Abstract
Mycobacterium tuberculosis secretes a large number of polypeptides with broad biological and immunological functions. We describe here the characterization of a 28-kDa acid phosphatase of M. tuberculosis (SapM) localized to the culture filtrate. The mature protein demonstrated biochemical characteristics similar to those of the bacterial nonspecific acid phosphatases. However, SapM yielded significant sequence homology to fungal acid phosphatases and not those of bacteria. Thus, SapM may represent a new class of bacterial nonspecific acid phosphatases.
The complete Mycobacterium tuberculosis genome was recently sequenced and annotated (8). However, of the 3,924 putative gene products, only a minority are assigned experimentally established physiological functions. Thus, continued analyses of the M. tuberculosis proteome are required to fully elucidate the biology of this intracellular pathogen. The most thoroughly studied gene products of M. tuberculosis are the culture filtrate proteins (CFPs) (reviewed in reference 6). The CFPs are proposed to participate in intracellular survival (6) and are a primary target of the host's protective T-cell response (21). Several laboratories have added some definition to the myriad of biological and enzymatic activities associated with the CFPs (4, 7, 15, 23). In the most comprehensive of these studies, Raynaud et al. (23) described 22 enzymatic activities. However, the molecular identities and characteristics of the enzymes responsible for these activities were not elucidated. One of the activities identified was that of an acid phosphatase.
One mechanism of intracellular survival employed by M. tuberculosis is the modulation of host cell activities, such as intracellular vesicle fusion, phagosome maturation, and phagosome acidification (reviewed in reference 27). Although the specific proteins of M. tuberculosis that modulate macrophage activity remain elusive, recent studies of other intracellular pathogens demonstrate that acid phosphatases are important to this aspect of microbial pathogenicity (2, 24, 25, 28). Thus, we believe that such an activity may also facilitate host cell modulation by M. tuberculosis. As a first step in testing this hypothesis, the presence of acid phosphatase activity in the culture filtrate of M. tuberculosis was confirmed and a 28-kDa protein possessing this activity was purified and characterized.
Purification and identification of the acid phosphatase.
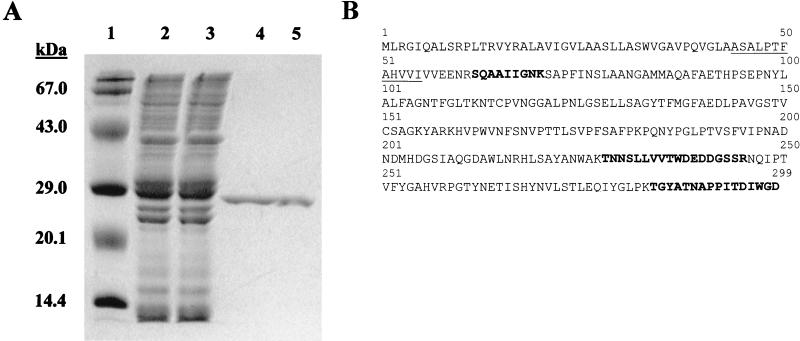
To identify and isolate a secreted acid phosphatase, a culture filtrate of M. tuberculosis cells grown to late log phase was harvested (9) and dialyzed against 0.1 M sodium acetate (pH 6.0). Initial evaluation of this culture filtrate for phosphatase activity, using p-nitrophenyl phosphate (pNPP) as the substrate (5), indicated the presence of weak activity (750 nmol of p-nitrophenol [pNP]/min/mg of protein) at pH 6.0. The culture filtrate was fractionated by cation-exchange chromatography, and a single asymmetric peak of acid phosphatase activity was eluted with about 0.3 M NaCl. Moreover, the acid phosphatase activity was enriched 70.6-fold. Final purification of this enzymatic activity was accomplished by hydrophobic-interaction chromatography. This resulted in the isolation of a single polypeptide with an apparent molecular mass of 28 kDa, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1A), and a specific acid phosphatase activity of 86,290 nmol of pNP/min/mg of protein.
FIG. 1.
Purification of acid phosphatase activity from culture filtrate of M. tuberculosis. (A) Coomassie blue-stained SDS-polyacrylamide gel (12% polyacrylamide) showing the purification of the M. tuberculosis acid phosphatase. Lane 1, molecular mass markers; lane 2, M. tuberculosis CFPs; lane 3, nonbinding proteins from cation-exchange chromatography; lane 4, a pool of fractions 12 to 30 from cation-exchange chromatography; lane 5, purified SapM after hydrophobic-interaction chromatography. (B) Amino acid sequence of the Rv3310 gene product (SapM). The solid line indicates the N-terminal amino acid sequence obtained from the mature protein. The bold sequences indicate individual peptides identified by MS-MS analysis of a tryptic digest of purified SapM. The boxes indicate His residues conserved in fungal acid phosphatase homologues.
The molecular identity of the 28-kDa acid phosphatase was determined via peptide mass fingerprinting. Peptides generated by trypsin digestion of the purified protein were separated by C18 reversed-phase high-performance liquid chromatography and analyzed by electrospray mass spectrometry (ES-MS) (12, 16). The molecular mass/charge ratio (m/z) and ES-MS-MS fragmentation patterns were determined for individual peptides, and these data were searched against the M. tuberculosis protein database by using the Sequest program (12). Three peptides with 901.4, 1,692.8, and 2,009.1 m/z (Fig. 1B) were matched to predicted trypsin fragments of a gene product annotated in the M. tuberculosis database as “hypothetical protein Rv3310.” The putative Rv3310 gene product possessed 299 amino acid residues with a theoretical molecular mass of 31,807 Da and an isoelectric point (pI) of 6.14.
To confirm the identity of the purified acid phosphatase, N-terminal sequencing was performed on the intact protein (29). This yielded a sequence of ASALPTFAHVVI (Fig. 1B) that corresponded to amino acids 44 to 55 of the putative Rv3310 gene product. Analysis of the deduced protein sequence revealed that the first 43 amino acids of the predicted Rv3310 gene product possessed characteristics of a typical prokaryotic signal sequence (31). Additionally, the theoretical molecular mass of the mature protein was calculated to be 27,365 Da, a value similar to that obtained by SDS-PAGE (Fig. 1A). These data provided strong evidence of a protein secreted via a sec-dependent mechanism. Thus, the 28-kDa acid phosphatase was designated SapM, for secreted acid phosphatase of M. tuberculosis.
Sequence homology of SapM.
The amino acid sequence of the full-length SapM was subjected to a BLASTP search (32) against the nonredundant protein database (National Center for Biotechnology Information). This resulted in the identification of three sequences with 28 to 31% identity and 44 to 51% similarity to SapM. These homologous sequences belonged to a phosphate-repressible acid phosphatase from Penicillium chrysogenum (Pc-PhoA) (14), a pH 6.0-optimum acid phosphatase from Aspergillus (niger) ficuum (Af-PhoA) (11), and a potential acid phosphatase of Kluyveromyces lactis (Kl-PhoX) (13). Interestingly, no significant homology to any prokaryotic acid phosphatases was found. SapM also lacked sequence motifs of phosphothreonine-serine and protein tyrosine phosphatases (3), metallophosphoesterases (19), and histidine phosphatases (22). However, SapM did possess two His residues (position 93 and 204) that were conserved in highly homologous regions of the fungal acid phosphatases.
Oligomeric state, pH optimum, and kinetics of SapM.
To determine whether SapM existed in a monomeric or oligomeric form, the purified protein (100 μg) was applied to a Sepharose 12 column (Amersham Pharmacia Biotech Inc.) and eluted with phosphate-buffered saline at a flow rate of 0.5 ml/min. A comparison of the SapM retention coefficient to those of protein standards (bovine serum albumin, 66 kDa; carbonic anhydrase, 29 kDa; cytochrome c, 12.4 kDa; and aprotinin, 6.5 kDa) yielded a relative molecular mass of 28.8 kDa. This value agreed well with the theoretical mass of the mature monomeric protein and that obtained by SDS-PAGE. Furthermore, the purified protein showed no oligomeric species by SDS-PAGE in cross-linking experiments with bis(sulfosuccinimidyl) suberate.
The pH optimum for the enzymatic hydrolysis of pNPP by SapM was determined using four buffer systems: 0.1 M sodium citrate (pH 3.0 to 5.0), 0.1 M sodium acetate (pH 5.5 to 6.5), 0.1 M Tris base (pH 7.0 to 8.0), and 0.1 M sodium bicarbonate (pH 8.5 to 9.0). The relative rate of hydrolysis of pNPP was determined by quantitation of released pNP (5) and Pi (20). Both methods yielded comparable results. Significant activity was observed between pH 5.5 and 8.0, and optimal activity was observed at pH 6.5 to 7.5. This optimal pH range is wider than that observed for other acid phosphatases, such as the homologous Af-PhoA (11) and those from Francisella tularensis (24) and Coxiella burnetii (2). However, it is similar to that observed for the bacterial nonspecific acid phosphatases (NSAPs) (26). The kinetic parameters for the hydrolysis of pNPP were determined using a 0.4 μM to 0.5 mM concentration range of pNPP in 0.1 M Tris buffer, pH 6.8. The Km was determined to be 0.43 mM, and the Vmax was 2.1 × 106 nmol of pNP/h/mg of protein.
Range of substrates and inhibitors of SapM.
Twenty-one compounds at a concentration of 1 mM were tested as substrates for SapM, and their rates of hydrolysis were compared to that of pNPP (Table 1). SapM demonstrated equivalent activity against α-naphthyl phosphate, another substrate commonly used to detect phosphatase activity. When tested with naturally occurring molecules of physiological significance to mycobacteria, the purified enzyme exhibited the highest activity against phosphoenolpyruvate, glycerophosphate, GTP, NADPH, phosphotyrosine, and trehalose-6-phosphate. In contrast, the enzyme exhibited poor activity against glucose-6-phosphate, phosphothreonine, and a number of nucleotides (NADP, ATP, AMP, and GMP). SapM was not active against cysteamine phosphate, a substrate specific for alkaline phosphatase. Similarly, no activity was detected against phospholipids or the phospholipase substrate p-nitrophenylphosphorylcholine. Some acid phosphatases, in particular a subgroup of histidine acid phosphatases, also possess phytase activity (18). However, the rate of hydrolysis of phytic acid by SapM was 15% of that for pNPP (Table 1). This low activity for phytic acid led us to conclude that SapM is not a phytase.
TABLE 1.
Substrate specificity of the M. tuberculosis acid phosphatase (SapM)a
| Substrate | % Activity |
|---|---|
| p-Nitrophenyl phosphate (pNPP) | 100 |
| α-Naphthyl phosphate | 104 |
| Phosphoenolpyruvate | 100 |
| Glycerophosphate | 89 |
| GTP | 72 |
| NADPH | 69 |
| Phosphotyrosine | 67 |
| Trehalose-6-phosphate | 64 |
| Phytic acid | 15 |
| Phosphatidic acid | <5 |
| p-Nitrophenylphosphorylcholine | <3 |
| MUPc | <3 |
| Glucose-6-phosphate | <3 |
| NADP | <3 |
| ATP | <3 |
| AMP | <3 |
| GMP | <3 |
| Phosphothreonine | <3 |
| Phosphatidylcholineb | <2 |
| Phosphatidylethanolamineb | <2 |
| Cysteamine phosphate | <2 |
Activity is based on the release of Pi.
0.1% Triton X-100 was included in the reaction mixture.
MUP, 4-methylumbelliferyl phosphate.
A more informative hypothesis of the activity of SapM can be derived from its susceptibility to different inhibitors. Using pNPP as the substrate, 16 potential phosphatase inhibitors were tested against SapM. The enzymatic activity of SapM was inhibited by low concentrations of several heavy metals (1 μM zinc chloride, 20 μM sodium molybdate, 0.3 mM magnesium chloride, and 0.5 mM copper sulfate) and moderately high concentrations (>8 mM) of EDTA. These results indicated the requirement of a specific metal cofactor for substrate hydrolysis and the displacement of the cofactor and inactivation by other heavy metals. SapM was resistant to sodium tartrate, a characteristic of other acid phosphatases that require iron for enzymatic activity (10). Additionally, the pH optimum of SapM is reflective of the pKa for the active site of a histidine acid phosphatase, an enzyme that forms a phosphohistidine intermediate and uses a metal cofactor (17). Although SapM lacked consensus sequences of histidine acid phosphatases, it did possess the “HD” motif (residues 204 and 205), shown to be involved in the catalytic activity of a number of such enzymes (22). Further evidence that the His residues of SapM might be important for catalytic activity is found in the conservation of this amino acid in two domains (residues 92 to 99 and residues 197 to 208) that are highly homologous to regions of the fungal acid phosphatases Pc-PhoA, Af-PhoA, and Kl-PhoX.
Subcellular localization of SapM.
Subcellular localization of SapM was investigated by assessing tartrate-resistant acid phosphatase activity in subcellular fractions (culture filtrate, cell wall, cell membrane, and cytosol) of M. tuberculosis H37Rv (Table 2). Tartrate was included in these reactions to minimize interference by acid phosphatases other than SapM. The acid phosphatase activity partitioned predominantly with the culture filtrate. However, a significant amount of activity was detected in the cell membrane and cell wall fractions, but not in the cytosol. In comparison, activity of isocitrate dehydrogenase, a cytosolic enzyme, was observed primarily in the cytosol, with minor activity detected in the culture filtrate (Table 2). The isocitrate dehydrogenase activity associated with the extracellular milieu was most likely due to some cellular autolysis of the culture (1). These data, combined with the sequence analysis of SapM, prove that this is a true secreted protein of M. tuberculosis.
TABLE 2.
Subcellular localization of tartrate-resistant acid phosphatase and SapM activity in M. tuberculosis
| Subcellular fraction | Acid phosphatase activitya (nmol of pNP/min/mg of protein) | Isocitrate dehydrogenase activityb (pmol of NADPH/ min/mg of protein) |
|---|---|---|
| Culture filtrate | 800 ± 70 | 8.4 ± 2.6 |
| Cell wall | 190 ± 30 | NAc |
| Cell membrane | 170 ± 20 | NAc |
| Cytosol | 30 ± 4 | 88.0 ± 8.4 |
Activity was determined in a microtiter plate assay.
Isocitrate dehydrogenase activity was determined as described by Andersen et al. (1).
NA, no activity was detected.
General conclusions.
SapM is the first mycobacterial acid phosphatase to be identified and characterized at a molecular level. Only one other gene product of M. tuberculosis (Rv2577) is predicted to be an acid phosphatase (8). However, Rv2577 does not possess an export signal sequence (8). Thus, SapM appears to be the only secreted acid phosphatase of M. tuberculosis, a hypothesis that is supported by our observations. This enzyme also fits the description of bacterial NSAPs, a broad group of phosphatases consisting of secreted proteins that function in an acidic-to-neutral pH range and use a wide variety of organic phosphoesters as substrates (26). However, the amino acid sequence of SapM does not possess the signature motifs used to define any of the three classes of bacterial NSAPs, and in fact, this enzyme shows the greatest homology to several acid phosphatases of fungal origin. This is not surprising given that the investigation of bacterial NSAPs is still maturing (26) and that the class C NSAPs were recognized only recently as a distinct group (30). Thus, it would appear that SapM represents a new class of bacterial NSAPs. This report also provides strong evidence that SapM is an NSAP that may function as a histidine phosphatase. However, the lack of available data on SapM homologues underscores the need to perform detailed mechanistic studies and site-directed mutagenesis to determine the mode of action of SapM. Similarly, SapM deletion mutants are required to elucidate the precise physiological function of SapM and whether it contributes to the pathogenicity of M. tuberculosis.
Acknowledgments
This work was supported by contract NO1 AI-75320 from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
We thank Marc Keen and Preston Hill for assistance with bacterial cultures and preparation of crude culture filtrates and Patrick Brennan for his critique of this work.
REFERENCES
- 1.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baca O G, Roman M J, Glew R H, Christner R F, Buhler J E, Aragon A S. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun. 1993;61:4232–4239. doi: 10.1128/iai.61.10.4232-4239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barford D, Das A K, Egloff M P. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. doi: 10.1146/annurev.biophys.27.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 5.Bowers G N, McComb R B, Upretti A. 4-Nitrophenyl phosphate characterization of high purity materials for measuring alkaline phosphatase activity in human serum. Clin Chem. 1981;27:135–143. [PubMed] [Google Scholar]
- 6.Braunstein M, Belisle J T. Genetics of protein secretion. In: Hatfull G F, Jacobs W R Jr, editors. Molecular genetics of mycobacteria. Washington, D.C.: ASM Press; 2000. pp. 203–220. [Google Scholar]
- 7.Clemens D L, Lee B-Y, Horwitz M A. Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J Bacteriol. 1995;177:5644–5652. doi: 10.1128/jb.177.19.5644-5652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Dobos K M, Swiderek K, Khoo K H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler H G, Gignac S M. Characterization and expression of tartrate-resistant acid phosphatase (TRAP) in hematopoietic cells. Leukemia. 1994;8:359–368. [PubMed] [Google Scholar]
- 11.Ehrlich K C, Montalbano B G, Mullaney E J, Dischinger H C, Ullah A H J. An acid phosphatase from Aspergillus ficuum has homology to Penicillium chrysogenum PhoA. Biochem Biophys Res Commun. 1994;204:63–68. doi: 10.1006/bbrc.1994.2426. [DOI] [PubMed] [Google Scholar]
- 12.Eng J K, McCormack A L, Yates J R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 13.Ferminan E, Dominguez A. The KIPHO5 gene encoding a repressible acid phosphatase in the yeast Kluyveromyces lactis: cloning, sequencing and transcriptional analysis of the gene, and purification and properties of the enzyme. Microbiology. 1997;143:2615–2625. doi: 10.1099/00221287-143-8-2615. [DOI] [PubMed] [Google Scholar]
- 14.Haas H, Redl B, Friedlin E, Stoffler G. Isolation and analysis of the Penicillium chrysogenum phoA gene encoding a secreted phosphate-repressible acid phosphatase. Gene. 1992;113:129–133. doi: 10.1016/0378-1119(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 15.Harth G, Clemens D L, Horwitz M A. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci USA. 1994;91:9342–9346. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellman U, Wernstedt C, Gonez J, Heldin C H. Improvement of an in-gel digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem. 1995;224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence G L, van Etten R L. The low-molecular-weight acid phosphatase from bovine liver: isolation, amino acid composition, and chemical modification studies. Arch Biochem Biophys. 1981;206:122–131. doi: 10.1016/0003-9861(81)90073-4. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell D B, Vogel K, Weimann B J, Pasamontes L, van Loon A P G M. The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology. 1997;143:245–252. doi: 10.1099/00221287-143-1-245. [DOI] [PubMed] [Google Scholar]
- 19.Mullaney E J, Ullah A H J. Conservation of the active site motif in Aspergillus niger (ficuum) pH 6.0 optimum acid phosphatase and kidney bean purple acid phosphatase. Biochem Biophys Res Commun. 1998;243:471–473. doi: 10.1006/bbrc.1998.8116. [DOI] [PubMed] [Google Scholar]
- 20.Murphy J, Riley J P. A modified single method for the determination of phosphate in neutral waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 21.Orme I M, Andersen P, Boom W H. T-cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 22.Ostanin K, Harms E H, Stevis P E, Kuciel R, Zhou M M, van Etten R L. Overexpression, site-directed mutagenesis, and mechanism of Escherichia coli acid phosphatase. J Biol Chem. 1992;267:22830–22836. [PubMed] [Google Scholar]
- 23.Raynaud C, Etienne C, Peyron P, Laneelle M A, Daffe M. Extracellular enzyme activities potentially involved in the pathogenicity of Mycobacterium tuberculosis. Microbiology. 1998;144:577–587. doi: 10.1099/00221287-144-2-577. [DOI] [PubMed] [Google Scholar]
- 24.Reilly T J, Baron G S, Nano F E, Kuhlenschmidt M S. Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J Biol Chem. 1996;271:10973–10983. doi: 10.1074/jbc.271.18.10973. [DOI] [PubMed] [Google Scholar]
- 25.Remaley A T, Das S, Campbell P I, Larocca G M, Pope M T, Glew R H. Characterization of Leishmania donovani acid phosphatases. J Biol Chem. 1985;260:880–886. [PubMed] [Google Scholar]
- 26.Rossolini G M, Schippa S, Riccio M L, Berlutti F, Macaskie L E, Thaller M C. Bacterial nonspecific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell Mol Life Sci. 1998;54:833–850. doi: 10.1007/s000180050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell D G. Mycobacterium and seduction of the macrophage. In: Ratledge C, Dale J, editors. Mycobacteria molecular biology and virulence. Oxford, United Kingdom: Blackwell Science; 1999. pp. 371–388. [Google Scholar]
- 28.Saha A K, Dowling J N, Lamarco K L, Das S, Remaley A T, Olomu N, Pope M T, Glew R H. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys. 1985;243:150–160. doi: 10.1016/0003-9861(85)90783-0. [DOI] [PubMed] [Google Scholar]
- 29.Shively J E, Miller P, Ronk M. Microsequence analysis of peptides and proteins. VI. A continuous flow reactor for sample concentration and sequence analysis. Anal Biochem. 1987;163:517–529. doi: 10.1016/0003-2697(87)90257-0. [DOI] [PubMed] [Google Scholar]
- 30.Thaller M C, Schippa S, Rossolini G M. Conserved sequence motifs among bacterial, eukaryotic, and archaeal phosphatases that define a new phosphohydrolase superfamily. Protein Sci. 1998;7:1647–1652. doi: 10.1002/pro.5560070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983;133:17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- 32.Worley K C, Wiese B A, Smith R F. BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genome Res. 1995;5:173–184. doi: 10.1101/gr.5.2.173. [DOI] [PubMed] [Google Scholar]