Abstract
Objective
The primary objective of the study was to investigate the correlation between high-sensitivity troponin I (hsTropI) levels during hospitalization and the prognostic outcome in patients with non-acute coronary syndrome (non-ACS) acute heart failure, over a follow-up period of one year. The secondary objective was to assess and characterize acute heart failure during index hospitalization.
Methods
High sensitivity troponin I value was noted both at the time of admission and discharge. The correlation of admission hsTropI along with other parameters and risk factors with in-hospital mortality was studied. Patients of index hospitalization after discharge were followed up for one year and the composite endpoint of cardiovascular death or re-hospitalization for heart failure was noted. The correlation between admission and discharge hsTropI values with the composite endpoint was then analyzed.
Results
Out of 350 patients, 38 (10.8 %) patients died during index hospitalization while 142 patients (46 %) developed composite outcomes during follow-up. Age, previous history of heart failure, atrial fibrillation, low left ventricular ejection fraction, systolic blood pressure, and high values of hsTropI above 99th percentiles were independent in-hospital mortality predictors. The value of hsTropI at the time of admission was not associated with poor composite outcome during follow-up. However, patients who showed an increasing trend of hsTropI value at the time of discharge were found to have a significant increase in the composite outcome.
Conclusion
High-sensitivity troponin I is a valuable biomarker that can predict in-hospital mortality and long-term follow-up outcomes in patients with acute heart failure. It plays a crucial role in developing improved strategies for heart failure surveillance and management in the community.
Keywords: Acute heart failure, Cardiac death, High sensitivity troponin I, Non-ACS, Re-hospitalization
Graphical abstract
1. Introduction
Heart failure (HF) is defined as the pathophysiological state in which an abnormality of cardiac function is responsible for the failure of the heart to pump blood to meet the requirements of the metabolizing tissues.1 The incidence of acute heart failure (AHF) requiring hospital admission is increasing constantly. Studies are now focusing on AHF due to its association with frequent hospitalization and increased healthcare expenses. The most notable among them are the ADHERE2 and OPTIMIZE-HF3 studies. However, only a few studies have investigated the causes, triggering factors, and prognostic significance of cardiac biomarkers such as high-sensitivity cardiac troponin I (hsTropI) in Indian patients outside the context of acute coronary syndrome (non-ACS) situations.
Cardiac troponins are structural proteins in the contractile apparatus of the myocyte that are released in blood circulation upon myocardial damage like infarction. Earlier assays that were used for measuring troponin levels were considered as a dichotomous test, but the advent of hsTropI assays in recent years which can detect cardiac troponin levels a hundred times lower than those detected by traditional methods has changed this paradigm. Hence hsTropI assay is now considered as a quantitative measure of cardiac myocyte injury not only in the setting of myocardial infarction but also in other conditions of myocardial stress such as heart failure.4 However, there is a lack of information regarding the importance of hsTropI in acute heart failure situations that are non-ACS, and there is little data on its dynamic values measured both at the time of admission and discharge. Hence, this study focused on investigating the predictive effect of high-sensitivity troponin I level by determining the likelihood of cardiovascular mortality and re-hospitalization for HF during one year of follow-up in patients who were admitted with non-ACS acute heart failure. In addition, the study also assessed and characterized acute heart failure during the index hospitalization.
2. Materials and methods
This was a single-center prospective observational study with one year of follow-up conducted in the department of cardiology of a government tertiary care center.
2.1. Inclusion criteria
2.2. Exclusion criteria
-
a)
Acute stroke
-
b)
End-stage CKD (eGFR<15 ml/min/1.73m2) or patients on hemodialysis
-
c)
Acute coronary syndrome (on a clinical and ECG basis)
-
d)
Active malignancy & ongoing chemotherapy
-
e)
Acute liver injury and sepsis
-
f)
Patients lost to follow-up or their outcome could not be determined due to lack of data.
-
g)
Patients who underwent cardiac surgeries or procedures (Ablation, cardioversion, percutaneous intervention) during the index hospitalization.
2.3. Definitions
2.3.1. Composite endpoints
The composite endpoints of the study were cardiovascular death and rehospitalization for heart failure during one-year follow-up. Death during follow-up was classified as cardiovascular (CV) or non-CV based on the primary cause of death. The primary cause was defined as the underlying disease or injury that initiated the train of events resulting in death. CV deaths include deaths that result from an acute myocardial infarction, sudden cardiac death, death due to heart failure (HF), death due to stroke, death due to CV procedures, death due to CV hemorrhage, and death due to other CV causes.7 Re-hospitalization for HF was considered when a patient was required to have an unscheduled hospital admission for a primary diagnosis of HF with a length of stay that either exceeds 24 h or crosses a calendar day. In addition to HF signs and symptoms, the patient should also receive treatment specifically directed at HF, including at least one of the following: 1) significant augmentation in oral diuretic therapy; 2) initiation of intravenous diuretic (even a single dose) or vasoactive agent (vasodilator, vasopressor, or inotropic therapy); or 3) mechanical circulatory support or fluid removal.7
2.3.2. Acute coronary syndrome
Diagnosis of ACS was mainly based on clinical presentation along with various supportive findings. First, the symptoms of patients admitted with AHF were screened for the presence of typical or atypical chest pain. Second ECG findings of significant ST-T changes (depression, elevation, or new onset dynamic changes) and echocardiographic findings of regional wall motion abnormality were noted for any significant variations suggesting ACS. Third, the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) principal or secondary diagnosis codes on discharge tickets or death certificates at the time of index hospitalization were noted.8 ACS-related codes such as unstable angina (I20.0), acute myocardial infarction (AMI) (I21. x), subsequent ST myocardial infarction (I22. x), and certain current complications following myocardial infarction (I23. x) and other acute ischemic heart diseases (I24. x) were excluded.
2.4. Data acquisition
The study was prior approved by the institutional ethical committee. Written consent was obtained from each patient. Data were collected using a pretested proforma meeting the objectives of the study. Hematological and biochemical tests along with hsTropI and other relevant investigations including echocardiography were performed. The values of hsTropI were measured twice during index hospitalization, one at the time of admission and another on the day of discharge. This was performed in a National Accreditation Board for Hospitals & Healthcare Providers (NABH) accredited lab and the same lab was used throughout the study. The lab was located on-site and was running in the Public-Private Partnership (PPP) model. The test was performed using an ELFA (enzyme-linked fluorescent assay) measurement procedure in a Vidas 3 analyzer (BioMérieux, 4th generation).9 Vidas troponin I is a quantitative test using a one-step immuno-enzymatic sandwich method and has very good analytical performance and precision and it is per the international standardized protocols. The 99th percentile of a presumably healthy population is defined at 19 ng/L. The coefficient of variation (CV, total imprecision at the 99th percentile) is equal to 7 % (recommended <10 %).9 This technology is FDA-approved and measures minimal changes between serial troponin I values.
2.5. Follow-up
Specialized medical and nursing personnel took care of the patients during their stay in the hospital and the same protocols of treatment were usually applied. Patients were followed every two months for one year for outcomes in terms of mortality and rehospitalization. Follow-up was done both via direct visit of the patient and telephonic interview. Regular telephonic interviews were conducted with patients and their relatives regarding the patient's functional status and drug compliance. In case it was informed that the primary outcome (death or rehospitalization) has happened outside at some other hospital, a review of documents was done either on the next visit or in some cases, via digital (messaging applications, e-mail).
2.6. Statistical analysis
Pair-wise comparison between various variables was done for different parameters using chi-square and student's t-test. The Range, Mean value, Standard Deviation (S.D.), Standard error of Mean, ‘t’ value, and ‘p’ values were calculated as per the applicability by using appropriate formulas. p-value <0.05 was regarded as statistically significant. Statistical Package of Social Sciences (SPSS) v. 27 was used for data entry and data analysis.
3. Results
Various demographic, clinical, and laboratory characteristics of the study population have been shown in [Table 1].
Table 1.
Demographic, clinical, and laboratory characteristics of the study population.
| VARIABLE | N (%) or MEAN ± SD |
|---|---|
| Age (years) | 60.3 ± 13.5 |
| Age categories | |
| <30 years | 5 (2 %) |
| 30–44 years | 41 (12 %) |
| 45–59 years | 109 (31 %) |
| 60–74 years | 134 (38 %) |
| ≥75 years | 61 (17 %) |
| Sex | |
| Male | 194 (55.4 %) |
| Female | 156 (44.6 %) |
| Risk Factors | |
| Diabetes | 103 (29.4 %) |
| Hypertension | 182 (52.0 %) |
| Dyslipidaemia | 142 (40.6 %) |
| Chronic kidney disease | 95 (27.1 %) |
| Coronary artery disease | 141 (40.3 %) |
| Smoking | 168 (48.0 %) |
| Previous Heart failure | 219 (62.6 %) |
| Atrial fibrillation | 101 (29.1 %) |
| Etiology | |
| PPCM | 11 (3.1 %) |
| CMP (systemic) | 64 (18.3 %) |
| CHD | 22 (6.3 %) |
| HCM | 10 (2.9 %) |
| RHD | 52 (14.9 %) |
| CMP (idiopathic) | 35 (10.0 %) |
| Ischemic | 140 (40.0 %) |
| Others | 16 (4.6 %) |
| SBP (mmHg) | 113 ± 28.54 |
| SBP <100 | 176 (50.3 %) |
| SBP 100-140 | 124 (35.4 %) |
| SBP >140 | 50 (14.3 %) |
| Serum creatinine (mg/dL) | 1.42 ± 0.908 |
| Serum sodium (mEq/L) | 139.7 ± 4.24 |
| Serum potassium (mEq/L) | 4.31 ± 0.61 |
| Hemoglobin (gm/dl) | 11.7 ± 2.26 |
| LVEF (%) | 41.72 ± 12.31 |
| LVEF <40 | 198 (56.6 %) |
| LVEF 40-50 | 72 (20.6 %) |
| LVEF >50 | 80 (22.8 %) |
| hsTropI at admission (ng/L) | 59.38 ± 80.63 |
| hsTropI at discharge (ng/L) | 40.14 ± 42.50 |
| Length of hospitalization (days) | 7.2 ± 4.01 |
Abbreviations: PPCM-Peripartum cardiomyopathy; CMP-Cardiomyopathy; CHD-Congenital heart disease; HCM-Hypertrophic cardiomyopathy; RHD-Rheumatic heart disease; SBP-Systolic blood pressure; LVEF-Left ventricular ejection fraction.
3.1. In - hospital outcome
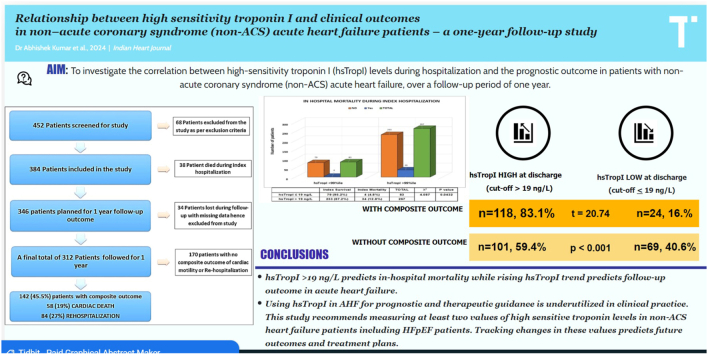
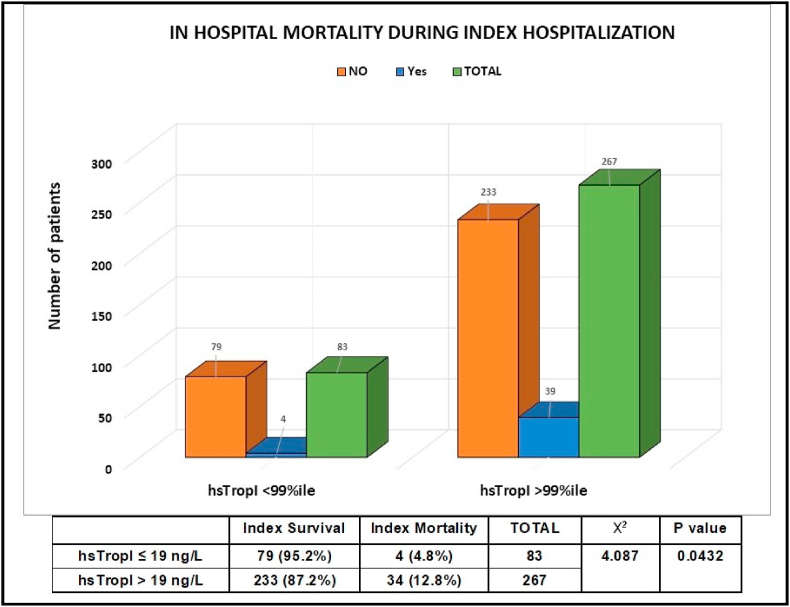
In this study, 38 (10.9 %) patients died in the index hospitalization while 312 (89.1 %) patients were discharged and were followed up for subsequent readmission and cardiac mortality [Fig. 1]. There was an increased in-hospital mortality noted during index hospitalization in the patients who had elevated hsTropI levels of >99th percentile during admission (p = 0.04) [Fig. 2]. Also, the mean value of hsTropI in the mortality group of patients was 102.70 ng/L (SD of 86.62, total number 38) as compared to 54.09 ng/L (SD of 49.80, total number 312) in the surviving patient group with a p-value of 0.001 which was statistically significant.
Fig. 1.
Flow chart and composite outcome of cardiac mortality and re-hospitalization.
Fig. 2.
Correlation between high sensitivity troponin I (hsTropI) values with index mortality.
3.2. Follow–up outcome
A total of 142 patients (46 %) developed composite endpoints of cardiac mortality (19 %) or rehospitalization (27 %) [Fig. 1]. The correlation between various demographic and clinical data with outcomes is shown in [Table 2]. Age, coronary artery disease (CAD), Hypertension, previous heart failure, atrial fibrillation, low systolic blood pressure (SBP), reduced left ventricular ejection fraction (LVEF), and prolonged duration of hospital stay were found to have a strong correlation with poor outcome. Patients of heart failure with preserved ejection fraction (HFpEF) (LVEF >50 %) were categorized into two groups based upon hsTropI levels at the time of admission (cut off based upon 99th percentile). 35 out of 58 (60.3 %) patients with elevated hsTropI values developed composite outcomes as compared to only 4 out of 17 (23.5 %) with normal hsTropI values. On comparison, the chi-square statistic was 7.138 with a p-value of 0.007 which was statistically very significant.
Table 2.
Comparison of various background clinical variables with the composite endpoint of cardiac mortality or rehospitalization.
| Composite Outcome (CV Death & Rehospitalization) |
X2 | p Value | |||
|---|---|---|---|---|---|
| Absent | Present | ||||
| AGE (YEARS) | <30 | 4 | 1 | 14.682 | 0.0054 |
| 30–44 | 25 | 16 | |||
| 45–59 | 61 | 37 | |||
| 60–74 | 61 | 50 | |||
| ≥75 | 19 | 38 | |||
| SEX | Male | 93 | 75 | 0.111 | 0.7393 |
| Female | 77 | 67 | |||
| CAD | Absent | 115 | 72 | 9.220 | 0.0024 |
| Present | 55 | 70 | |||
| DM | Absent | 128 | 99 | 1.210 | 0.2714 |
| Present | 42 | 43 | |||
| HTN | Absent | 99 | 61 | 7.228 | 0.0071 |
| Present | 71 | 81 | |||
| DLP | Absent | 110 | 79 | 2.658 | 0.1030 |
| Present | 60 | 63 | |||
| CKD | Absent | 133 | 104 | 1.054 | 0.3046 |
| Present | 37 | 38 | |||
| SMOKING | Absent | 98 | 73 | 1.212 | 0.2710 |
| Present | 72 | 69 | |||
| PREVIOUS HF | Absent | 97 | 29 | 42.997 | <0.001 |
| Present | 73 | 113 | |||
| AF | Absent | 128 | 83 | 10.026 | 0.0015 |
| Present | 42 | 59 | |||
| SBP CATEGORY (mmHg) | <100 | 64 | 82 | 14.269 | 0.0008 |
| 100–140 | 81 | 40 | |||
| >140 | 25 | 20 | |||
| LVEF CATEGORY (%) | <40 | 81 | 89 | 9.888 | 0.0071 |
| 40–50 | 37 | 30 | |||
| >50 | 52 | 23 | |||
| LENGTH OF HOSPITALIZATION | <7 DAYS | 102 | 56 | 13.047 | 0.0003 |
| ≥7 DAYS | 68 | 86 | |||
Abbreviations: CAD-Coronary artery disease; CV-Cardiovascular, DM-Diabetes mellitus; HTN-Hypertension; DLP-Dyslipidaemia; CKD-Chronic kidney disease; AF-atrial fibrillation; SBP-Systolic blood pressure; LVEF-Left ventricular ejection fraction.
Correlation between elevated levels of hsTropI (cut off based upon 99th percentile) during admission and at the time of discharge with various outcomes is shown in [Table 3]. Only elevated levels of hsTropI during discharge were significant for determining outcome. Similarly, on comparing the mean values, it was found that the value of hsTropI during admission does not significantly correlate with the future outcome (p = 0.0549). On the other hand, repeat hsTropI value performed at the time of discharge, had a statistically significant difference (p = 0.0002) in predicting poor composite outcome [Table 4]. High sensitivity troponin I levels were categorized into three categories based on the relative change in values as compared to admission levels. Those categories were – an increasing group with a change of value of more than +10 %, a static group with a change of value between −10 and +10 %, and a decreasing group with a change of value of more than −10 %. Most of the patients showing an increased trend in hsTropI value were found to develop various endpoints in a one-year follow-up [Table 3]. The trend as mentioned above of hsTropI was also documented for patients of HFpEF. Similar to other clinical subsets, an increasing trend of hsTropI values during the due course of hospitalization in patients of HFpEF was found to be a statistically significant predictor of poor outcomes. (The chi-square statistic was 11.105 with a p-value of 0.004)
Table 3.
Correlation between hsTropI values with various composite endpoint.
| hsTropI Values | CV Death |
X2 | p-Value | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| hsTropI (Admission) | ≤19 ng/L | 68 | 11 | 1.522 | 0.2170 |
| >19 ng/L | 186 | 47 | |||
| hsTropI (Discharge) | ≤19 ng/L | 83 | 10 | 5.377 | 0.021 |
| >19 ng/L | 171 | 48 | |||
| Change in hsTropI value | > −10 % | 130 | 17 | 10.648 | 0.0049 |
| −10 to +10 % | 42 | 10 | |||
| > +10 % | 82 | 31 | |||
|
HF Rehospitalization |
X2 | p-Value | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| hsTropI (Admission) | ≤19 ng/L | 59 | 20 | 0.139 | 0.709 |
| >19 ng/L | 169 | 64 | |||
| hsTropI (Discharge) | ≤19 ng/L | 79 | 14 | 9.487 | 0.002 |
| >19 ng/L | 149 | 70 | |||
| Change in hsTropI value | > −10 % | 120 | 27 | 13.858 | 0.001 |
| −10 to +10 % | 39 | 13 | |||
| > +10 % | 69 | 44 | |||
|
Composite (combined) Outcome |
X2 | p-Value | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| hsTropI (Admission) | ≤19 ng/L | 48 | 31 | 1.678 | 0.195 |
| >19 ng/L | 122 | 111 | |||
| hsTropI (Discharge) | ≤19 ng/L | 69 | 24 | 20.748 | <0.001 |
| >19 ng/L | 101 | 118 | |||
| Change in hsTropI value | > −10 % | 103 | 44 | 34.251 | <0.001 |
| −10 to +10 % | 29 | 23 | |||
| > +10 % | 38 | 74 | |||
Table 4.
Correlation between absolute hsTropI values with the composite endpoint.
| hsTropI value | Without composite outcome |
With composite outcome |
t-score | p value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Number | Mean | SD | Number | |||
| During Admission | 51.50 | 32.62 | 170 | 57.19 | 29.82 | 142 | 1.611 | 0.0549 |
| During Discharge | 34.45 | 29.65 | 170 | 46.94 | 37.68 | 142 | 3.503 | 0.0002 |
4. Discussion
4.1. In-hospital mortality during the index hospitalization
Old age was a significant factor in predicting poor outcomes in patients with heart failure during index hospitalization. A similar finding was seen in one of the recently published works by Venkatesh Munusamy et al.10 This strong association is not only because of aging alone but also due to the prolonged period of existence of untreated risk factors. There was no statistically significant difference in the outcome in male and female subgroups. Although a recent large rural population-based study conducted in north India, INDUS (INDia Ukieri Study) study showed rheumatic heart disease (RHD) as the most common cause of heart failure, this present study showed ischemic cardiomyopathy to be more common than RHD.11 This finding was consistent with the recent study performed by Sanjay G. et al which showed that the most common HF etiologies in Trivandrum Heart Failure Registry to be ischemic heart disease (72 %) followed by dilated cardiomyopathy.12 This finding related to etiology in this present study could be explained by the declining rate of RHD across the country and a significant increase in the burden of ischemic heart disease in India. The length of hospitalization in the present study was higher than those seen in the ADHERE2 and OPTIMIZE-HF3 studies done in developed countries and it was found to be an independent indicator of poor outcome.
It was also noted that patients who had higher levels of hsTropI during admission had longer stays and those who had a longer duration of stay also showed static or increase in the hsTropI levels during discharge. Hence there was a strong and significant correlation between hsTropI levels, duration of hospital stays, and composite outcome. This present study has also shown that patients who are admitted with a hsTropI value more than the baseline cut-off of the 99th percentile have a higher chance of in-hospital mortality during the index hospitalization. Similar findings were seen in Evans et al13 and Felker et al14 studies where they found a significant correlation between hsTropI level and cardiac mortality. There was no significant difference in in-hospital mortality when the ischemic and non-ischemic subgroups were compared.
4.2. Composite outcome
A total of 142 patients (46 %) developed composite endpoints. Cardiovascular mortality at one-year follow-up was 19 % while rehospitalization for HF was 27 %. Mortality and rehospitalization rate in the present study is similar to other studies recently published in India but it seems to be higher when compared to international data probably due to poor compliance by the patient along with a late presentation for treatment.10,12
The combined outcome was seen maximum in patients aged more than 60 years. This finding is attributed to a shorter life expectancy, poor compliance or side effects of the medications, and the presence of comorbidities that accelerate the progression of the disease. There was no significant difference in composite outcomes based on sex. Patients with CAD and hypertension had increased and statistically significant composite outcomes (p < 0.05). History of previous decompensated heart failure and the presence of atrial fibrillation were also very significant in predicting the outcome with p < 0.001. ACC/AHA guidelines have reported the paradigm that each decompensation episode additively and negatively affects the long-term prognosis. Patients with low SBP, low LVEF, and longer duration of hospital stay have a higher risk of rehospitalization and death during follow-up. This risk is similar to what was seen in the large cohort OPTIMIZE-HF study by Gheorghiade et al.15 In the subgroup of patients with HFpEF, high values of hsTropI (cut off >99th percentile of normal population) during admission and progressive increase of same at later stages was found to be a poor predictor of outcome in this study. This finding of elevated troponin in HFpEF is suggestive of ongoing subclinical myocardial injury that may explain the high morbidity and mortality associated with HFpEF. Similar findings were seen by Watson et al in their study related to biomarker profile in HFpEF.16
Many of the patients who were discharged after index hospitalization had elevated hsTropI levels at the time of admission above the baseline cut-off of the 99th percentile i.e. 233 out of 312 patients (74.6 %). On statistical analysis, there was no significant difference in the composite outcome based on this cut-off level. Also, the absolute value of hsTropI at the time of admission (mean 51.5 ng/L) did not show any independent increased risk of poor outcome. This finding of the present study seems to differ from the findings of Egger KM et al17 and Yan et al18 in which it was seen that hsTropI at the time of hospitalization was associated with poor clinical outcome, but there was little information on cardiac troponin levels measured at the time of discharge or about changes over time in cardiac troponin levels in above mentioned studies. High sensitivity troponin I levels measured at the time of discharge in this present study were categorized into three categories based upon the relative change in values as compared to admission levels (increasing group, static group, decreasing group). Most of the patients showing an increased trend in hsTropI value were found to develop composite endpoints in a 1-year follow-up (p < 0.001). Takashio et al are among the few studies in which serial changes in cardiac troponin levels using conventional or high-sensitivity assays during ADHF management were done.19 They also concluded that increased cardiac troponin level was associated with poor clinical outcomes, similar to the present study results. Hence increase in hsTropI levels during hospitalization was a significant independent risk predictor of poor outcome in patients with acute heart failure in terms of cardiac mortality and re-hospitalization.
5. Limitations
As this study was conducted in a single center, there is a possibility of referral bias. Additionally, there are known differences in high-sensitivity troponin I measurements between different vendors. Therefore, caution should be exercised when generalizing the results of this study to other vendors. Despite these limitations, the study is believed to reflect real-world practices accurately and highlights the clinical feasibility and usefulness of high-sensitivity troponin I measurement in non-ACS heart failure conditions.
6. Conclusion
The use of high-sensitivity troponin I is still mostly limited to clinical scenarios related to the diagnosis of suspected ACS. In this study, hsTropI >19 ng/L predicts in-hospital mortality, while rising hsTropI trend predicts follow-up outcome in acute heart failure. Therefore, it is suggested to measure highly sensitive troponin levels in all non-ACS heart failure patients at admission and discharge. Tracking changes in these values predicts future outcomes and guides treatment plans improving the quality of life as well as health economics.
What is already known?
➢Troponin assays have become more sensitive by more than a hundred times in the last decade. Troponin's relation to heart failure is now well understood. Nonetheless, high-sensitivity troponin is still mainly used for early detection of acute coronary syndrome in clinical practice.
What does this study add?
-
➢
The cut-off value of hsTropI > 19ng/L independent predictor of mortality during index hospitalization in both the ischemic and nonischemic acute heart failure patients, and a rising trend of hsTropI values noted at the time of discharge has a strong independent predictive value of future outcome in acute heart failure.
-
➢
This study recommends measuring at least two values of high sensitivity troponin I levels in non-ACS heart failure patients including HFpEF patients. Tracking the dynamics of these values helps in predicting future outcomes and formulating better treatment plans.
Financial support and sponsorship
Nil.
Ethical statement
All procedures performed in studies involving human participants were per the ethical standards of the institutional and/or national research committee.
Patient's consent
All patient's consent was taken for this research work and the identities of the patients have not been disclosed.
Prior publication
None.
Support
Nil.
Permissions
Nil.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Denolin H., Kuhn H., Krayenbuehl H.P., et al. The definition of heart failure. Eur Heart J. 1983 Jul;4(7):445–448. doi: 10.1093/oxfordjournals.eurheartj.a061500. PMID: 6628420. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow G.C., Adams K.F., Jr., Abraham W.T., et al. ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005 Feb 2;293(5):572–580. doi: 10.1001/jama.293.5.572. PMID: 15687312. [DOI] [PubMed] [Google Scholar]
- 3.Abraham W.T., Fonarow G.C., Albert N.M., et al. OPTIMIZE-HF investigators and coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008 Jul 29;52(5):347–356. doi: 10.1016/j.jacc.2008.04.028. PMID: 18652942. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy C.P., Raber I., Chapman A.R., et al. Myocardial injury in the era of high-sensitivity cardiac troponin assays: a practical approach for clinicians. JAMA Cardiol. 2019;4(10):1034–1042. doi: 10.1001/jamacardio.2019.2724. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood S.S., Wang T.J. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob Heart. 2013 Mar 1;8(1):77–82. doi: 10.1016/j.gheart.2012.12.006. PMID: 23998000; PMCID: PMC3756692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonagh T.A., Metra M., Adamo M., et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. Erratum in: Eur Heart J. 2021 Oct 14;: PMID: 34447992. [DOI] [PubMed] [Google Scholar]
- 7.Hicks K.A., Mahaffey K.W., Mehran R., et al. Standardized data collection for cardiovascular trials initiative (SCTI). 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018 Feb 27;137(9):961–972. doi: 10.1161/CIRCULATIONAHA.117.033502. PMID: 29483172. [DOI] [PubMed] [Google Scholar]
- 8.Kusnoor S.V., Blasingame M.N., Williams A.M., et al. A narrative review of the impact of the transition to ICD-10 and ICD-10-CM/PCS. JAMIA Open. 2019 Dec 26;3(1):126–131. doi: 10.1093/jamiaopen/ooz066. PMID: 32607494; PMCID: PMC7309233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masotti S., Prontera C., Musetti V., et al. Evaluation of analytical performance of a new high-sensitivity immunoassay for cardiac troponin I. Clin Chem Lab Med. 2018;56(3):492–501. doi: 10.1515/cclm-2017-0387. [DOI] [PubMed] [Google Scholar]
- 10.Munusamy V., Goenka L., Sharma M., et al. Clinical presentation and 2-year mortality outcomes in acute heart failure in a tertiary care hospital in South India: a retrospective cohort study. Journal of Clinical and Preventive Cardiology. 2019 Apr 1;8(2):56. [Google Scholar]
- 11.Chaturvedi V., Parakh N., Seth S., et al. Heart failure in India: the INDUS (India Ukieri study) study. Journal of the Practice of Cardiovascular Sciences. 2016 Jan 1;2(1):28. [Google Scholar]
- 12.Sanjay G., Jeemon P., Agarwal A., et al. In-hospital and three-year outcomes of heart failure patients in south India: the Trivandrum heart failure Registry. J Card Fail. 2018 Dec;24(12):842–848. doi: 10.1016/j.cardfail.2018.05.007. Epub 2018 Jun 7. PMID: 29885494; PMCID: PMC7263011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans J.D.W., Dobbin S.J.H., Pettit S.J., et al. High-sensitivity cardiac troponin and new-onset heart failure: a systematic review and meta-analysis of 67,063 patients with 4,165 incident heart failure events. JACC Heart Fail. 2018 Mar;6(3):187–197. doi: 10.1016/j.jchf.2017.11.003. Epub 2018 Jan 10. PMID: 29331272. [DOI] [PubMed] [Google Scholar]
- 14.Felker G.M., Mentz R.J., Teerlink J.R., et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015 Dec;17(12):1262–1270. doi: 10.1002/ejhf.341. Epub 2015 Sep 3. PMID: 26333655. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M., Abraham W.T., Albert N.M., et al. OPTIMIZE-HF Investigators and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006 Nov 8;296(18):2217–2226. doi: 10.1001/jama.296.18.2217. PMID: 17090768. [DOI] [PubMed] [Google Scholar]
- 16.Watson C.J., Gallagher J., Wilkinson M., et al. Biomarker profiling for risk of future heart failure (HFpEF) development. J Transl Med. 2021 Feb 9;19(1):61. doi: 10.1186/s12967-021-02735-3. PMID: 33563287; PMCID: PMC7871401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggers K.M., Jernberg T., Lindahl B. Cardiac troponin elevation in patients without a specific diagnosis. J Am Coll Cardiol. 2019 Jan 8;73(1):1–9. doi: 10.1016/j.jacc.2018.09.082. PMID: 30621937. [DOI] [PubMed] [Google Scholar]
- 18.Yan I., Börschel C.S., Neumann J.T., et al. High-sensitivity cardiac troponin I levels and prediction of heart failure: results from the BiomarCaRE consortium. JACC Heart Fail. 2020 May;8(5):401–411. doi: 10.1016/j.jchf.2019.12.008. Epub 2020 Mar 11. PMID: 32171759. [DOI] [PubMed] [Google Scholar]
- 19.Takashio S., Nagai T., Sugano Y., et al. NaDEF Investigators. Persistent increase in cardiac troponin T at hospital discharge predicts repeat hospitalization in patients with acute decompensated heart failure. PLoS One. 2017 Apr 5;12(4) doi: 10.1371/journal.pone.0173336. PMID: 28379962; PMCID: PMC5381770. [DOI] [PMC free article] [PubMed] [Google Scholar]