Abstract
Alkyl hydroperoxide reductase subunit C (AhpC) is the catalytic subunit responsible for alkyl peroxide metabolism. A Xanthomonas ahpC mutant was constructed. The mutant had increased sensitivity to organic peroxide killing, but was unexpectedly hyperresistant to H2O2 killing. Analysis of peroxide detoxification enzymes in this mutant revealed differential alteration in catalase activities in that its bifunctional catalase-peroxidase enzyme and major monofunctional catalase (Kat1) increased severalfold, while levels of its third growth-phase-regulated catalase (KatE) did not change. The increase in catalase activities was a compensatory response to lack of AhpC, and the phenotype was complemented by expression of a functional ahpC gene. Regulation of the catalase compensatory response was complex. The Kat1 compensatory response increase in activity was mediated by OxyR, since it was abolished in an oxyR mutant. In contrast, the compensatory response increase in activity for the bifunctional catalase-peroxidase enzyme was mediated by an unknown regulator, independent of OxyR. Moreover, the mutation in ahpC appeared to convert OxyR from a reduced form to an oxidized form that activated genes in the OxyR regulon in uninduced cells. This complex regulation of the peroxide stress response in Xanthomonas differed from that in other bacteria.
Increased rates of production and accumulation of reactive oxygen species (ROS), including H2O2, organic peroxide, and superoxide, are important components of active plant defense responses to microbial invasion (11). In addition, normal aerobic metabolism also generates large quantities of ROS (6, 7). For successful plant invasion, these ROS must be rapidly detoxified. Monofunctional catalases are major H2O2 scavenging enzymes in Xanthomonas (26), while detoxification of organic peroxides is more complex. We have identified in Xanthomonas alkyl hydroperoxide reductase genes (ahpC and ahpF [12, 16]) and a novel family of organic peroxide resistance genes (ohr [17]) which are involved in organic peroxide protection. Alkyl hydroperoxide reductase (AhpCF) is the best characterized microbial enzyme involved in organic peroxide metabolism. AhpCF consists of a catalytic 22-kDa C subunit (AhpC) and a reductase 52-kDa F subunit (AhpF) (19, 24). The ahpC gene has been highly conserved in evolution and is found in organisms ranging from bacteria to humans (5). Inactivation of ahpC in various bacterial mutants results in increased sensitivity to organic peroxide killing and to spontaneous mutagenesis (1, 3, 8, 20, 29). In addition, since mutants show additional alterations in oxidative stress response that range from increased sensitivity to hyperresistance to oxidative stress (1, 3, 21, 29), we have isolated and characterized Xanthomonas genes for both the catalytic (ahpC) and the reductase (ahpF) subunits (12, 16). ahpC has a unique form of regulation in which reduced OxyR represses ahpC expression while oxidized OxyR activates its expression (18).
Recently, we have shown that an ahpCE mutant with OxyR regulation separated from basal ahpC promoter activity has a lower aerobic growth rate and increased sensitivity to organic peroxide resistance (13). However, the lack of an ahpC knockout mutant in Xanthomonas has hampered analysis of the gene's physiological functions and its role in protection against peroxide stress. In this communication, we describe the construction and physiological characterization of an ahpC knockout mutant. The mutant showed atypical alterations in resistance to peroxide killing and deregulation of genes for peroxide scavenging enzymes.
Construction of an ahpC knockout mutant.
An ahpC mutant was constructed by integration into Xanthomonas campestris pv. phaseoli chromosome of a recombinant plasmid, pKSahpC1. Essentially, primers corresponding to amino acid residue numbers 63 to 70 and 126 to 133 of ahpC were used to amplify a 157-bp DNA fragment containing an internal coding region of ahpC that was subsequently cloned into pKSKm (16). The resultant plasmid, pKSahpC1, was electroporated into X. campestris pv. phaseoli, and transformants were selected for Kmr. This yielded the ahpC1 mutant. Southern analysis of genomic DNA digested with SacII from the mutant and probed with the ahpC probe showed a positive hybridization band with an increase of 3.5 kb compared to similarly digested DNA from the parental strain (data not shown). This pattern was consistent with the idea that pKSahpC1 had correctly integrated and disrupted the gene. Results of Western immunoblot analysis of lysates confirmed the lack of AhpC in the mutant (data not shown).
Altered sensitivity to peroxide killing in the mutant.
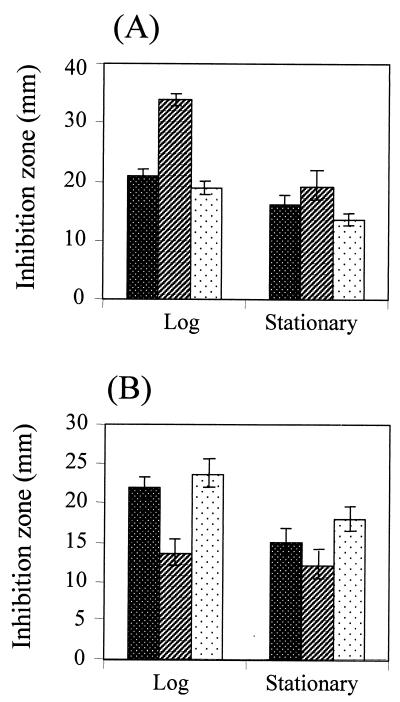
The levels of peroxide resistance in various ahpC-minus mutants differ widely (1, 3, 20, 21). Hence, levels of resistance to killing concentrations of organic peroxide and H2O2 in the ahpC1 mutant were determined during the exponential and stationary phases of growth. The mutant was more sensitive to tert-butyl hydroperoxide (tBOOH) killing than the parental strain during the exponential phase of growth (Fig. 1). This observation confirmed the important role of ahpC in protection against organic peroxide toxicity. A similar phenotype has been observed in other ahpC mutants (1, 20, 21, 24, 29). In contrast, Xanthomonas ahpC1 was more resistant to H2O2 killing (Fig. 1). Purified AhpCF is capable of using both H2O2 and organic peroxide as substrates (19). Thus, it was unexpected that inactivation of the gene for the catalytic subunit of the AhpCF would result in increased H2O2 resistance. Nonetheless, a similar phenotype has been observed in Bacillus subtilis ahpC (1, 3). The mutant was transformed with pahpC (an expression vector containing a functional ahpC gene [12, 16]). pahpC complemented alterations in peroxide resistance levels in the mutant. The mutant harboring pahpC1 had levels of resistance to tBOOH and H2O2 similar to those of the parental strain (Fig. 1).
FIG. 1.
Determination of levels of resistance to peroxide killing in the ahpC1 mutant and the parental strain. All Xanthomonas strains were grown aerobically in SB medium at 28°C. The exponential and stationary phases of growth were defined as 4 h (A550 of ∼0.5) and 30 h (A550 of ∼3.0) after inoculation, respectively. For determination of levels of resistance to peroxide killing, bacterial cells from the exponential or stationary phase were mixed with SB top agar and poured onto SB plates. Thus, 5 μl of 0.5 M tBOOH (A) or H2O2 (B) was spotted onto paper discs and placed on the cell lawn. The zone of growth inhibition around the disc was measured after 24 h of incubation. The experiments were repeated four times, and error bars indicate the standard error of the mean. ▩, parental strain; ▨, ahpC1 mutant; , mutant harboring pahpC.
In all bacteria thus far studied, levels of peroxide resistance significantly increase in the stationary phase (10, 27). Thus, we determined the levels of resistance to peroxide killing during the stationary phase. In the stationary phase, both the mutant and parental strains were more resistant to peroxide killing than in the exponential phase (Fig. 1). During the stationary phase, the mutant was also more resistant to H2O2 killing than the parental strain. However, differences in stationary-phase organic peroxide resistance levels between the mutant and the parental strain were less pronounced than differences during the exponential phase. The results suggested that AhpC was not an important factor in determining organic peroxide resistance levels during the stationary phase.
Altered levels of peroxide detoxification enzymes in the ahpC mutant.
The unusual phenotype of the mutant prompted us to determine the activities of various enzymes involved in oxidative stress protection and peroxide detoxification. The results in Table 1 show intricate changes in the activities of these enzymes. The activities of oxidative stress protection enzymes such as superoxide dismutase, glucose-6-phosphate dehydrogenase, glutathione reductase, and Ohr were similar in the mutant and the parental strains. In contrast, increased activities of the peroxide detoxification enzymes catalase (8-fold) and peroxidase (11-fold) were observed in the mutant (Table 1). These increases were due to inactivation of ahpC and could be complemented by pahpC (Table 1). Thus, lack of AhpC led to compensatory increase in the activities of these enzymes.
TABLE 1.
Determination of enzymes involved in peroxide resistance in various Xanthomonas strains
| X. campestris pv. phaseoli type | Enzyme
activity (U/mg of protein)a
|
||||
|---|---|---|---|---|---|
| Catalase | Peroxidase (103) | SOD | GR | G6PDH (103) | |
| Wild type | 4.9 | 1.6 | 5.3 | 1.9 | 27.5 |
| ahpC1 | 38.4 | 18.4 | 5.1 | 2.1 | 29.4 |
| ahpC1/pahpC1 | 2.6 | 1.1 | 5.5 | 1.8 | 28.4 |
| ahpC1 oxyR | 1.8 | 12.8 | 4.9 | 1.8 | 26.2 |
| oxyR | 2.1 | 1.3 | NDb | ND | ND |
Crude lysates from exponential-phase cultures of Xanthomonas strains were prepared as previously described (26). Assays to determine the activities of catalase (26), peroxidase (14), superoxide dismutase (SOD) (15), glutathione reductase (GR) (22), and glucose-6-phosphate dehydrogenase (G6PDH) (28) were performed as previously described. Values are means of four replicates.
ND, not done.
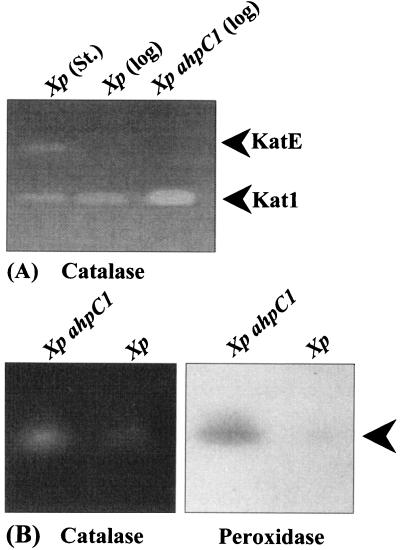
We have observed several forms of monofunctional catalases in Xanthomonas by using catalase activity gels (26), and analysis of cloned kat genes indicates that various forms of catalase are products of different genes (26; S. Mongkolsuk, unpublished data). Using catalase and peroxidase activity gels, we have shown that the levels of a major monofunctional catalase, Kat1 (26), increased severalfold in the ahpC strain compared to those in the parental strain (Fig. 2). Kat1 accounts for over 80% of total catalase activity, as judged by analysis of catalase activity gels (26). Increased Kat1 activity was responsible for the observed increase in total catalase activity (Table 1). Analysis of peroxidase activity gels showed only one positive activity band (Fig. 2). The intensity of this band was severalfold higher in the mutant. When a similar gel was stained for catalase activity, a catalase activity band was observed at the same position as the peroxidase activity band (Fig. 2). This suggested that the enzyme was a bifunctional catalase and peroxidase. Indeed, most of the bacterial peroxidases are bifunctional catalase-peroxidase enzymes (9). Analysis of catalase and peroxidase activity gels suggested that the bifunctional enzyme contributed less than 10% to total catalase activity (data not shown). Thus, the 11-fold increase in peroxidase activity (Table 1) could be assigned to increases in activity of the catalase-peroxidase bifunctional enzyme. The compensatory increases in activities of both enzymes were abolished in the mutant harboring pahpC. The levels of a third form of growth-phase-regulated monofunctional catalase (KatE [26]) were similar in the two strains (data not shown). Increased catalase activity in the mutant could account for the increased H2O2 resistance during the exponential and stationary phases.
FIG. 2.
Analysis of various forms of catalase and peroxidase in the ahpC1 mutant. (A) Forty micrograms of cell lysates prepared from exponential-phase (log) or stationary-phase (St) cultures of parental X. campestris pv. phaseoli (Xp) and exponential-phase ahpC1 mutant [Xp ahpC1 (log)] cultures was loaded into each lane. Lysate preparation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and renaturing of gels were performed as previously described (26). Catalase activity staining with diaminobenzidine was performed as described by Vattanaviboon and Mongkolsuk (26). The positions of Kat1 and KatE are shown. (B) Eighty micrograms of lysate from the exponential-phase ahpC1 mutant (Xp ahpC1) and the parental strain (Xp) was loaded into each lane. Gel electrophoresis was performed with a 9% polyacrylamide native gel (14). Subsequently, the gel was split into two halves, and each half was stained for peroxidase activity with diaminobenzidine (14) or for catalase activity (26). The arrowhead indicates the position of the catalase-peroxidase bifunctional enzyme.
The compensatory increases in monofunctional and bifunctional catalases were mediated by different regulators.
Compensatory alterations in gene expression resulting from either gene inactivation or altered gene expression are important reactions for bacterial survival under stressful conditions. In almost all cases, the regulation of these processes is unknown. OxyR is a peroxide sensor and a transcription regulator (23, 25). Thus, the role of OxyR in regulation of the catalase compensatory response in the ahpC1 mutant was investigated. An ahpC1 oxyR double mutant was constructed by transformation with chromosomal DNA from an X. campestris pv. phaseoli oxyR::Gmr mutant (18) into the ahpC1 mutant. Southern and Western analyses were used to confirm the integrity of the double mutant (data not shown). The levels and forms of catalases in the ahpC1 and the ahpC1 oxyR mutants were determined and are shown in Fig. 2 and Table 1. The ahpC1 oxyR double mutant showed levels of catalase similar to those of the oxyR mutant (Table 1), and these were 10-fold less than those in the ahpC1 mutant. Thus, oxyR mutation completely eliminated the compensatory increase in total catalase activity in the ahpC1 mutant. Surprisingly, the ahpC1 oxyR and ahpC1 mutants had comparable peroxidase levels. Both were 10-fold higher than those of the parental strain and the oxyR mutant (Table 1). Since oxyR mutation had no effect on the compensatory increase in the levels of bifunctional peroxidase and catalase in the ahpC1 mutant, the process had to be regulated by another unknown regulator. Thus, the data suggested that Xanthomonas has at least two regulators which responded to changes in levels of peroxide. Dual regulation of the catalase compensatory response could be a means of ensuring sustained activity even if one of the regulators was incapacitated, and the response could be vital to bacterial survival in the absence of a functional ahpC gene.
Mutation in ahpC altered expression of OxyR-regulated genes.
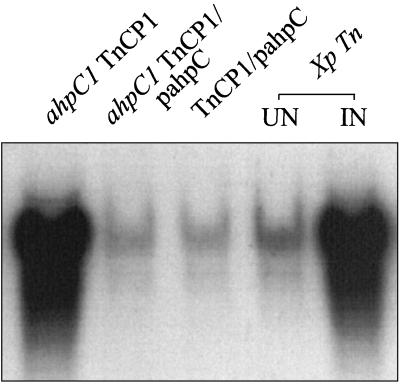
We wished to elucidate how OxyR could activate catalase expression responsible for the compensatory response in the ahpC1 mutant. OxyR can exist in either a reduced or oxidized form (23). In uninduced cells, OxyR exists in the reduced form (2, 30). Upon exposure to H2O2, highly conserved cysteine residues of OxyR are oxidized to form a disulfide bond, converting it to the oxidized form (30). In Xanthomonas, OxyR is required for oxidant-induced expression of catalase and ahpC genes (18). Oxidized OxyR probably activates kat1 expression. Since we could not directly determine the in vivo redox status of OxyR, an alternative approach was used. This was based on the fact that the ahpC promoter in Xanthomonas is transcriptionally activated by oxidized OxyR and repressed by reduced OxyR (13, 18). Thus, ahpC promoter activity can be used to reflect the redox status of OxyR. An experiment was designed to test whether the mutation in ahpC altered the redox status of OxyR. This was done by monitoring levels of a chloramphenicol acetyltransferase gene (cat) used as a reporter transcriptionally fused to the ahpC promoter in the ahpC1 mutant and the parental strain. The ahpC promoter fused to the cat gene was inserted into a mini-Tn10 transposon, resulting in TnCP1 (13). The construct was subsequently transposed into the parental strain. Chromosomal DNA of X. campestris pv. phaseoli TnCP1 was extracted and electroporated into the ahpC1 mutant. Integration of TnCP1 in the mutant and the parental strain was confirmed by PCR and Southern analysis (data not shown). Results of cat Northern analysis in strains containing TnCP1 are shown in Fig. 3. The parental strain containing TnCP1 showed low levels of uninduced cat expression. We have observed that menadione consistently induced ahpC expression in an oxyR-dependent manner (16, 18). Exposure to 50 μM menadione induced high levels of cat expression (Fig. 3). This result was consistent with the observations that reduced OxyR repressed the ahpC promoter and oxidized OxyR activated it (13). However, even in the absence of oxidant induction, high levels of cat mRNA were detected in the ahpC1 mutant containing TnCP1 (Fig. 3). The high cat mRNA level in the uninduced mutant was similar to the cat mRNA level in the oxidant-induced parental strain containing TnCP1 (Fig. 3). Complementation in the ahpC1 TnCP1 strain with pahpC resulted in uninduced cat mRNA levels similar to those of the uninduced parental strain containing TnCP1 (Fig. 3). Furthermore, activation of the ahpC promoter in the uninduced mutant required functional OxyR, since it was eliminated in oxyR-minus derivatives of the mutant (data not shown). These data and data from Table 1 support the idea that OxyR existed in an oxidized form in the uninduced ahpC1 mutant and that this oxidized OxyR was responsible for activation of genes in the OxyR regulon, including kat1. The question of how OxyR was converted to the oxidized form in uninduced cells remains unanswered. The physiological substrates of AhpC are not known. AhpC can metabolize a wide range of organic peroxides, such as nucleotide peroxides and lipid peroxides (19, 24). Our observations implied that the ahpC1 mutant probably accumulated various organic peroxides which converted OxyR from the reduced form to the oxidized form. This suggested, in turn, that various organic peroxides could act as intracellular signals to activate a global peroxide defense response via OxyR. We could not rule out that increased organic peroxide levels could lead to a transient increase in H2O2 levels sufficient to activate OxyR. Additional support for the role of organic peroxides and not H2O2 as the signal for activation of the OxyR-dependent compensatory response in the ahpC1 mutant came from the observation that the mutant had eightfold higher catalase levels than the parental strain. High catalase activity should efficiently prevent accumulation of H2O2 in the mutant. Moreover, addition of sodium pyruvate to SB medium did not affect the cat levels in the ahpC1 TnCP1 strain. At present, we cannot conclusively prove this hypothesis, because in our hands, the levels of organic peroxide could not be accurately determined. Definitive support for the theory must wait for accurate measurement of all organic peroxides in mutant and parental strains. In Xanthomonas, OxyR can function as a sensor for both H2O2 and organic peroxide. The proposed role of organic peroxides as signal molecules is novel but not unique to Xanthomonas. An analogous observation has been reported in B. subtilis ahpC mutants. Several groups have suggested that ahpC mutation can lead to accumulation of organic peroxides and result in inactivation of PerR, a peroxide-sensitive transcription repressor (1, 3, 4). This can lead to increased expression of genes in the PerR regulon (4). The role of organic peroxides as signal molecules is likely to be generally important in a wide range of bacteria.
FIG. 3.
Northern analysis of the ahpC promoter fused to cat in both the ahpC1 mutant and the parental strain. All Xanthomonas strains used in this study contained TnCP1 (13). Total RNA was extracted by a hot phenol method from the mutant (ahpC1 TnCP1), the mutant harboring pahpC (ahpC1 TnCP1/pahpC), the parental strain (Xp Tn, uninduced [UN] or induced with 50 μM menadione [IN]) and the parental strain harboring pahpC (TnCP1/pahpC). RNA (20 μg) was then loaded into each lane and separated on a formaldehyde agarose gel. Gel electrophoresis, blotting hybridization, washing, and preparation of the cat probe were performed as previously described (13).
Acknowledgments
We thank T. Flegel for critically reviewing the manuscript.
This research was supported by grants from Chulabhorn Research Institute to the Laboratory of Biotechnology, the Thailand Research Fund BRG 10-40 grant, and an NSTDA career development award (RCF 01-40-005) to S.M.
REFERENCES
- 1.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bsat N, Chen L, Helmann J D. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtiliscontains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 5.Chae H Z, Robison K, Poole L B, Church G, Storz G, Rhee S G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Flecha B, Demple B. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol. 1997;179:382–388. doi: 10.1128/jb.179.2.382-388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Flecha B, Demple B. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem. 1995;270:13681–13687. doi: 10.1074/jbc.270.23.13681. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg J T, Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 1988;7:2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klotz M G, Klassen G R, Loewen P C. Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol Biol Evol. 1997;14:951–958. doi: 10.1093/oxfordjournals.molbev.a025838. [DOI] [PubMed] [Google Scholar]
- 10.Kolter R, Siegele D A, Tormo A. The stationary phase of bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 11.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2from oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 12.Loprasert S, Atichartpongkun S, Whangsuk W, Mongkolsuk S. Isolation and analysis of the Xanthomonas alkyl hydroperoxide reductase gene and the peroxide sensor regulator genes ahpC and ahpF-oxyR-orfX. J Bacteriol. 1997;179:3944–3949. doi: 10.1128/jb.179.12.3944-3949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loprasert S, Faungthong M, Whangsuk W, Atichartpongkul S, Mongkolsuk S. Molecular and physiological analysis of an OxyR regulated ahpC promoter in Xanthomonas campestrispv. phaseoli. Mol Microbiol. 2000;37:1504–1514. doi: 10.1046/j.1365-2958.2000.02107.x. [DOI] [PubMed] [Google Scholar]
- 14.Loprasert S, Negoro S, Okada H. Cloning, nucleotide sequence, and expression in Escherichia coli of the Bacillus stearothermophilus peroxidase gene (perA) J Bacteriol. 1989;171:4871–4875. doi: 10.1128/jb.171.9.4871-4875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCord J M, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 16.Mongkolsuk S, Loprasert S, Whangsuk W, Fuangthong M, Atichartpongkun S. Characterization of transcription organization and analysis of unique expression patterns of an alkyl hydroperoxide reductase C gene (ahpC) and the peroxide regulator operon ahpF-oxyR-orfX from Xanthomonas campestrispv. phaseoli. J Bacteriol. 1997;179:3950–3955. doi: 10.1128/jb.179.12.3950-3955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestrispv. phaseoli. J Bacteriol. 1998;180:2636–2643. doi: 10.1128/jb.180.10.2636-2643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mongkolsuk S, Sukchawalit R, Loprasert S, Praituan W, Upaichit A. Construction and physiological analysis of a Xanthomonas mutant to examine the role of the oxyRgene in oxidant-induced protection against peroxide killing. J Bacteriol. 1998;180:3988–3991. doi: 10.1128/jb.180.15.3988-3991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole L B, Ellis H R. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry. 1996;35:56–64. doi: 10.1021/bi951887s. [DOI] [PubMed] [Google Scholar]
- 20.Rocha E R, Smith C J. Role of the alkyl hydroperoxide reductase (ahpCF) gene in oxidative stress defense of the obligate anaerobe Bacteroides fragilis. J Bacteriol. 1999;181:5701–5710. doi: 10.1128/jb.181.18.5701-5710.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman D R, Mdluli K, Hickey M J, Arain T M, Morris S L, Barry III C E, Stover C K. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science. 1996;272:1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 22.Smith I K, Vuerheller T L, Thorn C A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 23.Storz G, Imlay J A. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 24.Storz G, Jacobson F S, Tartaglia L A, Morgan R W, Silveira L A, Ames B N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storz G, Tartaglia L A, Ames B N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 26.Vattanaviboon P, Mongkolsuk S. Expression analysis and characterization of the mutant of a growth-phase- and starvation-regulated monofunctional catalase gene from Xanthomonas campestrispv. phaseoli. Gene. 2000;241:259–265. doi: 10.1016/s0378-1119(99)00483-7. [DOI] [PubMed] [Google Scholar]
- 27.Vattanaviboon P, Praituan W, Mongkolsuk S. Growth phase dependent resistance to oxidative stress in a phytopathogen Xanthomonas oryzaepv. oryzae. Can J Microbiol. 1995;41:1043–1047. [Google Scholar]
- 28.Wolf R E, Jr, Prather D M, Shea F M. Growth-rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coliK-12. J Bacteriol. 1979;139:1093–1096. doi: 10.1128/jb.139.3.1093-1096.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Dhandayuthapani S, Deretic V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosisto isoniazid. Proc Natl Acad Sci USA. 1996;93:13212–13216. doi: 10.1073/pnas.93.23.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]