Abstract
Objectives
Decisional conflict and regret about prenatal genetic screening and diagnostic tests may have important consequences in the current pregnancy and for future reproductive decisions. Identifying mechanisms that reduce conflict associated with the decision to use or decline these options is necessary for optimal patient counseling.
Methods
We conducted a cluster-randomized controlled trial of a shared decision-making tool (NEST) at the beginning of prenatal care. Enrolled patients completed follow-up surveys at the time of testing (QTT) and in the second–third trimester (QFF), including the Decision Conflict Scale (DCS). Total DCS scores were analyzed using a multivariate linear mixed-effect model.
Results
Of the total number of participants (n=502) enrolled, 449 completed the QTT and QFF surveys. The mean age of participants was 31.6±3.8, with most parous at the time of study participation (n=321; 71.7 %). Both the NEST (the intervention) and control groups had lower median total DCS scores at QFF (NEST 13.3 [1.7, 25.0] vs. control 16.7 [1.7, 25.0]; p=0.24) compared to QTT (NEST 20.8 [5.0, 25.0] vs. control 18.3 [3.3, 26.7]; p=0.89). Participants exposed to NEST had lower decisional conflict at QFF compared to control (β −3.889; [CI −7.341, −0.437]; p=0.027).
Conclusions
Using a shared decision-making tool at the start of prenatal care decreased decisional conflict regarding prenatal genetic testing. Such interventions have the potential to provide an important form of decision-making support for patients facing the unique type of complex and preference-based choices about the use of prenatal genetic tests.
Keywords: decisional regret, medical decision-making, prenatal genetic testing
Introduction
Prenatal genetic screening and diagnostic tests (collectively called prenatal genetic tests) contribute to obstetric outcomes by delivering high-quality prenatal care. Congenital abnormalities, including those caused by genetic conditions (e.g., Trisomies 21, 18, and 13), are a leading cause of infant mortality in the U.S. [1]. More than 4,000 infant deaths per year stemming from congenital abnormalities within the first year of life [1], [2], [3], [4], [5], [6]. Infants surviving longer are more likely to have long-term health conditions that impact their quality of life [7, 8]. The information obtained from these tests is significant for prenatal care as it may lead some patients to undergo additional procedures to optimize neonatal outcomes or, if a severe condition is identified, to end the pregnancy. Given the ramifications of these decisions, it is a priority to ensure that all pregnant patients can make informed decisions about prenatal genetic tests that reduce the potential for decisional conflict [9].
Decisional conflict is the uncertainty or conflict associated with using or declining a medical option that may result in a sense of loss, regret, or a challenge to one’s values [10], [11], [12], [13]. Decisional conflict may influence the current pregnancy, leading to psychological stressors for the patient that can delay implementing a medical decision. This is significant in prenatal care, where prenatal genetic testing decisions are time-sensitive, and delays by just a few days may prevent patients from accessing options that may align with their values and needs. In addition, studies demonstrate that decisional conflict and regret may have ramifications for the pregnant patient, family, and obstetric outcomes during the current pregnancy [14]. There are also concerns about the enduring nature of decisional conflict and regret for patients after pregnancy, with the potential to impact future decisions about whether and how to build a family after a negative experience from the prior pregnancy. The need to foster informed decision-making by all pregnant patients is a priority, particularly for those currently at risk for poor obstetric outcomes due to existing barriers to healthcare quality and access during pregnancy [15], [16], [17], [18].
Decisions about prenatal genetic tests are becoming increasingly more complex, presenting a challenge to ensure pregnant patients have the information and resources needed to make informed decisions about their use. For this reason, it is critical to identify mechanisms to minimize decisional conflict given the scientific evidence about the medical, ethical, legal, and social implications of doubt and uncertainty associated with prenatal genetic testing choices. We conducted this study to examine the impact of an intervention to support patients’ decisions about prenatal genetic tests.
Materials and methods
We conducted a cluster-randomized controlled trial of a point-of-care shared decision-making tool at two major healthcare systems, [the Cleveland Clinic and MetroHealth Medical Center], from August 2018 to December 2021. Clusters consisted of providers who practiced at that specific location. The intervention was implemented at the cluster level among patient-provider dyads at each study site.
The study was approved by the Cleveland Clinic Institutional Review Board (IRB), and all components of the study were performed in accordance with relevant human subject protection guidelines and regulations. All informed consent procedures were proposed and approved by the Cleveland Clinic IRB. A data safety and monitoring board reviewed and monitored research activities for the duration of the trial.
Participants included obstetric health providers and pregnant patients scheduled for a prenatal care visit at a participating cluster. Eligible participants were contacted by a recruitment letter describing the study, which included instructions to contact the research coordinator if they were interested in participating in the study. Informed consent was conducted by Research Coordinators and obtained from all participants before data collection.
Patients
Study inclusion criteria focused on pregnant patients, ages 18 years or older, who had an initial prenatal care appointment, scheduled to see one of the healthcare providers enrolled in the study, able to read and speak English, able to provide consent for research participation, and had a viable intrauterine pregnancy confirmed at the first prenatal visit.
Providers
Providers at the identified cluster sites were approached for recruitment. Inclusion criteria included board-certified obstetrician-gynecologists (OB/GYNs), certified nurse midwives (CMN), nurse practitioners (NP), or physician assistants (PA) who provided outpatient prenatal care at one of the study sites (together referenced as OB providers).
Procedures
Intervention
The intervention was a point-of-care shared decision-making instrument (NIPT Education Support Tool; NEST) administered via REDCap Survey on a tablet PC before the first prenatal care visit. The intervention was structured as a series of questions to help patients identify their educational needs and preferences about prenatal genetic tests before seeing their provider for the first prenatal visit. These items related to routine prenatal genetic screens and diagnostic tests. The intervention was designed to be used in tandem with educational resources and counseling provided by the healthcare provider. The items were developed with context experts in obstetrics, genetics, and medical decision-making. The intervention was composed of three sections (Section 1): a series of close-ended questions to ascertain patients’ familiarity with the different prenatal genetic screening and diagnostic tests; the conditions that can be detected using prenatal screening and testing, as well as terms used to describe these tests (Section 2); a series of close-ended questions to probe patients’ informational and decision-making priorities to set the agenda of the conversation about prenatal genetic testing; and (Section 3) open-ended questions for patients to personalize the discussion further. After completing the NEST, the responses were summed using REDCap, and two copies of the report summarizing the patients’ responses (the NEST Report) were printed. One copy was provided to the patient to reference any identified questions and informational priorities during the visit. A second copy was given to the provider to review to tailor the content and focus of the discussion with the patient about different testing options. The patient and provider were oriented to the NEST and the function of the NEST summation in individualized one-on-one educational sessions led by the Research Coordinator before study participation.
Providers were randomized by practice into one of two study arms: (1) the intervention arm utilizing the shared decision-making instrument or (2) the control arm utilizing standard practice patterns for education and counseling (usual care). All participating providers within a cluster were randomized to the same arm. A binary random number generator was used to assign intervention or control status at the practice level. The assignment was checked by a research team member who was blinded to cluster assignment to ensure a balance with respect to the number of clusters and providers in each of the study arms. Because the shared decision-making intervention was targeted to affect the patient-provider interaction, study participants were not blinded to which group (intervention or control) they were assigned.
Measures
Enrolled patients completed a self-administered baseline survey (Baseline Questionnaire or QBASE) before the first prenatal appointment [19, 20]. This instrument assessed baseline knowledge and attitudes regarding prenatal genetic screening and diagnostic testing using a modified version of the Multidimensional Measure of Informed Choice (MMIC) [21, 22]. The MMIC is a validated tool that uses close-ended and Likert-scale items to measure knowledge and attitudes with respect to prenatal genetic tests. The MMIC was modified to include questions about all routine prenatal genetic screens and diagnostic tests offered in the U.S. and validated before use. The instrument also contained Likert-scale items to identify attitudes about the testing options (eight 5-point Likert Scale items) and level of deliberation, also referred to as decision-making status (ten 5-point Likert Scale items). The instrument also assessed participants’ preferences for decision-making using the validated Denger Scale, with respect to preferring an “active role” in decisions (e.g., patient-centric approach), a “collaborative role” (e.g., shared decision-making or informed decision-making vs. passive role), or “passive role (e.g., provider-centric approach) [23]. Participants completed a self-administered survey at three additional points in time. The Post-Visit Questionnaire (QPV) was administered 1–7 days after the visit and measured knowledge and attitudes using the modified MMIC and a validated measure to assess the status of decision-making. A second survey, the time of test questionnaire (QTT), was administered once the patient initiated testing or the testing window passed for those who declined testing. This instrument used the same questions as the QBASE to measure knowledge and attitudes. It also included the Decision Conflict Scale (DCS) to measure decisional conflict specific to making decisions about prenatal genetic tests. A third and final survey, the final follow-up questionnaire (QFF), was administered in the mid-second and early-third trimester of pregnancy once all prenatal genetic testing was completed and resulted. It contained the same items as the QTT [24]. Survey items were developed and validated through input from content experts, pilot testing using cognitive interviews, and revisions based on this feedback. This final instrument was used for data collection. The survey was administered on a tablet PC provided by the research team and using REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies [19, 20]. The research coordinator conducted a formal chart review before each survey point of contact to exclude any participants who had experienced fetal loss or other significant obstetric complications from these surveys.
Statistical analysis
This is an analysis of the DCS responses (secondary outcome) from a trial to examine the impact of a point-of-care shared decision-making tool on shared decision-making (primary outcome). The OPTION scale determined the original sample size of the trial as a measure of shared decision-making as reported elsewhere [25, 26].
The data analyzed included participants who completed both QTT and QFF to determine the impact of the intervention over these two time points. Continuous measures were summarized using means and standard deviations for participant characteristics and compared using two-sample t-tests. Ordinal measures were summarized using medians and quartiles or frequencies and percentages and compared using Wilcoxon rank sum tests or Kruskal-Wallis test. Categorical factors were summarized using frequencies and percentages and were compared using Pearson’s chi-square tests. The total DCS scores at QTT and QFF were analyzed using the multivariable linear mixed-effect model. The model includes NEST vs. control as the fixed effect, provider cluster as the random intercepts, and adjusted other clinically relevant factors. Only significant factors were kept in the final models. Statistical significance was established at two-sided alpha of 0.05. All analyses were conducted using SAS 9.4 software.
Results
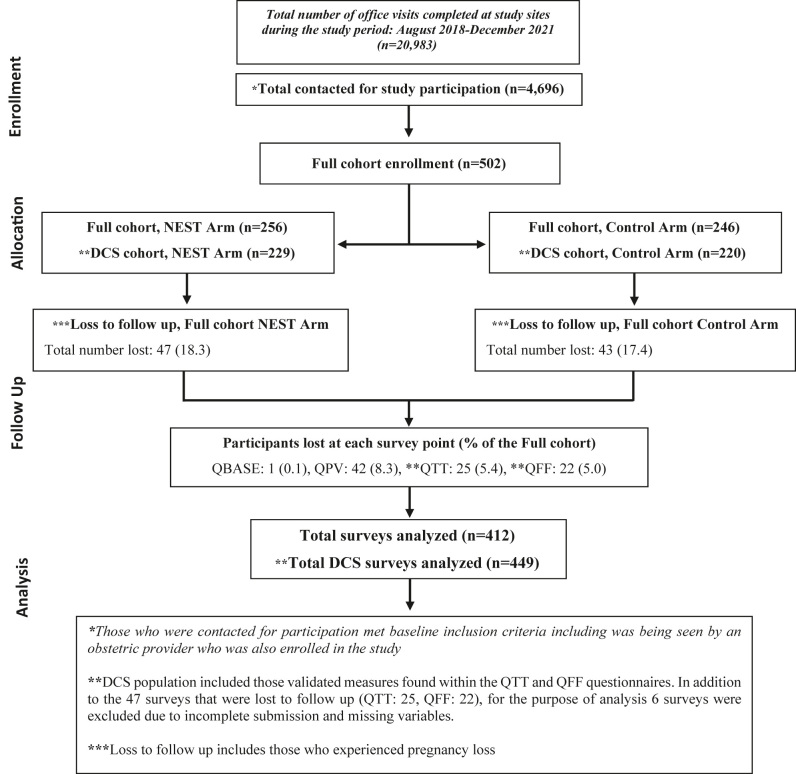
A total of 502 participants were enrolled, with 449 completing the DCS in both the initial QTT survey and the QFF survey (Figure 1). There were a total of 50 patient-provider dyads across 27 sites, with an average of 12 patient-participants per provider-participant. The mean age of participants was 31.6±3.8 (Table 1). The majority were <35 years old (n=353; 78.6 %), self-reported as white (n=390; 87.1 %), and non-Hispanic (n=434; 96.7 %). Most participants reported being parous at the time of study participation (n=321; 71.7 %).
Figure 1:
Flowchart for DCS cohort trial participants.
Table 1:
Demographics.
| Factor | Total (n=449) | NEST (n=229) | Control (n=220) | p-Value | ||
|---|---|---|---|---|---|---|
| n | Statistics | n | Statistics | |||
| What is your current age? | 31.6±3.8 | 229 | 31.6±4.0 | 220 | 31.6±3.6 | 0.99a |
| Age group | 229 | 220 | 0.48c | |||
| <35 | 353 (78.6) | 177 (77.3) | 176 (80.0) | |||
| ≥35 | 96 (21.4) | 52 (22.7) | 44 (20.0) | |||
| Race | 229 | 219 | 0.47d | |||
| White | 390 (87.1) | 197 (86.0) | 193 (88.1) | |||
| Black | 27 (6.0) | 12 (5.2) | 15 (6.8) | |||
| American native | 1 (0.22) | 1 (0.44) | 0 (0.00) | |||
| Asian | 17 (3.8) | 10 (4.4) | 7 (3.2) | |||
| Other | 13 (2.9) | 9 (3.9) | 4 (1.8) | |||
| Are you Hispanic or Latina? | 229 | 220 | 0.22c | |||
| No | 434 (96.7) | 219 (95.6) | 215 (97.7) | |||
| Yes | 15 (3.3) | 10 (4.4) | 5 (2.3) | |||
| Parity | 1.00 [0.00, 1.00] | 229 | 1.00 [0.00, 1.00] | 220 | 1.00 [0.00, 1.00] | 0.80b |
| Parity group | 229 | 220 | 0.52c | |||
| P0 | 168 (37.4) | 89 (38.9) | 79 (35.9) | |||
| P1+ | 281 (62.6) | 140 (61.1) | 141 (64.1) | |||
| Gravidity | 2.0 [1.00, 3.0] | 229 | 2.0 [1.00, 3.0] | 220 | 2.0 [1.00, 3.0] | 0.29b |
| Gravidity group | 229 | 219 | 0.52c | |||
| G1 | 127 (28.3) | 68 (29.7) | 59 (26.9) | |||
| G2+ | 321 (71.7) | 161 (70.3) | 160 (73.1) | |||
| What is the highest level of schooling that you have completed? | 229 | 220 | 0.26c | |||
| High school graduate or GED | 16 (3.6) | 11 (4.8) | 5 (2.3) | |||
| Associates degree, technical degree, or some college | 64 (14.3) | 37 (16.2) | 27 (12.3) | |||
| College graduate | 168 (37.4) | 80 (34.9) | 88 (40.0) | |||
| Graduate or professional degree | 201 (44.8) | 101 (44.1) | 100 (45.5) | |||
| What is your current marital status? | 229 | 220 | 0.57d | |||
| Single | 20 (4.5) | 8 (3.5) | 12 (5.5) | |||
| Currently married | 397 (88.4) | 202 (88.2) | 195 (88.6) | |||
| Divorced | 4 (0.89) | 2 (0.87) | 2 (0.91) | |||
| Never married | 2 (0.45) | 2 (0.87) | 0 (0.00) | |||
| Committed relationship | 26 (5.8) | 15 (6.6) | 11 (5.0) | |||
| Do you have a religious faith? | 227 | 220 | 0.19c | |||
| No | 141 (31.5) | 78 (34.4) | 63 (28.6) | |||
| Yes | 306 (68.5) | 149 (65.6) | 157 (71.4) | |||
| What is your faith? | 149 | 157 | 0.80d | |||
| Christian | 143 (46.7) | 67 (45.0) | 76 (48.4) | |||
| Catholic | 132 (43.1) | 68 (45.6) | 64 (40.8) | |||
| Jewish | 14 (4.6) | 5 (3.4) | 9 (5.7) | |||
| Hindu | 6 (2.0) | 3 (2.0) | 3 (1.9) | |||
| Buddhist | 1 (0.33) | 1 (0.67) | 0 (0.00) | |||
| Other | 10 (3.3) | 5 (3.4) | 5 (3.2) | |||
| Genetic counselor | 229 | 220 | 0.002 c | |||
| No | 417 (92.9) | 221 (96.5) | 196 (89.1) | |||
| Have genetic counselor | 32 (7.1) | 8 (3.5) | 24 (10.9) | |||
NEST, intervention. Statistics presented as mean±SD, median [P25, P75], n (column %). p-Values: at-test, bWilcoxon rank sum test, cPearson’s chi-square test, dFisher’s exact test. Values in bold indicate significant p-values.
Both the intervention and control groups had lower median total DCS scores at QFF (NEST 13.3 [1.7, 25.0] vs. control 16.7 [1.7, 25.0]; p=0.24) compared to QTT (NEST 20.8 [5.0, 25.0] vs. control 18.3 [3.3, 26.7]; p=0.89)) (Table 2). While total DCS scores were not significantly different between the intervention and control groups in univariate analyses, two specific items had statistically significant differences noted at QFF. Compared to control, a greater number of NEST participants reported positively to the statement, “I know the risks and side effects of each option” (NEST n=188; 84.3 % vs. control n=160; 76.6 %; p=0.042) and “I am clear about which is more important to me: the benefits or the risks and side effects” (NEST n=205; 91.9 % vs. control n=179; 85.2 %; p=0.028). While most of the component items at QTT demonstrated factors showed slightly lower conflict levels among the NEST group, none of these differences were statistically significant.
Table 2:
Decisional conflict at QTT (time of test) and QFF (final follow-up) NEST vs. control.
| QTT | QFF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NEST (n=229) | Control (n=220) | p-Value | NEST (n=229) | Control (n=220) | p-Value | |||||
| n | Statistics | n | Statistics | n | Statistics | n | Statistics | |||
| Total score | 228 | 20.8 [5.0, 25.0] | 219 | 18.3 [3.3, 26.7] | 0.89b | 224 | 13.3 [1.7, 25.0]a | 210 | 16.7 [1.7, 25.0] | 0.24b |
| DCS informed | 228 | 25.0 [8.3, 29.2] | 219 | 25.0 [8.3, 33.3] | 0.59b | 224 | 25.0 [0.00, 25.0] | 210 | 25.0 [0.00, 25.0] | 0.44b |
| DCS values clarity | 228 | 25.0 [0.00, 25.0] | 219 | 25.0 [0.00, 25.0] | 0.97b | 224 | 25.0 [0.00, 25.0] | 210 | 25.0 [0.00, 25.0] | 0.25b |
| DCS support | 228 | 16.7 [0.00, 25.0] | 219 | 16.7 [0.00, 25.0] | 0.85b | 224 | 8.3 [0.00, 25.0] | 210 | 8.3 [0.00, 25.0] | 0.19b |
| DCS effective decision | 228 | 18.8 [0.00, 25.0] | 219 | 12.5 [0.00, 25.0] | 0.42b | 224 | 0.00 [0.00, 25.0] | 210 | 12.5 [0.00, 25.0] | 0.093b |
| I know which options are available to me. | 228 | 211 (92.5) | 219 | 194 (88.6) | 0.15c | 224 | 208 (92.9) | 210 | 184 (87.6) | 0.065c |
| I know the benefits of each option. | 228 | 195 (85.5) | 217 | 181 (83.4) | 0.54c | 224 | 197 (87.4) | 210 | 182 (86.7) | 0.69c |
| I know the risks and side effects of each option. | 228 | 165 (72.4) | 219 | 166 (75.8) | 0.41c | 223 | 188 (84.3) | 209 | 160 (76.6) | 0.042 c |
| I am clear about which benefits matter most to me. | 228 | 199 (87.3) | 218 | 186 (84.9) | 0.47c | 224 | 204 (91.1) | 210 | 179 (85.2) | 0.059c |
| I am clear about which risks and side effects matter most to me. | 228 | 190 (83.3) | 218 | 190 (83.3) | 0.64c | 224 | 203 (90.6) | 209 | 179 (85.2) | 0.060c |
| I am clear about which is more important to me: the benefits or the risks and side effects. | 227 | 195 (85.9) | 219 | 183 (83.6) | 0.49c | 223 | 205 (91.9) | 210 | 179 (85.2) | 0.028 c |
| I have enough support from others to make a choice. | 227 | 213 (93.8) | 219 | 198 (90.4) | 0.18c | 224 | 209 (93.3) | 209 | 190 (90.9) | 0.35c |
| I am choosing without pressure from others. | 228 | 221 (96.9) | 219 | 209 (95.4) | 0.41c | 224 | 213 (95.1) | 208 | 191 (91.8) | 0.17c |
| I have enough advice to make a choice. | 228 | 201 (88.2) | 219 | 186 (84.9) | 0.32c | 222 | 199 (89.6) | 210 | 183 (87.1) | 0.42c |
| I am clear about the best choice for me. | 228 | 204 (89.5) | 219 | 188 (85.8) | 0.24c | 224 | 207 (92.4) | 210 | 188 (89.5) | 0.29c |
| I feel sure about what to choose. | 228 | 198 (87.6) | 218 | 182 (83.5) | 0.22c | 224 | 206 (92.0) | 209 | 187 (89.5) | 0.37c |
| I feel i have made an informed choice. | 228 | 203 (89.0) | 219 | 195 (89.0) | 0.99c | 224 | 209 (93.3) | 210 | 185 (88.1) | 0.061c |
| My decision shows what is important to me. | 228 | 205 (89.9) | 219 | 191 (87.2) | 0.37c | 223 | 205 (91.9) | 209 | 181 (86.6) | 0.073c |
| I expect to stick with my decision. | 227 | 206 (90.7) | 219 | 204 (93.2) | 0.35c | 224 | 216 (96.4) | 210 | 194 (92.4) | 0.065c |
| I am satisfied with me decision. | 228 | 209 (91.7) | 219 | 201 (91.8) | 0.97c | 224 | 212 (94.6) | 210 | 191 (91.0) | 0.14c |
NEST, intervention; DCS, decisional conflict scale; QTT, time of test; QFF, final follow-up. Statistics presented as median [P25, P75], n (column %). p-Values: bWilcoxon rank sum test, cPearson’s chi-square test. Patient responses were pre-populated in likert-scale format, 1/2 of the responses were combined into a YES (strongly agree, agree), and other responses were combined into an NO (strongly disagree, disagree). Values in bold indicate significant p-values.
In multivariable analyses, participants exposed to NEST had statistically significant lower decisional conflict at QFF compared to control (β −3.889; [CI −7.341, −0.437]; p=0.027) though, at QTT, DCS scores were similar between the intervention and control groups (β −0.996; [CI (−4.169, 2.177); p=0.54) (Table 3). Several factors were associated with lower decision conflict levels at QTT and QFF. At earlier stages of the decision-making process (QTT), higher educational levels were associated with significantly lower levels of decisional conflict. Participants with an associate degree or similar level of education (β −10.474; [CI −19.806, −1.142]; p=0.028) had lower decisional conflict levels than those with a high school diploma or professional degree. The strongest association was noted among those with those who had graduated from college (β −14.046; [CI −23.662, −6.256]; p<0.002) or with a graduate or professional degree (β −14.959; [CI -23.662, −6.256]; p<0.001). Decision-making status (0=had not considered options vs. 4=had thoroughly considered options) was also a factor. Participants who had considered the benefits, risks, and alternatives to prenatal genetic testing before making their choice about use had lower levels of decisional conflict (β −4.803; [CI −6.527, −3.079]; p<0.001). Attitudes regarding prenatal diagnostic testing played a role, with participants with more positive attitudes about diagnostic testing noting higher levels of decisional conflict (β 4.487; CI 1.364, 7.609]; p=0.005. Those factors that were significant at QTT were no longer significant at later stages of the decision-making process. At QFF, only higher baseline knowledge levels (0–100 scale) were associated with lower conflict levels, with participants with higher knowledge levels at baseline having lower conflict levels (for every 10 points increase of knowledge levels, β −1.601; [CI −2.258, −0.943]; p<0.001). The type of test taken (screen vs. diagnostic) or the test result was not associated with conflict levels among NEST or control groups at either time point.
Table 3:
Multivariable linear mixed effect models predicting total DCS scores.
| Variable | Level | Beta | 95 % CI | p-Value |
|---|---|---|---|---|
| Model 1: QTT DCS score, n=445 | ||||
|
| ||||
| Group | Control | – | -Reference- | |
| Group | NEST | −0.996 | (−4.169, 2.177) | 0.54 |
| What is the highest level of schooling that you have completed? | High school graduate or GED | – | -Reference- | |
| What is the highest level of schooling that you have completed? | Associates degree, technical degree, or some college | −10.474 | (−19.806, −1.142) | 0.028 |
| What is the highest level of schooling that you have completed? | Graduate or professional degree | −14.959 | (−23.662, −6.256) | <0.001 |
| What is the highest level of schooling that you have completed? | College graduate | −14.046 | (−22.814, −5.278) | 0.002 |
| Diagnostic by score>=9 QB | Negative | – | -Reference- | |
| Diagnostic by score>=9 QB | Positive | 4.487 | (1.364, 7.609) | 0.005 |
| QB Decision making status mean score (0–4) (1 unit=1 in decision making status score) |
1 unit increasea | −4.803 | (−6.527, −3.079) | <0.001 |
|
| ||||
| Model 2: QFF DCS score, n=434 | ||||
|
| ||||
| Group | Control | – | -Reference- | |
| Group | NEST | −3.889 | (−7.341, −0.437) | 0.027 |
| Knowledge score Qbase (1 unit=10 % in correction rate) |
1 unit increasea | −1.601 | (−2.258, −0.943) | <0.001 |
NEST, intervention; DCS, decisional conflict scale; Qbase, baseline; QTT, time of test; QFF, final follow-up; CI, confidence interval. Only group variable and significant covariates were kept in the final models. aThe average by which the dependent variable increases when the independent variable increases one unit and other independent variables are held constant. Values in bold indicate significant p-values.
Participants’ opinions concerning decision-making preferences were associated with levels of decisional conflict (Table 4). At both time points, participants’ decision-making preferences influenced decisional conflict in a U-shaped fashion, with more definitive opinions about an approach to decision-making associated with lower decisional conflict compared to those who were unsure or undecided about their preferred approach. For instance, participants who had a strong opinion (e.g., “strongly agree” (0 on a Likert Scale), “agree” (1), “disagree” (3), or “strongly disagree” (4)) about approaches decision-making (e.g., a collaborative shared decision-making approach, a patient-focused informed decision-making approach, or a provider-focused approach), had lower levels of decisional conflict. In contrast, participants who did not have a strong opinion regarding decision-making approaches (e.g., “Neither disagree or agree”; Likert scale 2) demonstrated higher levels of decisional conflict at both QTT and QFF.
Table 4:
Decision making preferences and decisional conflict.
|
Factor |
QTT | QFF | |||||
|---|---|---|---|---|---|---|---|
| n | Median [P25, P75] | p-Value | n | Median [P25, P75] | p-Value | ||
| I prefer to leave decisions about using a prenatal genetic test to my doctor, midwife, or a genetic counselor. | Strongly agree | 12 | 10.8 [0.83, 24.2] | <0.001 | 10 | 13.3 [0.00, 28.6] | 0.009 |
| Agree | 41 | 23.3 [11.7, 26.7] | 40 | 21.7 [4.2, 25.8] | |||
| Neither agree or disagree | 103 | 25.0 [6.7, 28.3] | 100 | 21.7 [2.5, 26.7] | |||
| Disagree | 197 | 21.7 [6.7, 26.7] | 191 | 16.1 [1.8, 25.0] | |||
| Strongly disagree | 86 | 5.8 [0.00, 21.7] | 85 | 8.3 [0.00, 18.3] | |||
| I prefer for my doctor, midwife, or a genetic counselor to make a decision about using a prenatal genetic test for me but only after hearing my opinions about it. | Strongly agree | 10 | 12.5 [0.00, 28.3] | 0.004 | 10 | 12.5 [3.3, 28.3] | 0.011 |
| Agree | 52 | 23.3 [8.6, 26.7] | 49 | 18.3 [3.3, 25.0] | |||
| Neither agree or disagree | 101 | 21.7 [5.0, 28.3] | 97 | 21.7 [0.00, 26.7] | |||
| Disagree | 197 | 21.7 [6.7, 26.7] | 193 | 16.7 [3.3, 25.0] | |||
| Strongly disagree | 79 | 6.7 [0.00, 23.3] | 77 | [0.00, 16.7] | |||
| I prefer to work together with my doctor, midwife, or genetic counselor to make a decision about using a prenatal genetic test. | Strongly agree | 192 | 15.0 [3.3, 25.0] | 0.045 | 185 | 10.0 [1.7, 23.3] | 0.008 |
| Agree | 199 | 23.3 [6.7, 28.3] | 193 | 16.7 [3.3, 25.0] | |||
| Neither agree or disagree | 28 | 24.2 [5.8, 25.8] | 28 | 24.2 [12.5, 30.8] | |||
| Disagree | 12 | 19.2 [1.7, 27.5] | 12 | 24.2 [4.2, 31.7] | |||
| Strongly disagree | 9 | 16.7 [5.0, 25.0] | 9 | 11.7 [0.00, 16.7] | |||
| I like to make decisions about using a prenatal genetic test by myself using a prenatal genetic test by myself before talking to my doctor, midwife or genetic counselor | Strongly agree | 48 | 15.0 [4.2, 26.7] | 0.018 | 45 | 11.7 [3.3, 25.0] | 0.75 |
| Agree | 187 | 23.3 [5.0, 28.3] | 183 | 15.0 [3.3, 25.0] | |||
| Neither agree or disagree | 102 | 18.3 [6.7, 25.0] | 97 | 16.7 [1.7, 25.0] | |||
| Disagree | 84 | 12.1 [1.7, 25.0] | 83 | 11.7 [1.7, 25.0] | |||
| Strongly disagree | 19 | 6.7 [0.00, 16.7] | 19 | 11.7 [0.00, 56.7] | |||
| I like to make a decision about using a prenatal genetic test by myself before talking to my spouse or partner, family member, or friend. | Strongly agree | 13 | 10.0 [0.00, 20.0] | 0.023 | 13 | 21.7 [1.7, 53.3] | 0.57 |
| Agree | 41 | 11.7 [5.0, 25.0] | 42 | 11.7 [3.3, 25.0] | |||
| Neither agree or disagree | 56 | 25.0 [5.0, 27.5] | 52 | 17.5 [0.00, 25.0] | |||
| Disagree | 237 | 23.3 [6.7, 26.7] | 233 | 16.7 [1.7, 25.0] | |||
| Strongly disagree | 93 | 15.0 93.3, 25.0] | 87 | 11.7 [0.00, 25.0] | |||
| I like to make a decision about using a prenatal genetic test after talking to my spouse or partner, family member, or friend. | Strongly agree | 154 | 14.2 [3.3, 25.0] | 0.082 | 146 | 11.7 [1.7, 25.0] | 0.55 |
| Agree | 214 | 23.3 [6.7, 26.7] | 210 | 16.7 [3.3, 25.0] | |||
| Neither agree or disagree | 42 | 11.7 [1.7, 25.0] | 40 | 17.5 [0.00, 25.0] | |||
| Disagree | 16 | 19.0 [9.6, 25.8] | 17 | 16.7 [0.00, 25.0] | |||
| Strongly disagree | 14 | 15.0 93.3, 43.3] | 14 | 16.7 [6.7, 44.2] | |||
QTT, time of test; QFF, final follow-up; p-values, Kruskal-Wallis test. Values in bold indicate significant p-values.
Discussion
The findings of this study demonstrate that decision-making interventions introduced early in the prenatal care episode may decrease patients’ decisional conflict regarding the use of prenatal genetic tests. It also provides insight into additional approaches to preparing patients to make such decisions. For instance, orienting patients to prepare them to consider the scope and nature of the decision process before discussing concepts specific to prenatal genetic screening and diagnostic testing may be beneficial. These findings are significant given the priority to reduce decisional conflict for pregnant patients facing a series of complex and personal decisions about pregnancy, disability, and parenthood during prenatal care.
This study demonstrated a way to reduce decisional conflict and regret for patients considering prenatal genetic testing, as participants randomized to the intervention had lower decisional conflict indicators when deciding whether to initiate testing and later in pregnancy once the testing process was completed. This may be a function of investing in a preparatory stage before patients engage in directed discussions about prenatal genetic testing with their provider, giving the opportunity to consider their values, preferences, and needs before considering the medical aspects of the decision. This is significant because there are several well-documented reasons to reduce decisional conflict and regret in prenatal genetic testing decisions, including the chance of individuals being more likely to change their mind about a healthcare decision, delay their decision, and express decisional regret [27], [28], [29], [30]. Studies also document that patients who receive unexpected or positive results during the prenatal genetic testing process may experience anxiety and stress that can negatively impact their health and well-being [31], [32], [33]. In addition, the stress and anxiety associated with such experiences can make it difficult for patients to navigate a series of different probabilities and choices. Barriers to information acquisition and decision-making are particularly salient in the context of prenatal genetic testing, a setting in which time-sensitive decisions must be made to enable patients’ access to testing or procedures that impact obstetric outcomes. In addition, the decision-making landscape is increasingly complex, given the expanded capability of testing, advances in maternal-fetal and neonatal interventions to improve neonatal outcomes, and restrictions to abortion access [6], [7], [8, 34], [35], [36]. If a serious fetal genetic condition is identified, urgency is required for patients to consider their options, setting the stage for decisional conflict and regret if made without the information and support needed to navigate options that align with one’s values. There is also the concern about the impact of decisional regret on parents’ experience. This has been described as “watchful waiting” by parents who may be looking for the initial signs of a prenatally diagnosed genetic condition, impacting bonding and relationships with the newborn [11].
In this study, we noted overall lower levels of decisional conflict among the intervention group compared to the control group in the late second and early third trimester of the pregnancy, marking the final stages of the testing process. These differences indicate an important impact of the study intervention on patients at the end of the antenatal decision-making pathway. At QFF, participants exposed to the intervention at the onset of prenatal care reported feeling more familiar with each testing option’s risks and side effects and personal and decision-making priorities when considering prenatal genetic testing compared to control. Similar trends with higher satisfaction and lower conflict were observed among NEST participants for each of the remaining component measures of decisional conflict compared to the control. While some of the individual DCS factors were not statistically significant, they may represent critical clinical differences for patients and providers as they may represent additional modifiable factors important to patients in the decision-making process. These findings also highlight the importance of establishing patient-centric Minimally Important Difference (MID) in decisional regret that communicates what is most important to patients in improving outcomes [37]. This is particularly relevant to these types of highly preference-based decisions that are situated in a shifting landscape of post-diagnosis management options and have a significant impact on obstetric outcomes.
The study findings raise important questions about how best to provide personalized support for patients as they navigate their prenatal genetic testing options, including potentially modifiable factors that may reduce decisional conflict. This study describes the type of interventions that may improve decision-making and support the informed consent process, particularly as the complexity of these prenatal genetic testing decisions increases. This includes tools that focus on a preparatory stage when patients consider the nature and scope of decisions and how their values play into those decisions before learning about specific chromosomal conditions and ways to identify them. Studies show that reducing decisional conflict may not be a function of the nature or volume of educational information provided to the patient [38]. In addition, our study noted that baseline educational levels only played a role in reducing decisional conflict at earlier stages of the decision-making process and less of a role later in the pregnancy once testing was completed. Instead, it may be a matter of when and how that information is provided to patients and satisfaction with the counseling process [38]. For instance, lower decisional regret levels were noted when patients received tailored counseling before a major decision had to be made about prenatal genetic testing [39]. Furthermore, lower levels of post-test decisional conflict, anxiety, and regret are also associated more with improvements in the quality of pre-test counseling [39].
While efforts focused on patients’ knowledge of genetic risk and assessments can improve decisions, theythere may be other key factors in the informed decision-making process. In efforts to support patients’ decision-making, there may be a role to broaden the focus of interventions beyond information exchange and other aspects of the decision-making process. On one hand, it may not be feasible or effective to focus educational efforts on presenting detailed medical information about genetic conditions that can be identified or current testing modalities to identify said conditions. This may increase the chance of “information overload” and not address the patients’ preferences that evolve in conjunction with the nature and volume of information that may be obtained in the process. In addition, we observed that the impact of baseline educational levels on decisional conflict damped over time. On the other hand, there may be other aspects of the decision-making process that patients would prefer to be oriented to as they navigate the unique aspects of the decision-making process. Studies have demonstrated the role of values clarification in prenatal genetic testing decisions [34]. There may be additional factors to consider as well. For instance, our study highlights that there may also be a role for orienting patients to the decision-making process before introducing concepts related to prenatal genetic risk and assessment. This includes discussing with patients how they approach decision-making may be associated with regret and providing the opportunity to clarify their preferences before they initiate testing and at the various stages of the decision-making process.
There are some limitations to consider when interpreting the results of this cluster RCT, recognizing both the strengths and limitations of this approach. We conducted this study in accordance with the CONSORT guidelines for cluster RCTs with a cohort of patients and providers who elected to participate in this study and completed the longitudinal assessments over the course of the pregnancy. We selected this methodological approach given the unique aspects of prenatal care delivery, which may span over several weeks, taking steps to prevent contamination between the control and intervention groups. In doing so, we recognized the chance of unbalanced participant characteristics and variability across clusters. While our recruitment approach was developed to reach a broad demographic representation, most study patient-participants were >35 years of age, self-described as Caucasian, with more than one prior pregnancy, higher education levels, and higher health literacy levels. These factors may affect generalizability. Those who elected to participate may have fundamental differences from those who did, not just with respect to their understanding of the concepts but also to their acceptance of participating in the direct observation approach. Thus, the perspectives and experiences of these research participants may reflect self-selection bias, particularly for those who may have a specific approach to exploring decision-making needs and preferences regarding prenatal genetic testing and participation in study during pregnancy. In addition, providers and patients represent two large, urban, academic medical centers, which is also an important limitation to consider. Despite these limitations, this study sheds light on steps that can be instituted to ensure that patients make prenatal genetic testing decisions free of decisional regret.
Conclusions
It is important to develop patient-centered approaches to support pregnant patients as they consider initiating prenatal genetic tests and make prenatal care decisions, particularly as these choices are becoming increasingly complex due to advances in prenatal genomics. These approaches should include mechanisms that educate patients about the testing process and how their values, beliefs, and preferences regarding those options may influence the outcome of their decisions. Further research is needed to understand how best to provide patients with the information and resources necessary to navigate this uniquely complex and personal process in a way that aligns with their goals and needs as patients, parents, and members of a family.
Acknowledgments
We would like to thank Patricia Agatisa, PhD, and Uma Perni, MD, for their contributions to this project.
Footnotes
Research ethics: This study has complied with all relevant national regulations and institutional policies of the Cleveland Clinic. It has been approved by the authors’ Institutional Review Board. The study has been conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. All authors met the following criteria: (1) substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; (2) drafting the work or revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: Dr. Rose is a consulting ethicist for Blue Cross Blue Shield Association (national nonprofit). The remaining authors state no conflict of interest.
Research funding: This study was funded by a grant from the National Human Genome Research Institute (R01HG010092).
Data availability: The raw data can be obtained on request from the corresponding author.
Trial registration: The trial was registered in clinicaltrials.gov.
References
- 1.Almli LM, Ely DM, Ailes EC, Abouk R, Grosse SD, Isenburg JL, et al. Infant mortality attributable to birth defects — United States, 2003–2017. Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6902a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Murphy SL, Kochanek KD, Bastian BA. National vital statistics reports: from the centers for disease control and prevention, national center for health statistics. Vol. 62. Natl Vital Stat Rep.; 2016. Division of vital statistics. Deaths: final data for 2013; pp. 1–119. [Google Scholar]
- 3.Berg JW, Appelbaum PS, Lidz CW, Parker LS. Informed consent: legal theory and clinical practice. Oxford University Press; 2001. [Google Scholar]
- 4.Faden RR, Beauchamp TL. A history and theory of informed consent. Oxford University Press; 1986. [PubMed] [Google Scholar]
- 5.Tymitz K, Lidor A, Lidor A. The Institute of medicine: crossing the quality chasm. In: Tichansky D, Morton J, Jones D, editors. The SAGES manual of quality, outcomes and patient safety. Boston, MA: Springer; 2011. [Google Scholar]
- 6.Romano PS, Hussey P, Ritley D. Selecting quality and resource use measures: a decision guide for community quality collaboratives. AHRQ. 2010;9:0073. [Google Scholar]
- 7.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. National vital statistics reports: from the centers for disease control and prevention, national center for health Statistics. Vol. 60. Natl Vital Stat Rep; 2011. Births: final data for 2009; pp. 1–72. [PubMed] [Google Scholar]
- 8.Osterman MJ, Martin JA. National vital statistics reports: from the centers for disease control and prevention, national center for health statistics. Vol. 67. Natl Vital Stat Rep.; 2018. System timing and adequacy of prenatal care in the United States, 2016; pp. 1–14. [PubMed] [Google Scholar]
- 9.Lewis C, Hill M, Chitty LS. A qualitative study looking at informed choice in the context of non-invasive prenatal testing for aneuploidy. Prenat Diagn. 2016;36:875–81. doi: 10.1002/pd.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aetna . Serum and urine marker screening for fetal aneuploidy. 2020. [Google Scholar]
- 11.Fowles JB, Terry P, Xi M, Hibbard J, Bloom CT, Harvey L. Measuring self-management of patients’ and employees’ health: further validation of the Patient Activation Measure (PAM) based on its relation to employee characteristics. Patient Educ Counsel. 2009;77:116–22. doi: 10.1016/j.pec.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, Wineinger NE, Steinhubl SR. The influence of wireless self-monitoring program on the relationship between patient activation and health behaviors, medication adherence, and blood pressure levels in hypertensive patients: a substudy of a randomized controlled trial. J Med Internet Res. 2016;18:e116. doi: 10.2196/jmir.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shively MJ, Gardetto NJ, Kodiath MF, Kelly A, Smith TL, Stepnowsky C, et al. Effect of patient activation on self-management in patients with heart failure. J Cardiovasc Nurs. 2013;28:20–34. doi: 10.1097/jcn.0b013e318239f9f9. [DOI] [PubMed] [Google Scholar]
- 14.Stortz SK, Mulligan S, Snipes M, Hippman C, Shridhar NN, Stoll K, et al. A randomized controlled trial on the effect of standardized video education on prenatal genetic testing choices: uptake of genetic testing. Am J Perinatol. 2021;40:267–73. doi: 10.1055/s-0041-1727229. [DOI] [PubMed] [Google Scholar]
- 15.Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14 doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James J, Hibbard J, Agres T, Lott R, Dentzer S. Health policy brief: patient engagement. Health Aff. 2013;33:1–6. [Google Scholar]
- 17.Schnock KO, Snyder JE, Fuller TE, Duckworth M, Grant M, Yoon C, et al. Acute care patient portal intervention: portal use and patient activation. J Med Internet Res. 2019;21:e13336. doi: 10.2196/13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey L, Fowles JB, Xi M, Terry P. When activation changes, what else changes? The relationship between change in patient activation measure (PAM) and employees’ health status and health behaviors. Patient Educ Counsel. 2012;88:338–43. doi: 10.1016/j.pec.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect. 2001;4:99–108. doi: 10.1046/j.1369-6513.2001.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis C, Hill M, Skirton H, Chitty LS. Development and validation of a measure of informed choice for women undergoing non-invasive prenatal testing for aneuploidy. Eur J Hum Genet. 2015;24:809–16. doi: 10.1038/ejhg.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45:941–50. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor AM. User manual – decisional conflict scale. Ottawa: Ottawa Hospital Research Institute; 1993. [Google Scholar]
- 25.Collart C, Craighead C, Yao M, Chien E, Rose S, Frankel RM, et al. Identifying Strategies to improve shared decision-making for pregnant patients’ decisions about prenatal genetic screens and diagnostic tests. [Manuscript submitted for publication]
- 26.Elwyn G, Hutchings H, Edwards A, Rapport F, Wensing M, Cheung W, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect. 2005;8:34–42. doi: 10.1111/j.1369-7625.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner-Lin A, Barg FK, Kellom KS, Stumm KJ, Pilchman L, Tomlinson AN, et al. Couple’s narratives of communion and isolation following abnormal prenatal microarray testing results. Qual Health Res. 2016;26:1975–87. doi: 10.1177/1049732315603367. [DOI] [PubMed] [Google Scholar]
- 28.Bernhardt BA, Soucier D, Hanson K, Savage MS, Jackson L, Wapner RJ. Women’s experiences receiving abnormal prenatal chromosomal microarray testing results. Genet Med. 2013;15:139–45. doi: 10.1038/gim.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harding E, Hammond J, Chitty LS, Hill M, Lewis C. Couples experiences of receiving uncertain results following prenatal microarray or exome sequencing: a mixed-methods systematic review. Prenat Diagn. 2020;40:1028–39. doi: 10.1002/pd.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agatisa PK, Mercer MB, Mitchum A, Coleridge MB, Farrell RM. Patient-centered obstetric care in the age of cell-free fetal DNA prenatal screening. J Patient Exp. 2017;5:26–33. doi: 10.1177/2374373517720482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becerra-Perez MM, Menear M, Turcotte S, Labrecque M, Légaré F. More primary care patients regret health decisions if they experienced decisional conflict in the consultation: a secondary analysis of a multicenter descriptive study. BMC Fam Pract. 2016;17 doi: 10.1186/s12875-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Making. 2010;30:701–11. doi: 10.1177/0272989x10386231. [DOI] [PubMed] [Google Scholar]
- 33.Hillman SC, Skelton J, Quinlan‐Jones E, Wilson A, Kilby MD. “If it helps.” the use of microarray technology in prenatal testing: patient and partners reflections. Am J Med Genet. 2013;161:1619–27. doi: 10.1002/ajmg.a.35981. [DOI] [PubMed] [Google Scholar]
- 34.Kuppermann M, Pena S, Bishop JT, Nakagawa S, Gregorich SE, Sit A, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing. JAMA. 2014;312:1210. doi: 10.1001/jama.2014.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant AS, Norton ME, Nakagawa S, Bishop JT, Pena S, Gregorich SE, et al. Variation in women’s understanding of prenatal testing. Obstet Gynecol. 2015;125:1306–12. doi: 10.1097/aog.0000000000000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beulen L, van den Berg M, Faas BH, Feenstra I, Hageman M, van Vugt JM, et al. The effect of a decision aid on informed decision-making in the era of non-invasive prenatal testing: a randomised controlled trial. Eur J Hum Genet. 2016;24:1409–16. doi: 10.1038/ejhg.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312:1342–3. doi: 10.1001/jama.2014.13128. [DOI] [PubMed] [Google Scholar]
- 38.Hartwig TS, Borregaard Miltoft C, Malmgren CI, Tabor A, Jørgensen FS. High risk—what’s next? A survey study on decisional conflict, regret, and satisfaction among high-risk pregnant women making choices about further prenatal testing for fetal aneuploidy. Prenat Diagn. 2019;39:635–42. doi: 10.1002/pd.5476. [DOI] [PubMed] [Google Scholar]
- 39.Gammon BL, Jaramillo C, Riggan KA, Allyse M. Decisional regret in women receiving high risk or inconclusive prenatal cell-free DNA screening results. J Matern Neonatal Med. 2018;33:1412–18. doi: 10.1080/14767058.2018.1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]