) and the complete MRS (p=0·044; appendix p 4). Compared with a CCRS that included microbiological tests and a clinical diagnosis of tuberculosis, a single stool qPCR had a sensitivity of 57·6% (95% CI 51·3–63·7; figure 3A) versus 49·0% (42·7–55·3; figure 3C) for stool Xpert Ultra (p=0·00090 on paired comparison; table 3). The specificity of the stool qPCR in the control population was 96·9% (95% CI 92·2–99·1), which did not differ from the specificity of stool Xpert Ultra of 98·4% (94·3–99·8; figure 3D) on paired comparison (p=0·68; table 4).
Figure 3: Diagnostic accuracy of the stool qPCR (A and B) and stool Xpert Ultra (C and D) undertaken on stool specimens for the diagnosis of tuberculosis.
(A) Sensitivity of the stool qPCR test compared with the CMRS and CCRS in participants with tuberculosis. (B) Specificity of the stool qPCR in the control population without tuberculosis. (C) Sensitivity of the stool Xpert test compared with the CMRS and CCRS in participants with tuberculosis. (D) Specificity of the stool Xpert test in the control population without tuberculosis. The analysis for both assays was completed in the entire cohort and restricted to people living with HIV, HIV-non-reactive participants, adolescents, and adults with a valid stool qPCR or stool Xpert result, respectively. qPCR=quantitative PCR. CMRS=composite microbiological reference standard. CCRS=clinical composite reference standard. PLHIV=people living with HIV. HIV NR=non-reactive HIV. TP=true positive. FN=false negative. FP=false positive. TN=true negative.
Table 3:
Paired comparisons of stool qPCR and stool Xpert Ultra sensitivity compared with a composite microbiological reference standard, a confirmed or clinical tuberculosis diagnosis, and stool qPCR
| Stool qPCR sensitivity (%, 95% CI) | Stool Xpert Ultra sensitivity (%, 95% CI) | p value | |
|---|---|---|---|
|
| |||
| Positive by CMRS * | |||
| HIV positive (N=97) | 75·3% (65·5–83·5) | 62·9% (52·5–72·5) | 0·0060 |
| HIV negative (N=62) | 88·7% (78·1–95·3) | 90·3% (80·1–96·4) | 1·00 |
| Adult (N=137) | 81·8% (74·3–87·8) | 73·7% (65·5–80·9) | 0·029 |
| Adolescent (N=23) | 73·9% (51·6–89·8) | 69·6% (47·1–86·8) | 1·00 |
| Complete (N=160) | 80·6% (73.6–86·4) | 73·1% (65·6–79·8) | 0·025 |
| Diagnosed with tuberculosis | |||
| HIV positive (N=137) | 56·2% (47·5–64·7) | 45·3% (36·7–54·0) | 0·0023 |
| HIV negative (N=113) | 59·3% (49·6–68·4) | 54·0% (44·4–63·4) | 0·24 |
| Adult (N=187) | 66·8% (59·6–73·5) | 55·6% (48·2–62·9) | 0·00033 |
| Adolescent (N=65) | 30·8% (19·9–43·4) | 29·2% (18·6–41·8) | 1·00 |
| Complete (N=252) | 57·5% (51·2–63·7) | 48·8% (42·5–55·2) | 0·00090 |
qPCR=quantitative PCR.
Composite microbiological reference standard (CMRS): positive if sputum culture, sputum Xpert Ultra, or lipoarabinomannan in people living with HIV is positive; participants not required to have had all reference tests undertaken.
Table 4:
Paired comparisons of stool qPCR and stool Xpert Ultra specificity in a control population without tuberculosis
| Stool qPCR specificity (%, 95% CI) | Stool Xpert Ultra specificity (%, 95% CI) | p value | |
|---|---|---|---|
|
| |||
| HIV positive (N=11) | 90·9% (58·7–99·8) | 90·9% (58·7–99·8) | 1·00 |
| HIV negative (N=111) | 97·3% (92·3–99·4) | 99·1% (95·1–1·0) | 0·62 |
| Adult (N=46) | 95·7% (85·2–99·5) | 97·8% (88·5–99·9) | 1·00 |
| Adolescent (N=79) | 97·5% (91·2–99·7) | 98·7% (93·1–1·0) | 1·00 |
| Complete (N=125) qPCR=quantitative PCR. |
96·8% (92·0–99·1) | 98·4% (94·3–99·8) | 0·68 |
In subgroup analyses, the sensitivity of qPCR was 75·8% (95% CI 66·1–83·8) in people living with HIV and 87·3% (76·5–94·4) in participants without HIV compared with the CMRS. This sensitivity was higher than that for stool Xpert Ultra in people living with HIV (63·3%, 52·9–72·8, figure 3C; p=0·0060, table 3) but no different from stool Xpert Ultra in people without HIV (90·5%, 80·4–96·4, figure 3C; p=1·00, table 3). When compared with the CMRS, the sensitivity of the stool qPCR was 70·8% (95% CI 48·9–87·4) in adolescents compared with 82·0% (74·6–88·0) in adults (figure 3A). On paired comparison, the sensitivity of stool qPCR was superior to stool Xpert Ultra in adults (p=0·029, table 3). The specificity within all subgroups was greater than 95·7% for qPCR and 97·8% for stool Xpert Ultra, apart from the HIV-positive subgroup for which the small number of controls limited analytical power (figure 3B, D), with no difference by paired comparisons (table 4). All sensitivity estimates and paired comparisons in subgroups were similar when compared with the complete MRS (appendix pp 4, 8). Details of participants with an isolated positive stool qPCR and control participants with positive stool qPCR or stool Xpert are shown in the appendix (pp 5–6).
Among participants with confirmed or clinical tuberculosis, 205 of 268 (76%) had valid results for all diagnostic tests (appendix pp 9–10). In participants with tuberculosis who had valid results for all diagnostic tests undertaken, tuberculosis was microbiologically confirmed by at least one diagnostic test in 143 (70%). Among the subgroup with microbiologically confirmed tuberculosis, the most common pattern observed was positivity by all microbiological tests (90 of 143 [63%]), followed by stool qPCR only (14 of 143 [10%]). In participants without HIV (appendix pp 9–10), 94 of 119 (79%) had valid results for all diagnostic tests, and at least one test was positive in 68 of 94 (72%) participants. Of these results, eight of 68 (12%) were positive by stool qPCR alone. Among people living with HIV (appendix pp 9–10), 95 of 147 (65%) had valid results for all diagnostic reference tests including lipoarabinomannan, and 78 (82%) were positive by at least a single test. The most common pattern was positivity by M tuberculosis culture, stool Xpert Ultra, stool qPCR, and sputum Xpert Ultra (24 of 78 [31%]), followed by all testing including lipoarabinomannan (18 of 78 [23%]). In the 78 participants with HIV who had tuberculosis and valid results for all diagnostic tests, three (4%) had qPCR as the only positive test result compared with eight (10%) for whom only lipoarabinomannan was positive.
Discussion
In three African countries with a high burden of tuberculosis and HIV infection, we evaluated a novel qPCR diagnostic platform using stool specimens for the diagnosis of tuberculosis in adolescents and adults with pulmonary tuberculosis. The sensitivity of the stool qPCR assay was equivalent to sputum Xpert Ultra, using a predefined equivalency margin of 10%, when compared to a reference standard of sputum culture. Further, the sensitivity of the stool qPCR assay was superior to the sensitivity of the current standard stool PCR, Xpert Ultra, when compared with the CMRS within the complete cohort, and when limited to adults and people living with HIV.
The sensitivity of the stool qPCR assay and stool Xpert Ultra assays did not differ when compared with the sputum Xpert Ultra and sputum culture individually. This observation reflects the suboptimal sensitivity of these sputum-based individual tests as standalone reference standards, particularly in people living with HIV who have paucibacillary sputum.19 Critically, the specificity of qPCR did not differ from stool Xpert Ultra in the control population. Considering the high specificity of the qPCR, the additional yield of 17–21% gained compared with individual reference tests was notable. Compared with the robust CMRS there was still an additional yield of 7%. These results suggest the additive value of this stool-based diagnostic compared with traditional diagnostics for tuberculosis. The stool qPCR test provided additive microbiological confirmation in adults in whom respiratory culture and sputum Xpert Ultra were otherwise negative.
The sensitivity of the stool qPCR in this study was similar to that in earlier reports of stool Xpert MTB/RIF in adults,20–22 which showed a sensitivity ranging from 84·6% to 94·8%, but was significantly higher than stool Xpert Ultra in this study. Findings from a study of adults living with HIV also suggested value in stool diagnostics and showed an additional yield of 4·1% (95% CI 1·6–6·6) with stool Xpert Ultra.12 The sensitivity of other stool PCR tests has ranged from 70% to 97%;23–25 nevertheless, interpretation of previously published data is limited by small cohort sizes, use of different PCR techniques, and comparison to heterogeneous reference standards.
The number of participants with tuberculosis who did not provide a stool or sputum sample was similar in our study (eight participants for stool and seven for sputum). A stool test that performs similarly to sputum Xpert Ultra presents an attractive option for patients who struggle with sputum production or in settings in which sputum induction is unsuccessful or not available.8 In areas with high levels of M tuberculosis drug resistance, the DNA isolated for stool qPCR can additionally be used for targeted next-generation sequencing that accurately predicts results from sputum phenotypic drug susceptibility tests for first-line and second-line tuberculosis drugs.26
There are limitations to non-design locked PCR tests, predominantly related to heterogeneity in performance between laboratories and the risk for contamination affecting results. The index test in this study performed similarly across three clinical and research laboratories in Eswatini, Mozambique, and Tanzania, suggesting that these barriers can be overcome. However, for clinical use in non-reference laboratories, the DNA isolation technique used for this assay would need to be automated to reduce cost and time, and limit the risk for contamination. The main difference between the stool qPCR assay and the stool Xpert Ultra assay is the multistep soil DNA isolation kit, originally modified to optimise DNA isolation from stool for helminth diagnosis.27 Incorporation of these steps within a stool-specific cartridge would likely result in the tests having similar performance. Use of two concordant replicates in the qPCR to reduce test variability and additional low-cost approaches to improve sensitivity, such as sample preservation in DNA transport media, are also important considerations for commercialisation.28,29 On the basis of the additional diagnostic yield of the qPCR assay, health-care systems could benefit from strategic investment in automated stool-specific commercial assays to optimise performance with this specimen type. Other limitations of the study included challenges with sputum collection in asymptomatic controls, which is a limitation of sputum as a specimen. Tuberculosis was excluded in these participants through longitudinal follow-up. Due to limitations with laboratory infrastructure and sputum quantity, some participants did not complete M tuberculosis culture; however, the combined microbiological reference standard was arguably more robust than culture alone.
Although stool is recommended as a first-line tuberculosis diagnostic specimen only in children, findings from this study suggest that analysis of stool would improve routine diagnostic yield for adults and adolescents as well. Additional evaluation of this assay as a complement to traditional sputum specimens in people with presumed tuberculosis is warranted.
Supplementary Material
Research in context.
Evidence before this study
Tuberculosis is conventionally diagnosed by molecular diagnostics or culture of sputum; however, this specimen can be difficult to obtain from individuals who are unable to produce sputum. By contrast, stool is available from almost all patients as an alternative diagnostic specimen. Stool-based molecular diagnostics are now recommended by WHO in children being assessed for active tuberculosis. Stool can also be a useful tool for the diagnosis of tuberculosis in adults but is currently not recommended by WHO for this population. We searched PubMed from database inception to June 15, 2023, for articles containing the terms “Mycobacterium tuberculosis” and “polymerase chain reaction” in combination with “stool” or “feces” in any language, limited to adolescents and adults. We found eight articles that evaluated the performance of molecular diagnostics for the detection of M tuberculosis using PCR testing of stool specimens. The quality of the data was poor, and four of the eight articles were considered to have a high risk of bias. The sensitivity ranged from 54·2% to 94·8%, and specificity ranged from 91% to 100% when compared to sputum culture as the reference standard. No articles compared the performance of stool-based molecular diagnostics with sputum-based Xpert-MTB/RIF Ultra, the current PCR reference standard in adolescents and adults.
Added value of this study
This prospective, multinational, diagnostic accuracy study was designed to evaluate the diagnostic accuracy of a novel quantitative PCR (qPCR) compared with the Xpert-MTB/RIF Ultra assay undertaken on stool and sputum samples from adolescents and adults with tuberculosis and controls without tuberculosis. The stool qPCR had a sensitivity of 93·7% (95% CI 87·4–97·4) compared with detection of M tuberculosis growth by sputum culture, 88·1% (81·3–93·0) compared with detection of M tuberculosis-specific DNA by sputum Xpert-MTB/RIF Ultra, and 80·4% (73·4–86·2) compared with a robust composite microbiological reference standard. The qPCR assay performed superiorly to stool Xpert-MTB/RIF Ultra and confirmed tuberculosis in 27 (17%) additional participants with tuberculosis who were not identified by sputum Xpert-MTB/RIF Ultra and 29 (21%) not identified by sputum culture. The stool qPCR sensitivity (93·7%, 95% CI 87·4–97·4) was equivalent to sputum Xpert-MTB/RIF Ultra sensitivity (94·8%, 89·1–98·1) when compared with detection of M tuberculosis growth by sputum culture.
Implications of all the available evidence
The novel stool qPCR assay evaluated in this study for the diagnosis of active pulmonary tuberculosis in adults and adolescents has superior diagnostic accuracy compared with the current standard for tuberculosis PCR diagnostics from stool, Xpert-MTB/RIF Ultra, and is equivalent to Xpert-MTB/RIF Ultra undertaken with sputum samples.
Acknowledgments
This study was supported by the NIH Allergy and Infectious Diseases (R01AI137527–01A1; AMM) and NIH Fogarty International Center (1K01TW011482–01A1; AK).
Footnotes
Declaration of interests
CL has received funding for this work from the German Center for Infection Research (DZIF); consulting fees from Insmed; and speakers’ honoraria from Insmed, Gilead, GSK, and Janssen outside of the scope of this work. AMM has received honoraria from Janssen for participation in a data and safety monitoring board outside of the scope of this work. All other authors declare no competing interests.
Data sharing
Individual participant data that underlie the results in the text, tables, and figures, and the data analysis plan, will be available after de-identification. The data will be available from 6 months to 10 years after publication. Data will be shared with investigators providing a methodologically sound protocol for individual patient meta-analysis that will be reviewed by relevant ethics boards. Requests may be submitted to Anna Maria Mandalakas (anna.mandalakas@bcm.edu), which will be followed by review at the site level, and requestors will need to sign country-specific data access agreements.
References
- 1.World Health Organization. Global tuberculosis report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed Dec 13, 2022).
- 2.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med 2018; 6: 299–314. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The End TB Strategy. 2015. https://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1 (accessed Dec 13, 2022).
- 4.Bacha JM, Ngo K, Clowes P, et al. Why being an expert - despite Xpert-remains crucial for children in high TB burden settings. BMC Infect Dis 2017; 17: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection. 2020. https://apps.who.int/iris/bitstream/handle/10665/332862/9789240007307-eng.pdf?sequence=1&isAllowed=y (accessed Dec 13, 2022). [PubMed]
- 6.Zifodya JS, Kreniske JS, Schiller I, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev 2021; 2: CD009593. [DOI] [PubMed] [Google Scholar]
- 7.Mathebula U, Emerson C, Agizew T, et al. Improving sputum collection processes to increase tuberculosis case finding among HIV-positive persons in Botswana. Public Health Action 2020; 10: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhana A, Hamada Y, Kengne AP, et al. Tuberculosis screening among HIV-positive inpatients: a systematic review and individual participant data meta-analysis. Lancet HIV 2022; D: e233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO consolidated guidelines on tuberculosis: module 5: management of tuberculosis in children and adolescents. https://www.who.int/publications-detail-redirect/9789240046764 (accessed Dec 13, 2022). [PubMed]
- 10.Kay AW, Ness T, Verkuijl SE, et al. Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children. Cochrane Database Syst Rev 2022; D: CD013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay AW, González Fernández L, Takwoingi Y, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev 2020; 8: CD013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Haas P, Nhung NV, Hng NT, et al. Introduction of the Simple One-Step stool Xpert Ultra method to detect TB in children and adults. Int J Tuberc Lung Dis 2023; 27: 19–27. [DOI] [PubMed] [Google Scholar]
- 13.Ulmar D, Ornstein GG. Gastric examination in pulmonary tuberculosis with negative sputum: diagnostic importance. J Am Med Assoc 1933; 101: 835. [Google Scholar]
- 14.DiNardo AR, Kay AW, Maphalala G, et al. Diagnostic and treatment monitoring potential of a stool-based quantitative polymerase chain reaction assay for pulmonary tuberculosis. Am J Trop Med Hyg 2018; DD: 310–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ness TE, Meiwes L, Kay A, et al. Optimizing DNA extraction from pediatric stool for diagnosis of tuberculosis and use in next-generation sequencing applications. Microbiol Spectr 2022; 0: e02269–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma G, Kashyap P. Decontamination method for tuberculosis: a review. J Tuberc 2021; 4: 1025. [Google Scholar]
- 17.Abbot. DetermineTM TB LAM Ag. https://www.globalpointofcare.abbott/en/product-details/determine-tb-lam.html (accessed Dec 30, 2022).
- 18.Liu JP, Hsueh HM, Hsieh E, Chen JJ. Tests for equivalence or non-inferiority for paired binary data. Stat Med 2002; 21: 231–45. [DOI] [PubMed] [Google Scholar]
- 19.Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman SMM, Maliha UT, Ahmed S, et al. Evaluation of Xpert MTB/RIF assay for detection of Mycobacterium tuberculosis in stool samples of adults with pulmonary tuberculosis. PLoS One 2018; 13: e0203063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngadaya E, Kimaro G, Sandi E, et al. Evaluation of stool GeneXpert MTB/RIF for the diagnosis of pulmonary tuberculosis among presumptive patients in Tanzania. J Clin Tuberc Other Mycobact Dis 2020; 21: 100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu R, Liang Q, Shang Y, et al. GeneXpert of stool versus gastric lavage fluid for the diagnosis of pulmonary tuberculosis in severely ill adults. Infection 2019; 47: 611–16. [DOI] [PubMed] [Google Scholar]
- 23.Abaye GE, Abebe T, Worku A, Tolessa D, Ameni G, Mihret A. Detection of Mycobacterium tuberculosis from the stool of HIV seropositive individuals suspected of pulmonary tuberculosis. PLoS One 2017; 12: e0177529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordova J, Shiloh R, Gilman RH, et al. Evaluation of molecular tools for detection and drug susceptibility testing of Mycobacterium tuberculosis in stool specimens from patients with pulmonary tuberculosis. J Clin Microbiol 2010; 48: 1820–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Khéchine A, Henry M, Raoult D, Drancourt M. Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology (Reading) 2009; 155: 2384–89. [DOI] [PubMed] [Google Scholar]
- 26.Sibandze DB, Kay A, Dreyer V, et al. Rapid molecular diagnostics of tuberculosis resistance by targeted stool sequencing. Genome Med 2022; 14: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mejia R, Vicuña Y, Broncano N, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg 2013; 88: 1041–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chibolela M, de Haas P, Klinkenberg E, et al. Use of stool swabs in molecular transport media increases access to Xpert Ultra testing for TB in children. Int J Tuberc Lung Dis 2023; 27: 612–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sessolo A, Musisi E, Kaswabuli S, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra and culture assays to detect Mycobacterium Tuberculosis using OMNIgene-sputum processed stool among adult TB presumptive patients in Uganda. PLoS One 2023; 18: e0284041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results in the text, tables, and figures, and the data analysis plan, will be available after de-identification. The data will be available from 6 months to 10 years after publication. Data will be shared with investigators providing a methodologically sound protocol for individual patient meta-analysis that will be reviewed by relevant ethics boards. Requests may be submitted to Anna Maria Mandalakas (anna.mandalakas@bcm.edu), which will be followed by review at the site level, and requestors will need to sign country-specific data access agreements.