This comparative effectiveness research study examines associations between opioid use disorder treatment pathways and overdose and opioid-related acute care use as proxies for opioid use disorder recurrence.
Key Points
Question
What is the real-world effectiveness of different treatment pathways for opioid use disorder?
Findings
In this comparative effectiveness research study of 40 885 adults with opioid use disorder that compared 6 different treatment pathways, only treatment with buprenorphine or methadone was associated with reduced risk of overdose and serious opioid-related acute care use compared with no treatment during 3 and 12 months of follow-up.
Meaning
Methadone and buprenorphine were associated with reduced overdose and opioid-related morbidity compared with opioid antagonist therapy, inpatient treatment, or intensive outpatient behavioral interventions and may be used as first-line treatments for opioid use disorder.
Abstract
Importance
Although clinical trials demonstrate the superior effectiveness of medication for opioid use disorder (MOUD) compared with nonpharmacologic treatment, national data on the comparative effectiveness of real-world treatment pathways are lacking.
Objective
To examine associations between opioid use disorder (OUD) treatment pathways and overdose and opioid-related acute care use as proxies for OUD recurrence.
Design, Setting, and Participants
This retrospective comparative effectiveness research study assessed deidentified claims from the OptumLabs Data Warehouse from individuals aged 16 years or older with OUD and commercial or Medicare Advantage coverage. Opioid use disorder was identified based on 1 or more inpatient or 2 or more outpatient claims for OUD diagnosis codes within 3 months of each other; 1 or more claims for OUD plus diagnosis codes for opioid-related overdose, injection-related infection, or inpatient detoxification or residential services; or MOUD claims between January 1, 2015, and September 30, 2017. Data analysis was performed from April 1, 2018, to June 30, 2019.
Exposures
One of 6 mutually exclusive treatment pathways, including (1) no treatment, (2) inpatient detoxification or residential services, (3) intensive behavioral health, (4) buprenorphine or methadone, (5) naltrexone, and (6) nonintensive behavioral health.
Main Outcomes and Measures
Opioid-related overdose or serious acute care use during 3 and 12 months after initial treatment.
Results
A total of 40 885 individuals with OUD (mean [SD] age, 47.73 [17.25] years; 22 172 [54.2%] male; 30 332 [74.2%] white) were identified. For OUD treatment, 24 258 (59.3%) received nonintensive behavioral health, 6455 (15.8%) received inpatient detoxification or residential services, 5123 (12.5%) received MOUD treatment with buprenorphine or methadone, 1970 (4.8%) received intensive behavioral health, and 963 (2.4%) received MOUD treatment with naltrexone. During 3-month follow-up, 707 participants (1.7%) experienced an overdose, and 773 (1.9%) had serious opioid-related acute care use. Only treatment with buprenorphine or methadone was associated with a reduced risk of overdose during 3-month (adjusted hazard ratio [AHR], 0.24; 95% CI, 0.14-0.41) and 12-month (AHR, 0.41; 95% CI, 0.31-0.55) follow-up. Treatment with buprenorphine or methadone was also associated with reduction in serious opioid-related acute care use during 3-month (AHR, 0.68; 95% CI, 0.47-0.99) and 12-month (AHR, 0.74; 95% CI, 0.58-0.95) follow-up.
Conclusions and Relevance
Treatment with buprenorphine or methadone was associated with reductions in overdose and serious opioid-related acute care use compared with other treatments. Strategies to address the underuse of MOUD are needed.
Introduction
The increasing burden of opioid use disorder (OUD) has resulted in increased opioid-related morbidity and mortality, with 47 600 overdose deaths in 2017 alone.1,2,3 From 2002 to 2012, hospitalization costs attributable to opioid-related overdose increased by more than $700 million annually.4 Associated health complications, such as hepatitis C infection, HIV infection, and serious injection-related infections, are also increasing.5,6,7 In addition, as rates of opioid-related death have increased despite decreases in prescription opioid supply, there is an increasing recognition that greater attention must be paid to improving access to effective OUD treatment.8,9
Medication for opioid use disorder (MOUD) is effective and improves mortality, treatment retention, and remission, but most people with OUD remain untreated.10,11,12,13,14,15 Many parts of the United States lack access to buprenorphine prescribers, and only a few addiction treatment programs offer all forms of MOUD.16,17,18 This lack of access has resulted in a treatment gap of an estimated 1 million people with OUD untreated with MOUD annually.19
Nationally representative, comparative effectiveness studies of MOUD compared with nonpharmacologic treatment are limited. One prior study12 compared MOUD with psychosocial treatments but was limited to a Massachusetts Medicaid population. Studies20,21,22,23 examining OUD treatment among nationally representative populations have examined trends in MOUD initiation, patterns of OUD treatment, and effectiveness of different types of MOUD at reducing overdose using Medicaid and commercial claims data. However, none of those studies20,21,22,23 compared the effectiveness of MOUD with nonpharmacologic treatments in a national sample. Despite better access to medical care, only a few commercially insured patients are treated with MOUD, and psychosocial-only treatments continue to be common, suggesting that greater understanding of the comparative effectiveness of these different treatments is needed.21
In this study, we used a large, nationally representative database of commercially insured and Medicare Advantage (MA) individuals to evaluate the effectiveness of MOUD compared with nonpharmacologic treatment. This retrospective comparative effectiveness study was designed to inform treatment decisions made by policy makers, insurers, practitioners, and patients.
Methods
We conducted a comparative effectiveness research study using the OptumLabs Data Warehouse, which includes medical, behavioral health, and pharmacy claims for commercial and MA enrollees.24 The database represents a diverse mixture of ages, races/ethnicities, and geographic regions across the United States. Our analysis used deidentified administrative claims data. The window for identification of OUD for this study was January 1, 2015, to September 30, 2017. The study used claims data from October 3, 2014, to December 31, 2017, to allow for a 90-day period to ensure a nonopioid clean period and a minimum of 90 days of follow-up for all individuals with diagnosed OUD. Data analysis was performed from April 1, 2018, to June 30, 2019. Because this study involved analysis of preexisting, deidentified data, the Chesapeake Institutional Review Board deemed it exempt from institutional review board approval. This study followed the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guideline.25
Cohort Selection
We defined OUD as 1 or more inpatient or 2 or more outpatient claims for International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes for opioid dependence that occurred within 3 months of each other; 1 or more claims for diagnosis codes for opioid dependence, opioid use, or opioid abuse plus diagnosis codes for an encounter related to opioid overdose or an injection-related infection, opioid-related inpatient detoxification or residential services; or claims for MOUD or detoxification (eFigure 1 in the Supplement). Cohort inclusion required presence of OUD and age of 16 years or older; commercial or MA medical, pharmacy, and behavioral coverage; and continuous enrollment for 3 months before and after OUD treatment initiation date. For those in the no treatment group, a treatment initiation index date was selected at random that matched the treated groups (eAppendix 1 in the Supplement).
Treatment Pathways
We examined treatments received in the 3 months after OUD diagnosis during the first 90 days after cohort entry to identify patterns of treatment (eFigure 2 in the Supplement). We categorized individuals into 1 of 6 mutually exclusive pathway designations based on initial treatment: (1) no treatment, (2) inpatient detoxification or residential services, (3) intensive behavioral health (intensive outpatient or partial hospitalization), (4) buprenorphine or methadone, (5) naltrexone, and (6) only nonintensive behavioral health (outpatient counseling) (eAppendix 2 in the Supplement). In addition, we examined mean duration of MOUD treatment in days.
Classification of treatment pathways was informed by detailed exploration of the sequence of treatment modalities provided to patients using medical and pharmacy claims (eFigure 3 in the Supplement). For this study, consistent with an intent-to-treat design, patients were assigned to the initial treatment received.
Outcomes
Our primary outcomes were overdose or serious opioid-related acute care use, defined as an emergency department or hospitalization with a primary opioid diagnosis code. Overdose was identified based on diagnosis codes from claims for health care encounters. These encounters may include both fatal and nonfatal overdose (lack of mortality data preclude that determination). For actively treated individuals, the index date was the date of first treatment. For untreated individuals, the index date was set randomly based on the distribution of time to first treatment among actively treated individuals. Risk for adverse outcomes started 1 day after the index date; however, because the time sequence for adverse events that occurred during an initial inpatient treatment could not be reliably established, risk of adverse outcomes started 1 day after inpatient discharge. Time to event was calculated as (event date – index date + 1), which is consistent with an intent-to-treat analysis for all treatment pathways. Individuals were censored at the earlier outcome, health plan disenrollment, or 12 months. We selected overdose and opioid-related acute care use as negative clinical outcomes, which likely indicate recurrence of OUD. These outcomes may underestimate the prevalence of OUD recurrence because they represent severe consequences of ongoing use.
A secondary outcome was admission to inpatient detoxification or readmission for those who initiated treatment with inpatient detoxification or residential services. All outcomes were evaluated for 3 months and 12 months after treatment initiation. In the absence of an event, patients were followed up until the earliest date of health plan disenrollment or end of the respective period.
Statistical Analysis
We used Cox proportional hazards regression models to estimate the hazard ratios (HRs) for primary and secondary outcomes, adjusting for age, sex, race/ethnicity, insurance type, baseline cost rank, mental health and medical comorbidities, and injection-related infections or overdose at study inclusion. For medical comorbidities, we used a modified Elixhauser index that excluded mental health subcomponents because they were classified separately.26 All analyses were conducted using an intent-to-treat approach that attributed patient outcomes to their initial treatment category. We conducted a subanalysis of patients who received methadone or buprenorphine, stratifying by duration of MOUD treatment as 1 to 30 days, 31 to 180 days, or more than 180 days.
For the secondary outcome of admission to inpatient detoxification, we conducted a subanalysis in which patients in the no treatment and nonintensive behavioral health groups were removed from the sample. These 2 treatment pathways were, by definition, required to not have any treatment (no treatment group) or any treatment other than outpatient behavioral health treatment (nonintensive behavioral health group) in the first 3 months of follow-up, which made them systematically different from the other pathways evaluated for this outcome.
Analysis of survival for all outcomes was performed using unadjusted Kaplan-Meier curves and adjusted Cox proportional hazards regression (PHREG procedure, SAS Enterprise Guide, version 7.13 [SAS Institute Inc]) under both 3-month and 12-month time windows to examine potential survivorship bias and informative censoring. For the unadjusted analysis, the log-rank test is reported; 95% Wald CIs are reported for the adjusted HRs (AHRs). The proportionality assumption was assessed visually and tested by including treatment pathway as a time-dependent covariate in the Cox proportional hazards regression model. Hazards appeared to be proportional during 3 months, but there was evidence of nonproportionality for the behavioral health outpatient pathway during the 12-month time window.
Results
Cohort Characteristics
A total of 40 885 individuals with OUD (mean [SD] age, 47.73 [17.25] years; 22 172 [54.2%] male; 30 332 [74.2%] white) were identified. A total of 23 636 (57.8%) were commercially insured, and 17 249 (42.2%) were enrolled in MA plans. Of those with MA, 10 322 (25.2%) were younger than 65 years. Non–substance use disorder mental health comorbidities in the 3 months before the index date were found in 10 942 individuals (45.1%) in the cohort. Depression (9733 [23.8%]) and anxiety (10 704 [26.2%]) were most common (Table 1).
Table 1. Patient Characteristicsa.
| Characteristic | Total | No Treatment | Inpatient Detoxification or Residential Services | BH IOP | MOUD | BH Other | |
|---|---|---|---|---|---|---|---|
| Buprenorphine or Methadone | Naltrexone | ||||||
| Total sample | 40 885 (100) | 2116 (5.2) | 6455 (15.8) | 1970 (4.8) | 5123 (12.5) | 963 (2.4) | 24 258 (59.3) |
| Age, mean (SD), y | 47.73 (17.25) | 44.85 (18.66) | 39.22 (15.38) | 31.28 (12.19) | 42.58 (13.93) | 38.10 (14.01) | 53.05 (16.36) |
| Follow-up duration, mean (SD), d | 293.2 (91.3) | 285.0 (93.9) | 284.3 (96.1) | 291.1 (93.2) | 281.8 (94.7) | 282.5 (94.5) | 299.3 (88.1) |
| Age group, y | |||||||
| 16-25 | 5978 (14.6) | 437 (20.7) | 1837 (28.5) | 948 (48.1) | 578 (11.3) | 247 (25.6) | 1931 (8.0) |
| 26-34 | 5350 (13.1) | 354 (16.7) | 1124 (17.4) | 404 (20.5) | 1194 (23.3) | 197 (20.5) | 2077 (8.6) |
| 35-44 | 6070 (14.8) | 332 (15.7) | 1089 (16.9) | 290 (14.7) | 1172 (22.9) | 206 (21.4) | 2981 (12.3) |
| 45-54 | 7208 (17.6) | 300 (14.2) | 1059 (16.4) | 188 (9.5) | 995 (19.4) | 167 (17.3) | 4499 (18.5) |
| 54-64 | 8897 (21.8) | 318 (15.0) | 983 (15.2) | 117 (5.9) | 817 (15.9) | 108 (11.2) | 6554 (27) |
| ≥65 | 7382 (18.1) | 375 (17.7) | 363 (5.6) | 23 (1.2) | 367 (7.2) | 38 (3.9) | 6216 (25.6) |
| Sex | |||||||
| Female | 18 713 (45.8) | 797 (37.7) | 2482 (38.5) | 662 (33.6) | 1971 (38.5) | 387 (40.2) | 12 414 (51.2) |
| Male | 22 172 (54.2) | 1319 (62.3) | 3973 (61.5) | 1308 (66.4) | 3152 (61.5) | 576 (59.8) | 11 844 (48.8) |
| Insurance type | |||||||
| Commercial | 23 636 (57.8) | 1299 (61.4) | 5062 (78.4) | 1889 (95.9) | 3630 (70.9) | 841 (87.3) | 10 915 (45) |
| Medicare Advantage | |||||||
| Age <65 y | 10 322 (25.2) | 457 (21.6) | 1067 (16.5) | 63 (3.2) | 1147 (22.4) | 91 (9.4) | 7497 (30.9) |
| Age ≥65 y | 6927 (16.9) | 360 (17.0) | 326 (5.1) | 18 (0.9) | 346 (6.8) | 31 (3.2) | 5846 (24.1) |
| Race/ethnicity | |||||||
| White | 30 332 (74.2) | 1485 (70.2) | 4976 (16.4) | 1552 (78.8) | 4044 (78.9) | 791 (82.1) | 17 484 (72.1) |
| Hispanic | 3388 (8.3) | 192 (9.1) | 511 (15.1) | 158 (8.0) | 338 (6.6) | 47 (4.9) | 2142 (8.8) |
| Black | 4991 (12.2) | 317 (15.0) | 628 (12.6) | 161 (8.2) | 468 (9.1) | 68 (7.1) | 3349 (13.8) |
| Other or unknown | 2174 (5.3) | 122 (5.8) | 340 (15.6) | 99 (5.0) | 273 (5.3) | 57 (5.9) | 1283 (5.3) |
| Elixhauser index score excluding mental health, mean (SD) | 1.75 (2.35) | 1.25 (2.15) | 1.00 (1.67) | 0.51 (1.15) | 0.88 (1.49) | 0.94 (1.41) | 2.30 (2.60) |
| Any mental health diagnosis | 18 218 (44.6) | 585 (27.6) | 3078 (47.7) | 933 (47.4) | 2060 (40.2) | 620 (64.4) | 10 942 (45.1) |
| Depression | 9733 (23.8) | 270 (12.8) | 1670 (25.9) | 552 (28.0) | 965 (18.8) | 398 (41.3) | 5878 (24.2) |
| Anxiety | 10 704 (26.2) | 274 (12.9) | 1921 (29.8) | 554 (28.1) | 1329 (25.9) | 391 (40.6) | 6235 (25.7) |
| ADHD | 1774 (4.3) | 33 (1.6) | 402 (6.2) | 159 (8.1) | 272 (5.3) | 77 (8.0) | 831 (3.4) |
| PTSD | 1462 (3.6) | 41 (1.9) | 245 (3.8) | 104 (5.3) | 153 (3.0) | 69 (7.2) | 850 (3.5) |
| Alcohol | 4166 (10.2) | 174 (8.2) | 961 (14.9) | 471 (23.9) | 225 (4.4) | 496 (51.5) | 1839 (7.6) |
| Bipolar disorder | 3138 (7.7) | 102 (4.8) | 556 (8.6) | 183 (9.3) | 290 (5.7) | 146 (15.2) | 1861 (7.7) |
| Psychosis | 1526 (3.7) | 76 (3.6) | 268 (4.2) | 76 (3.9) | 87 (1.7) | 40 (4.2) | 979 (4) |
| IDU infection | 5556 (13.6) | 249 (11.8) | 330 (5.1) | 66 (3.4) | 151 (2.9) | 31 (3.2) | 4729 (19.5) |
| Hepatitis C | 2018 (4.9) | 64 (3.0) | 181 (2.8) | <29 (<1.7) | 121 (2.4) | <11 (<1.1) | 1623 (6.7) |
| Opioid overdose | 2135 (5.2) | 249 (11.8) | 267 (4.1) | 84 (4.3) | 86 (1.7) | 27 (2.8) | 1422 (5.9) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BH IOP, intensive behavioral health (intensive outpatient or partial hospitalization); BH other, only nonintensive behavioral health (outpatient counseling); IDU, injection drug use; MOUD, medication for opioid use disorder; PTSD, posttraumatic stress disorder.
Data are presented as number (percentage) of patients unless otherwise indicated.
The most common treatment pathway was nonintensive behavioral health (24 258 [59.3%]), followed by inpatient detoxification or residential services (6455 [15.8%]) and buprenorphine or methadone (5123 [12.5%]). Not receiving any treatment was more common (2116 [5.2%]) than naltrexone (963 [2.4%]) or intensive behavioral health (1970 [4.8%]). Mean (SD) length of stay in inpatient detoxification or residential services was 7.47 (10.35) days. For the 5048 in that group who had at least 6 months of continuous enrollment, mean (SD) length of stay was 7.56 (10.99) days. For the 3098 in that group who had at least 12 months of continuous enrollment, mean (SD) length of stay was 7.64 (12.24) days.
Maintaining continuous commercial health insurance was challenging in this cohort; 19 685 (48.1%) were disenrolled by 12 months after the index date. Individuals receiving nonintensive behavioral health had the lowest disenrollment (11 037 [45.5%]), and those receiving MOUD treatment with buprenorphine or methadone (2755 [53.8%]) and MOUD treatment with naltrexone (520 [54.0%]) had the highest disenrollment rates. No differences were found between those who maintained enrollment and those who were disenrolled with regard to race/ethnicity, comorbidities, or markers of severity of OUD, including those with a history of an injection-related infection, hepatitis C infection, or overdose. It was not possible to distinguish disenrollment attributable to death from disenrollment for other reasons (eg, health insurance options offered by employers). Details on demographic characteristics and comorbidities by treatment group for individuals who were disenrolled are provided in the eTable in the Supplement.
Recurrence Outcomes
During the 3-month follow-up period, 707 participants (1.7%) experienced an overdose, and 773 (1.9%) had a serious opioid-related acute care use episode. Only individuals receiving MOUD treatment with buprenorphine or methadone were less likely to experience an overdose compared with those receiving no treatment (AHR, 0.24; 95% CI, 0.14-0.41) (Table 2 and Figure 1A). Inpatient detoxification or residential services (AHR, 0.82; 95% CI, 0.57-1.19), naltrexone (AHR, 0.59; 95% CI, 0.29-1.20), nonintensive behavioral health services (AHR, 0.92; 95% CI, 0.67-1.27), or intensive behavioral health services (AHR, 0.81; 95% CI, 0.50-1.32) were not significantly associated with overdose.
Table 2. Adjusted Hazard Ratios for Overdose and Serious Opioid-Related Acute Care Use by Initial Treatment Group Compared With No Treatmenta.
| Variable | Adjusted Hazard Ratio (95% CI) | |
|---|---|---|
| 3 Months | 12 Months | |
| Overdose | ||
| No treatment | 1 [Reference] | 1 [Reference] |
| Inpatient detoxification or residential services | 0.82 (0.57-1.19) | 1 (0.79-1.25) |
| BH IOP | 0.81 (0.50-1.32) | 0.75 (0.56-1.02) |
| MOUD treatment with buprenorphine or methadone | 0.24 (0.14-0.41) | 0.41 (0.31-0.55) |
| MOUD treatment with naltrexone | 0.59 (0.29-1.20) | 0.73 (0.48-1.11) |
| BH other | 0.92 (0.67-1.27) | 0.69 (0.56-0.85) |
| ED or inpatient stay | ||
| No treatment | 1 [Reference] | 1 [Reference] |
| Inpatient detoxification or residential services | 1.05 (0.76-1.45) | 1.20 (0.96-1.50) |
| BH IOP | 0.84 (0.54-1.30) | 0.90 (0.67-1.20) |
| MOUD treatment with buprenorphine or methadone | 0.68 (0.47-0.99) | 0.74 (0.58-0.95) |
| MOUD treatment with naltrexone | 1.15 (0.69-1.92) | 1.07 (0.75-1.54) |
| BH other | 0.59 (0.44-0.80) | 0.60 (0.48-0.74) |
Abbreviations: BH IOP, intensive behavioral health (intensive outpatient or partial hospitalization); BH other, only nonintensive behavioral health (outpatient counseling); ED, emergency department; MOUD, medication for opioid use disorder.
The hazard ratios were adjusted for age, sex, race/ethnicity, insurance type, baseline medical (modified Elixhauser index score) and mental health comorbidities (depression, anxiety, posttraumatic stress disorder, and attention-deficit/hyperactivity disorder), evidence of overdose or infections related to intravenous drug use, and cost rank.
Figure 1. Probability of Opioid Overdose and Acute Care Use During the 3-Month Follow-up Period.
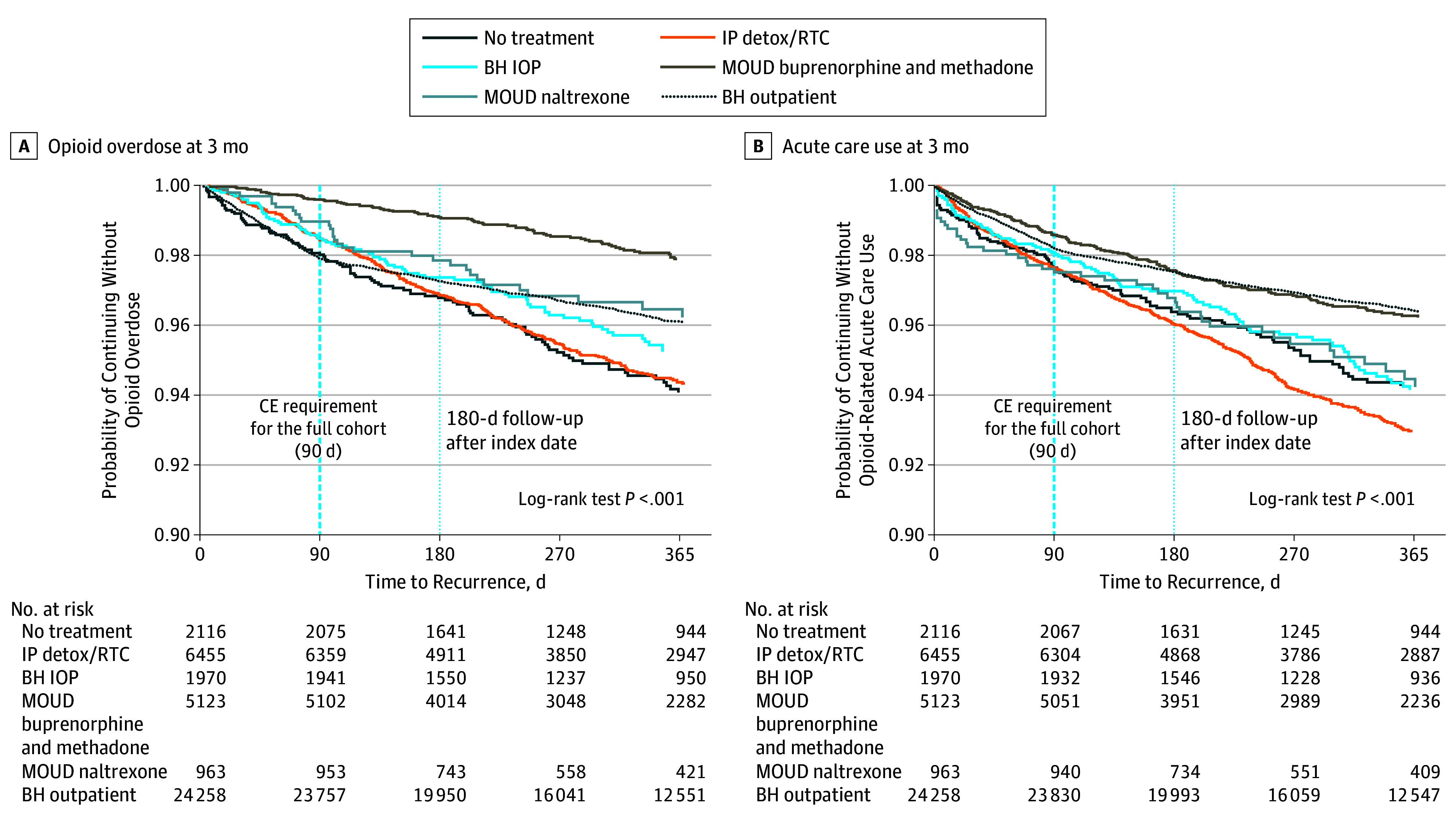
BH indicates behavioral health; CE, continuous enrollment; BH IOP, intensive behavioral health (intensive outpatient or partial hospitalization); IP detox/RTC, inpatient detoxification or residential services; and MOUD, medication for opioid use disorder.
MOUD treatment with buprenorphine or methadone was also protective against serious opioid-related acute care use during the 3-month follow-up period (AHR, 0.68; 95% CI, 0.47-0.99) (Table 2 and Figure 1B). Inpatient detoxification or residential services treatment, naltrexone, and intensive behavioral health services were not significantly associated with serious opioid-related acute care use during 3 months (inpatient detoxification or residential services: AHR, 1.05; 95% CI, 0.76-1.45; naltrexone: AHR, 1.15; 95% CI, 0.69-1.92; intensive behavioral health: AHR, 0.84; 95% CI, 0.54-1.30). Nonintensive behavioral health services were associated with a reduction in serious opioid-related acute care use (AHR, 0.59; 95% CI, 0.44-0.80). Receiving MOUD treatment with buprenorphine or methadone continued to be protective against overdose (AHR, 0.41; 95% CI, 0.31-0.55) and serious opioid-related acute care use (AHR, 0.74; 95% CI, 0.58-0.95) at 12 months.
Compared with MOUD treatment with buprenorphine or methadone, all treatment groups were more likely to have a posttreatment admission to inpatient detoxification. Patients who initiated treatment with inpatient detoxification or residential services were most likely to return within 3 months (AHR, 3.76; 95% CI, 2.98-4.74) and 12 months (AHR, 3.48; 95% CI, 3.02-4.01). However, treatment with naltrexone or intensive behavioral health services was also associated with a higher risk of subsequent detoxification admission during the 3-month (naltrexone: AHR, 2.64; 95% CI, 1.84-3.78; intensive behavioral health: AHR, 2.19; 95% CI, 1.63-2.96) and 12-month (naltrexone: AHR, 1.98; 95% CI, 1.55-2.52; intensive behavioral health: AHR, 2.08; 95% CI, 1.73-2.50) follow-up periods.
MOUD Treatment Duration
Treatment duration for MOUD was relatively short. During 12 months, the mean (SD) treatment duration for naltrexone was 74.41 (70.15) days and 149.65 (119.37) days for buprenorphine or methadone. Individuals who received longer-duration MOUD treatment with buprenorphine or methadone had lower rates of overdose (Figure 2A) or serious opioid-related acute care use (Figure 2B).
Figure 2. Probability of Opioid Overdose and Acute Care Use During the 12-Month Follow-up Period.
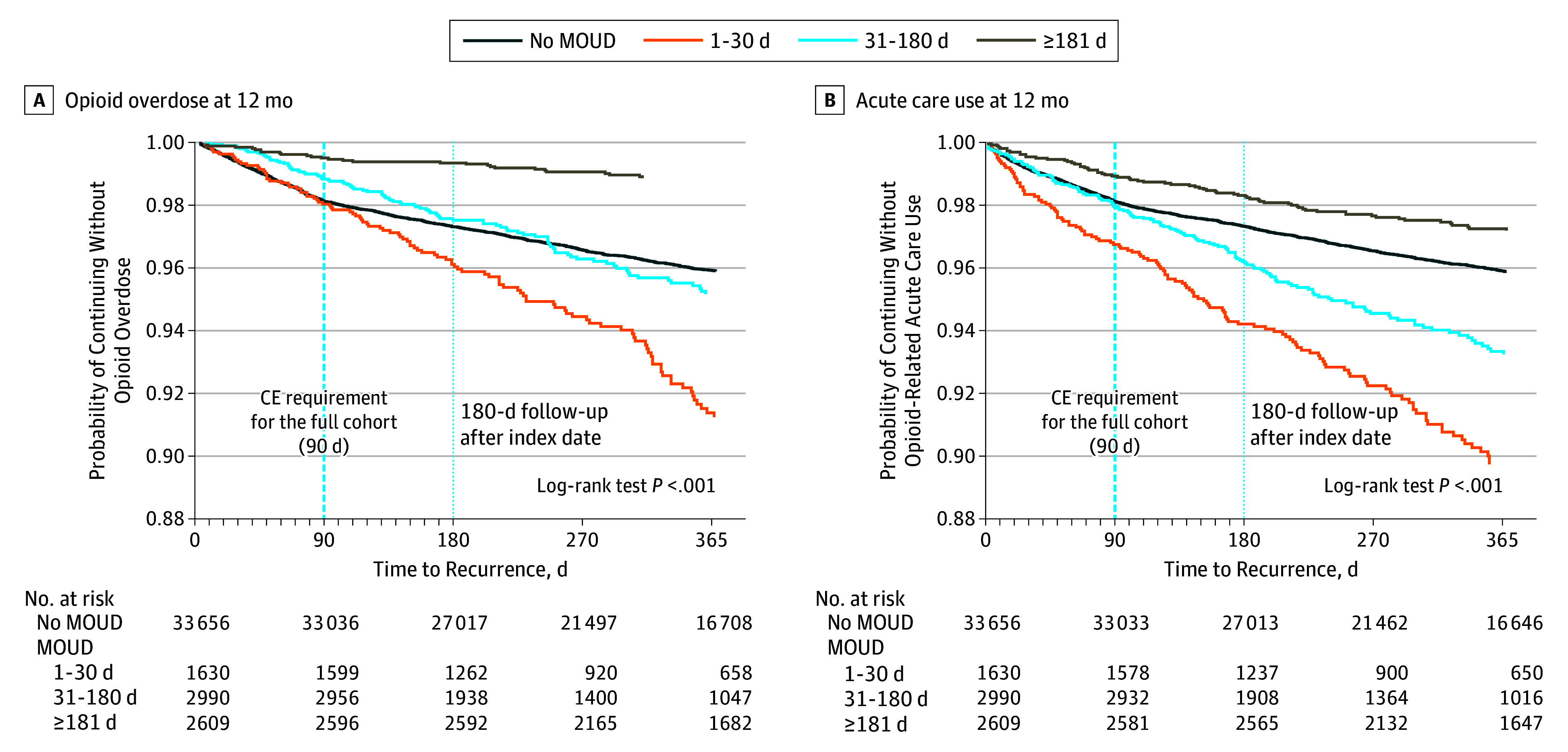
CE indicates continuous enrollment; MOUD, medication for opioid use disorder.
At the end of 12 months, 1198 (3.6%) of those who received no MOUD had an overdose, and 1204 (3.6%) had serious opioid-related acute care use; 105 (6.4%) of those who received MOUD treatment with buprenorphine or methadone for 1 to 30 days had an overdose, and 133 (8.2%) had serious opioid-related acute care use; 101 (3.4%) of those who received MOUD treatment with buprenorphine or methadone for 31 to 180 days had an overdose, and 148 (5.0%) had serious opioid-related acute care use; and 28 (1.1%) of those who received MOUD treatment with buprenorphine or methadone for more than 180 days had an overdose, and 69 (2.6%) had serious opioid-related acute care use.
Discussion
In a national cohort of 40 885 insured individuals between 2015 and 2017, MOUD treatment with buprenorphine or methadone was associated with a 76% reduction in overdose at 3 months and a 59% reduction in overdose at 12 months. To our knowledge, this was the largest cohort of commercially insured or MA individuals with OUD studied in a real-world environment with complete medical, pharmacy, and behavioral health administrative claims.
Treatment with buprenorphine or methadone was associated with a 32% relative rate of reduction in serious opioid-related acute care use at 3 months and a 26% relative rate of reduction at 12 months compared with no treatment. In contrast, detoxification, intensive behavioral health, and naltrexone treatment were not associated with reduced overdose or serious opioid-related acute care use at 3 or 12 months.
Despite the known benefit of MOUD treatment with buprenorphine or methadone, only 12.5% initiated these evidence-based treatments. Most individuals in this cohort initiated treatment with psychosocial services alone or inpatient detoxification, both of which are less effective than MOUD. It is possible that individuals accessed public sector treatments that were not captured in our data, particularly for methadone, which was not covered by Medicare and may not have been covered without co-payment for all commercial plans during this time. Low rates of MOUD use among an insured population highlight the need for strategies to improve access to and coverage for MOUD treatment.
Our results demonstrate the importance of treatment retention with MOUD. Individuals who received methadone or buprenorphine for longer than 6 months experienced fewer overdose events and serious opioid-related acute care use compared with those who received shorter durations of treatment or no treatment. These findings are consistent with prior research11,15,27,28,29 demonstrating high rates of recurrent opioid use if MOUD treatment is discontinued prematurely. Despite the benefit of MOUD in our study, treatment duration was relatively short. Given the chronic nature of OUD and the evidence that longer treatment duration may be associated with improved outcomes, patient-centered MOUD treatment models explicitly focused on engagement and retention are needed. Low-threshold treatment, which aims to reduce barriers to entry and is tailored to the needs of high-risk populations,30 may be a strategy to improve retention; however, to our knowledge, no rigorous studies have evaluated these models to date.31,32 In addition, patient-centered MOUD care, which allows participants to determine the services they need rather than requirements, such as mandatory counseling, are noninferior to traditional treatment.32
Numerous barriers limit sustained engagement in MOUD, including a lack of access to waivered practitioners, high co-payments, prior authorization requirements, and other restrictions on use. Previous studies33,34 have demonstrated that restrictions on use for MOUD are associated with limited access and harm. Addiction treatment programs in states that require Medicaid prior authorizations for buprenorphine are less likely to offer buprenorphine, and the more restrictions on use in state Medicaid programs, the fewer treatment programs that offer buprenorphine.33 Requiring prior authorization for higher doses of buprenorphine may also result in increased recurrence rates among patients.34 Our finding that MOUD treatment with buprenorphine or methadone was associated with lower overdose and serious opioid-related acute care use supports expanded coverage of these medications without restrictions on use.
Our findings are also consistent with analyses showing that MOUD treatment with buprenorphine or methadone is significantly associated with reduced overdose and recurrence of opioid use compared with no treatment or non-MOUD treatment. A previous cohort study15 of individuals in Massachusetts demonstrated a reduction in overdose-related mortality associated with treatment with buprenorphine (AHR, 0.62; 95% CI, 0.41-0.92) or methadone (AHR, 0.41; 95% CI, 0.24-0.70), results that are similar to our finding of an AHR of 0.41 (95% CI, 0.31-0.55) for overdose at 12 months for methadone or buprenorphine. A large meta-analysis11 examining mortality when individuals were in or out of treatment with buprenorphine or methadone similarly showed decreased overdose mortality during treatment. A study12 examining proxies for recurrent OUD among Massachusetts Medicaid enrollees found that treatment with buprenorphine or methadone was associated with lower recurrence rates and costs. No studies, to our knowledge, have examined the effect of different OUD treatment pathways on overdose and serious opioid-related acute care use among a national sample of commercially insured and MA enrollees.
Our finding that MOUD treatment with naltrexone was not protective against overdose or serious opioid-related acute care use is consistent with other studies15,35 that found naltrexone to be less effective than MOUD treatment with buprenorphine. The mean (SD) treatment duration for naltrexone in this cohort was longer than prior observational studies at 74.41 (70.15) days.
The findings that nonintensive behavioral health treatment was associated with a reduced risk of overdose at 12 months but not 3 months and a reduced risk of opioid-related acute care use was surprising. Although we attempted to control for differences among various treatment groups, individuals referred to nonintensive behavioral health may represent a less complex patient population than those who receive MOUD treatment or are referred to intensive behavioral health or inpatient treatment.
Strengths and Limitations
Specifically, we identified a research question a priori that was meaningful, had clinical and policy implications, and was concise and unambiguous. Our study design’s strengths are the large, nationally representative sample and complete claims data, which allowed us to adequately identify appropriate patients and interventions. In addition, we used a conservative definition of OUD and of proxies for OUD recurrence to limit inclusion of individuals who did not have OUD or of outcomes that did not represent clinically significant recurrence.
This study has limitations. The limitations of our study design include the lack of clinical information in claims data or outcomes that occurred outside a health care encounter (eg, fatal overdoses or active use without medical complication). As with any observational study, there is the possibility that unmeasured patient characteristics were associated with treatment assignment and outcomes, possibly biasing estimates of outcomes associated with MOUD treatment groups. It is also possible that individuals selected for different treatments differed by characteristics that were also associated with the outcomes. We were able to control for many patient characteristics, such as race/ethnicity, sex, insurance type, and comorbidities, but selection bias is possible. Another limitation is the degree of sample attrition during the 12-month follow-up period. However, we attempted to assess potential bias from informative censoring in 2 ways.36 First, we compared the baseline characteristics of censored and uncensored cases. These distributions were similar, suggesting that, at least on the basis of observable characteristics, censored cases were not statistically different from uncensored cases. Second, we examined the proportionality of HRs. Visual inspection of the HRs indicated that they were proportional for the 3-month period but could not be assumed to be proportional for the 12-month period. Another limitation is the risk of immortal time bias by requiring 3-month enrollment for inclusion; however, we believed it was important to require 3 months of follow-up to adequately measure outcomes. In addition, assessment of community mortality with claims data is characterized by high degrees of measurement error. Traditional instrumental variable methods for addressing immortal time bias cannot be applied to survival models because of their nonlinear functional form.
Conclusions
In a national sample of commercial insurance and MA enrollees with OUD, treatment with buprenorphine or methadone was associated with reductions in overdose and serious opioid-related acute care use, but only a few individuals were treated with these medications. These findings suggest that opportunities exist for health plans to reduce restrictions on use for MOUD and the need for treatment models that prioritize access to and retention of MOUD treatment.
eAppendix 1. Cohort Selection
eAppendix 2. Supplementary Methods
eFigure 1. Definition of OUD
eFigure 2. Cohort Inclusion and Timeline
eFigure 3. Alluvial Flow of OUD Treatment Pathways in the Initial Cohort
eTable. Censoring by Baseline Characteristics
References
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths–United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi: 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson WT, Kazbour C, Nassau T, et al. Brief report: nonfatal overdose events among persons who inject drugs: findings from seven national HIV behavioral surveillance cities 2009 & 2012. J Acquir Immune Defic Syndr. 2017;75(suppl 3):S341-S345. doi: 10.1097/QAI.0000000000001426 [DOI] [PubMed] [Google Scholar]
- 3.Burnett JC, Broz D, Spiller MW, Wejnert C, Paz-Bailey G. HIV infection and HIV-associated behaviors among persons who inject drugs—20 cities, United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):23-28. doi: 10.15585/mmwr.mm6701a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu DJ, McCarthy EP, Stevens JP, Mukamal KJ. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001-12. Addiction. 2017;112(9):1558-1564. doi: 10.1111/add.13795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175-181. doi: 10.2105/AJPH.2017.304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cranston K, Alpren C, John B, et al. ; Amy Board . Notes from the field: HIV diagnoses among persons who inject drugs–Northeastern Massachusetts, 2015-2018. MMWR Morb Mortal Wkly Rep. 2019;68(10):253-254. doi: 10.15585/mmwr.mm6810a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir MA, Slater J, Jandoc R, Koivu S, Garg AX, Silverman M. The risk of infective endocarditis among people who inject drugs: a retrospective, population-based time series analysis. CMAJ. 2019;191(4):E93-E99. doi: 10.1503/cmaj.180694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. 2019;2(2):e187621. doi: 10.1001/jamanetworkopen.2018.7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019;71:183-188. doi: 10.1016/j.drugpo.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The National Academies of Science, Engineering, and Medicine. Medications for opioid use disorder save lives. March 20, 2019. http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 26, 2019. [PubMed]
- 11.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, Barton BA. Risk factors for relapse and higher costs among Medicaid members with opioid dependence or abuse: opioid agonists, comorbidities, and treatment history. J Subst Abuse Treat. 2015;57:75-80. doi: 10.1016/j.jsat.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hser YI, Evans E, Huang D, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111(4):695-705. doi: 10.1111/add.13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss RD, Potter JS, Griffin ML, et al. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug Alcohol Depend. 2015;150:112-119. doi: 10.1016/j.drugalcdep.2015.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi: 10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham AJ, Adams GB, Bradford AC, Bradford WD. County-level access to opioid use disorder medications in Medicare Part D (2010-2015). Health Serv Res. 2019;54(2):390-398. doi: 10.1111/1475-6773.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Health. 2019;35(1):108-112. doi: 10.1111/jrh.12307 [DOI] [PubMed] [Google Scholar]
- 18.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication treatment for opioid use disorders in substance use treatment facilities. Health Aff (Millwood). 2019;38(1):14-23. doi: 10.1377/hlthaff.2018.05162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-e63. doi: 10.2105/AJPH.2015.302664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001-2014. JAMA Pediatr. 2017;171(8):747-755. doi: 10.1001/jamapediatrics.2017.0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90-96. doi: 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wollschlaeger BA, Willson TM, Montejano LB, Ronquest NA, Nadipelli VR. Characteristics and treatment patterns of US commercially insured and Medicaid patients with opioid dependence or abuse. J Opioid Manag. 2017;13(4):207-220. doi: 10.5055/jom.2017.0389 [DOI] [PubMed] [Google Scholar]
- 23.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34-39. doi: 10.1016/j.drugalcdep.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.OptumLabs . OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Cambridge, MA: OptumLabs; May 2019.
- 25.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report, part I. Value Health. 2009;12(8):1044-1052. doi: 10.1111/j.1524-4733.2009.00600.x [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 27.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303-1310. doi: 10.1001/jama.283.10.1303 [DOI] [PubMed] [Google Scholar]
- 28.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238-1246. doi: 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O’Connor PG. Primary care–based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(12):1947-1954. doi: 10.1001/jamainternmed.2014.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mofizul Islam M, Topp L, Conigrave KM, Day CA. Defining a service for people who use drugs as ‘low-threshold’: what should be the criteria? Int J Drug Policy. 2013;24(3):220-222. doi: 10.1016/j.drugpo.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 31.Edland-Gryt M, Skatvedt AH. Thresholds in a low-threshold setting: an empirical study of barriers in a centre for people with drug problems and mental health disorders. Int J Drug Policy. 2013;24(3):257-264. doi: 10.1016/j.drugpo.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz RP, Kelly SM, Mitchell SG, et al. Patient-centered methadone treatment: a randomized clinical trial. Addiction. 2017;112(3):454-464. doi: 10.1111/add.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews CM, Abraham AJ, Grogan CM, Westlake MA, Pollack HA, Friedmann PD. Impact of Medicaid restrictions on availability of buprenorphine in addiction treatment programs. Am J Public Health. 2019;109(3):434-436. doi: 10.2105/AJPH.2018.304856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark RE, Baxter JD, Barton BA, Aweh G, O’Connell E, Fisher WH. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence. Health Serv Res. 2014;49(6):1964-1979. doi: 10.1111/1475-6773.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siannis F, Copas J, Lu G. Sensitivity analysis for informative censoring in parametric survival models. Biostatistics. 2005;6(1):77-91. doi: 10.1093/biostatistics/kxh019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Cohort Selection
eAppendix 2. Supplementary Methods
eFigure 1. Definition of OUD
eFigure 2. Cohort Inclusion and Timeline
eFigure 3. Alluvial Flow of OUD Treatment Pathways in the Initial Cohort
eTable. Censoring by Baseline Characteristics


