ABSTRACT
Introduction
Previous studies have demonstrated a correlation between the serum uric acid‐to‐high‐density lipoprotein cholesterol ratio (UHR) and insulin resistance (IR) in individuals with type 2 diabetes mellitus. However, no existing studies have investigated the relationship between IR and UHR in the general population. Therefore, the primary objective of this study was to investigate the correlation between UHR and IR in the general American population.
Methods
A sample of 8,817 participants was selected from the 2013 to 2020 National Health and Nutrition Examination Survey (NHANES). Homeostatic model assessment of insulin resistance (HOMA‐IR) was used to assess insulin resistance. Multiple logistic regression, generalized smooth curve fitting, and subgroup analysis were used to assess the association between IR and UHR.
Results
Multiple logistic regression analysis indicated a significant correlation between insulin resistance and UHR, with odds ratios (OR) of 1.07 (95% CI = 1.03–1.11) in males and 1.18 (95% CI = 1.13–1.25) in females. A non‐linear relationship and saturation effect between IR risk and UHR were observed, characterized by an inverted L‐shaped curve and a critical inflection point at 8.82. It was found that the area under the ROC curve (AUC) of UHR was significantly larger (AUC = 0.703 for males and 0.747 for females, all P < 0.01) compared with the use of UA or HDL‐C alone. Subgroup analysis showed that this independent association remain consistent regardless of race, age, BMI, diabetes, moderate activities, education level, alcohol drinking, and gender.
Conclusion
Elevated UHR demonstrates a significant correlation with insulin resistance, so it can be used as a potential indicator of insulin resistance within the American population.
Keywords: Insulin resistance, Obesity, Uric acid
Elevated UHR demonstrates a significant association with insulin resistance within the American population. UHR is more effective in detecting IR compared with the use of HDL‐C or UA alone. A non‐linear association between IR risk and UHR was discovered.
INTRODUCTION
Insulin resistance is widely recognized as a significant contributing factor in various pathological conditions, including diabetes, atherosclerosis, hypertension and metabolic syndrome (MetS). Therefore, an accurate measurement of insulin resistance is of the utmost importance. The hyperinsulinemic–euglycemic clamp is considered as the gold standard for determining insulin resistance. However, its routine clinical application is hindered by issues related to replicability, cost, accessibility and reproducibility 1 , 2 , 3 , 4 , 5 . As an alternative, HOMA‐IR is considered as an index that is used widely in adults 6 . Although HOMA‐IR is commonly adopted in adults, its reliance on fasting plasma insulin measurements poses challenges within clinical settings. Consequently, there is a demand for a diagnostic test with accuracy, cost‐effectiveness and simplicity in predicting insulin resistance.
It has been found that uric acid contributes to the development of insulin resistance and atherosclerosis through mechanisms such as reduced nitric oxide production, endothelial dysfunction, and the promotion of vascular smooth muscle proliferation 7 . Furthermore, low levels of HDL‐C have been implicated in the pathogenesis of insulin resistance and metabolic syndrome 8 , 9 , 10 , 11 , 12 . Recently, the UHR has emerged as a potential marker for increased inflammation 13 . Xu et al. 14 advocated the utilization of UHR as a valuable diagnostic instrument for detecting insulin resistance in individuals diagnosed with type 2 diabetes. Moreover, it has been observed that UHR exhibits a significant correlation with fasting plasma glucose and HbA1c levels, thus serving as a valuable indicator for evaluating the control of type 2 diabetes mellitus in males 15 .
However, despite the aforementioned findings, the relationship between UHR and insulin resistance in non‐diabetic individuals and in the general population remains unclear. Furthermore, considering the racial disparities in the levels of uric acid and HDL‐C, the association between UHA and IR may vary from race to race 16 . In this study, data from the NHANES was utilized to explore the potential association between the UHR and IR within the American population.
METHODS
Study population
The data analyzed in this study were obtained from NHANES (2013–2020), with a stratified, multi‐stage probability and complex sample of an uninstitutionalized population in America. The cross‐sectional surveys were conducted by NCHS. Further information regarding NHANES methods can be accessed at www.cdc.gov/nchs/NHANEs/.
The study focused exclusively on participants who were 12 years old or more (n = 32,232). 23,415 participants were eliminated: (1) missing data on fasting insulin (FINS), serum UA, HDL‐C, or fasting plasma glucose (FPG); (2) severe diseases such as stroke, liver disease, heart disease, kidney disease, and inflammatory disease. Consequently, our final analysis involved 8,817 participants aged 12–80 years old (Figure 1).
Figure 1.
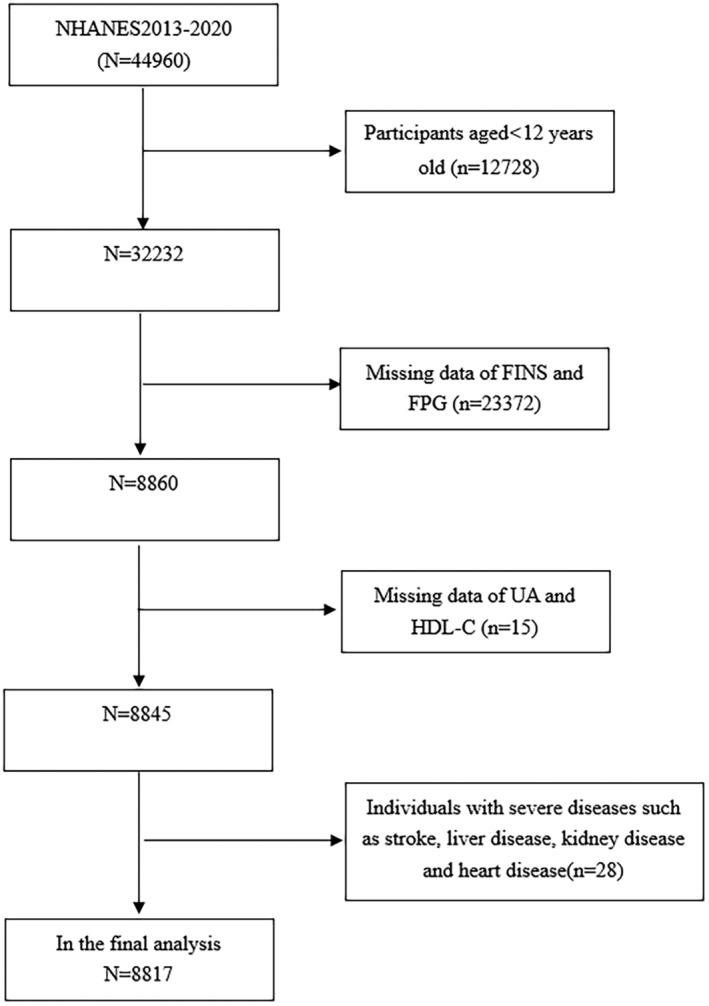
Flowchart of the sample selection from the 2013 to 2020 NHANES.
The implementation of NHANES was granted approval by NCHS Ethics Review Board, and all subjects provided written informed consent 17 .
Anthropometric measurements
The following data were collected at admission, such as history of diabetes, alcohol intake, race, physical activity, education, and physical measurements including weight, waist circumference, height, and blood pressure. Normal weight was defined as BMI <25 kg/m2, obesity or overweight were defined as BMI ≥25 kg/m2.
TC, HbA1c, LDL‐C, FINS, UA, FPG, TG, creatinine, albumin, and HDL‐C in blood samples were collected. Less than 3% of values were missing in total. Multiple imputation was performed for the missing values. eGFR was estimated with the Modification of Diet in Renal Diseases 18 . The detailed measuring method and acquisition process of each variable are available at www.cdc.gov/nchs/nhanes.
Assessment of insulin resistance
The HOMA‐IR formula was used to assess IR, and the HOMA‐IR was calculated by multiplying the FPG (mmol/L) by FINS (IU/L) divided by 22.5 2 . Insulin resistance was defined as a HOMA‐IR value equal to or >3.80 for adults (age ≥ 18 years old) and 4.47 for adolescents (12 ≤ age ≤ 17 years old) 19 , 20 .
Statistical analysis
The UHR (%) was determined by dividing UA (mg/dL) by HDL‐C (mg/dL) and multiplying the result by 100. It is worth noting that there were gender disparities in UA, HDL, and UHR, and separate analyses were necessary for males and females. The assessment of normality for continuous variables involved expressing them as either median and interquartile range or mean ± standard deviation. In order to evaluate the differences between the two groups, the t‐test or the Mann–Whitney U test was adopted for continuous variables, while chi‐square tests were used for categorical variables. Furthermore, the association between UHR and metabolic risk factors was explored using Spearman's correlation. The subjects were divided into groups based on their UHR levels (≤9.59, 9.59–12.28, 12.28–16.08, ≥16.08 in the male group, ≤6.34, 6.34–8.22, 8.22–10.79, ≥10.79 in the female group). Variables demonstrating clinical significance and statistical significance in the univariate analysis (P < 0.05) were incorporated into the multivariate analyses. The association between UHR quartiles and the presence of IR was assessed with binary logistic regression models. Model 1, no covariate was adjusted; In Model 2, adjustment was made for BMI and age; based on Model 2, the race, moderate activities, diabetes, SBP, education level, WC, alcohol drinking, HbA1c, DBP, serum albumin, eGFR as covariates were added to Model 3. Subgroup analysis stratified by BMI (<25 and ≥25 kg/m2), gender (male and female), diabetes (yes and no), age (<50 and ≥50 years), moderate activities (yes and no), education level (high school or above and less than high school), and alcohol drinking (yes and no) were conducted 21 , 22 , 23 , 24 . To examine the potential effect modification within subgroups, interaction terms were employed between subgroup indicators, followed by likelihood ratio tests. To ascertain potential non‐linear patterns in the likelihood of IR based on UHR levels, generalized smooth curve fitting techniques were employed. ROC curve analysis was conducted to evaluate the diagnostic efficacy of UHR in detecting IR. The statistical analysis was performed using EmpowerStats software and R, with significance determined at a threshold of P < 0.05.
RESULTS
Characteristics of participants
As shown in Table 1, the prevalence of insulin resistance reached 28.7% in females and 28.5% in males, respectively. The age, HOMA‐IR, proportion of individuals with diabetes, WC, BMI, SBP, DBP, HbA1c, FPG, FINS, TC, TG, UA, and UHR levels were all higher in IR subjects than those in non‐IR subjects for both genders (P < 0.001). Furthermore, the proportion of moderate activities and HDL‐C levels were lower in patients with insulin resistance than those in non‐IR patients for both genders.
Table 1.
Baseline characteristics of the study population stratified by insulin resistance, and gender
| Male | P‐value | Female | P‐value | |||
|---|---|---|---|---|---|---|
| IR positive | IR negative | IR positive | IR negative | |||
| N | 1,219 | 3,058 | 1,304 | 3,236 | ||
| Age, years | 48.3 ± 20.2 | 43.0 ± 21.1 | <0.001 | 47.2 ± 19.7 | 43.1 ± 20.8 | <0.001 |
| Race, % | ||||||
| Mexican American | 18.7 | 14.0 | <0.001 | 22.0 | 14.2 | <0.001 |
| Other Hispanic | 9.3 | 9.9 | 13.3 | 10.4 | ||
| Non‐Hispanic White | 38.7 | 36.8 | 29.3 | 37.1 | ||
| Non‐Hispanic Black | 20.1 | 21.5 | 23.3 | 20.8 | ||
| Other race | 13.3 | 17.9 | 12.1 | 17.5 | ||
| Moderate activities, % | ||||||
| Yes | 37.3 | 42.5 | 0.008 | 35.1 | 44.6 | <0.001 |
| No | 62.7 | 57.5 | 64.9 | 55.4 | ||
| Diabetes | ||||||
| Yes | 26.3 | 8.1 | <0.001 | 24.5 | 5.8 | <0.001 |
| No | 73.7 | 91.9 | 75.5 | 94.2 | ||
| Education level | ||||||
| Less than high school | 22.5 | 23.8 | 0.410 | 26.1 | 19.0 | <0.001 |
| High school or above | 77.5 | 76.2 | 73.9 | 81.0 | ||
| Alcohol drinking, % | ||||||
| Current or ever | 93.9 | 93.8 | 1.000 | 87.3 | 86.2 | 0.650 |
| Never | 6.1 | 6.2 | 12.7 | 13.8 | ||
| Body mass index, kg/m2 | 33.1 ± 7.2 | 25.9 ± 5.0 | <0.001 | 34.9 ± 8.2 | 26.8 ± 6.6 | <0.001 |
| Waist circumference, cm | 112.0 ± 16.8 | 92.5 ± 14.6 | <0.001 | 109.5 ± 17.0 | 90.6 ± 15.3 | <0.001 |
| Systolic blood pressure, mmHg | 127.7 ± 16.4 | 122.4 ± 18.0 | <0.001 | 125.7 ± 18.8 | 118.3 ± 19.4 | <0.001 |
| Diastolic blood pressure, mmHg | 71.5 ± 14.6 | 68.2 ± 14.0 | <0.001 | 69.0 ± 12.8 | 66.6 ± 12.3 | <0.001 |
| Hemoglobin A1c, mmol/L | 6.3 ± 1.5 | 5.5 ± 0.8 | <0.001 | 6.3 ± 1.6 | 5.5 ± 0.6 | <0.001 |
| FPG, mmol/L | 6.3 (5.7, 7.5) | 5.6 (5.2, 5.9) | <0.001 | 6.1 (5.6, 7.3) | 5.3 (5.0, 5.7) | <0.001 |
| FINS, ng/mL | 21.8 (17.0, 31.8) | 7.3 (5.0, 10.2) | <0.001 | 21.6 (17.0, 29.4) | 7.9 (5.5, 10.8) | <0.001 |
| HOMA‐IR | 6.38 (4.84, 9.89) | 1.86 (1.25, 2.67) | <0.001 | 5.98 (4.68, 8.57) | 1.91 (1.29, 2.66) | <0.001 |
| Albumin, g/dL | 42.2 ± 3.4 | 43.4 ± 3.5 | <0.001 | 40.1 ± 3.4 | 41.6 ± 3.5 | <0.001 |
| Creatinine, μmol/L | 82.2 (70.7, 96.4) | 82.2 (71.6, 93.7) | 0.464 | 61.9 (53.0, 72.5) | 62.8 (54.8, 72.5) | 0.964 |
| eGFR, mL/min per 1.73 m2 | 88 (70, 109) | 90 (76, 111) | <0.001 | 92 (74, 113) | 93 (75, 113) | 0.182 |
| Uric acid, μmol/L | 381.1 ± 82.2 | 349.1 ± 76.3 | <0.001 | 316.2 ± 81.8 | 275.8 ± 69.9 | <0.001 |
| Total cholesterol, mmol/L | 4.72 ± 1.11 | 4.65 ± 1.08 | 0.067 | 4.84 ± 1.11 | 4.88 ± 1.10 | 0.226 |
| Triglycerides, mmol/L | 1.58 (1.08, 2.33) | 1.03 (0.71, 1.48) | <0.001 | 1.47 (1.04, 2.01) | 0.93 (0.68, 1.34) | 1 |
| HDL‐cholesterol, mmol/L | 1.12 ± 0.29 | 1.35 ± 0.37 | <0.001 | 1.29 ± 0.32 | 1.60 ± 0.43 | <0.001 |
| LDL‐cholesterol, mmol/L | 2.77 ± 0.91 | 2.74 ± 0.92 | 0.315 | 2.81 ± 0.90 | 2.77 ± 0.93 | 0.157 |
| UHR | 15.8 ± 5.4 | 12.2 ± 4.6 | <0.001 | 11.3 ± 4.4 | 8.1 ± 3.2 | <0.001 |
Values are mean ± SD or number (%). P < 0.05 was deemed significant (comparison between IR positive and IR negative).
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HDL‐c, high density lipoprotein cholesterol; LDL‐c, low density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; UHR, serum uric acid‐to‐high‐density lipoprotein cholesterol ratio.
Correlation between clinical parameters and UHR
The correlation between metabolic parameters and UHR was analyzed using Spearman's correlation and the results are shown in Table 2. The analysis revealed positive correlation between UHR and LDL‐C, BMI, HbA1c, WC, TG, SBP, FPG, DBP, FINS, and HOMA‐IR in all subjects.
Table 2.
Spearman's correlation of UHR levels with clinical and biochemical parameters
| Variable | Male | Female | ||
|---|---|---|---|---|
| r | P | r | P | |
| BMI | 0.476 | <0.001 | 0.463 | <0.001 |
| WC | 0.457 | <0.001 | 0.460 | <0.001 |
| SBP | 0.081 | <0.001 | 0.162 | <0.001 |
| DBP | 0.102 | <0.001 | 0.075 | <0.001 |
| HbA1c | 0.130 | <0.001 | 0.255 | <0.001 |
| TC | −0.002 | 0.910 | −0.068 | <0.001 |
| TG | 0.513 | <0.001 | 0.451 | <0.001 |
| LDL‐C | 0.113 | <0.001 | 0.117 | <0.001 |
| FPG | 0.179 | <0.001 | 0.272 | <0.001 |
| FINS | 0.408 | <0.001 | 0.444 | <0.001 |
| HOMA‐IR | 0.405 | <0.001 | 0.462 | <0.001 |
Correlation between UHR and IR
Table 3 shows the binary logistic analysis for the correlation between UHR quartiles with insulin resistance in subjects. In the unadjusted model, UHR was positively correlated with IR (OR = 1.15 in male and 1.27 in female). The relationship still existed in model 2 (OR = 1.07 in male and 1.18 in female) and Model 3 (OR = 1.07 in male and 1.18 in female). In order to further investigate the relationship between IR status and UHR, smooth curve fittings and a generalized additive model were adopted (Table 4 and Figure 2). Among all participants, the correlation between UHR and IR risk exhibited an inverted L‐shaped curve, with inflection points at 8.82.
Table 3.
Association of the insulin resistance with UHR quartiles
| Crude model | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Male | ||||||
| UHR | 1.15 (1.13, 1.17) | <0.001 | 1.07 (1.05, 1.09) | <0.001 | 1.07 (1.03–1.11) | <0.001 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 3.37 (1.53–7.42) | 0.002 | 1.38 (1.06–1.78) | 0.016 | 0.97 (0.55–1.72) | 0.926 |
| Q3 | 4.89 (2.28–10.48) | <0.001 | 1.46 (1.13–1.88) | 0.004 | 1.37 (1.09–2.18) | 0.034 |
| Q4 | 21.35 (10.24–44.53) | <0.001 | 2.52 (1.96–3.24) | <0.001 | 2.17 (1.26–3.71) | 0.005 |
| Female | ||||||
| UHR | 1.27 (1.25, 1.30) | <0.001 | 1.18 (1.15, 1.20) | <0.001 | 1.18 (1.13–1.25) | <0.001 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 2.10 (1.64–2.69) | <0.001 | 1.59 (1.22–2.07) | 0.001 | 1.96 (1.00–3.82) | 0.048 |
| Q3 | 4.13 (3.27–5.22) | <0.001 | 2.42 (1.88–3.11) | <0.001 | 3.88 (2.04–7.36) | <0.001 |
| Q4 | 11.77 (9.36–14.80) | <0.001 | 5.23 (4.08–6.70) | <0.001 | 8.44 (4.40–16.20) | <0.001 |
Crude model: adjusted for none. Model 1: adjusted for age and BMI. Model 2: adjusted for age, BMI, race, moderate activities, diabetes, education level, drinking, WC, SBP, DBP, HbA1c, eGFR, serum albumin.
Table 4.
Threshold effect analysis of UHR on insulin resistance using the two‐piecewise linear regression model
| UHR | Adjusted OR (95% CI) P value |
|---|---|
| Fitting by the standard linear model | 1.105 (1.073, 1.138), <0.001 |
| Fitting by the two‐piecewise linear model | |
| Inflection point | 8.82 |
| AC <8.82 | 1.444 (1.246, 1.672), <0.001 |
| AC >8.82 | 1.071 (1.036, 1.107), <0.001 |
| Log likelihood ratio | <0.001 |
Figure 2.
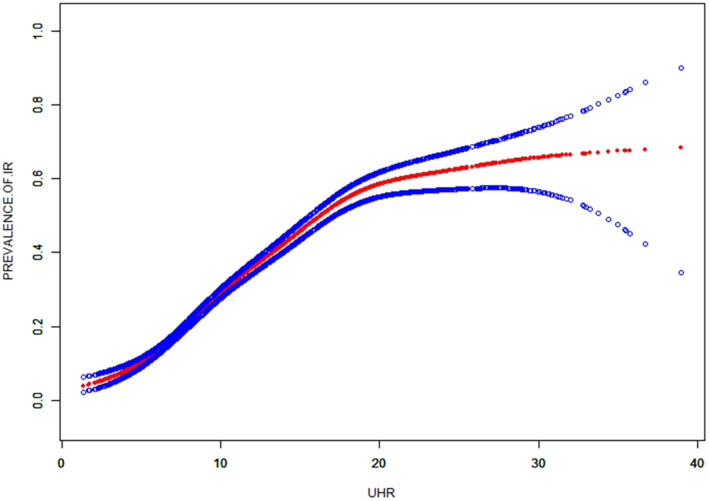
The smooth curve fit for the association between UHR and prevalence of IR.
Subgroup analysis on the correlation between IR and UHR
In order to assess the impact of subgroups on the relationship between IR and UHR, subgroup analyses were conducted (Table 5). The results indicated that the p values in subgroups were below 0.005. UHR exhibited an independent correlation with IR, and this correlation remained consistent regardless of race, age, BMI, diabetes, moderate activities, education level, alcohol drinking, and gender. Furthermore, upon employing smooth curve fittings to characterize the non‐linear association, it was observed that the positive correlation between IR and UHR levels persisted in the majority of groups (Figure 3).
Table 5.
Association between UHR and insulin resistance stratified by gender, age, race, and BMI
| OR (95% CI), P value | P for interaction | |
|---|---|---|
| Stratified by gender | ||
| Male | 1.07 (1.03–1.11), <0.001 | 0.160 |
| Female | 1.18 (1.13–1.25), <0.001 | |
| Stratified by race | ||
| Mexican American | 1.11 (1.02, 1.21), 0.021 | 0.376 |
| Other Hispanic | 1.16 (1.04, 1.28), 0.005 | |
| Non‐Hispanic White | 1.07 (1.02, 1.13), 0.007 | |
| Non‐Hispanic Black | 1.12 (1.05, 1.20), 0.001 | |
| Other race | 1.14 (1.06, 1.23), <0.001 | |
| Stratified by age | ||
| Age <50 years old | 1.15 (1.10, 1.21), <0.001 | 0.526 |
| Age ≥50 years old | 1.09 (1.05, 1.13), <0.001 | |
| Stratified by BMI | ||
| BMI <25 kg/m2 | 1.11 (1.01, 1.22), 0.032 | 0.241 |
| BMI ≥25 kg/m2 | 1.11 (1.08, 1.14), <0.001 | |
| Stratified by diabetes | ||
| Non‐diabetes | 1.12 (1.08, 1.15), <0.001 | 0.811 |
| Diabetes | 1.07 (1.01, 1.15), 0.030 | |
| Stratified by moderate activities | ||
| No | 1.09 (1.05, 1.13), <0.001 | 0.109 |
| Yes | 1.15 (1.09, 1.21), <0.001 | |
| Stratified by education level | ||
| Less than high school | 1.06 (1.01, 1.13), 0.043 | 0.194 |
| High school or above | 1.13 (1.09, 1.16), <0.001 | |
| Stratified by alcohol drinking | ||
| Current or ever drinking | 1.12 (1.08, 1.15), <0.001 | 0.279 |
| Never | 1.04 (1.01, 1.10), 0.021 | |
Gender, age, BMI, race, moderate activities, diabetes, education level (not adjusted for in the subgroup analyses), drinking, WC, SBP, DBP, HbA1c, eGFR, and serum albumin were adjusted.
Figure 3.
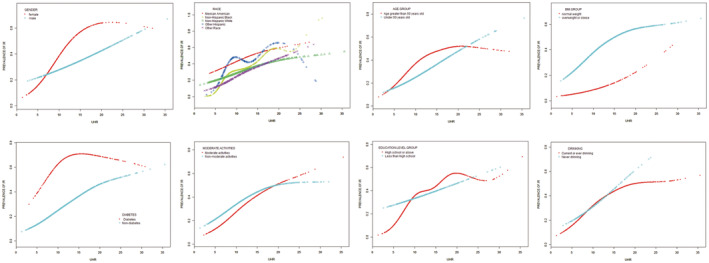
Subgroup analysis for the association between UHR and prevalence of insulin resistance by gender, age, race, BMI, diabetes, moderate activities, education level, and alcohol drinking.
Predictive value of UHR for IR
The ROC curve in Figure 4 presents the diagnostic performance of UA, UHR, and HDL‐C in identifying insulin resistance. Table 6 demonstrates that the AUC for UHR in the ROC analysis was 0.703 (95% CI: 0.686–0.720) for males and 0.747 (95% CI: 0.731–0.762) for females, which exceeds that for HDL‐C and UA (P < 0.001), suggesting that UHR may serve as a superior indicator of IR compared with HDL‐C or UA alone, although its diagnostic accuracy remains somewhat limited.
Figure 4.
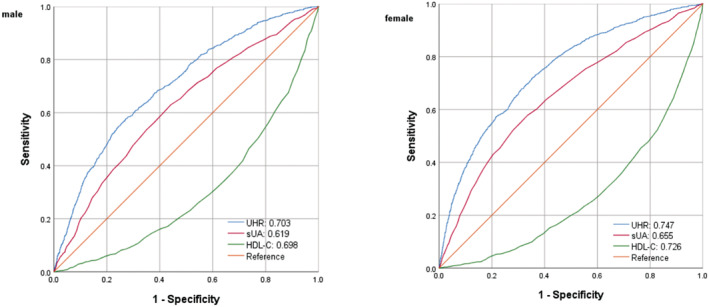
ROC analysis of UHR, UC, and HDL‐C to IR among an American population.
Table 6.
The results of ROC analysis of UHR for the diagnosis of insulin resistance stratified by gender, diabetes, race, and BMI
| Nutritional indices | Cut‐off | Sensitivity (%) | Specificity (%) | Youden's index | AUC | 95% CI |
|---|---|---|---|---|---|---|
| Male | 14.4 | 56.0 | 74.5 | 0.305 | 0.703 | 0.686–0.720 |
| Female | 9.0 | 67.8 | 68.8 | 0.366 | 0.747 | 0.731–0.762 |
| Diabetes | ||||||
| Male | 10.8 | 82.8 | 37.7 | 0.205 | 0.604 | 0.557–0.652 |
| Female | 8.5 | 74.6 | 52.9 | 0.275 | 0.667 | 0.618–0.716 |
| Non diabetes | ||||||
| Male | 14.6 | 56.2 | 76.7 | 0.329 | 0.713 | 0.693–0.733 |
| Female | 8.6 | 71.6 | 65.4 | 0.370 | 0.751 | 0.733–0.769 |
| Age < 50 years old | ||||||
| Male | 14.0 | 64.3 | 73.0 | 0.373 | 0.742 | 0.720–0.765 |
| Female | 9.1 | 65.5 | 72.0 | 0.375 | 0.752 | 0.730–0.774 |
| Age ≥ 50 years old | ||||||
| Male | 14.8 | 48.3 | 76.4 | 0.247 | 0.667 | 0.641–0.692 |
| Female | 8.6 | 73.6 | 61.8 | 0.354 | 0.734 | 0.711–0.758 |
| BMI < 25 kg/m2 | ||||||
| Male | 11.0 | 52.8 | 61.6 | 0.144 | 0.600 | 0.535–0.646 |
| Female | 7.4 | 65.2 | 62.0 | 0.272 | 0.652 | 0.600–0.704 |
| BMI ≥ 25 kg/m2 | ||||||
| Male | 15.0 | 55.2 | 68.4 | 0.236 | 0.649 | 0.629–0.670 |
| Female | 10.4 | 56.4 | 74.0 | 0.304 | 0.705 | 0.686–0.725 |
| Male | ||||||
| Mexican American | 12.8 | 67.4 | 65.4 | 0.328 | 0.708 | 0.668–0.749 |
| Other Hispanic | 14.7 | 56.6 | 71.3 | 0.279 | 0.663 | 0.605–0.722 |
| Non‐Hispanic White | 14.4 | 60.4 | 73.7 | 0.341 | 0.716 | 0.689–0.744 |
| Non‐Hispanic Black | 10.6 | 82.4 | 53.4 | 0.358 | 0.727 | 0.691–0.763 |
| Other race | 17.1 | 42.9 | 84.1 | 0.270 | 0.671 | 0.623–0.719 |
| Female | ||||||
| Mexican American | 9.1 | 67.5 | 69.6 | 0.371 | 0.739 | 0.702–0.776 |
| Other Hispanic | 7.8 | 79.2 | 53.7 | 0.329 | 0.728 | 0.683–0.773 |
| Non‐Hispanic White | 8.7 | 75.9 | 65.9 | 0.418 | 0.765 | 0.737–0.793 |
| Non‐Hispanic Black | 7.9 | 77.2 | 59.6 | 0.368 | 0.732 | 0.699–0.766 |
| Other race | 10.4 | 59.9 | 83.7 | 0.436 | 0.774 | 0.733–0.815 |
The authors used diabetes, BMI, and race as the stratification variables to further evaluate the diagnostic performance of the UHR among the different subgroups. The AUC of UHR for IR in the different diabetes, BMI, age and race subgroups were all higher than 0.6, which had a certain accuracy and certain predictive or screening value for insulin resistance in American populations.
DISCUSSION
Our study provides strong evidence that UHR is positively correlated with the risk of insulin resistance and an increase in HOMA‐IR among an American population. This relationship remains consistent regardless of diabetes, gender, race, BMI, physical activities, age, education level, and alcohol drinking. In addition, a non‐linear association between IR risk and UHR was discovered. Significantly, our ROC analysis demonstrates that the use of UHR is more effective in detecting insulin resistance compared with the use of HDL‐C or uric acid alone, and the AUC of UHR for IR in the different diabetes, BMI and race subgroups were all higher than 0.6, indicating that UHR is a sensitive and specific marker for insulin resistance.
Recent prospective studies conducted in the adult population have provided evidence that hyperuricemia is a predictive factor for diabetes and insulin resistance 25 , 26 , 27 . After follow up for 15 years, Krishnan et al. 26 discovered that hyperuricemia increases the risk of developing type 2 diabetes mellitus by 1.87 times and insulin resistance by 1.36 times. In another cross‐sectional study, Niu et al. 28 revealed that serum uric acid plays a mediating role in the development of insulin resistance induced by obesity in obese adolescents and children. Moreover, an elevation in HDL‐C is widely recognized as a protective factor against IR 29 . However, recent studies have proposed that the combination of uric acid and HDL‐C may serve as a more sensitive and novel biomarker for assessing inflammatory and metabolic disorders 30 . Currently, there is limited literature available on the relationship between UHR and insulin resistance. In a cross‐sectional study conducted by Xu et al. 14 involving a small sample size of 2,545 patients with type 2 diabetes mellitus in China, it was found that an increased UHR could potentially indicate the occurrence of insulin resistance. However, diabetes and race differences in this study were not fully considered, which led to this association only being applied to patients with type 2 diabetes mellitus in China. Due to race variations in both UA and HDL‐C levels, the relationship between UHA and IR may differ by ethnicity 16 . Similarly, in our large population‐based study conducted in the general American population, we have confirmed a positive correlation between IR and UHR. Additionally, this association remains consistent in non‐diabetes individuals. Furthermore, we performed an ROC analysis and observed that UHR exhibited greater effectiveness in detecting IR when compared with HDL‐C or UA alone, suggesting its superior performance in this regard.
Previous studies have indicated the efficacy of UHR in the prediction of metabolic syndrome. In a study conducted by Kocak et al., 30 it was observed that serum UHR exhibited significant predictive capabilities for MetS in individuals with diabetes mellitus in Turkey. Similarly, Yazdi et al. 31 identified UHR as a potential screening and diagnostic tool for assessing the risks of metabolic syndrome in Iranians without diabetes mellitus. In addition, Kocak et al. 15 demonstrated that UHR surpassed the established criteria, such as uric acid, in its effectiveness as a marker for MetS. Moreover, Kosekli et al. 32 conducted a study within a singular institution, elucidating a correlation between nonalcoholic liver disease and UHR. However, this study is the first to examine the relationship between UHR and the risk of IR or elevated HOMA‐IR in a general American population.
Moreover, this study also assessed the diagnostic value of the UHR for insulin resistance in different subgroups, and the results showed that the UHR was useful even in subjects with differing gender, diabetes, BMI, and race subgroups. In addition, the UHR exhibits simplicity and feasibility for determination. Therefore, the UHR is feasible for screening and identifying insulin resistance in a general American population. Furthermore, we have made an intriguing discovery of a previously unreported non‐linear correlation between IR and UHR. It is likely that there is a saturating effect of IR risk when UHR reaches 8.82. Our findings have the potential to provide new insights into the treatment and prevention of insulin resistance.
Possible mechanistic explanations exist for the correlation between UHR and IR, as demonstrated by studies showing that elevated levels of uric acid induce oxidative stress in adipocytes through the downregulation of adiponectin and the upregulation of monocyte chemotactic protein‐1 33 . This pro‐oxidative effect may contribute to the accumulation of adipose tissue 34 , 35 , thus leading to the development of IR 36 . Moreover, the decrease in nitric oxide levels caused by uric acid can hinder the uptake of glucose by skeletal muscle, thereby worsening insulin resistance 34 . Furthermore, research has indicated that the reduction of uric acid levels through the administration of xanthine oxidase inhibitors and uricosuric agents can effectively reverse IR in conditions such as fructose‐induced leptin receptor‐mediated obesity and MetS 33 , 36 , 37 , 38 . HDL‐C possesses various beneficial effects including reverse cholesterol transport, which can mitigate atherosclerosis, as well as anti‐thrombotic, vasodilatory, anti‐inflammatory, and antiapoptotic properties 39 . This study suggests that UHR, a combination of the inflammatory response and lipid metabolism, may serve as a potential indicator for insulin resistance.
The strengths of our study lie in the extensive sample size and the nationwide representativeness of the United States. In addition, we have taken into account various confounding factors, including diabetes, age, drinking status, gender, physical activity, and BMI. Nevertheless, there are certain constraints in this study. First of all, cross‐sectional studies do not allow us to establish a cause‐and‐effect relationship between UHR and IR. Secondly, we suggest utilizing HOMA‐IR as a means to assess IR. However, it should be noted that HOMA‐IR has been found to be correlated with fasting plasma glucose, primarily reflecting liver IR rather than muscle IR 40 . Consequently, additional investigations are warranted to explore the association between UHR and IR, utilizing the gold standard hyperinsulinemic–euglycemic clamp technique. Thirdly, it is crucial to verify the connection between UHR and IR in diverse populations and ethnicities, as this study solely focused on the American population.
CONCLUSION
The UHR demonstrates a positive correlation with insulin resistance in the American population. It may be an effective indicator to identify insulin resistance in American population and to prevent disease progression.
DISCLOSURE
The authors declare that they have no conflict of interest.
Approval of the research protocol: The NCHS Ethics Review Board has approved the implementation of NHANES, and all participants have provided written informed consent.
Informed consent: The written informed consent of all subjects was obtained following the Declaration of Helsinki.
Registry and the registration no. of the study/trial: Data: Jan 2011 Protocol #2011‐17.
Animal studies: N/A.
ACKNOWLEDGMENTS
The authors thank the NHANES database for providing the data source for this study.
Contributor Information
Xiaohai Zhou, Email: zhouxiaohai1990@163.com.
Jing Xu, Email: 47914057@qq.com.
REFERENCES
- 1. Tam CS, Xie W, Johnson WD, et al. Defining insulin resistance from hyperinsulinemic‐euglycemic clamps. Diabetes Care 2012; 35: 1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 3. Espinel‐Bermudez MC, Robles‐Cervantes JA, del Sagrario Villarreal‐Hernandez L, et al. Insulin resistance in adult primary care patients with a surrogate index, Guadalajara, Mexico, 2012. J Investig Med 2015; 63: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borai A, Livingstone C, Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem 2007; 44: 324–342. [DOI] [PubMed] [Google Scholar]
- 5. Rudvik A, Mansson M. Evaluation of surrogate measures of insulin sensitivity – correlation with gold standard is not enough. BMC Med Res Methodol 2018; 18: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke JP, Hale DE, Hazuda HP, et al. A quantitative scale of acanthosis nigricans. Diabetes Care 1999; 22: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 7. Meshkani R, Zargari M, Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol 2011; 48: 79–88. [DOI] [PubMed] [Google Scholar]
- 8. Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol 2011; 46: 200–215. [DOI] [PubMed] [Google Scholar]
- 9. von Eckardstein A, Sibler RA. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr Opin Lipidol 2011; 22: 26–32. [DOI] [PubMed] [Google Scholar]
- 10. Han T, Cheng Y, Tian S, et al. Changes in triglycerides and high‐density lipoprotein cholesterol may precede peripheral insulin resistance, with 2‐h insulin partially mediating this unidirectional relationship: A prospective cohort study. Cardiovasc Diabetol 2016; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rachek LI. Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci 2014; 121: 267–292. [DOI] [PubMed] [Google Scholar]
- 12. Karhapaa P, Malkki M, Laakso M. Isolated low HDL cholesterol. An insulin‐resistant state. Diabetes 1994; 43: 411–417. [DOI] [PubMed] [Google Scholar]
- 13. Kurtkulagi O, Tel BMA, Kahveci G, et al. Hashimoto's thyroiditis is associated with elevated serum uric acid to high density lipoprotein‐cholesterol ratio. Rom J Intern Med 2021; 59: 403–408. [DOI] [PubMed] [Google Scholar]
- 14. Zhou X, Xu J. Association between serum uric acid‐to‐high‐density lipoprotein cholesterol ratio and insulin resistance in patients with type 2 diabetes mellitus. J Diabetes Investig 2023; 15: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aktas G, Kocak MZ, Bilgin S, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male 2020; 23: 1098–1102. [DOI] [PubMed] [Google Scholar]
- 16. Chen X, You J, Zhou M, et al. The association between serum uric acid and creatine phosphokinase in the general population: NHANES 2015–2018. BMC Cardiovasc Disord 2023; 23: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zipf G, Chiappa M, Porter KS, et al. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat 2013; 1: 1–37. [PubMed] [Google Scholar]
- 18. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 19. Caporaso NE, Jones RR, Stolzenberg‐Solomon RZ, et al. Insulin resistance in healthy U.S. adults: findings from the National Health and Nutrition Examination Survey (NHANES). Cancer Epidemiol Biomarkers Prev 2020; 29: 157–168. [DOI] [PubMed] [Google Scholar]
- 20. Lee JA, Laurson KR. Obesity and insulin resistance screening tools in American adolescents: National Health and nutrition examination survey (NHANES) 1999–2010. Can J Diabetes 2016; 40: 311–317. [DOI] [PubMed] [Google Scholar]
- 21. Zeng P, Cai X, Yu X, et al. Markers of insulin resistance associated with non‐alcoholic fatty liver disease in non‐diabetic population. Sci Rep 2023; 13: 20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Murro E, Di Giuseppe G, Soldovieri L, et al. Physical activity and type 2 diabetes: in search of a personalized approach to improving beta‐cell function. Nutrients 2023; 15: 4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortega FB, Ruiz JR, Hurtig‐Wennlof A, et al. Physical activity attenuates the effect of low birth weight on insulin resistance in adolescents: findings from two observational studies. Diabetes 2011; 60: 2295–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arjmand B, Ebrahimi Fana S, Ghasemi E, et al. Metabolic signatures of insulin resistance in non‐diabetic individuals. BMC Endocr Disord 2022; 22: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Juraschek SP, McAdams‐Demarco M, Miller ER, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community‐based study population. Am J Epidemiol 2014; 179: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krishnan E, Pandya BJ, Chung L, et al. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15‐year follow‐up study. Am J Epidemiol 2012; 176: 108–116. [DOI] [PubMed] [Google Scholar]
- 27. Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care 2009; 32: 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niu Y, Tang Q, Zhao X, et al. Obesity‐induced insulin resistance is mediated by high uric acid in obese children and adolescents. Front Endocrinol (Lausanne) 2021; 12: 773820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anan F, Yonemochi H, Masaki T, et al. High‐density lipoprotein cholesterol and insulin resistance are independent and additive markers of left ventricular hypertrophy in essential hypertension. Hypertens Res 2007; 30: 125–131. [DOI] [PubMed] [Google Scholar]
- 30. Kocak MZ, Aktas G, Erkus E, et al. Serum uric acid to HDL‐cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras 1992; 65: 9–15. [DOI] [PubMed] [Google Scholar]
- 31. Yazdi F, Baghaei MH, Baniasad A, et al. Investigating the relationship between serum uric acid to high‐density lipoprotein ratio and metabolic syndrome. Endocrinol Diabetes Metab 2022; 5: e00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kosekli MA, Kurtkulagii O, Kahveci G, et al. The association between serum uric acid to high density lipoprotein‐cholesterol ratio and non‐alcoholic fatty liver disease: the abund study. Rev Assoc Med Bras 1992; 67: 549–554. [Google Scholar]
- 33. Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011; 60: 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: A danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Semin Nephrol 2011; 31: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee H, Lee YJ, Choi H, et al. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 2009; 284: 10601–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose‐induced metabolic syndrome. Am J Physiol Renal Physiol 2006; 290: F625–F631. [DOI] [PubMed] [Google Scholar]
- 38. Sanchez‐Lozada LG, Tapia E, Bautista‐Garcia P, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose‐induced metabolic syndrome. Am J Physiol Renal Physiol 2008; 294: F710–F718. [DOI] [PubMed] [Google Scholar]
- 39. Nagao M, Nakajima H, Toh R, et al. Cardioprotective effects of high‐density lipoprotein beyond its anti‐atherogenic action. J Atheroscler Thromb 2018; 25: 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]


