Abstract
Purpose
The burden of herpes zoster (HZ) is substantial and numerous chronic underlying conditions are known as predisposing risk factors for HZ onset. Thus, a comprehensive study is needed to synthesize existing evidence. This study aims to comprehensively identify these risk factors.
Methods
A systematic literature search was done using MEDLINE via PubMed, EMBASE and Web of Science for studies published from January 1, 2003 to January 1, 2023. A random-effects model was used to estimate pooled Odds Ratios (OR). Heterogeneity was assessed using the I2 statistic. For sensitivity analyses basic outlier removal, leave-one-out validation and Graphic Display of Heterogeneity (GOSH) plots with different algorithms were employed to further analyze heterogeneity patterns. Finally, a multiple meta-regression was conducted.
Results
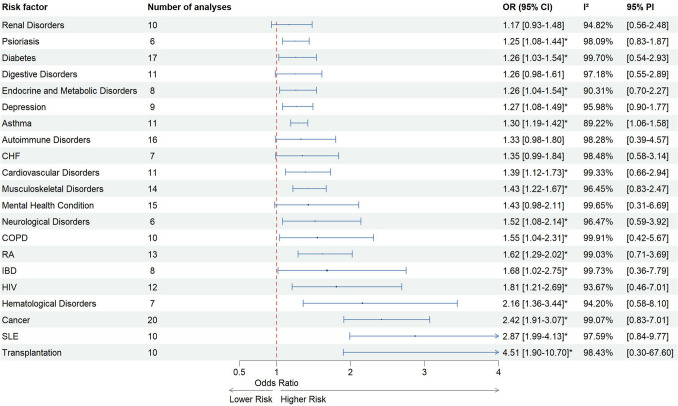
Of 6392 considered records, 80 were included in the meta-analysis. 21 different conditions were identified as potential risk factors for HZ: asthma, autoimmune disorders, cancer, cardiovascular disorders, chronic heart failure (CHF), chronic obstructive pulmonary disorder (COPD), depression, diabetes, digestive disorders, endocrine and metabolic disorders, hematological disorders, HIV, inflammatory bowel disease (IBD), mental health conditions, musculoskeletal disorders, neurological disorders, psoriasis, renal disorders, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and transplantation. Transplantation was associated with the highest risk of HZ (OR = 4.51 (95% CI [1.9–10.7])). Other risk factors ranged from OR = 1.17–2.87, indicating an increased risk for all underlying conditions. Heterogeneity was substantial in all provided analyses. Sensitivity analyses showed comparable results regarding the pooled effects and heterogeneity.
Conclusions
This study showed an increased risk of HZ infections for all identified factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-023-02156-y.
Keywords: Herpes zoster, Risk factors, Meta-analysis, Systematic review, Random-effects model, Meta-regression
Introduction
Herpes zoster (HZ) infection is caused by reactivation of varicella zoster virus (VZV), which has established latency in the sensory ganglia after primary infection with VZV [1]. HZ causes a painful unilateral blistering dermatomal rash. It is thought to cause nerve damage that can lead to postherpetic neuralgia (PHN). PHN is a dermatomal nerve pain that can continue for months or years [2]. HZ substantially impacts the Quality of Life as well as psychological and physical functioning aspects of patients’ lives [3, 4]. In addition to neurologic complications, ophthalmic, vascular, and visceral complications of HZ are also evident [5]. These complications lead to increased healthcare costs and an economic burden in older adults [6].
The incidence rate (IR) of HZ ranges between 3 and 5 per 1000 person-years (PY) in North America, Europe and Pacific Asia [7]. In Germany, the overall IR of HZ varied between 6.76 (95% CI 6.71–6.82) per 1000 PY in 2006 and 7.52 (95% confidence interval (CI) 7.47–7.58) per 1000 PY in 2012 [8]. During the last years the incidence of HZ infection was increasing [7]. Furthermore, the incidence of HZ is rising with age [9]. As a result, the number of HZ infections will increase in the upcoming years due to the demographic change in developed countries [10].
In addition to age, patients with immunocompromising conditions have an elevated risk of developing HZ. This includes both immunocompromising diseases and immunosuppressive medications [11, 12]. A recent German claims data analysis showed, that patients suffering from asthma, chronic heart disease, chronic obstructive pulmonary disorder (COPD), depression or rheumatoid arthritis (RA) on average had a 30% higher chance of developing acute HZ compared to those without any underlying condition. Among these conditions, RA had the highest odds ratio, ranging from 1.37 to 1.57 for all age groups [13].
Previous meta-analyses by Marra et al. [12] and Kawai et al. [14] reviewed data up to 2019. Since then, additional relevant studies examining risk factors for HZ have been published. Our aim is extending already existing evidence by broadening the search and including studies from 2019 to 2022. Furthermore, our methodology addresses concerns raised by Marra et al. [12] and Kawai et al. [14], providing a more accurate representation of the association between exposure and outcome. We specifically focus on heterogeneity patterns, using different models to elucidate those. The objective of this meta-analysis is to identify different risk factors for HZ to determine the importance of certain possibly underestimated risks regarding HZ infections. This could provide helpful information for health care professionals to identify patients at elevated risk in all age groups.
Methods
Systematic review
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [15] (see supplementary information [SI], S1–2).
Search strategy and study selection
The electronic databases MEDLINE via PubMed, EMBASE and Web of Science were used to identify relevant articles reporting HZ infections and associated risk factors. The search strategy included search terms associated with HZ, epidemiological effect size estimators and underlying conditions. Details of the search strategy are provided in SI S3. In addition, reference lists of identified studies were searched manually for further publications. The searches were limited to English- and German-language studies published from January 1, 2003 until January 1, 2023.
Eligible studies were case–control or cohort studies that assessed the association between HZ and underlying diseases. All other publication types (letters, editorials, comments, case reports and articles without full text, e.g. conference abstracts) were excluded. Studies with a focus on the impact of immunosuppressive or antirheumatic medications, including biologics and corticosteroids as well as studies investigating only family history, age, race or sex as potential risk factors for HZ were excluded as well, since these studies analyzed mostly too narrow subgroups. Two authors (MS and MLH) independently screened titles and abstracts, and full-text articles were screened based on predetermined inclusion and exclusion criteria. Discrepancies were resolved by consensus. A third reviewer (WG) was involved, if consensus could not be reached.
Data extraction
Data were extracted by two review authors (MS and MLH) using an Excel spreadsheet. The following information was extracted from the included studies: details about the study design and analysis, data base (questionnaire, claims, data linkage or medical records), country, study period, and population.
Numerous researchers emphasize the importance of including a substantial number of studies in a meta-analysis to ensure reliability of inferential outcomes [16, 17]. When the number of studies (k) is small, conventional thresholds for statistical significance (p < 0.05) often cannot be met. Permutation tests, which were used later in the analyses, can only achieve significance when k > 4 [18]. To address this issue and enhance reliability and interpretability of our results, we chose to include at least k ≥ 5 studies for each risk factor in our meta-analyses. Since there were not at least five studies for every disease found in our systematic review, we grouped them into superordinate risk factor groups if k < 5. The risk factors for HZ were categorized into 21 risk factor groups: asthma, autoimmune disorders (e.g. primary Sjörgen’s syndrome, multiple sclerosis, vasculitis), cancer, cardiovascular disorders, chronic heart failure (CHF), COPD, depression, diabetes, digestive disorders, endocrine and metabolic disorders, hematological disorders, HIV, inflammatory bowel disease (IBD), mental health conditions, musculoskeletal disorders, neurological disorders, psoriasis, renal disorders, RA, systemic lupus erythematosus (SLE) and transplantation. If a study reported more than one risk factor of interest, it was included in all analyses of each corresponding group. When risk factors are referred to in the following, the aforementioned risk factor groups are meant.
As effect estimates, odds ratios (OR) were extracted. If authors did not report OR, the absolute case numbers (HZ positive and HZ negative cases, cases with and without underlying condition, total study population) were extracted, to calculate the OR as a standard measure for the meta-analysis. Furthermore, characteristics of the study population (e.g. gender, age, follow-up period) were obtained.
Quality assessment
Two independent reviewers (MS and AF) used the Newcastle–Ottawa Quality Assessment Scale (NOS) [19], a standard tool from the Cochrane Collaboration Non-Randomized Studies Working Group, to assess the methodological quality of the studies. This scale evaluates three main categories: selection of the study sample (four items), comparability of the sample groups (two points), and ascertainment of exposure (for case–control and cross-sectional studies) or outcome (for cohort studies) (three points). Case–control and cohort studies were rated out of a total of nine points. A predefined threshold of six points was chosen as fair quality, a threshold of seven or more was chosen to indicate good methodological quality [19]. Those studies below the threshold of five points were categorized as poor quality and excluded for meta-analysis. Furthermore, NOS was combined with a critical revision of the appropriateness of statistical adjustment for confounding (e.g. matching, stratification, use of multivariate models).
Meta-analysis
Statistical analyses
All statistical analyses were conducted in R, version 4.3.1, using the meta, dmetar and metafor packages [20–23]. To ensure consistency and transparency of effect sizes for heterogenous studies, recalculation is recommended [24]. However, not all studies provided complete raw data that would have enabled the recalculation of effect sizes. In such cases, the reported effect estimates were used. This approach allowed the inclusion of studies that might otherwise have been excluded due to data unavailability. Furthermore, multiple effect size estimates for the same risk factor (group) in the same study were included as separate effect sizes in the meta-analysis. For pooled OR and their corresponding standard errors (SE) a log transformation was implemented to normalize the distribution of the data.
In anticipation of substantial heterogeneity with varying sample sizes and potential deviations from normality across studies, random-effects models using the Hartung–Knapp and Sidik–Jonkman adjustment were applied across all analyses for every risk factor. This adjustment widens the confidence interval to reflect uncertainty in the estimation of between-study heterogeneity [25–27]. Prediction intervals (95% PI) were computed around the pooled effect sizes to delineate the range within which the actual effects of analogous future trials are likely to reside [28]. A two-sided significance level of α = 0.05 was adopted for all analyses.
Heterogeneity was estimated using the I2 statistics [29]. Following general recommendations for the interpretation of I2 statistics, a value of 25% was considered as insignificant or low, a value of 50% as moderate, and 75% as substantial heterogeneity [24, 30]. To investigate further variations in heterogeneity between studies, meta-analyses on resampled sets of effect sizes were conducted. This allows to create graphical representations of between-study heterogeneity, known as Graphical Display of Study Heterogeneity (GOSH) plots [31].
Subgroup analyses
To examine potential sources of heterogeneity, subgroup analyses were conducted. We applied the rule of thumb that at least 10 studies must be available per risk factor to achieve sufficient statistical power in our subgroup analyses [32]. Subgroup analyses were done for predefined moderators; including study design (cohort study or case–control study), study year (2003–2008, 2011–2016 or 2017–2022), region (Europe, Northern America, Asia or Middle East) and sample size (< 100,000, 100,000–999,999 or ≥ 1,000,000).
Sensitivity analyses
Further sensitivity analyses that excluded statistical outliers were conducted if substantial heterogeneity (I2 ≥ 75%) was detected. With this basic outlier removal studies were excluded if their 95% CI was outside the range of the pooled OR [22].
To assess the impact of individual studies on the overall outcome, “leave-one-out” influence analyses were conducted. This process involved recalculating the pooled effect estimate iteratively while omitting one study at a time. Furthermore, diagnostic plots, containing externally standardized residuals, DFFITS values, Cook’s distance, covariance ratio, leave-one-out τ2 and Q values as well as hat values and study weights where used in combination with Baujat plots to identify influential studies [22, 33]. The study which had the largest impact on the effect estimates and the study which had the largest impact on heterogeneity was subsequently excluded.
To assess potential publication bias, contour-enhanced funnel plots and Egger’s tests of the intercept were used to evaluate potential asymmetry. If publication bias was identified, the Duval and Tweedie trim-and-fill method was used to rectify publication bias [34].
Meta-regression
Furthermore, multiple meta-regression analyses were conducted to explore potential sources of heterogeneity. Multiple meta-regression analyses were conducted for each risk factor, incorporating multiple predictors (βk) to account for potential variations in effect sizes and heterogeneity. Predefined moderators from the subgroup analyses were used. To assess the presence of multicollinearity among the predictor variables, correlation matrix analyses were performed. Hierarchical multiple meta-regression was done, by including the covariates in a stepwise procedure. The best-fitting model for each risk factor was chosen based on the Akaike Information Criterion (AIC) values, with preference given to models demonstrating the lowest AIC. To assess the validity of the coefficients capturing the underlying data patterns, permutation tests were employed [35].
Results
Study characteristics
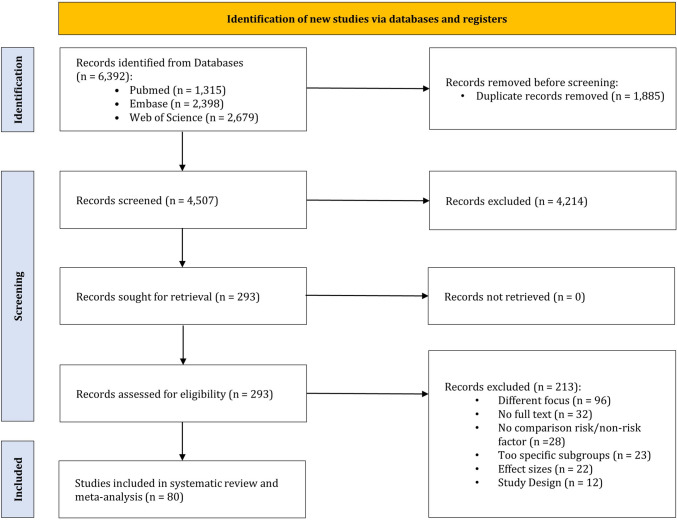
The initial database search identified 6392 records. Following the removal of duplicates and the screening of titles and abstracts, 293 studies remained for full-text analysis. Reasons for exclusion after full-text screening were: (i) different focus, no focus on underlying risk factors, or risk factors could not be assigned to one of our risk factors (n = 96), (ii) conference abstracts or no full text available (n = 32), (iii) no comparison between risk factor and non-risk factor (n = 28), (iv) analysis of subgroups with risk specific drug use or other overly specific subgroups (n = 23), (v) different effect sizes or calculation of odds ratios is not possible with available data (n = 22) and (vi) study designs other than cohort or case–control studies (n = 12). Ultimately, 80 studies met all eligibility criteria and were included in the analysis. The study selection process and the rationale for exclusions are presented in Fig. 1. 56 of the included studies were cohort studies [13, 36–90] and 24 case–control studies [88, 91–113].
Fig. 1.
PRISMA flowchart
An overview of the distribution of studies on risk factors can be found in Table 1. The most frequent evidence was found for patients with cancer (n = 20). Details of all grouped risk factors can be found in SI S4.
Table 1.
Distribution of risk factors among included studies
| Risk factor | Total number of analyses in all studies | Studies including analyses of risk factorsa |
|---|---|---|
| Cancer | 20 | [45, 49, 51, 52, 57, 86, 89, 93, 103] |
| Diabetes | 17 | [13, 38, 41, 45, 49, 51, 52, 56, 59, 73, 82, 92, 94, 99, 100, 103] |
| Autoimmune disorders | 16 | [40, 45, 49, 51, 52, 77, 86, 90, 92, 103] |
| Mental health condition | 15 | [38, 44, 74, 85, 106, 109, 110, 113, 114] |
| Musculoskeletal disorders | 14 | [51, 52, 81, 86, 95–97, 99, 103, 107] |
| RA | 13 | [13, 49, 51, 52, 69, 80, 86, 88, 89, 92] |
| HIV | 12 | [37, 45–47, 63, 71, 82, 86, 89, 92, 94, 103] |
| Asthma | 11 | [13, 43, 45, 51, 52, 68, 92, 101–103, 112] |
| Cardiovascular disorders | 11 | [13, 45, 49, 51, 52, 99, 103, 111] |
| Digestive disorders | 11 | [38, 53, 55, 58, 61, 65, 72, 90, 103] |
| COPD | 10 | [13, 45, 51, 52, 67, 75, 85, 92, 99, 103], |
| Transplantation | 10 | [36, 48, 51, 52, 82, 86, 89, 92] |
| SLE | 10 | [39, 42, 49, 51, 52, 66, 69, 86, 89, 92] |
| Renal disorders | 10 | [49, 51, 52, 62, 84, 86, 87, 92, 104, 105] |
| Depression | 9 | [13, 51, 52, 91, 92, 99, 106, 109, 110] |
| Endocrine and metabolic disorders | 8 | [50–52, 86, 99] |
| IBD | 8 | [51, 52, 55, 64, 72, 86, 89, 92] |
| CHF | 7 | [13, 45, 51, 52, 83, 103, 111] |
| Hematological disorders | 7 | [60, 92, 94, 98] |
| Neurological disorders | 6 | [51, 52, 54, 78, 79, 103] |
| Psoriasis | 6 | [51, 52, 76, 86, 89, 108] |
RA, rheumatoid arthritis; COPD, chronic obstructive pulmonary disorder; SLE, systemic lupus erythematosus; IBD, inflammatory bowel disease; CHF, chronic heart failure
aPlease note that a single study may include both analyses of multiple risk factors and multiple analyses of the same risk factor
The included studies were from Asia (n = 44), Northern America (n = 20), Europe (n = 13) and the Middle East (n = 3). Most studies were from Taiwan (n = 30) [40, 41, 43, 44, 50, 54, 58–63, 68, 76–79, 81, 83–85, 87, 90, 96–98, 100, 104, 105, 115] and the US (n = 19) [36, 37, 39, 46, 47, 55, 57, 64, 73, 75, 80, 88, 89, 99, 101, 102, 106, 112, 113]. In sum a total study population of 796,796,295 was included, with 10,904,736 HZ cases reported in the included studies. Sample sizes varied substantially from n = 94 to n = 51,022,838. The age of participants ranged from 3 months to 103 years with a median age of 52.5 years across all studies. The frequency of women ranged from 0 to 100% with a median of 54.7% across all studies.
Quality assessment
The quality of the included studies, based on the Newcastle Ottawa scale, ranged from six to nine for cohort and from five to nine for case–control studies. Most (91%) of the cohort studies showed good overall quality and a sufficient adjustment for confounding, as well as most of the case–control studies (92%). Since no study was below the predefined threshold of five points, all were included for further analyses.
Main analyses
Pooled results showed an increased risk of HZ infections for all included risk factors and are presented in Fig. 2. Data demonstrated a noteworthy association between all analyzed risk factors and HZ, ranging from a pooled OR of 1.17 (95% CI [0.93–1.48]) for renal disorders up to 2.87 for SLE (95% CI [1.99–4.13]).
Fig. 2.
Pooled analysis for risk of herpes zoster
Between-study heterogeneity was high for all risk factors, varying from I2 = 89.22% for asthma to I2 = 99.91% for COPD. Prediction intervals, estimating the range within which future observations are expected to fall with a 95% level of confidence, showed quite precise estimates (e.g. g = 1.06–1.58 for asthma), but were also notably broad and thus, indicating a considerable degree of uncertainty in estimating future values (e.g. g = 0.30–67.60 for transplantation). Detailed forest plots for all risk factors not shown here can be found in SI S5.
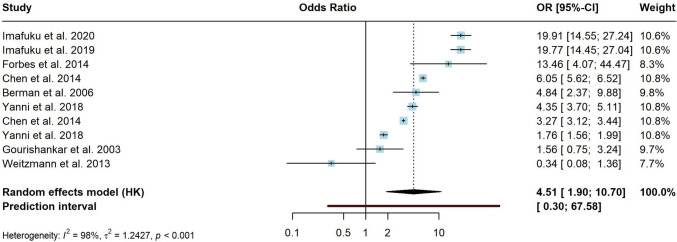
Transplantation (Fig. 3) was associated with the highest risk of HZ with a pooled OR = 4.51 (95% CI [1.9–10.7]). However, the effects between studies varied substantially, as evidenced by high between-study heterogeneity (I2 = 98.4%). The prediction interval ranged from g = 0.3–67.58. The heterogeneity remained substantial (I2 = 97.3%) after sensitivity analyses with basic outlier removal and leave-one-out analysis, but reduced the pooled effect size to OR = 3.55 (95% CI [1.3–9.8]). GOSH diagnostics did not provide a unimodal, symmetrical or contiguous distribution; thus, effect sizes were still quite heterogenous.
Fig. 3.
Forest plot for risk of herpes zoster in transplantation subgroup. The transplantation subgroup summarizes allogenic, bone marrow, (solid) organ and hematopoietic stem cell transplantation
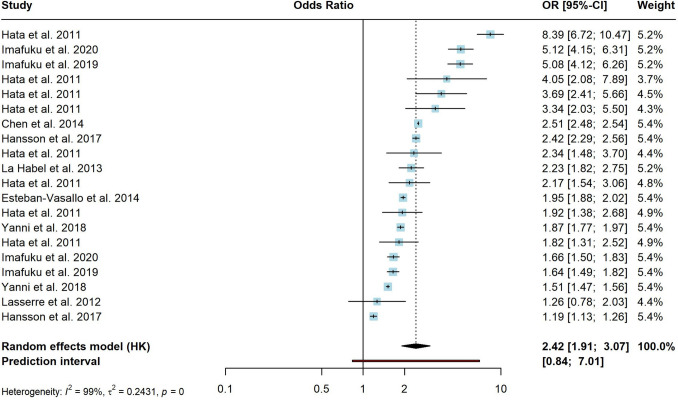
The risk factor with most available evidence, various forms of malignancies and cancer, were associated with a significant higher risk of HZ, indicating a pooled OR of 2.42 (95% CI [1.91–3.07]) (Fig. 4). Reported effect sizes differed substantially between studies (I2 = 99.1%). Prediction interval ranged between g = 0.84–7.01. After sensitivity analyses, between-study heterogeneity was still high (I2 = 95.3%), but an increased risk of HZ remained (OR = 2.21; 95% CI [1.9–2.6]).
Fig. 4.
Forest plot for risk of herpes zoster in cancer subgroup. The cancer subgroup summarizes any solid malignancy, hematological malignancies, solid organ malignancies, brain tumor, lung cancer, breast cancer, esophageal cancer, gastric cancer, colorectal cancer, gynecologic cancer and malignant lymphoma
Subgroup analyses
The subgroup analyses only revealed a significant difference between studies when testing for regional differences (asthma, digestive disorder, SLE and transplantation, see SI S6 for further details). However, all conducted Q tests for within-subgroup heterogeneity showed significance, therefore, indicating, that there is excess variability in the subgroups.
Sensitivity analyses
Since heterogeneity was high for all risk factors and subgroup analyses revealed a clear tendency for heterogeneity within the subgroups, five different sensitivity analyses were done for each of the risk factors. Even after outlier removal, for all risk factors the pooled estimates still provided OR ≥ 1 for developing a HZ infection.
For cancer, cardiovascular disorders, COPD, diabetes, digestive disorders, IBD, mental health disorders, musculoskeletal disorders, neurological disorders, renal disorders, RA and SLE the basic outlier removal emerged as the most effective method in mitigating heterogeneity (see Table 2 for further details and SI S7 for corresponding plots). Systematically eliminating data points whose confidence interval did not overlap with the confidence interval of the pooled effect exerted a profound influence on the results. After basic outlier removal, all pooled OR decreased compared to the unadjusted random effects model. The effect was highest for mental health conditions (adj. OR = 1.16, 95% CI [1.0–1.3], I2 = 87.85%). This reduction indicates that by excluding extreme data points, consistency and robustness of the findings is enhanced, leading to a clearer representation of the risk patterns within the studied populations. Influence analyses, based on the “leave-one-out” approach was done, iteratively reassessing the results and excluding one study at a time. These revealed the greatest impact on the heterogeneity of asthma, autoimmune disorders, COPD, endocrine and metabolic disorders, hematological disorders, neurological disorders, transplantation and psoriasis. Remarkably, “leave-one-out” analysis increased the pooled effect estimates simultaneously, with the highest value for autoimmune disorders (prev. OR = 1.33, adj. OR = 1.46, 95% CI [1.3–1.7]; I2 = 92.55%, resp.). GOSH diagnostics helped reducing the heterogeneity for depression, HIV and CHF. For psoriasis, heterogeneity could even be reduced to 0%, but showed wide confidence intervals for I2 (adj. OR = 1.21, 95% CI [1.1–1.3], I2 = 0% (95% CI [0–85.00])).
Table 2.
Pooled effects of risk factors on HZ, sensitivity analyses
| Risk factor | Effect size | Heterogeneity | Excluded studies | ||||
|---|---|---|---|---|---|---|---|
| nk | OR | 95% CI | I2 (%) | 95% CI | 95% PI | ||
| Asthma | |||||||
| Random effects model, unadjusted | 11 | 1.30 | [1.19–1.42]a | 89.22 | [83–93.00]a | 0.06–0.46 | – |
| Basic outlier removal | 11 | 1.30 | [1.20–1.40]a | 89.22 | [83–93.00]a | 0.06–0.46 | – |
| Influence analysis („leave-one-out”) | 10 | 1.27 | [1.20–1.40]a | 65.59 | [33–82.00]a | 1.08–1.48 | [45] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 10 | 1.27 | [1.20–1.40]a | 65.59 | [33–82.00]a | 1.08–1.48 | [45] |
| Gaussian mixture model (GMM) clustering | 10 | 1.28 | [1.20–1.40]a | 89.66 | [83–94.00]a | 1.05–1.56 | [68] |
| K-means clustering | 10 | 1.30 | [1.20–1.40]a | 90.11 | [84–94.00]a | 1.06–1.60 | [52] |
| Autoimmune disorders | |||||||
| Random effects model, unadjusted | 16 | 1.33 | [0.98–1.80] | 98.28 | [98–99.00]a | − 0.95–1.52 | – |
| Basic outlier removal | 13 | 1.40 | [1.20–1.60]a | 91.11 | [87–94.00]a | − 0.14–0.81 | [40, 45, 77] |
| Influence analysis („leave-one-out”) | 14 | 1.46 | [1.30–1.70]a | 92.55 | [89–95.00]a | 0.84–2.54 | [45, 77] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 9 | 1.49 | [1.20–1.90]a | 96.22 | [94–97.00]a | 0.71–3.09 | [49, 51, 52, 77, 89, 92] |
| Gaussian mixture model (GMM) clustering | 9 | 1.49 | [1.20–1.90]a | 96.22 | [94–97.00]a | 0.71–3.09 | [49, 51, 52, 77, 89, 92] |
| K-means clustering | 15 | 1.53 | [1.30–1.80]a | 95.35 | [94–97.00]a | 0.82–2.84 | [89] |
| Cancer | |||||||
| Random effects model, unadjusted | 20 | 2.42 | [1.91–3.07]a | 99.07 | [99–99.00]a | − 0.18–1.95 | – |
| Basic outlier removal | 13 | 2.21 | [1.90–2.60]a | 95.93 | [94–97.00]a | 0.43–1.16 | [49, 86, 93, 116, 117] |
| Influence analysis („leave-one-out”) | 18 | 2.24 | [1.80–2.80]a | 97.58 | [97–98.00]a | 0.90–5.53 | [49, 89] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 13 | 2.45 | [2.00–3.10]a | 97.26 | [96–98.00]a | 1.09–5.55 | [49, 52, 86, 89, 93, 103] |
| Gaussian mixture model (GMM) clustering | 13 | 2.45 | [2.00–3.10]a | 97.26 | [96–98.00]a | 1.09–5.55 | [49, 52, 86, 89, 93, 103] |
| K-means clustering | 19 | 2.25 | [1.80–2.80]a | 99.05 | [99–99.00]a | 0.94–5.37 | [103] |
| Cardiovascular disorders | |||||||
| Random effects model, unadjusted | 11 | 1.39 | [1.12–1.73]a | 99.33 | [99–99.00]a | − 0.42–1.08 | – |
| Basic outlier removal | 8 | 1.30 | [1.00–1.60]a | 92.62 | [88–96.00]a | − 0.44–0.96 | [13, 45] |
| Influence analysis („leave-one-out”) | 9 | 1.37 | [1.10–1.80]a | 98.62 | [98–99.00]a | 0.64–2.94 | [13, 45] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 7 | 1.26 | [0.92–1.70] | 99.39 | [99–100.00]a | 0.50–3.17 | [45, 103, 111] |
| Gaussian mixture model (GMM) clustering | 7 | 1.26 | [0.92–1.70] | 99.39 | [99–100.00]a | 0.50–3.17 | [45, 103, 111] |
| K-means clustering | 10 | 1.40 | [1.10–1.80]a | 99.40 | [99–100.00]a | 0.64–3.07 | [45] |
| CHF | |||||||
| Random effects model, unadjusted | 7 | 1.35 | [0.99–1.84] | 98.48 | [98–99.00]a | − 0.55–1.15 | – |
| Basic outlier removal | 6 | 1.24 | [0.93–1.70] | 91.74 | [85–96.00]a | − 0.46–0.90 | [45] |
| Influence analysis („leave-one-out”) | 6 | 1.24 | [0.93–1.70] | 91.74 | [85–96.00]a | 0.63–2.45 | [45] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 5 | 1.32 | [0.93–1.90] | 76.13 | [42–90.00]a | 0.64–2.73 | [83, 111] |
| Gaussian mixture model (GMM) clustering | 5 | 1.32 | [0.93–1.90] | 76.13 | [42–90.00]a | 0.64–2.73 | [83, 111] |
| K-means clustering | 5 | 1.26 | [0.85–1.90] | 93.35 | [87–96.00]a | 0.49–3.24 | [45, 111] |
| COPD | |||||||
| Random effects model, unadjusted | 10 | 1.55 | [1.04–2.31]a | 99.91 | [100–100.00]a | − 0.86–1.74 | – |
| Basic outlier removal | 9 | 1.38 | [1.10–1.70]a | 99.08 | [99–99.00]a | − 0.28–0.92 | [75] |
| Influence analysis („leave-one-out”) | 9 | 1.38 | [1.10–1.70]a | 99.08 | [99–99.00]a | 0.76–2.51 | [75] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 8 | 1.34 | [1.10–1.70]a | 99.19 | [99–99.00]a | 0.70–2.54 | [45, 85] |
| Gaussian mixture model (GMM) clustering | 8 | 1.34 | [1.10–1.70]a | 99.19 | [99–99.00]a | 0.70–2.54 | [45, 85] |
| K-means clustering | 9 | 1.38 | [1.10–1.70]a | 99.08 | [99–99.00]a | 0.76–2.51 | [85] |
| Depression | |||||||
| Random effects model, unadjusted | 9 | 1.27 | [1.08–1.49]a | 95.98 | [94–97.00]a | − 0.10–0.57 | – |
| Basic outlier removal | 8 | 1.23 | [1.10–1.40]a | 96.18 | [94–97.00]a | − 0.08–0.50 | [106] |
| Influence analysis („leave-one-out”) | 8 | 1.21 | [1.10–1.40]a | 84.52 | [71–92.00]a | 0.98–1.48 | [99] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 6 | 1.20 | [1.10–1.30]a | 82.43 | [63–92.00]a | 0.96–1.50 | [13, 51, 52] |
| Gaussian mixture model (GMM) clustering | 6 | 1.20 | [1.10–1.30]a | 82.43 | [63–92.00]a | 0.96–1.50 | [13, 51, 52] |
| K-means clustering | 8 | 1.23 | [1.10–1.40]a | 96.18 | [94–97.00]a | 0.93–1.64 | [13] |
| Diabetes | |||||||
| Random effects model, unadjusted | 17 | 1.26 | [1.03–1.54]a | 99.70 | [100–100.00]a | − 0.62–1.08 | – |
| Basic outlier removal | 13 | 1.21 | [1.10–1.40]a | 96.33 | [95–97.00]a | − 0.22–0.60 | [45, 49, 73, 82] |
| Influence analysis („leave-one-out”) | 14 | 1.26 | [1.10–1.40]a | 99.38 | [99–99.00]a | 0.76–2.09 | [49, 73, 82] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 15 | 1.24 | [1.00–1.50] | 99.74 | [100–100.00]a | 0.52–2.99 | [13, 45] |
| Gaussian mixture model (GMM) clustering | 15 | 1.24 | [1.00–1.50] | 99.74 | [100–100.00]a | 0.52–2.99 | [13, 45] |
| K-means clustering | 15 | 1.29 | [1.10–1.50]a | 99.69 | [100–100.00]a | 0.76–2.18 | [41, 100] |
| Digestive disorders | |||||||
| Random effects model, unadjusted | 11 | 1.26 | [0.98–1.61] | 97.18 | [96–98.00]a | − 0.60–1.06 | – |
| Basic outlier removal | 6 | 1.36 | [1.20–1.60]a | 73.86 | [40–89.00]a | 0.04–0.57 | [55, 61, 72, 90] |
| Influence analysis („leave-one-out”) | 9 | 1.20 | [0.92–1.60] | 96.68 | [95–98.00]a | 0.53–2.75 | [58, 72] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 7 | 1.14 | [0.78–1.70] | 98.24 | [98–99.00]a | 0.37–3.48 | [38, 53, 65, 72] |
| Gaussian mixture model (GMM) clustering | 7 | 1.14 | [0.78–1.70] | 98.24 | [98–99.00]a | 0.37–3.48 | [38, 53, 65, 72] |
| K-means clustering | 9 | 1.16 | [0.91–1.50] | 96.69 | [95–98.00]a | 0.53–2.54 | [38, 58] |
| Endocrine and metabolic disorders | |||||||
| Random effects model, unadjusted | 8 | 1.26 | [1.04–1.54]a | 90.31 | [83–94.00]a | − 0.35–0.82 | – |
| Basic outlier removal | 7 | 1.33 | [1.10–1.60]a | 88.96 | [80–94.00]a | − 0.18–0.76 | [86] |
| Influence analysis („leave-one-out”) | 6 | 1.29 | [1.10–1.50]a | 86.50 | [73–93.00]a | 0.79–2.09 | [51, 86] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 7 | 1.33 | [1.10–1.60]a | 88.96 | [80–94.00]a | 0.83–2.13 | [52] |
| Gaussian mixture model (GMM) clustering | 7 | 1.33 | [1.10–1.60]a | 88.96 | [80–94.00]a | 0.83–2.13 | [52] |
| K-means clustering | 7 | 1.29 | [1.00–1.60]a | 91.67 | [85–95.00]a | 0.67–2.49 | [50] |
| Hematological disorders | |||||||
| Random effects model, unadjusted | 7 | 2.16 | [1.36–3.44]a | 94.20 | [90–96.00]a | − 0.55–2.09 | – |
| Basic outlier removal | 7 | 2.16 | [1.40–3.40]a | 94.20 | [90–96.00]a | − 0.55–2.09 | – |
| Influence analysis („leave-one-out”) | 5 | 2.19 | [1.50–3.30]a | 81.02 | [56–92.00]a | 0.76–6.32 | [60, 118] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 5 | 2.19 | [1.50–3.30]a | 81.02 | [56–92.00]a | 0.76–6.32 | [92, 98] |
| Gaussian mixture model (GMM) clustering | 5 | 2.19 | [1.50–3.30]a | 81.02 | [56–92.00]a | 0.76–6.32 | [92, 98] |
| K-means clustering | 5 | 2.43 | [1.50–4.00]a | 94.94 | [91–97.00]a | 0.62–9.50 | [92] |
| HIV | |||||||
| Random effects model, unadjusted | 12 | 1.81 | [1.21–2.69]a | 93.67 | [91–96.00]a | − 0.77–1.95 | – |
| Basic outlier removal | 10 | 1.43 | [1.00–2.00]a | 87.19 | [78–92.00]a | − 0.61–1.32 | [89, 118] |
| Influence analysis („leave-one-out”) | 10 | 1.69 | [1.10–2.50]a | 87.43 | [79–93.00]a | 0.52–5.52 | [86, 92] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 8 | 1.64 | [0.90–3.00] | 92.07 | [87–95.00]a | 0.28–9.60 | [82, 86, 89, 118] |
| Gaussian mixture model (GMM) clustering | 8 | 1.64 | [0.90–3.00] | 92.07 | [87–95.00]a | 0.28–9.60 | [82, 86, 89, 118] |
| K-means clustering | 9 | 1.55 | [1.10–2.10]a | 87.12 | [78–93.00]a | 0.61–3.92 | [63, 71, 86] |
| IBD | |||||||
| Random effects model, unadjusted | 8 | 1.68 | [1.02–2.75]a | 99.73 | [100–100.00]a | − 1.02–2.05 | – |
| Basic outlier removal | 6 | 1.50 | [1.30–1.80]a | 97.38 | [96–98.00]a | − 0.05–0.86 | [55, 64] |
| Influence analysis („leave-one-out”) | 7 | 1.86 | [1.10–3.10]a | 99.75 | [100–100.00]a | 0.41–8.57 | [64] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 7 | 1.38 | [1.10–1.80]a | 98.02 | [97–99.00]a | 0.66–2.87 | [52] |
| Gaussian mixture model (GMM) clustering | 7 | 1.38 | [1.10–1.80]a | 98.02 | [97–99.00]a | 0.66–2.87 | [52] |
| K-means clustering | 7 | 1.38 | [1.10–1.80]a | 98.02 | [97–99.00]a | 0.66–2.87 | [52] |
| Mental health condition | |||||||
| Random effects model, unadjusted | 15 | 1.43 | [0.98–2.11] | 99.65 | [100–100.00]a | − 1.18–1.90 | – |
| Basic outlier removal | 13 | 1.16 | [1.00–1.30]a | 87.85 | [81–92.00]a | − 0.19–0.48 | [106, 114] |
| Influence analysis („leave-one-out”) | 14 | 1.23 | [1.00–1.50]a | 91.08 | [87–94.00]a | 0.66–2.28 | [114] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 9 | 1.64 | [0.83–3.20] | 99.80 | [100–100.00]a | 0.18–14.50 | [44, 74, 109, 110, 114] |
| Gaussian mixture model (GMM) clustering | 9 | 1.64 | [0.83–3.20] | 99.80 | [100–100.00]a | 0.18–14.50 | [44, 74, 109, 110, 114] |
| K-means clustering | 14 | 1.23 | [1.00–1.50]a | 91.08 | [87–94.00]a | 0.66–2.28 | [110] |
| Musculoskeletal disorders | |||||||
| Random effects model, unadjusted | 14 | 1.43 | [1.22–1.67]a | 96.45 | [95–97.00]a | − 0.19–0.90 | – |
| Basic outlier removal | 11 | 1.3 | [1.20–1.40]a | 88.21 | [81–93.00]a | 0.00–0.52 | [51, 52, 86] |
| Influence analysis („leave-one-out”) | 12 | 1.37 | [1.20–1.60]a | 91.37 | [87–94.00]a | 0.78–2.39 | [52, 86] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 9 | 1.45 | [1.10–1.90]a | 93.61 | [90–96.00]a | 0.66–3.23 | [52, 81, 99, 103] |
| Gaussian mixture model (GMM) clustering | 9 | 1.45 | [1.10–1.90]a | 93.61 | [90–96.00]a | 0.66–3.23 | [52, 81, 99, 103] |
| K-means clustering | 12 | 1.34 | [1.20–1.50]a | 96.48 | [95–97.00]a | 0.88–2.04 | [86, 107] |
| Neurological disorders | |||||||
| Random effects model, unadjusted | 6 | 1.52 | [1.08–2.14]a | 96.47 | [94–98.00]a | − 0.53–1.37 | – |
| Basic outlier removal | 5 | 1.32 | [1.10–1.60]a | 88.15 | [75–94.00]a | − 0.26–0.82 | [79] |
| Influence analysis („leave-one-out”) | 5 | 1.32 | [1.10–1.60]a | 88.15 | [75–94.00]a | 0.77–2.27 | [79] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 5 | 1.32 | [1.10–1.60]a | 88.15 | [75–94.00]a | 0.77–2.27 | [51] |
| Gaussian mixture model (GMM) clustering | 5 | 1.32 | [1.10–1.60]a | 88.15 | [75–94.00]a | 0.77–2.27 | [51] |
| K-means clustering | 4 | 1.35 | [1.00–1.80]a | 91.00 | [80–96.00]a | 0.61–2.97 | [51, 54] |
| Psoriasis | |||||||
| Random effects model, unadjusted | 6 | 1.25 | [1.08–1.44]a | 98.09 | [97–99.00]a | − 0.18–0.63 | – |
| Basic outlier removal | 5 | 1.16 | [1.10–1.30]a | 59.33 | [0 -85.00]a | − 0.10–0.40 | [89] |
| Influence analysis („leave-one-out”) | 4 | 1.21 | [1.10–1.30]a | 0.00 | [0–85.00] | 1.04–1.42 | [86, 89] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 6 | 1.25 | [1.10–1.40]a | 98.09 | [97–99.00]a | 0.83–1.87 | – |
| Gaussian mixture model (GMM) clustering | 6 | 1.25 | [1.10–1.40]a | 98.09 | [97–99.00]a | 0.83–1.87 | – |
| K-means clustering | 4 | 1.21 | [1.10–1.30]a | 0.00 | [0–85.00] | 1.04–1.42 | [51, 86] |
| Renal disorders | |||||||
| Random effects model, unadjusted | 10 | 1.17 | [0.93–1.48] | 94.82 | [92–97.00]a | − 0.59–0.91 | – |
| Basic outlier removal | 8 | 1.15 | [1.00–1.30] | 84.85 | [72–92.00]a | − 0.22–0.51 | [49, 60] |
| Influence analysis („leave-one-out”) | 8 | 1.28 | [1.00–1.60] | 90.00 | [83–94.00]a | 0.65–2.51 | [62, 118] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 9 | 1.18 | [0.91–1.50] | 95.39 | [93–97.00]a | 0.53–2.64 | [52] |
| Gaussian mixture model (GMM) clustering | 9 | 1.26 | [1.00–1.50] | 89.47 | [82–94.00]a | 0.69–2.28 | [52] |
| K-means clustering | 8 | 1.27 | [1.00–1.60] | 90.79 | [84–95.00]a | 0.66–2.43 | [52, 60] |
| RA | |||||||
| Random effects model, unadjusted | 13 | 1.62 | [1.29–2.02]a | 99.03 | [99–99.00]a | − 0.34–1.30 | – |
| Basic outlier removal | 10 | 1.62 | [1.40–1.90]a | 93.26 | [90–96.00]a | − 0.02–0.99 | [69, 88, 89] |
| Influence analysis („leave-one-out”) | 10 | 1.74 | [1.40–2.10]a | 97.16 | [96–98.00]a | 0.98–3.10 | [69, 88, 89] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 5 | 2.08 | [1.70–2.50]a | 95.61 | [92–98.00]a | 1.25–3.47 | [13, 49, 51, 86, 88, 89, 92] |
| Gaussian mixture model (GMM) clustering | 5 | 2.08 | [1.70–2.50]a | 95.61 | [92–98.00]a | 1.25–3.47 | [13, 49, 51, 86, 88, 89, 92] |
| K-means clustering | 12 | 1.75 | [1.50–2.10]a | 98.56 | [98–99.00]a | 0.96–3.17 | [51] |
| SLE | |||||||
| Random effects model, unadjusted | 10 | 2.87 | [1.99–4.13]a | 97.59 | [97–98.00]a | − 0.17–2.28 | – |
| Basic outlier removal | 6 | 2.91 | [2.10–4.00]a | 91.97 | [85–96.00]a | 0.14–2.00 | [39, 66, 69, 92] |
| Influence analysis („leave-one-out”) | 8 | 2.93 | [2.10–4.10]a | 96.95 | [96–98.00]a | 1.05–8.23 | [39, 66] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 6 | 2.63 | [1.40–4.90]a | 94.15 | [90–97.00]a | 0.44–15.90 | [49, 51, 52, 92] |
| Gaussian mixture model (GMM) clustering | 6 | 2.63 | [1.40–4.90]a | 94.15 | [90–97.00]a | 0.44–15.90 | [49, 51, 52, 92] |
| K-means clustering | 9 | 3.18 | [2.30–4.40]a | 96.76 | [95–98.00]a | 1.09–9.29 | [89] |
| Transplantation | |||||||
| Random effects model, unadjusted | 10 | 4.51 | [1.90–10.70]a | 98.43 | [98–99.00]a | − 1.20–4.21 | – |
| Basic outlier removal | 7 | 3.68 | [2.00–6.80]a | 98.27 | [98–99.00]a | − 0.26–2.87 | [51, 52, 82] |
| Influence analysis („leave-one-out”) | 8 | 3.55 | [1.30–9.80]a | 97.27 | [96–98.00]a | 0.19–64.80 | [89, 117] |
| GOSH-Diagnostics Connectivity (DBSCAN) clustering | 9 | 5.59 | [2.70–12.00]a | 98.58 | [98–99.00]a | 0.56–56.10 | [52] |
| Gaussian mixture model (GMM) clustering | 9 | 5.59 | [2.70–12.00]a | 98.58 | [98–99.00]a | 0.56–56.10 | [52] |
| K-means clustering | 8 | 5.15 | [2.30–12.00]a | 98.75 | [98–99.00]a | 0.45–59.30 | [48, 52] |
nk, number of studies; OR, odds ratio; CI, confidence interval; PI, prediction interval
aIndicates significant values p < 0.005
Publication bias analyses
Examination of the funnel plots revealed a potential issue of publication bias within all conducted analyses. For 12 of 21 risk factors, Egger’s test revealed a negative intercept, which suggests that smaller studies included in our analysis may exhibit a systematic tendency to report larger effect sizes than larger studies. Such bias could lead to an overestimation of the true effect estimate, and it underscores the importance of interpreting our meta-analysis results with caution. The negative intercept was highest for diabetes, COPD and RA. For asthma, digestive disorders, musculoskeletal disorders, renal disorders and SLE the intercept was close to zero, indicating less publication bias. Positive intercept, i.e., demonstrating a potential underestimation of the true effect, was largest for the mental health conditions. However, for all analyzed risk factors, the results of Egger’s test for funnel plot asymmetry yielded non-significant values (p > 0.05) indicating that there might be no evidence for publication bias within the selected studies (see Table 3). Even though results of the Egger’s test were non-significant, the trim-and-fill procedure was applied as a precautionary measure to assess the impact of potentially missing studies on the overall effect size estimation. However, results of the trim-and-fill procedure were similar to previous results. Since heterogeneity was still quite high in all risk factors, results of the trim-and-fill procedure were not very robust (data available on request).
Table 3.
Egger's test results for publication bias
| Risk factor | Intercept | 95% CI | t value | p value |
|---|---|---|---|---|
| Asthma | 0.647 | [− 1.99–3.28] | 0.482 | 0.642 |
| Autoimmune disorders | − 3.278 | [− 9.04–2.48] | − 1.116 | 0.283 |
| Cancer | − 1.852 | [− 7.46–3.76] | − 0.647 | 0.526 |
| Cardiovascular disorders | − 4.191 | [− 16.15–7.77] | − 0.687 | 0.509 |
| CHF | 1.614 | [− 10.40–13.63] | 0.263 | 0.803 |
| COPD | − 8.910 | [− 45.18–27.36] | − 0.482 | 0.643 |
| Depression | 2.688 | [− 3.08–8.46] | 0.913 | 0.392 |
| Diabetes | − 10.285 | [− 15.74–4.83] | − 1.886 | 0.079 |
| Digestive disorders | − 0.387 | [− 5.94–5.17] | − 0.136 | 0.895 |
| Endocrine and metabolic disorders | 2.038 | [− 0.78–4.85] | 1.419 | 0.206 |
| Hematological disorders | − 2.006 | [− 8.91–4.90] | − 0.569 | 0.594 |
| HIV | − 2.349 | [− 4.72–0.02] | − 1.943 | 0.081 |
| IBD | − 3.261 | [− 29.32–22.80] | − 0.245 | 0.814 |
| Mental health condition | 6.213 | [− 4.09–16.52] | 1.182 | 0.258 |
| Musculoskeletal disorders | 0.916 | [− 2.83–6.42] | 0.388 | 0.705 |
| Neurological disorders | 1.796 | [− 5.57–9.16] | 0.478 | 0.658 |
| Psioriasis | − 3.240 | [-12.33–5.85] | − 0.699 | 0.523 |
| RA | − 7.584 | [− 14.72–0.45] | − 2.082 | 0.061 |
| Renal disorders | 0.003 | [− 3.95–3.96] | 0.001 | 0.999 |
| SLE | − 0.928 | [− 6.61–4.76] | − 0.320 | 0.757 |
| Transplantation | 2.305 | [− 4.77–9.38] | 0.638 | 0.541 |
Meta-regression
A comprehensive meta-regression analysis was conducted to explore potential sources of heterogeneity across the included studies. This aimed to identify the factors that might contribute to variations in effect sizes and heterogeneity. Correlation matrices (see SI S9) showed that there are correlations among variables; however, these correlations do not appear to be substantial enough to justify removing any of these variables from meta-regression analysis. A permutation test was incorporated to ensure the robustness of the findings. While publication year, sample size, region and study design were explored as potential moderators, only two of these moderators (region and year) demonstrated a statistically significant influence on any of the analyzed risk factors. In total, the meta-regression model could explain heterogeneity for psoriasis (R2 = 100.00%), endocrine and metabolic disorders (R2 = 98.63%), cardiovascular disorders (R2 = 72.78%) and CHF (R2 = 71.88%), but showed no significance for any predictor after permutation testing. Results of the meta-regression and potential moderators are shown in the SI S8.
For CHF, the model indicated a substantial level of residual heterogeneity (τ2 = 0.0263), contributing to a notable unaccounted variability (I2 = 83.95%). The meta-regression results revealed significant associations for publication year (p = 0.0053). These findings suggest that the publication year of the included studies might have statistically significant impacts on the outcome. However, the permutation test could not confirm the robustness of these associations (p > 0.05).
Within the IBD group substantial unexplained variability in effect sizes was observed (I2 = 96.14%), with the meta-regression model explaining 53.24% of this heterogeneity. Publication year showed a significant negative association with effect sizes (p = 0.0007), while study design, region and sample size did not exhibit significant associations (p > 0.05). Permutation testing could not verify the robustness of these findings, as moderator effects were not statistically significant (p = 0.1160).
For mental health conditions significant unexplained variability in effect sizes was observed, with our model explaining a moderate portion of this heterogeneity (R2 = 41.97%). Moderator effects were evident, as indicated by significant tests for residual heterogeneity and moderators. Specifically, publication year demonstrated significant association with effect sizes. Permutation testing supported the moderator effect showing significance.
Within the SLE subgroup, our analysis suggests that geographical location (region) significantly influences effect sizes even after permutation testing, while other factors (publication year, study design, and sample size) did not exhibit statistically significant associations after permutation testing.
Discussion
In this meta-analysis, we aimed to summarize and quantify a range of risk factors associated with HZ incidence. Our findings show, that patients with immunosuppressed conditions, such as transplantation (OR = 4.51) or cancer (OR = 2.42), have the highest risk of HZ. The presence of autoimmune disorders such as RA, SLE, IBD, psoriasis and HIV also increases the risk of HZ. In addition, our analysis underscores the significance of various comorbidities, including renal disorders, hematological disorders, endocrine and metabolic disorders, cardiovascular disorders, CHF, COPD, diabetes, asthma, mental health conditions and depression, in elevating HZ risk.
Two previous meta-analyses examining risk factors for HZ corroborate our results. Marra et al. 2020 determined HIV as the disease with the highest risk for HZ (RR = 3.22; 95% CI [2.4–4.33]), whereas in our pooled analyses, HIV was only associated with an OR = 1.81 (95% CI [1.21–2.69]). Kawai et al. 2017 estimate SLE as disease with the highest risk (RR = 2.1; 95% CI = 1.40–3.15), which is in line with the results by Marra et al. (RR = 2.08; 95% CI [1.56–2.78]) and our results (OR = 2.87; 95% CI [1.99–4.13]). However, both meta-analyses focused not only on diseases as potential risk factors, but also included family history, race, gender and age. They found that, family history of HZ is a risk factor of HZ (OR = 2.48; 95% CI [1.70–3.60] [12] resp. OR = 3.59; 95% CI [2.39–5.40] [14]), indicating a genetic inclination due to the absence of temporal links among cases in relatives. Female gender (OR = 1.19; 95% CI [1.14–1.24] [12] resp. RR = 1.31; 95% CI [1.27–1.34] [14] and older age (RR = 1.65; 95% CI [1.37–1.97] [12]) were also associated with an increased risk of HZ.
Varicella-zoster virus usually remains dormant in sensory ganglia due to cell-mediated immunity (CMI) [119, 120]. Most HZ risk factors relate to weakened CMI. Patients with autoimmune diseases have an elevated HZ risk due to their compromised immunity and medication [8, 121, 122]. For example, individuals with diabetes mellitus have reduced VZV-specific CMI [123]. In addition, depression, characterized by an inflammatory response, is associated with a decreased VZV-specific cell-mediated immune response [124].
Reactivation of VZV is typically associated with a decline in cell-mediated immunity, placing older and immunocompromised (IC) individuals at a higher risk of developing HZ and its complications, such as postherpetic neuralgia (PHN) and VZV vasculopathy [8, 121, 122]. A systematic review reported HZ incidence rates (IR) between 6 and 8 per 1000 PY in 60 years and between 8 and 12 per 1000 PY at age 80 [2]. Studies have consistently observed significantly higher IRs in IC individuals. For instance, Weitzman et al. reported a HZ IR of 12.8 per 1000 PY in IC subjects, compared to 3.5 per 1000 PY in the general population [125, 126]. In a large analysis of German health insurance data, Hillebrand et al. found a HZ IR approximately 75% higher in IC patients than in immunocompetent individuals [126]. Another German claims data analysis revealed higher HZ incidences with decreasing immune status and higher prevalences of complications and healthcare resource utilization [8]. These findings highlight the substantial impact of HZ on immunocompromised patients, with the disease burden being most pronounced in severely immunocompromised individuals.
In the context of substantial variation in pooled OR with large confidence intervals, it is prudent to approach those estimates cautiously. To unravel the origins of this variability, further research is indispensable. For instance, exploring whether the relationship between diabetes mellitus and HZ risk is influenced by variations in glycemic control levels is of paramount importance. In addition, discrepancies in the impact of psychological disorders on HZ risk may stem from divergent disease definitions, including factors, such as acute versus chronic stress, stress severity, duration, and cumulative exposure. Future research should prioritize investigating modifiable risk factors like physical activity, dietary patterns, and environmental exposures to comprehensively understand their roles in HZ incidence [2].
Furthermore, funnel plot asymmetry can originate from variations in between-study heterogeneity, which was often unaccounted for in previous meta-analyses. Smaller studies may ensure precise treatment adherence, potentially yielding higher observed effects, while larger studies might encounter challenges in maintaining treatment fidelity, leading to lower observed effects. Thus, a thorough examination of study characteristics is warranted to assess this alternative explanation. Moreover, it is not uncommon for lower-quality studies to exhibit larger effect sizes due to increased susceptibility to bias. The resource-intensive nature of larger studies can result in more robust methodologies but can also introduce funnel plot asymmetry even in the absence of publication bias. Finally, it is important to recognize that funnel plot asymmetry can occasionally occur purely by chance, highlighting the need for a comprehensive evaluation of potential causes beyond publication bias [127].
Considering the substantial heterogeneity and diverse findings, Bayesian meta-analysis presents an alternative approach [128, 129]. It accommodates prior knowledge and handle complex relationships and uncertainties effectively, providing a versatile tool to address the multifaceted aspects of HZ risk factors. In addition, Bayesian techniques offer probabilistic statements about the effects, enhancing interpretability when traditional frequentist statistics fall short capturing uncertainties [130, 131].
Limitations
Notwithstanding, the following limitations need to be considered when interpreting the results. All selected studies were observational studies, which can introduce bias, due to the study design. Although adjusting for key variables, those studies often relied on administrative or electronic medical records, and raising concerns about potential exposure to misclassification due to coding errors or unaccounted confounders [132]. However, cumulative risk was challenging to determine due to varying study designs and populations or study locations, since most of the studies were conducted in the northern hemisphere. An inherent limitation in this meta-analysis stems from the variability in available data across the selected studies. While efforts were made to recalculate effect estimates, not all studies provided complete raw data for this purpose. Consequently, precalculated effect estimates were used for studies with limited data availability, aiming to encompass the widest scope of evidence. It is essential to note that when one study investigated multiple risk factors or multiple types of disease (e.g. lung cancer and breast cancer), each factor was counted individually in the specific risk factors, thus potentially inflating heterogeneity. These factors underline the importance of considering the potential impact of data availability and study count methodology on the interpretation of results.
Notably, this study extends beyond previous meta-analyses, encompassing the latest literature published over the past decade. Furthermore, conducting not only classical random effects models but also using other methods, e.g. sensitivity analyses and meta-regression to quantitatively synthesize these findings and providing a robust estimate for understanding the multifaceted risk factors contributing to HZ occurrence.
Conclusion
This meta-analysis revealed an increased risk of HZ for all considered risk factors (SLE, hematological disorders, transplantation, asthma, diabetes, cardiovascular disorders, CHF, COPD, musculoskeletal disorders, neurological disorders, digestive disorders, HIV, autoimmune disorders, cancer, mental health conditions, depression, rheumatoid arthritis, renal disorders, psoriasis, endocrine and metabolic disorders, IBD). Further analyses considering the high level of heterogeneity are needed to provide more robust pooled estimates.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the study conception and design. MS performed the statistical analysis. MS and MLH collected and extracted the raw data. MS and AF did the quality assessment of included studies. The first draft of the manuscript was written by MS. DL, JAG and JS critically revised previous versions of the manuscript. WG supervised the study. All authors reviewed and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open access funding provided by Bielefeld University.
Availability of data and materials
The Corresponding author has access to all data included into the analysis. Requests should be submitted to the corresponding author in the first instance.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
References
- 1.Hope-Simson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014 doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curran D, Schmidt-Ott R, Schutter U, Simon J, Anastassopoulou A, Matthews S. Impact of herpes zoster and postherpetic neuralgia on the quality of life of Germans aged 50 or above. BMC Infect Dis. 2018;18:496. doi: 10.1186/s12879-018-3395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizukami A, Sato K, Adachi K, et al. Impact of herpes zoster and post-herpetic neuralgia on health-related quality of life in Japanese adults aged 60 years or older: results from a prospective, observational cohort study. Clin Drug Invest. 2018;38:29–37. doi: 10.1007/s40261-017-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanpää M. Neurological complications of herpes zoster. In: Watson CPN, Gershon AA, Oxman MN, editors. Herpes zoster: postherpetic neuralgia and other complications. Cham: Springer; 2017. pp. 61–75. [Google Scholar]
- 6.Schmidt-Ott R, Schutter U, Simon J, et al. Incidence and costs of herpes zoster and postherpetic neuralgia in German adults aged ≥ 50 years: a prospective study. J Infect. 2018;76:475–482. doi: 10.1016/j.jinf.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63:221–226. doi: 10.1093/cid/ciw296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder C, Enders D, Schink T, Riedel O. Incidence of herpes zoster amongst adults varies by severity of immunosuppression. J Infect. 2017;75:207–215. doi: 10.1016/j.jinf.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 9.van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 2021;17:1714–1732. doi: 10.1080/21645515.2020.1847582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputo M, Horn J, Karch A, et al. Herpes zoster incidence in Germany—an indirect validation study for self-reported disease data from pretest studies of the population-based German National Cohort. BMC Infect Dis. 2019;19:99. doi: 10.1186/s12879-019-3691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marra F, Lo E, Kalashnikov V, Richardson K. Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3:ofw205. doi: 10.1093/ofid/ofw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7:ofaa005. doi: 10.1093/ofid/ofaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batram M, Witte J, Schwarz M, et al. Burden of herpes zoster in adult patients with underlying conditions: analysis of german claims data, 2007–2018. Dermatol Ther (Heidelb) 2021;11:1009–1026. doi: 10.1007/s13555-021-00535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–1821. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ Br Med J. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guolo A. Higher-order likelihood inference in meta-analysis and meta-regression. Stat Med. 2012;31:313–327. doi: 10.1002/sim.4451. [DOI] [PubMed] [Google Scholar]
- 17.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 18.Viechtbauer W, López-López JA, Sánchez-Meca J, Marín-Martínez F. A comparison of procedures to test for moderators in mixed-effects meta-regression models. Psychol Methods. 2015;20:360–374. doi: 10.1037/met0000023. [DOI] [PubMed] [Google Scholar]
- 19.Wells CI, O’Grady G, Bissett IP. Acute colonic pseudo-obstruction: a systematic review of aetiology and mechanisms. World J Gastroenterol. 2017;23:5634–5644. doi: 10.3748/wjg.v23.i30.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017.
- 21.Schwarzer G. Meta: an R package for meta-analysis; 2007.
- 22.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010. 10.18637/jss.v036.i03.
- 23.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Dmetar: companion R package for the guide ‘Doing meta-analysis in R’; 2019.
- 24.Cochrane. Cochrane handbook for systematic reviews of interventions version 6.4; 2023. Available from: URL: www.training.cochrane.org/handbook.
- 25.IntHout J, Ioannidis JPA, Borm GF. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian–Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 27.Sidik K, Jonkman JN. A simple confidence interval for meta-analysis. Stat Med. 2002;21:3153–3159. doi: 10.1002/sim.1262. [DOI] [PubMed] [Google Scholar]
- 28.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olkin I, Dahabreh IJ, Trikalinos TA. GOSH—a graphical display of study heterogeneity. Res Synth Methods. 2012;3:214–223. doi: 10.1002/jrsm.1053. [DOI] [PubMed] [Google Scholar]
- 32.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Cham: Springer; 2015. [Google Scholar]
- 33.Baujat B, Mahé C, Pignon J-P, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 34.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 36.Berman JN, Wang M, Berry W, Neuberg DS, Guinan EC. Herpes zoster infection in the post-hematopoietic stem cell transplant pediatric population may be preceded by transaminitis: an institutional experience. Bone Marrow Transpl. 2006;37:73–80. doi: 10.1038/sj.bmt.1705191. [DOI] [PubMed] [Google Scholar]
- 37.Blank LJ, Polydefkis MJ, Moore RD, Gebo KA. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr. 2012;61:203–207. doi: 10.1097/QAI.0b013e318266cd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cadogan SL, Mindell JS, Breuer J, Hayward A, Warren-Gash C. Prevalence of and factors associated with herpes zoster in England: a cross-sectional analysis of the Health Survey for England. BMC Infect Dis. 2022;22:513. doi: 10.1186/s12879-022-07479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakravarty EF, Michaud K, Katz R, Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2013;22:238–244. doi: 10.1177/0961203312470186. [DOI] [PubMed] [Google Scholar]
- 40.Chen J-Y, Wang L-K, Feng P-H, et al. Risk of shingles in adults with primary Sjogren’s syndrome and treatments: a nationwide population-based cohort study. PLoS ONE. 2015;10:e0134930. doi: 10.1371/journal.pone.0134930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H-H, Lin I-C, Chen H-J, Yeh S-Y, Kao C-H. Association of herpes zoster and type 1 diabetes mellitus. PLoS ONE. 2016;11:e0155175. doi: 10.1371/journal.pone.0155175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D, Li H, Xie J, Zhan Z, Liang L, Yang X. Herpes zoster in patients with systemic lupus erythematosus: clinical features, complications and risk factors. Exp Ther Med. 2017;14:6222–6228. doi: 10.3892/etm.2017.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S-J, Huang K-H, Tsai W-C, Lin C-L, Cheng Y-D, Wei C-C. Asthma status is an independent risk factor for herpes zoster in children: a population-based cohort study. Ann Med. 2017;49:504–512. doi: 10.1080/07853890.2017.1309060. [DOI] [PubMed] [Google Scholar]
- 44.Chung W-S, Lin H-H, Cheng N-C. The incidence and risk of herpes zoster in patients with sleep disorders: a population-based cohort study. Medicine (Baltimore) 2016;95:e2195. doi: 10.1097/MD.0000000000002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteban-Vasallo MD, Domínguez-Berjón MF, Gil-Prieto R, Astray-Mochales J, Gil de Miguel A. Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: a population-based study from primary care in Madrid (Spain). Hum Vaccin Immunother. 2014;10:1650–60. 10.4161/hv.28620. [DOI] [PMC free article] [PubMed]
- 46.Gebo KA, Kalyani R, Moore RD, Polydefkis MJ. The incidence of, risk factors for, and sequelae of herpes zoster among HIV patients in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2005;40:169–174. doi: 10.1097/01.qai.0000178408.62675.b0. [DOI] [PubMed] [Google Scholar]
- 47.Glesby MJ, Hoover DR, Tan T, et al. Herpes zoster in women with and at risk for HIV: data from the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2004;37:1604–1609. doi: 10.1097/00126334-200412150-00013. [DOI] [PubMed] [Google Scholar]
- 48.Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transpl. 2004;4:108–115. doi: 10.1046/j.1600-6143.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 49.Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39:537–544. doi: 10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh W-C, Chen C-H, Cheng Y-C, et al. The risk of herpes zoster in women with polycystic ovary syndrome: a retrospective population-based study. Int J Environ Res Public Health. 2022 doi: 10.3390/ijerph19053094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imafuku S, Dormal G, Goto Y, Jégou C, Rosillon D, Matsuki T. Risk of herpes zoster in the Japanese population with immunocompromising and chronic disease conditions: results from a claims database cohort study, from 2005 to 2014. J Dermatol. 2020;47:236–244. doi: 10.1111/1346-8138.15214. [DOI] [PubMed] [Google Scholar]
- 52.Imafuku S, Matsuki T, Mizukami A, et al. Burden of herpes zoster in the Japanese population with immunocompromised/chronic disease conditions: results from a cohort study claims database from 2005–2014. Dermatol Ther (Heidelb) 2019;9:117–133. doi: 10.1007/s13555-018-0268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin YJ, Park B, Park I-S, Choi HG. Increased risk of herpes zoster in patients with peptic ulcers: a longitudinal follow-up study using a national sample cohort. Medicine (Baltimore) 2020;99:e19318. doi: 10.1097/MD.0000000000019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ke D-S, Hsu C-Y, Lin C-L, Hsu C-Y, Kao C-H. Herpes zoster in patients with sciatica. BMC Musculoskelet Disord. 2020;21:813. doi: 10.1186/s12891-020-03847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan N, Patel D, Trivedi C, et al. Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol. 2018;16:1919. doi: 10.1016/j.cgh.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi D, Shimbo T, Noto H, Eto H, Takahashi O, Higuchi T. Low level of hemoglobin A1c and the increased incidence of herpes zoster: longitudinal study. Eur J Clin Microbiol Infect Dis. 2019;38:1539–1545. doi: 10.1007/s10096-019-03584-1. [DOI] [PubMed] [Google Scholar]
- 57.Habel La, Ray GT, Silverberg MJ, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:82–90. doi: 10.1158/1055-9965.EPI-12-0815. [DOI] [PubMed] [Google Scholar]
- 58.Lai S-W, Lin C-L, Liao K-F. Chronic pancreatitis correlates with increased risk of herpes zoster in a population-based retrospective cohort study. J Hepatob Pancreat Sci. 2018;25:412–417. doi: 10.1002/jhbp.575. [DOI] [PubMed] [Google Scholar]
- 59.Lai S-W, Lin C-L, Liao K-F. Real-world database investigating the association between diabetes mellitus and herpes zoster in Taiwan. Medicine (Baltimore) 2019;98:e15463. doi: 10.1097/MD.0000000000015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai S-W, Lin C-L, Liao K-F. Splenectomy associated with increased risk of herpes zoster in a population-based cohort study. Int J Evid Based Healthc. 2020;18:241–246. doi: 10.1097/XEB.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 61.Lai S-W, Liao K-F, Lin C-L, Liu C-S, Hwang B-F. Association between cirrhosis and herpes zoster in a cohort study in Taiwan. Int J Clin Pract. 2021;75:e14677. doi: 10.1111/ijcp.14677. [DOI] [PubMed] [Google Scholar]
- 62.Lai S-W, Kuo Y-H, Lin C-L, Liao K-F. Risk of herpes zoster among patients with predialysis chronic kidney disease in a cohort study in Taiwan. Int J Clin Pract. 2020;74:e13566. doi: 10.1111/ijcp.13566. [DOI] [PubMed] [Google Scholar]
- 63.Lee Y-C, Hung C-C, Tsai M-S, et al. Incidence and risk factors of herpes zoster in human immunodeficiency virus-positive patients initiating combination antiretroviral therapy in Taiwan. J Microbiol Immunol Infect. 2018;51:38–44. doi: 10.1016/j.jmii.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420–429. doi: 10.1111/apt.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludvigsson JF, Choung RS, Marietta EV, Murray JA, Emilsson L. Increased risk of herpes zoster in patients with coeliac disease-nationwide cohort study. Scand J Public Health. 2018;46:859–866. doi: 10.1177/1403494817714713. [DOI] [PubMed] [Google Scholar]
- 66.Mok CC, Ho LY, Tse SM, Chan KL, To CH. Prevalence and risk factors of herpes zoster infection in patients with rheumatic diseases not receiving biologic or targeted therapies. Clin Rheumatol. 2022 doi: 10.1007/s10067-022-06450-2. [DOI] [PubMed] [Google Scholar]
- 67.Munoz-Quiles C, Lopez-Lacort M, Diez-Domingo J. Risk and impact of herpes zoster among COPD patients: a population-based study, 2009–2014. BMC Infect Dis. 2018 doi: 10.1186/s12879-018-3121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng Y-H, Fang H-Y, Wu B-R, et al. Adult asthma is associated with an increased risk of herpes zoster: a population-based cohort study. J Asthma. 2017;54:250–257. doi: 10.1080/02770903.2016.1211142. [DOI] [PubMed] [Google Scholar]
- 69.Ryu HJ, Han J-O, Lee SA, et al. Risk factors for herpes zoster in patients with rheumatic diseases: a nationwide cohort study in Korea. Rheumatology (Oxford) 2021;60:2427–2433. doi: 10.1093/rheumatology/keaa636. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt SAJ, Sørensen HT, Langan SM, Vestergaard M. Perceived psychological stress and risk of herpes zoster: a nationwide population-based cohort study. Br J Dermatol. 2021;185:130–138. doi: 10.1111/bjd.19832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinayobye JD, Hoover DR, Shi Q, Mutimura E, Cohen HW, Anastos K. Prevalence of shingles and its association with PTSD among HIV-infected women in Rwanda. BMJ Open. 2015;5:e005506. doi: 10.1136/bmjopen-2014-005506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soh H, Chun J, Han K, et al. Increased risk of herpes zoster in young and metabolically healthy patients with inflammatory bowel disease: a nationwide population-based study. Gut Liver. 2019;13:333–341. doi: 10.5009/gnl18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suaya JA, Chen S-Y, Li Q, Burstin SJ, Levin MJ. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis. 2014;1:ofu049. doi: 10.1093/ofid/ofu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takao Y, Okuno Y, Mori Y, Asada H, Yamanishi K, Iso H. Associations of perceived mental stress, sense of purpose in life, and negative life events with the risk of incident herpes zoster and postherpetic neuralgia. Am J Epidemiol. 2018;187:251–259. doi: 10.1093/aje/kwx249. [DOI] [PubMed] [Google Scholar]
- 75.Thompson-Leduc P, Ghaswalla P, Cheng WY, et al. Chronic obstructive pulmonary disease is associated with an increased risk of herpes zoster: a retrospective United States claims database analysis. Clin Respir J. 2022;16:826–834. doi: 10.1111/crj.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai S-Y, Chen H-J, Lio C-F, et al. Increased risk of herpes zoster in patients with psoriasis: a population-based retrospective cohort study. PLoS ONE. 2017;12:e0179447. doi: 10.1371/journal.pone.0179447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai S-Y, Lin C-L, Wong Y-C, et al. Increased risk of herpes zoster following dermatomyositis and polymyositis: a nationwide population-based cohort study. Medicine (Baltimore) 2015;94:e1138. doi: 10.1097/MD.0000000000001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tung Y-C, Tu H-P, Tsai W-C, et al. Increased incidence of herpes zoster and postherpetic neuralgia in adult patients following traumatic brain injury: a nationwide population-based study in Taiwan. PLoS ONE. 2015;10:e0129043. doi: 10.1371/journal.pone.0129043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tung Y-C, Tu H-P, Wu M-K, et al. Higher risk of herpes zoster in stroke patients. PLoS ONE. 2020;15:e0228409. doi: 10.1371/journal.pone.0228409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veetil BMA, Myasoedova E, Matteson EL, Gabriel SE, Green AB, Crowson CS. Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65:854–861. doi: 10.1002/acr.21928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S, Wei JC-C, Huang J-Y, Perng W-T, Zhang Z. The risk of herpes zoster among patients with ankylosing spondylitis: a population-based cohort study in Taiwan. Int J Rheum Dis. 2020;23:181–188. doi: 10.1111/1756-185X.13650. [DOI] [PubMed] [Google Scholar]
- 82.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013;67:463–469. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 83.Wu P-H, Lin Y-T, Lin C-Y, Huang M-Y, Chang W-C, Chang W-P. A nationwide population-based cohort study to identify the correlation between heart failure and the subsequent risk of herpes zoster. BMC Infect Dis. 2015;15:17. doi: 10.1186/s12879-015-0747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu MY, Hsu YH, Su CL, Lin YF, Lin HW. Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis. 2012;60:548–552. doi: 10.1053/j.ajkd.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Yang YW, Chen YH, Wang KH, Wang CY, Lin HW. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: a population-based study. CMAJ. 2011;183:E275–E280. doi: 10.1503/cmaj.101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yanni EA, Ferreira G, Guennec M, et al. Burden of herpes zoster in 16 selected immunocompromised populations in England: a cohort study in the Clinical Practice Research Datalink 2000–2012. BMJ Open. 2018;8:e020528. doi: 10.1136/bmjopen-2017-020528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu T-M, Li C-Y, Chuang Y-W, et al. Risk of severe herpes zoster infection in patients with polycystic kidney disease: a nation-wide cohort study with propensity score matching analysis. Int J Clin Pract. 2021;75:e13675. doi: 10.1111/ijcp.13675. [DOI] [PubMed] [Google Scholar]
- 88.Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–1438. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 89.Chen S-Y, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42:325–334. doi: 10.1007/s15010-013-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J-Y, Cheng T-J, Chang C-Y, et al. Increased incidence of herpes zoster in adult patients with peptic ulcer disease: a population-based cohort study. Int J Epidemiol. 2013;42:1873–1881. doi: 10.1093/ije/dyt213. [DOI] [PubMed] [Google Scholar]
- 91.Choi HG, Kim E-J, Lee YK, Kim M. The risk of herpes zoster virus infection in patients with depression: a longitudinal follow-up study using a national sample cohort. Medicine (Baltimore) 2019;98:e17430. doi: 10.1097/MD.0000000000017430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case–control study. BMJ. 2014;348:g2911. doi: 10.1136/bmj.g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansson E, Forbes HJ, Langan SM, Smeeth L, Bhaskaran K. Herpes zoster risk after 21 specific cancers: population-based case–control study. Br J Cancer. 2017;116:1643–1651. doi: 10.1038/bjc.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heymann AD, Chodick G, Karpati T, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008;36:226–230. doi: 10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 95.Hsu C-Y, Ke D-S, Lin C-L, Kao C-H. Association between de Quervain syndrome and herpes zoster: a population-based cohort study. BMJ Open. 2021;11:e046891. doi: 10.1136/bmjopen-2020-046891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsu C-Y, Ke D-S, Lin C-L, Kao C-H. Plantar fascial fibromatosis and herpes zoster. PLoS ONE. 2021;16:e0259942. doi: 10.1371/journal.pone.0259942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsu C-Y, Ke D-S, Lin C-L, Kao C-H. Association between lateral epicondylitis and the risk of herpes zoster development. Postgrad Med. 2021;133:96–101. doi: 10.1080/00325481.2020.1816713. [DOI] [PubMed] [Google Scholar]
- 98.Hu SC-S, Lin C-L, Lu Y-W, et al. Lymphopaenia, anti-Ro/anti-RNP autoantibodies, renal involvement and cyclophosphamide use correlate with increased risk of herpes zoster in patients with systemic lupus erythematosus. Acta Derm Venereol. 2013;93:314–318. doi: 10.2340/00015555-1454. [DOI] [PubMed] [Google Scholar]
- 99.Joesoef RM, Harpaz R, Leung J, Bialek SR. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc. 2012;87:961–967. doi: 10.1016/j.mayocp.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ke C-C, Lai H-C, Lin C-H, et al. Increased risk of herpes zoster in diabetic patients comorbid with coronary artery disease and microvascular disorders: a population-based study in Taiwan. PLoS ONE. 2016;11:e0146750. doi: 10.1371/journal.pone.0146750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim B-S, Mehra S, Yawn B, et al. Increased risk of herpes zoster in children with asthma: a population-based case–control study. J Pediatr. 2013;163:816–821. doi: 10.1016/j.jpeds.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwon HJ, Bang DW, Kim EN, et al. Asthma as a risk factor for zoster in adults: a population-based case–control study. J Allergy Clin Immunol. 2016;137:1406–1412. doi: 10.1016/j.jaci.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lasserre A, Blaizeau F, Gorwood P, et al. Herpes zoster: family history and psychological stress-case–control study. J Clin Virol. 2012;55:153–157. doi: 10.1016/j.jcv.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 104.Lin SY, Liu JH, Lin CL, et al. A comparison of herpes zoster incidence across the spectrum of chronic kidney disease, dialysis and transplantation. Am J Nephrol. 2012;36:27–33. doi: 10.1159/000339004. [DOI] [PubMed] [Google Scholar]
- 105.Lin S-Y, Liu J-H, Yeh H-C, et al. Association between herpes zoster and end stage renal disease entrance in chronic kidney disease patients: a population-based cohort study. Eur J Clin Microbiol Infect Dis. 2014;33:1809–1815. doi: 10.1007/s10096-014-2143-6. [DOI] [PubMed] [Google Scholar]
- 106.Marin M, Harpaz R, Zhang J, Wollan PC, Bialek SR, Yawn BP. Risk factors for herpes zoster among adults. Open Forum Infect Dis. 2016;3:ofw119. doi: 10.1093/ofid/ofw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Min C, Bang WJ, Oh DJ, Sim S, Choi HG. Association between herpes zoster and osteoporosis: a nested case–control study using a national sample cohort. Biomed Res Int. 2019;2019:4789679. doi: 10.1155/2019/4789679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Min C, Yoo DM, Kim M, Choi HG. Increased risk of herpes zoster in patients with psoriasis: a longitudinal follow-up study using a national sample cohort. Australas J Dermatol. 2021;62:183–189. doi: 10.1111/ajd.13534. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt SAJ, Langan SM, Pedersen HS, et al. Mood disorders and risk of herpes zoster in 2 population-based case–control studies in Denmark and the United Kingdom. Am J Epidemiol. 2018;187:1019–1028. doi: 10.1093/aje/kwx338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidt SAJ, Vestergaard M, Pedersen HS, et al. Partner bereavement and risk of herpes zoster: results from two population-based case–control studies in Denmark and the United Kingdom. Clin Infect Dis. 2017;64:572–579. doi: 10.1093/cid/ciw840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seo H-M, Cha M-J, Han JH, et al. Reciprocal relationship between herpes zoster and cardiovascular diseases: a nationwide population-based case–control study in Korea. J Dermatol. 2018;45:1312–1318. doi: 10.1111/1346-8138.14597. [DOI] [PubMed] [Google Scholar]
- 112.Wi C-I, Kim B-S, Mehra S, Yawn BP, Park MA, Juhn YJ. Risk of herpes zoster in children with asthma. Allergy Asthma Proc. 2015;36:372–378. doi: 10.2500/aap.2015.36.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang JX, Joesoef RM, Bialek S, Wang C, Harpaz R. Association of physical trauma with risk of herpes zoster among medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007–1011. doi: 10.1093/infdis/jis937. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y-W, Chen Y-H, Lin H-W. Risk of herpes zoster among patients with psychiatric diseases: a population-based study. J Eur Acad Dermatol Venereol. 2011;25:447–453. doi: 10.1111/j.1468-3083.2010.03811.x. [DOI] [PubMed] [Google Scholar]
- 115.Hsu C-Y, Ke D-S, Lin C-L, Kao C-H. To investigate the risk of herpes zoster in women with endometriosis: a Taiwan national population-based cohort study. Front Med (Lausanne) 2021;8:584322. doi: 10.3389/fmed.2021.584322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Imafuku S, Matsuki T, Mizukami A, et al. [Duplikat] burden of herpes zoster in the japanese population with immunocompromised/chronic disease conditions: results from a cohort study claims database from 2005–2014. Dermatol Ther (Heidelb) 2019;9:117–133. doi: 10.1007/s13555-018-0268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Imafuku S, Dormal G, Goto Y, Jégou C, Rosillon D, Matsuki T. [Duplikat] Risk of herpes zoster in the Japanese population with immunocompromising and chronic disease conditions: Results from a claims database cohort study, from 2005 to 2014. J Dermatol. 2020;47:236–244. doi: 10.1111/1346-8138.15214. [DOI] [PubMed] [Google Scholar]
- 118.Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. [Duplikat] Quantification of risk factors for herpes zoster: population based case–control study. BMJ. 2014;348:g2911. doi: 10.1136/bmj.g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arvin AM. Varicella–Zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transpl. 2000;6:219–230. doi: 10.1016/S1083-8791(00)70004-8. [DOI] [PubMed] [Google Scholar]
- 120.Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109:S13–S17. [PubMed] [Google Scholar]
- 121.Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis. 2011;11:173. doi: 10.1186/1471-2334-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ultsch B, Köster I, Reinhold T, et al. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ. 2013;14:1015–1026. doi: 10.1007/s10198-012-0452-1. [DOI] [PubMed] [Google Scholar]
- 123.Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009;200:1606–1610. doi: 10.1086/644646. [DOI] [PubMed] [Google Scholar]
- 124.Irwin MR, Levin MJ, Carrillo C, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun. 2011;25:759–766. doi: 10.1016/j.bbi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weitzman D, Shavit O, Cohen R, Chodick G, Shalev V. Epidemiology of herpes zoster and its complication: a population based study in Israel. Pharmacoepidemiol Drug Saf. 2012;21:94. doi: 10.1002/pds.3324. [DOI] [PubMed] [Google Scholar]
- 126.Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E. Incidence of herpes zoster and its complications in Germany, 2005–2009. J Infect. 2015;70:178–186. doi: 10.1016/j.jinf.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 127.Page MJ, Sterne JAC, Higgins JPT, Egger M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods. 2021;12:248–259. doi: 10.1002/jrsm.1468. [DOI] [PubMed] [Google Scholar]
- 128.Röver C. Bayesian random-effects meta-analysis using the Bayes meta R package. J Stat Soft. 2020; 10.18637/jss.v093.i06.
- 129.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams DR, Rast P, Bürkner P-C. Bayesian meta-analysis with weakly informative prior distributions; 2018.
- 131.McNeish D. On using Bayesian methods to address small sample problems. Struct Equ Model. 2016;23:750–773. doi: 10.1080/10705511.2016.1186549. [DOI] [Google Scholar]
- 132.Funk MJ, Landi SN. Misclassification in administrative claims data: quantifying the impact on treatment effect estimates. Curr Epidemiol Rep. 2014;1:175–185. doi: 10.1007/s40471-014-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Corresponding author has access to all data included into the analysis. Requests should be submitted to the corresponding author in the first instance.