Summary
Tuberculosis (TB) is the leading infectious cause of morbidity and mortality globally. Despite available tools for preventing, finding, and treating TB, many people with TB remain undiagnosed. In high-incidence settings, TB transmission is ubiquitous within the community, affecting both high-risk groups and the general population. In fact, most people who develop TB come from the general population. To disrupt the chain of transmission that sustains the TB epidemic, we need to find and treat everyone with infectious TB as early as possible, including those with minimal symptoms or subclinical TB who are unlikely to present for care. Important elements of an effective active case-finding strategy include effective social mobilisation and community engagement, using sensitive screening tools that can be used at scale, and embracing population-wide screening in high-incidence (‘hot spot’) areas. We require a better description of feasible delivery models, ‘real-life’ impact and cost effectiveness to enable wider implementation.
Keywords: Tuberculosis, High-incidence settings, Population-wide active case finding
Background
Robert Koch discovered Mycobacterium tuberculosis in 18821 and formulated the classic Koch's postulates to establish the causative relationship between a microorganism and a specific disease, which he also applied to tuberculosis (TB).2 M. tuberculosis spreads through the air when individuals with active pulmonary TB cough, sneeze, talk or breathe, releasing infectious aerosol droplets. Any person can be infected when inhaling these infectious droplets, which can remain in the air for long periods in spaces with poor ventilation. Sharing of poorly ventilated airspaces, overcrowded living conditions, and close contact with infectious individuals all increase the risk of TB transmission.3
For many years, we have had tools to help end TB, but progress has been very slow. Basic diagnostic methods have been available for 125 years, including microscopy and culture,4 as have chest radiology to visualise pulmonary disease.5 BCG vaccine has helped to reduce TB-related mortality in young children for more than 100 years.6 Still, it has limited efficacy in preventing infectious forms of TB, which drives the community transmission that sustains the epidemic. Effective drugs to treat TB have been available for seven decades, following the discovery of streptomycin7 and the first use of isoniazid and streptomycin combination therapy in the 1950s before the development of multi-drug ‘short-course’ (6-month duration) rifampicin-based regimens in the 1960s.8 In 1995, the DOTS strategy model was launched by WHO, focusing on diagnosing people with infectious TB (using quality-assured sputum microscopy), ensuring that they commence and complete standardised treatment for TB, and tackling barriers in program implementation.9 Effective treatment rapidly reduces infectiousness and prevents ongoing TB transmission. This allowed ambulatory treatment to be delivered in the community, coupled with directly observed therapy (DOT), to support treatment adherence, improve treatment outcomes and prevent further transmission of TB.
The availability of these tools strengthened prospects for TB control,9 but despite their incorporation into the global TB control strategy, TB remains a persistent global health challenge and the leading infectious disease killer in the world. In 2022, 10.6 million individuals contracted TB, and 1.3 million died of the disease.10 Although a lot of emphasis has been placed on inequalities in the global response to the COVID-19 pandemic, it is arguable that there is no disease where the failure to translate the benefits of scientific innovation and long-standing knowledge into global public health gains is starker. The situation is complicated by persistently low case detection rates in high-incidence settings,10 especially in people with minimally symptomatic or asymptomatic disease,11 which probably makes a major contribution to TB transmission within communities. The situation is further complicated by TB-associated stigma, the lack of universal health care access and the catastrophic costs associated with TB in most high-incidence settings, as well as the fact that there is a massive global reservoir of TB infection from which future cases may arise.12, 13, 14
In this paper, we review the characteristics of the TB epidemic in high-incidence settings and explore the role of population-wide active case finding as an unused tool to help end TB.
TB transmission in high-incidence settings and implications for screening strategies
Setting-specific TB transmission differences
TB is one of the oldest diseases known to humanity and has a global distribution, with nearly 90% of all persons with TB located in 30 high-burden countries.15 It is well-established that TB incidence and prevalence are higher within specific high-risk groups. These include people who have been in close contact with people with pulmonary TB disease, those with compromised immune systems such as people living with human immunodeficiency virus (HIV), people with severe undernutrition or poorly controlled diabetes, those with silica or cigarette smoke exposure and people in crowded, stressful conditions such as prisoners and refugees.10,16, 17, 18 Although TB incidence and vulnerability are higher among high-risk groups, it is only in low-incidence settings where they constitute the majority of cases (Table 1).
Table 1.
Key differences between countries with a high and low TB incidence—from a TB control perspective.
| Characteristic | Incidence category19 |
|
|---|---|---|
| Low (<10/100,000 population) | Higha (≥100/100,000 population) | |
| Local TB transmission | Very limited and not sustained with low lifetime of infection | Occurring ‘everywhere’ with high lifetime risk infection, and multiple infection episodes |
| Caseload | Mostly small clusters with only a few people with TB, rapid cluster recognition and containment possible | Large and sustained clusters without cluster recognition capacity (low resource health care system); comprise ∼90% of global incidence. |
| Risk population | Close contacts of people with infectious TB; migrants from high TB incidence settings, immunocompromised people. Mainly reactivation of TB infection acquired in the distant past |
Everyone, anywhere, at any time. Only a minority of people with TB belong to a high-risk group that can be easily recognised and identified for targeted active case finding. In practice, the majority are found within the general population and without easily recognisable risk factors. Most disease resulting from recent transmission, occurring within a relatively short timeframe after infection or re-infection |
| Health system capacity | Adequate resources and high capacity | Limited resources and low capacity; unable to cope with high caseload. Multiple barriers to access all steps of the care cascade |
| Public awareness | Recognised as a serious infectious disease and major public health threat; Stigma less prevalent, but can be a concern | Living with disease often normalised; Not recognised as a major public health threat; Often highly stigmatised, but setting specific |
| Case finding approaches | Relying on people who are ill to present for assessment (passive case finding) is adequate given low case load and limited risk of spread. | Passive case finding is not sufficient to find all people with infectious TB and reduce transmission. Active case finding is a key strategy but requires careful consideration of feasibility. |
TB; tuberculosis.
Can also be considered ‘TB endemic’.
In high-incidence settings where TB transmission is endemic and persistent within the community, the risk of TB infection is ubiquitous, and the majority of people who develop TB are not in discrete high-risk groups. In Peru, a high prevalence of multidrug-resistant TB was reported in a general population without any identifiable risk factors such as HIV, diabetes, persons working or admitted in prison, health care workers, persons with a recent and prolonged admission to a hospital, and alcohol and drug abuse.20 Similar findings were made in Vietnam, where only 50% were smokers and <10% had diabetes.21 While the prevalence of TB within these high-risk subpopulations is substantially elevated when compared to the general population, the absolute number of people with TB in the high-risk group is constrained by their relatively smaller population size. A recent systematic review revealed that the main factors associated with geospatial TB clusters are adverse socioeconomic conditions and high population density, as found in dense urban or peri-urban slum areas.22 The WHO recommends systematic screening to be conducted in targeted populations, particularly those with structural risk factors for TB.17 However, focusing exclusively on high-risk subpopulations will inadvertently neglect the majority of individuals without apparent high-risk characteristics, who develop TB in higher absolute numbers than high-risk groups even though their individual TB risk is less–and contributes to ongoing epidemic spread within communities.
Most cases arise from recent infection (including recent re-infection)
In low-incidence settings, TB mainly occurs sporadically and rarely results in sustained transmission in the community, mitigating the risk of significant disease outbreaks. In high-incidence settings, however, most people develop TB following recent infection; primary or re-infection occurring during the past 1–2 years.23,24 This pattern of disease following recent infection is in stark contrast to the experience in low-incidence settings, where people without recent travel to a TB endemic setting develop TB following reactivation of TB infection acquired in the distant past.25, 26, 27 This distinction in the relative contribution of recent TB transmission to overall TB case numbers in different settings is important and has significant implications for TB control strategies.
In high-incidence settings where community-level TB transmission is widespread and ongoing, the treatment of TB infection will only provide transient benefits.28 This is because individuals who complete treatment for TB infection remain at risk of reinfection and subsequent future progression to active disease. In high-incidence settings, previous TB infection confers limited protection against subsequent re-infection and TB disease progression.29 This is akin to the dynamics of infectious diseases like COVID-19, where prior infection does not provide long-term protection from reinfection and where people with minimal or no symptoms may spread the infection. Therefore, without universal population-wide active TB case finding, many people with TB will unknowingly spread TB infection within the community, including to those who have previously completed preventive treatment.24
Barriers to TB diagnosis and treatment
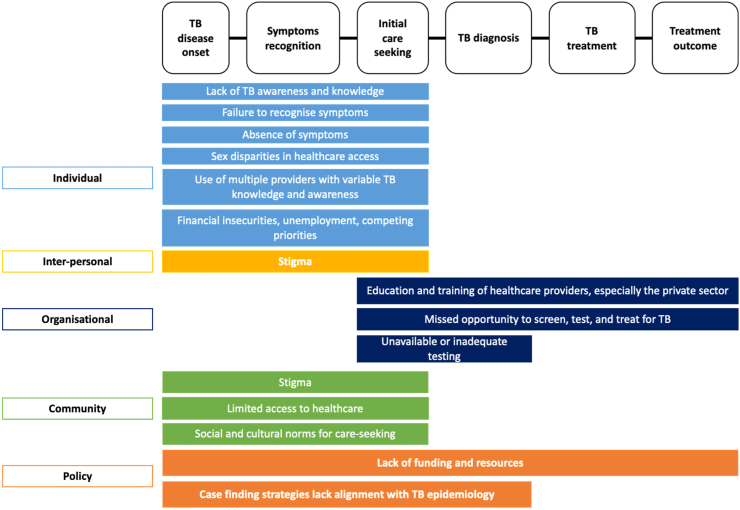
Ongoing TB transmission is sustained by people with untreated infectious TB who may not even be aware that they are ill. WHO estimates over a third of people with TB were undiagnosed or underreported.10 There are several reasons why individuals with infectious TB are not diagnosed and adequately treated (Fig. 1). First, a significant barrier to diagnosis is that half of people with TB do not manifest symptoms that would have prompted care-seeking.31 Even when symptoms are present, individuals may not recognise them as being attributable to TB. Symptoms such as cough, fatigue, and mild fever can be misconstrued as indications of less severe illnesses, delaying the seeking of medical attention.32 Second, limited or difficult access to TB services, especially in resource-limited settings, hinders individuals from seeking the necessary medical attention. Even when symptoms are recognised as concerning, some lack access to health care services, resulting in delayed presentation, self-medication, or consulting with traditional healers.33, 34, 35
Fig. 1.
Barriers to the diagnosis and effective treatment of people with infectious TB in the community. The left column represents the five levels of the socio-ecological framework.30 The top row represents the steps in the TB care cascade. Abbreviation: TB; tuberculosis.
In addition, healthcare providers may not suspect TB when patients present with symptoms that are not specific to TB, leading to delayed or missed diagnoses that facilitate ongoing transmission.32,36 Even when TB is suspected, misdiagnosis can occur if the appropriate tests, such as chest X-ray or molecular TB tests, are not readily available, not done or appropriately interpreted. The introduction of rapid molecular tests with better sensitivity than sputum smear examination is a major advance, but tests remain centralised in many settings, which limits access. Molecular testing relies on good laboratory infrastructure and a robust healthcare ‘ecosystem’, with adequate staffing levels, sample transportation systems, prompt communication of test results, and subsequent follow-up to ensure treatment initiation and support.37 In settings with limited resources, the absence of a robust healthcare ‘ecosystem’ hampers timely TB diagnosis and treatment.38 Effective TB treatment may be compromised if healthcare providers with inadequate training (mainly in private practice) provide sub-optimal and non-standard regimens, leading to suboptimal outcomes with potential amplification of resistance. Economic, logistical, or societal factors, such as TB-associated stigma or catastrophic costs associated with TB care, can also prevent individuals from accessing and receiving appropriate care, thus contributing to ongoing transmission that sustains the TB epidemic.33
Breaking the chain of TB transmission in high-incidence settings
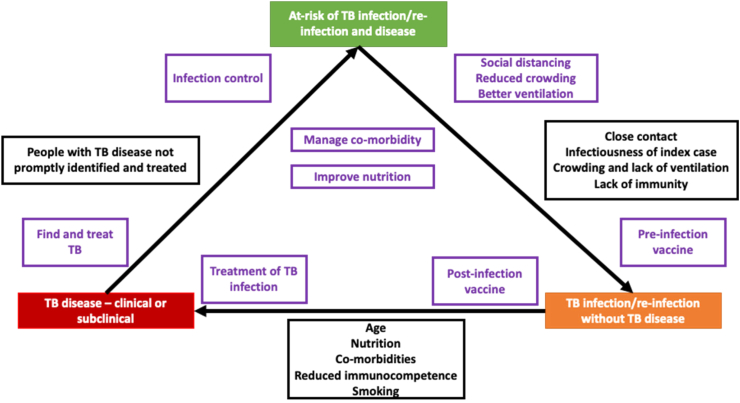
To end TB, it is essential to disrupt the chain of transmission that sustains the epidemic. There are several opportunities to achieve this objective. In particular, we will consider each contributing factor described in Fig. 2.
Fig. 2.
Relevant risk factors and interventions to consider along the ‘TB triangle’. The figure shows the risk factors (black boxes) and interventions for TB (purple boxes) at different phases of TB infection and disease. Abbreviation: TB; tuberculosis.
Preventing primary infection and re-infection
The initial phase of this strategy entails implementing measures to protect uninfected individuals. This need for protection also applies to people who have previously been infected and have either received treatment (for past TB disease or TB infection) or have successfully avoided progression to TB disease. These measures encompass infection prevention and control practices such as cough triage and respiratory isolation of people with presumptive TB,39 improving ventilation in enclosed spaces to minimise the risk of airborne transmission (especially in health care facilities where people with respiratory symptoms congregate), use of personal respiratory protection, and timely and effective treatment for TB disease to stop transmission.39,40
In a systematic review of 7 before-and-after studies, the use of surgical masks by persons with infectious TB resulted in a 14.8% decrease in TB infection in a hospital setting.40 With COVID-19, universal mask-wearing was promoted as a strategy to limit community aerosol transmission. Although this would not be feasible as a long-term intervention, universal mask-wearing in high-risk environments, such as clinic waiting rooms, requires consideration. Improved natural and mechanical ventilation could also contribute to a reduction in TB infection.36,41 Bacillus Calmette-Guérin (BCG) is the only vaccine currently available that offers protection against TB, but this is mainly restricted to primary progressive TB in childhood.42,43 Although BCG has shown some protection against TB infection (as measured by IGRA), this is insufficient to disrupt the chain of transmission at the population level.44 Recently, a promising TB vaccine candidate (VPM1002) has entered clinical trials to assess its effectiveness in preventing TB infection.45,46
Preventing those with infection from developing disease
Preventing TB infection from progressing to active disease presents another opportunity for breaking the chain of transmission. Achieving this involves providing TB preventive treatment (TPT) to individuals who test positive for TB infection but do not have evidence of active disease. Over the past decade, shorter rifamycin-based regimens, 3 months of once-weekly isoniazid plus rifapentine (3HP), 3 months of daily rifampicin plus isoniazid (3RH), 4 months of daily rifampicin (4R), and 1 month of daily isoniazid plus rifapentine (1HP) for people living with HIV are alternatives to the traditional 6–9 month isoniazid regimen, which enhance patient treatment completion.47 These options should simplify TPT provision, but there are multiple barriers to TPT uptake that need to be considered.48 Challenges that underlie persistent policy-practice gaps include the absence of feasible cost-effective TPT implementation models, conducive regulatory and funding frameworks, differing perceptions about risk-benefit ratios among TB control staff and different TPT recipient groups, the absence of a biomarker identifying those at greatest risk who stand to benefit most. Post-exposure vaccines may also provide protection, but this has not been demonstrated. The M72/AS01E subunit vaccine is currently being evaluated for this application, with phase 3 trials assessing its safety and effectiveness in preventing TB disease in individuals with TB infection.49 Additionally, reducing vulnerability and addressing comorbidities that may exacerbate the risk of TB activation is important. This could be done by prompt initiation of antiretroviral therapy after HIV diagnosis,50 better control of diabetes,51 smoking cessation, offering social protection support to improve living conditions, poverty elimination,52 food assistance,53 and access to quality healthcare for comorbidities among those at risk for TB disease.54,55
Person-centred approach to case management and prevention
Active case finding strategies should place a strong emphasis on ensuring that every person with TB starts appropriate treatment promptly and is supported throughout the treatment journey to achieve a sustained (relapse-free) cure. Integration of contact investigation activities and TB preventive treatment services during active case finding is important to identify secondary cases and protect vulnerable individuals, especially young children excluded from population-wide screening. Furthermore, the intersection between non-communicable diseases and TB has been well-recognised, and population-wide case-finding activities also provide a platform to explore synergies.55, 56, 57
Population-wide active case finding to end TB
In high-incidence settings, finding and treating all, or nearly all, individuals with infectious TB by screening the community at regular intervals until the prevalence within the community is low enough to reduce endemic transmission to a level that will allow more targeted interventions to be successful is critical. To do that effectively, the general population should be systematically screened.
The Western Pacific Region is at the forefront of efforts to adopt historically effective population-wide screening strategies using modern tools and standards. In the ACT3 study, population-wide screening for TB was conducted using a molecular test performed on spontaneously expectorated sputum annually over three years to identify and treat people with TB in Ca Mau province, Vietnam.58 Those with positive test results received chest X-rays and were further assessed by TB doctors for TB diagnosis and treatment. After the three-year intervention, the prevalence of TB in the intervention sites was 44% lower than in the control sites. The ACT3 study successfully established an evidence base for mass screening, suggesting that when executed effectively, it can significantly reduce the prevalence of TB disease and the incidence of both TB infection and TB disease at the population level. In the Marshall Islands, Kiribati, and the Federated States of Micronesia, mass screening, treatment and prevention interventions delivered over the past 5 years have demonstrated the feasibility and gathered practical experiences in geographically isolated settings.59,60 Key considerations from these experiences are discussed below and summarised in Table 2.
Table 2.
Key action domains and elements of population-wide active TB case finding—reflecting on experiences from Vietnam and Kiribati as country exemplars.
| Action domain | Key elements | Specific activities–drawing on experiences from Vietnam and Kiribati |
|---|---|---|
| Prepare | Stakeholder engagement and community mobilisation |
|
| Human resource infrastructure |
|
|
| Health system capacity |
|
|
| Implement ‘universal test & treat’ intervention | Screening test coverage |
|
| Diagnostic confirmation |
|
|
| Case notification and linkage to quality care |
|
|
| Treatment support |
|
|
| Refine and report | Iterative refinement |
|
| Report key outcomes |
|
CXR; chest X-ray, NTP; National TB Programme, TB; tuberculosis, TPT; TB preventive therapy, WHO; World Health Organization.
Social mobilisation and community engagement
Effective active case finding begins with social mobilisation and the identification of local champions. Engaging community leaders, healthcare workers, and local networks in designing, planning, and implementing interventions is essential to foster a sense of ownership and collective responsibility to improve the situation within the community. Community members can be further empowered by raising their awareness about the disease, its detrimental impact on the community, the availability of new tools and the importance of early detection and treatment. Through these engagements, individuals are more likely to participate in screening activities and access healthcare services.
Deployment of sensitive and scalable screening tools
To identify all people with TB disease, it is essential to use sensitive screening and diagnostic tests. Screening algorithms based on symptoms are likely to miss a large number of people with infectious TB due to inherent subjectivity in symptom reporting62 and the high prevalence of subclinical TB disease, particularly among people proactively screened in the community.31,63 Despite being relatively simple and inexpensive, sputum smear microscopy requires a high-quality sputum specimen, has low sensitivity and is labour-intensive, resulting in many missed diagnoses and delayed treatment initiation.64 Chest radiographs have reasonable sensitivity and specificity (≥85%) for TB detection, and artificial intelligence (AI)-assisted interpretation of chest radiograph images has shown promise in large-scale, low-cost population-wide TB screening.17 When integrated with molecular rapid diagnostic tests, this approach offers an efficient and accurate diagnostic strategy.
Detecting and treating TB disease early and effectively
Linkage to care and efficient case management is crucial to breaking the chain of TB transmission. Early diagnosis through scaled-up active case finding implies that diagnosis and treatment are ‘front-loaded’ to increase population impact. It should be recognised that the spectrum of TB disease will be different among people identified by active case finding compared to passive case finding programmes, with a greater proportion of subclinical disease. Patients with post-TB radiographic changes or other chronic lung disease may also be flagged through chest radiograph screening, which will require careful clinical consideration and awareness of their increased risk for reinfection and relapse disease. In Kiribati, where the PEARL study60 is evaluating the feasibility and impact of implementing a population-wide TB test, treat and prevent intervention in a high transmission setting, the reach of the screening intervention requires close communication with the local TB program and matching with the existing TB case management capacity.
Population-wide screening is vital for impact
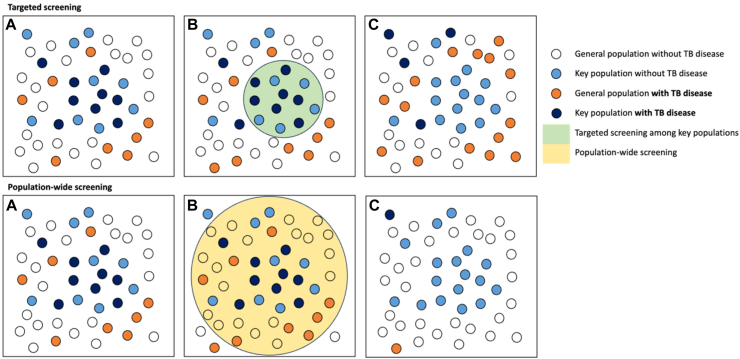
Active case finding can be implemented using diverse modalities and focusing on various population groups; however, in high-incidence (‘hot spot’) areas, population-wide screening is vital for impact. Systematic screening for TB disease in high-risk groups may be more effective in countries with low TB incidence where the epidemic is concentrated among key populations. However, in high-incidence settings, TB transmission is ubiquitous. In these settings, TB transmission and disease risk transcends the boundaries of specific population groups, and virtually everyone is at risk.65 Therefore, as supported by current WHO guidelines, universal population-wide screening should at least be considered in settings with an estimated prevalence of >500/100,000 population (Fig. 3). The rationale behind universal population-wide screening is simple. By testing everyone, even those who might not exhibit typical symptoms or belong to known risk groups, we stand a better chance of detecting and treating everyone with current or future infectious TB early enough to interrupt ongoing transmission within the community. Screening of at-risk populations, such as incarcerated individuals, migrants, and immunocompromised individuals, can be conducted in tandem to reduce their specific disease risk but is not expected to have a major transmission impact within the community.
Fig. 3.
The population impact of targeted screening vs population-wide screening for TB disease. This figure illustrates the potential impact of targeted and population-wide screening for TB disease. Boxes A represent hypothetical scenarios in high-incidence settings where TB disease is not confined to at-risk groups. Boxes B represent the scope and reach of targeted screening (green circle) and population-wide screening (yellow circle). Boxes C represent the potential impact of the different screening approaches. The number of smaller circles does not accurately reflect the population size or the precise transmission risk resulting from the different screening methods deployed. Abbreviation: TB; tuberculosis.
For active case-finding strategies to be effective, they must be implemented with high coverage and intensity to change TB epidemiology effectively.66 So, how do we contextualise population-wide screening for it to be feasible and widely deployed? First, acknowledging that the success of active case-finding activities hinges on strong political support and community acceptance. Implementation in lower-middle-income countries, such as Vietnam and Kiribati, underscores the multifaceted nature of commitments and considerations that are as critical for success.
Large-scale strategic funding that is beyond what TB programs receive currently is imperative, with such initiatives operating as high-level strategic interventions independent of, but closely linked to, the daily operations of National TB Programmes (NTPs). When assessing cost-effectiveness and suitability for investment, it is imperative to extend the focus on effectiveness to impacts at the broader population (transmission control) level. Multisectoral engagement, training and capacity building, and strategic involvement of community health workers, non-governmental organisations and members of the community are integral to the success of population-wide screening. Implementation science methods can be applied to understand the barriers and facilitators to implement population-wide TB screening and how that can also strengthen the broader health infrastructure. Second, leveraging epidemiological data is crucial for guiding and bolstering the adoption of the intervention. The WHO advocates for systematic screening in regions with an estimated TB prevalence of ≥0.5%.17 Therefore, these areas should be prioritised for population-wide screening. Moreover, locations with persistently high case detection, despite repeated active case-finding activities, or those where case attributes indicate endemicity,17 warrant consideration for population-wide screening, regardless of symptoms or risk factors. Third, piloting population-wide screening in these areas, followed by a rigorous evaluation of outcomes, can provide valuable insights for scale-up.
Conclusion
Ending TB requires a comprehensive and multi-faceted strategy that integrates socio-economic development, reduced inequality, health system strengthening, multi-sectoral collaboration, adequate funding for TB control, and unwavering political commitment. However, until we develop an effective vaccine that could break the transmission cycle, finding and treating as many people with infectious TB as possible is the only way to reduce transmission and resultant disease in high-incidence settings; awaiting more data on the complementary effect of better TB prevention through the widespread use of newer short-course TPT. Population-wide active case finding has multiple context-specific challenges to consider, but we have the tools to get it done. It is also an equitable approach that reaches the unreached and respects the right of every person to be free from TB infection and disease.
Contributors
BJM, GM, and TA conceptualised the paper. TA, AKJT, and JH drafted the manuscript. TA, AKJT, YZ, MQ, JH, FM, BJM, and GM critically revised the manuscript. All authors contributed to the final version of the manuscript, reviewed, and approved the manuscript.
Declaration of interests
Guy B. Marks is the President of IUATLD. The rest authors have nothing to declare.
Acknowledgements
We express our gratitude to the study and field teams of ACT 3, ACT 5, and PEARL for their invaluable contributions in implementing population-wide active case finding in Vietnam and Kiribati. Their efforts have given us a greater appreciation of the possibilities and the challenges, significantly enriching our collective experience and wisdom.
Contributor Information
Thu-Anh Nguyen, Email: thuanh.nguyen@sydney.edu.au, thuanh.nguyen@sydneyvietnaminstitute.org.
Alvin Kuo Jing Teo, Email: alvin.teo@sydney.edu.au.
Yanlin Zhao, Email: zhaoyl@chinacdc.cn.
Mamel Quelapio, Email: mameldquelapio@gmail.com.
Jeremy Hill, Email: jeremy.hill@sydney.edu.au.
Fukushi Morishita, Email: morishitaf@who.int.
Ben J. Marais, Email: ben.marais@sydney.edu.au.
Guy B. Marks, Email: g.marks@unsw.edu.au.
References
- 1.Koch R. The etiology of tuberculosis. Rev Infect Dis. 1982;4:1270–1274. [PubMed] [Google Scholar]
- 2.Walker L., LeVine H., Jucker M. Koch's postulates and infectious proteins. Acta Neuropathol. 2006;112:1–4. doi: 10.1007/s00401-006-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchyard G., Kim P., Shah N.S., et al. What we know about tuberculosis transmission: an overview. J Infect Dis. 2017;216:S629–S635. doi: 10.1093/infdis/jix362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop P.J., Neumann G. The history of the Ziehl-Neelsen stain. Tubercle. 1970;51:196–206. doi: 10.1016/0041-3879(70)90073-5. [DOI] [PubMed] [Google Scholar]
- 5.Mould R.F. The early history of X-ray diagnosis with emphasis on the contributions of physics 1895-1915. Phys Med Biol. 1995;40:1741. doi: 10.1088/0031-9155/40/11/001. [DOI] [PubMed] [Google Scholar]
- 6.Luca S., Mihaescu T. History of BCG vaccine. Maedica (Bucur) 2013;8:53–58. [PMC free article] [PubMed] [Google Scholar]
- 7.Schatz A., Bugle E., Waksman S.A. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Clin Orthop Relat Res. 1944;55:66–69. doi: 10.1097/01.blo.0000175887.98112.fe. [DOI] [PubMed] [Google Scholar]
- 8.Fox W. Whither short-course chemotherapy? Br J Dis Chest. 1981;75:331–357. doi: 10.1016/0007-0971(81)90022-x. [DOI] [PubMed] [Google Scholar]
- 9.Raviglione M.C., Pio A. Evolution of WHO policies for tuberculosis control, 1948-2001. Lancet. 2002;359:775–780. doi: 10.1016/s0140-6736(02)07880-7. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 2023. Global tuberculosis report 2023. [Google Scholar]
- 11.Emery J.C., Dodd P.J., Banu S., et al. Estimating the contribution of subclinical tuberculosis disease to transmission—an individual patient data analysis from prevalence surveys. medRxiv. 2022 doi: 10.1101/2022.06.09.22276188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trauer J.M., Moyo N., Tay E.-L., et al. Risk of active tuberculosis in the five years following infection ... 15%? Chest. 2016;149:516–525. doi: 10.1016/j.chest.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Cardona P.-J. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection. 2009;37:80–86. doi: 10.1007/s15010-008-8087-y. [DOI] [PubMed] [Google Scholar]
- 14.Menzies N.A., Wolf E., Connors D., et al. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect Dis. 2018;18:e228–e238. doi: 10.1016/S1473-3099(18)30134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; Geneva: 2021. WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB) 2021-2025.https://apps.who.int/iris/handle/10665/341980 [Google Scholar]
- 16.Roy Chowdhury R., Vallania F., Yang Q., et al. A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature. 2018;560:644–648. doi: 10.1038/s41586-018-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . World Health Organization; Geneva: 2021. WHO consolidated guidelines on tuberculosis. Module 2: screening–systematic screening for tuberculosis disease.https://www.who.int/publications/i/item/9789240001503 [PubMed] [Google Scholar]
- 18.Mia M.M., Hasan M., Pory F.S. Occupational exposure to livestock and risk of tuberculosis and brucellosis: a systematic review and meta-analysis. One Health. 2022;15 doi: 10.1016/j.onehlt.2022.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization Regional Office for the Western Pacific . World Health Organization Regional Office for the Western Pacific; Manila: 2022. Western Pacific regional framework to end TB 2021-2030.https://apps.who.int/iris/handle/10665/352278 [Google Scholar]
- 20.Otero L., Krapp F., Tomatis C., et al. High prevalence of primary multidrug resistant tuberculosis in persons with No known risk factors. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callum J., Nguyen P.T.B., Martinez E., et al. Prevalence and genetic basis of first-line drug resistance of Mycobacterium tuberculosis in Ca Mau, Vietnam. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00122-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teibo T.K.A., Andrade R.L.P., Rosa R.J., Tavares R.B.V., Berra T.Z., Arcêncio R.A. Geo-spatial high-risk clusters of Tuberculosis in the global general population: a systematic review. BMC Public Health. 2023;23:1586. doi: 10.1186/s12889-023-16493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardell E.A. Time to revise our tuberculosis infection-latency-disease model in high-burden settings. Clin Infect Dis. 2021;72:2016–2017. doi: 10.1093/cid/ciaa866. [DOI] [PubMed] [Google Scholar]
- 24.Marks G.B., Ho J., Nguyen P.T.B., et al. A direct measure of tuberculosis incidence — effect of community screening. N Engl J Med. 2022;386:1380–1382. doi: 10.1056/NEJMc2114176. [DOI] [PubMed] [Google Scholar]
- 25.Cardona P.-J. Reactivation or reinfection in adult tuberculosis: is that the question? Int J Mycobacteriol. 2016;5:400–407. doi: 10.1016/j.ijmyco.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Behr M.A., Edelstein P.H., Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. doi: 10.1136/bmj.k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houben R.M.G.J., Dodd P.J. The global burden of latent tuberculosis infection: a Re-estimation using mathematical modelling. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . World Health Organization; Geneva: 2020. WHO operational handbook on tuberculosis: module 1: prevention: tuberculosis preventive treatment.https://apps.who.int/iris/handle/10665/331525 [Google Scholar]
- 29.Verver S., Warren R.M., Beyers N., et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–1435. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 30.Golden S.D., Earp J.A.L. Social ecological approaches to individuals and their contexts: twenty years of health education & behavior health promotion interventions. Health Educ Behav. 2012;39:364–372. doi: 10.1177/1090198111418634. [DOI] [PubMed] [Google Scholar]
- 31.Frascella B., Richards A.S., Sossen B., et al. Subclinical tuberculosis disease—a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis. 2021;73:e830–e841. doi: 10.1093/cid/ciaa1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Htet K.K.K., Chongsuvivatwong V., Aung S.T. Sensitivity and specificity of tuberculosis signs and symptoms screening and adjunct role of social pathology characteristics in predicting bacteriologically confirmed tuberculosis in Myanmar. Trop Med Health. 2021;49:3. doi: 10.1186/s41182-020-00292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo A.K.J., Singh S.R., Prem K., Hsu L.Y., Yi S. Duration and determinants of delayed tuberculosis diagnosis and treatment in high-burden countries: a mixed-methods systematic review and meta-analysis. Respir Res. 2021;22:251. doi: 10.1186/s12931-021-01841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teo A.K.J., Morishita F., Prem K., et al. Where are the missing people affected by tuberculosis? A programme review of patient-pathway and cascade of care to optimise tuberculosis case-finding, treatment and prevention in Cambodia. BMJ Glob Health. 2023;8 doi: 10.1136/bmjgh-2022-010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tupasi T.E., Radhakrishna S., Co V.M., et al. Bacillary disease and health seeking behavior among Filipinos with symptoms of tuberculosis: implications for control. Int J Tuberc Lung Dis. 2000;4:1126–1132. [PubMed] [Google Scholar]
- 36.Zawahir S., Le H., Nguyen T.A., et al. Standardised patient study to assess tuberculosis case detection within the private pharmacy sector in Vietnam. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camelique O., Scholtissen S., Dousset J.-P., Bonnet M., Bastard M., Hewison C. Mobile community-based active case-finding for tuberculosis among older populations in rural Cambodia. Int J Tuberc Lung Dis. 2019;23:1107–1114. doi: 10.5588/ijtld.18.0611. [DOI] [PubMed] [Google Scholar]
- 38.Parsons L.M., Somoskövi Á., Gutierrez C., et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . World Health Organization; Geneva: 2022. WHO operational handbook on tuberculosis: module 1: prevention: infection prevention and control.https://www.who.int/publications/i/item/9789240055889 [Google Scholar]
- 40.Fox G.J., Redwood L., Chang V., Ho J. The effectiveness of individual and environmental infection control measures in reducing the transmission of Mycobacterium tuberculosis: a systematic review. Clin Infect Dis. 2021;72:15–26. doi: 10.1093/cid/ciaa719. [DOI] [PubMed] [Google Scholar]
- 41.Knibbs L.D., Morawska L., Bell S.C., Grzybowski P. Room ventilation and the risk of airborne infection transmission in 3 health care settings within a large teaching hospital. Am J Infect Control. 2011;39:866–872. doi: 10.1016/j.ajic.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 43.Mangtani P., Abubakar I., Ariti C., et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 44.Roy A., Eisenhut M., Harris R.J., et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cotton M.F., Madhi S.A., Luabeya A.K., et al. Safety and immunogenicity of VPM1002 versus BCG in South African newborn babies: a randomised, phase 2 non-inferiority double-blind controlled trial. Lancet Infect Dis. 2022;22:1472–1483. doi: 10.1016/S1473-3099(22)00222-5. [DOI] [PubMed] [Google Scholar]
- 46.Manjula S. Clinical Trial Registry India; India: 2023. A phase III, randomized, double-blind, three arm placebo controlled trial to evaluate the efficacy and safety of two vaccines VPM1002 and immuvac in preventing tuberculosis (TB) in healthy household contacts of newly diagnosed sputum positive pulmonary TB patients (clinical trial register)https://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=27411&EncHid=&modid=&compid=%27,%2727411det%27 [Google Scholar]
- 47.Sterling T.R., Njie G., Zenner D., et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Recomm Rep. 2020;69:1–11. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surie D., Interrante J.D., Pathmanathan I., et al. Policies, practices and barriers to implementing tuberculosis preventive treatment-35 countries, 2017. Int J Tuberc Lung Dis. 2019;23:1308–1313. doi: 10.5588/ijtld.19.0018. [DOI] [PubMed] [Google Scholar]
- 49.Tuberculosis Vaccine Initiative . TBVI; 2022. Pipeline of vaccines.https://www.tbvi.eu/what-we-do/pipeline-of-vaccines/ [Google Scholar]
- 50.Suthar A.B., Lawn S.D., del Amo J., et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee P.-H., Fu H., Lai T.-C., Chiang C.-Y., Chan C.-C., Lin H.-H. Glycemic control and the risk of tuberculosis: a cohort study. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter D.J., Glaziou P., Lönnroth K., et al. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1. Lancet Global Health. 2018;6:e514–e522. doi: 10.1016/S2214-109X(18)30195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhargava A., Bhargava M., Meher A., et al. Nutritional support for adult patients with microbiologically confirmed pulmonary tuberculosis: outcomes in a programmatic cohort nested within the RATIONS trial in Jharkhand, India. Lancet Global Health. 2023;11:e1402–e1411. doi: 10.1016/S2214-109X(23)00324-8. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization . 2012. WHO policy on collaborative TB/HIV activities : guidelines for national programmes and other stakeholders. Politique de l’OMS pour les activités conjointes de lutte contre la tuberculose et le VIH : principes directeurs à l’intention des programmes nationaux et autres partenaires; p. 36. [Google Scholar]
- 55.Marais B.J., Lönnroth K., Lawn S.D., et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis. 2013;13:436–448. doi: 10.1016/S1473-3099(13)70015-X. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization . World Health Organization; Geneva: 2022. Framework for collaborative action on tuberculosis and comorbidities. [Google Scholar]
- 57.Byrne A.L., Marais B.J., Mitnick C.D., et al. Feasibility and yield of screening for non-communicable diseases among treated tuberculosis patients in Peru. Int J Tuberc Lung Dis. 2018;22:86–92. doi: 10.5588/ijtld.17.0381. [DOI] [PubMed] [Google Scholar]
- 58.Marks G.B., Nguyen N.V., Nguyen P.T.B., et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med. 2019;381:1347–1357. doi: 10.1056/NEJMoa1902129. [DOI] [PubMed] [Google Scholar]
- 59.Ragonnet R., Williams B.M., Largen A., et al. Estimating the long-term effects of mass screening for latent and active tuberculosis in the Marshall Islands. Int J Epidemiol. 2022;51:1433–1445. doi: 10.1093/ije/dyac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coleman M., Hill J., Timeon E., et al. Population-wide active case finding and prevention for tuberculosis and leprosy elimination in Kiribati: the PEARL study protocol. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phuong N.T.B., Anh N.T., Van Son N., et al. Effect of two alternative methods of pooling sputum prior to testing for tuberculosis with genexpert MTB/RIF. BMC Infect Dis. 2019;19:347. doi: 10.1186/s12879-019-3778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon C., Dowdy D.W., Esmail H., MacPherson P., Schumacher S.G. Screening for tuberculosis: time to move beyond symptoms. Lancet Respir Med. 2019;7:202–204. doi: 10.1016/S2213-2600(19)30039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esmail H., Macpherson L., Coussens A.K., Houben R.M.G.J. Mind the gap—managing tuberculosis across the disease spectrum. eBioMedicine. 2022;78 doi: 10.1016/j.ebiom.2022.103928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teo A.K.J., Ork C., Eng S., et al. Determinants of delayed diagnosis and treatment of tuberculosis in Cambodia: a mixed-methods study. Infect Dis Poverty. 2020;9:49. doi: 10.1186/s40249-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marks G.B., Horsburgh C.R., Fox G.J., Nguyen T.A. Epidemiological approach to ending tuberculosis in high-burden countries. Lancet. 2022;400:1750–1752. doi: 10.1016/S0140-6736(22)01433-7. [DOI] [PubMed] [Google Scholar]
- 66.Burke R.M., Nliwasa M., Feasey H.R.A., et al. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health. 2021;6:e283–e299. doi: 10.1016/S2468-2667(21)00033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]