Abstract
Background
This article introduces the updated version of the Iranian guideline for the diagnosis and treatment of hypertension in adults. The initial version of the national guideline was developed in 2011 and updated in 2014. Among the reasons necessitating the update of this guideline were the passage of time, the incompleteness of the scopes, the limitation of the target group, and more important is the request of the ministry of health in Iran.
Method
The members of the guideline updating group, after reviewing the original version and the new evidence, prepared 10 clinical questions regarding hypertension, and based on the evidence found from the latest scientific documents, provided recommendations or suggestions to answer these questions.
Result
According to the updated guideline, the threshold for office prehypertension diagnosis should be considered the systolic blood pressure (SBP) of 130-139 mmHg and/or the diastolic blood pressure (DBP) of 80-89 mmHg, and in adults under 75 years of age without comorbidities, the threshold for office hypertension diagnosis should be SBP ≥ 140 mmHg and or DBP ≥ 90 mmHg.
The goal of treatment in adults who lack comorbidities and risk factors is SBP < 140 mmHg and DBP < 90 mmHg. The first-line treatment recommended in people with prehypertension is lifestyle modification, while for those with hypertension, pharmacotherapy along with lifestyle modification. The threshold to start drug therapy is determined at SBP ≥ 140 mmHg and or DBP ≥ 90 mmHg, and the first-line treatment is considered a drug or a combined pill of antihypertensive drugs, including ACEIs, ARBs, thiazide and thiazide-like agents, or CCBs.
At the beginning of the pharmacotherapy, the Guideline Updating Group members suggested studying serum electrolytes, creatinine, lipid profile, fasting sugar, urinalysis, and an electrocardiogram. Regarding the visit intervals, monthly visits are suggested at the beginning of the treatment or in case of any change in the type or dosage of the drug until achieving the treatment goal, followed by every 3-to-6-month visits. Moreover, to reduce further complications, it was suggested that healthcare unit employees use telehealth strategies.
Conclusions
In this guideline, specific recommendations and suggestions have been presented for adults and subgroups like older people or those with cardiovascular disease, diabetes mellitus, chronic kidney disease, and COVID-19.
Keywords: Hypertension, Blood Pressure, Clinical practice Guideline, Updating, Diagnosis, Treatment
Background
According to the conducted research, the volume of scientific information is increasing exponentially, and approximately 75 clinical trials and 11 systematic reviews are published daily [1]. Dealing with this growing amount of information and utilizing it to make the best clinical decisions requires keeping the available resources, especially clinical guidelines, up-to-date [2]. Clinical guidelines are a set of instructions aimed at optimizing patient care and are prepared based on the systematic review of available resources and taking into account the advantages and disadvantages of other care options [3]. Considering the importance of clinical guidelines, updating them is essential to guarantee the validity of the guidelines and maintain their benefits for patients, healthcare providers, and other beneficiaries [4].
There is no single and uniform opinion about the optimal time interval for updating clinical guidelines. Some resources have suggested this interval be between 3 and 5 years [5, 6]. On the other hand, updating guidelines by adopting a time-based approach is not necessarily a suitable method for all clinical guidelines due to the fact that the rate of changes in scientific resources is not the same. According to some researchers, the priority-setting approach (updating by taking into account the priority of updating clinical guidelines whose related documents and scientific evidence have changed sufficiently) is a more efficient method than the time-based method; however, this method requires active monitoring of scientific resources [7].
One of the most important health problems, the management of which is highly important due to being based on accurate and up-to-date instructions, is hypertension [8]. Essential hypertension is one of the main causes of disability and death and has a proven role in causing cardiovascular events [9]; nevertheless, it is possible to control it. Considering that one of the known obstacles in society regarding the management of this disease is related to the physicians' lack of knowledge and awareness or their failure to follow the available guideline for the control and care of this disease [10], it seems necessary to offer physicians a set of clinical solutions (which naturally cause the least harm and damage to the patients) based on the latest scientific evidence.
In different countries, various clinical strategies are developed for the prevention, control, and treatment of blood pressure that are updated at regular intervals. Some of the most important clinical strategies include the guideline for the prevention, detection, evaluation, and management of high blood pressure in adults [10] presented by the American Heart Association, guideline for the management of arterial hypertension presented by the European Society of Cardiology and the European Society of Hypertension [11], guideline for diagnosis, risk assessment, prevention, and the treatment of hypertension in adults provided by the Canadian Hypertension Education Program [12], and the guideline for hypertension in adults provided by the National Institute for Health and Healthcare Excellence in England [13].
In Iran, with the aim of providing an evidence-based approach for the prevention, treatment, and control of hypertension based on existing international solutions, and considering the results of regional research, Iran’s social situation, and its healthcare needs, the first version of this guideline was compiled in 2011 and updated in 2014 with the cooperation of professors from various Iranian universities and organizations along with foreign experts who were all active in preparing guidelines for their countries or regions.
In the initial version of the guideline, it was decided that it should be revised every 3 years [14]. Since maintaining the dynamics of clinical guidelines is undeniable due to the changes that occur over time in medical evidence (especially in diagnostic and treatment methods, the incidence and prevalence of diseases, and patients' lifestyle and their conditions), as well as taking into account an important official request of the Office of Health Technology Assessment, Standardization, and Tariff of the Iranian Ministry of Health (MOH) in this regard, and considering some of the limitations of the previous versions and the extent of novel developments in the management and treatment of hypertension, the MOH decided to update the guideline for the diagnosis and treatment of hypertension in Iran. As a result, important information sources in accordance with the latest scientific documents accepted by prominent international scientific communities regarding the diagnosis, and treatment of hypertension have been developed based target groups.
Methods
The process of updating the guideline was initiated following discussion of the members of the Steering Committee, and establishing the Guideline Updating Group (GUG). At the same stage, the External Review Group (ERG) was determined and the conflicts of interest of all groups were carefully evaluated. The GUG consisted of 38 experts in related fields from various universities of medical sciences, members of the national network of research in Iran, research institutes, research centers, and scientific associations, as well as the staff involved in providing relevant services. The systematic review group (SRG), who were independent of the GUG, were selected through a call and their conflicts of interest were investigated. The process of updating this guideline was accomplished in three phases. In the first stage, the scope of the guideline was determined considering the initial version of the guideline, and questions in the field of scope were collected to develop the Population, Intervention, Comparison, and Outcomes (PICOs). In the second phase, systematic review was done. The PICOs were sent to the SR group who did extensive review and grading to develop the evidence and in the third phase recommendations and suggestions were prepared. Finally, guideline was written and the corresponding algorithm was drawn.
The first and third phases were conducted during 23 online meetings of the GUG with the cooperation of each member of this group, while the second phase was done by the systematic review group.
Participating groups in updating the guideline
The participating groups in developing this guideline consist of:
Steering Committee (SC): This committee included the project executive, main collaborators, and the officials of the Office of Health Technology Assessment, Standardization, and Tariff of the Ministry of Health. It was in charge of selecting the members of other groups, holding the meetings and implementing the steps of updating the guideline, monitoring the time and finalizing the guideline.
Guideline Updating Group (GUG): This group consisted of experts from national universities of medical sciences and related scientific societies or research institutes. Members of the National Network of CVD Research participated too. The participants were specialists, namely internists, cardiologists, endocrinologists, nephrologists, neurologists, pediatricians, obstetricians and gynecologists, nutritionists, epidemiologists, and pharmacologists, Health economists as well as general physicians, nurses, and staff working in the healthcare system. These individuals were selected based on their expertise, and interest from all over the country. A methodologist facilitated the GUG group meetings.
Systematic Review Group (SRG): The members of this group were independent from the GUG members and were experts in the field of systematic review and meta-analysis. This group was responsible for searching, developing and summarizing the evidence.
External Review Group (ERG): It included a number of experts in various fields related to the subject as well as influential people in policymaking in this field that were all responsible to evaluate the updated guideline. All were external to the GUG or other groups in developing this guideline.
Declaration of conflicts of interest
To identify the types of conflicts of interest (e.g., finance, work, research, and consultancy), the standard conflict of interest approved by the Ministry of Health of Iran was signed by all members of the groups involved in updating the guideline. There were no cases of conflict of interest in the completed forms; however, according to prior planning, it was decided that if any conflict of interest was identified, while maintaining the confidentiality of information, the cases would be managed by the SC and then possible measures.
Scope of guidelines and questions
The scope of the guideline included the functional area, the target group (i.e., individuals who might be affected by the recommendations), and the results obtained from the guideline. The functional scope of the last updated guideline, according to the GUG members, did not deal with diagnosis.
The target group of the guideline, according to the decision of the GUG group, was initially people with hypertension and included subgroups of children, pregnant women, the elderly, individuals with cardiovascular diseases (CVDs), diabetes mellitus (DM), chronic kidney disease (CKD), and COVID-19. Nevertheless, at the end of the first phase of the process, considering the vast extent and spectrum of blood pressure in children and pregnant women, the GUG agreed that these two subgroups be excluded, and it was decided that separate guideline be developed for these two subgroups in the future. As a result, the target group of this guideline was considered as male and female adults (18 years old and above) with hypertension.
To determine and rank the outcomes, the members of the SC group prepared an initial list of outcomes proposed in the initial version of the hypertension guideline in 2011 and other outcomes in the latest published hypertension guideline at global level. Afterward, the members of the GUG were asked to review these outcomes and provide their proposed outcomes via an electronic form sent to them. following, the lists of primary and proposed outcomes were reviewed by GUG members in a meeting and the final outcomes were determined and ranked.
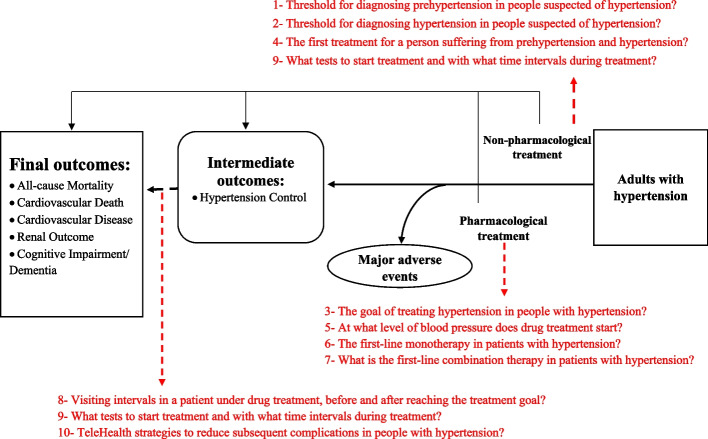
Next, during another process, primary or clinical questions and PICO questions were extracted after removing unrelated and repetitive items and merging similar ones, eventually, ten PICO questions were finalized and provided to the SR group. The members of the working group also created an analytical framework (Fig. 1) that showed the impact of interventions on intermediate and final outcomes and specified the order of the 10 PICO questions for better visualization and placing them along the path of patient management. To determine the values and preferences of patients with hypertension, qualitative research was performed in the form of two sessions for patients and physicians. A session of focus group discussion (FGD) that was held with the participation of 7 patients suffering from different levels of hypertension. During this session, the participants' preferences for diagnosis, medicinal and non- medicinal therapy (i.e., lifestyle), and required training were extracted. In addition to that, the values, preferences, and views of general practitioners regarding the problems of patients with hypertension were also determined during a virtual FGD session with the participation of 5 general practitioners who had a clinical practice in the field of hypertension. Their preferences were extracted in four axes consisting of diagnosis, treatment, use and expectation of the Iranian hypertension guideline. The values and preferences of physicians and patients were considered in the design of PICO questions and in later phases of developing this guideline (Fig. 1).
Fig. 1.
Analytical framework of hypertension management in adults
Search for evidence
All PICO questions were submitted to the SR group. The SR group performed an extensive systematic search for each PICO question in PubMed, Embase, and Cochrane Library databases with a time limit for the last 6 years. At first, an Umbrella Systematic Review was conducted on all previous systematic reviews. In this way, the latest information related to new systematic reviews was found, and since there was no new systematic review for one of the PICO questions, a new one was conducted. Moreover, the evidence used in preparing the recent hypertension guideline was reviewed. The identified systematic reviews were evaluated in terms of their up-to-datedness, the degree of compliance with the PICO questions, the assessment of their methodology by the AMSTAR tool, the provision of sufficient information to evaluate the certainty of the evidence, and reporting evidence on different outcomes and in the subgroups of the PICOs; subsequently, the most appropriate ones were selected.
Evidence to recommendations
After searching for scientific evidence through a systematic review, certainty in and quality of evidence were rated by the standard GRADE method. In this process, the risk of bias, imprecision, indirectness, and inconsistency were investigated. Additionally, another overview was conducted by the SC to examine other decision-making criteria in the framework of the Evidence to Decision tables, and the evidence related to the criteria of certainty, patients' values and preferences, health benefits and harms, resources, costs, acceptability, the feasibility of implementation, and health equity indicators were discussed.
Compilation of recommendations and suggestions
To compile the recommendations, 9 two-hour online meetings were held with the members of the GUG group. In each meeting, one or two PICO question was introduced and then its related evidence provided by the SR group was presented in the form of a GRADE evidence profile by the head of SR group. Subsequently, the members of the GUG group, with the guidance of the methodologists, completed the evidence-to-decision tables.
The recommendations of this guideline were divided into two groups based on the strength of the evidence in the recommendation. The strength of recommendations indicated the extent to which the GUG was confident that the desirable effects of the recommendations (e.g., beneficial health outcomes) outweigh the potential undesirable effects (e.g., side effects). The classification of the recommendations in this hypertension guideline was as follows:
Strong recommendation: A recommendation for which the GUG was confident that the favorable effects of adhering to it would outweigh its adverse effects.
Suggestion or Conditional Recommendation: A recommendation for which there was greater uncertainty regarding the quality of the evidence, the balance of benefits and harms, values and preferences, and the use of resources; however, the GUG concluded that the positive effects of adhering to it probably outweighed its negative effects. These cases were presented as "Suggestions". In some cases, when there was extensive discussion on the evidence, a consensus of GUG members on the recommendation was reached
External evaluation
The initial report of the guideline along with the AGREE Reporting Checklist was provided to the ERG (an independent group from the GUG) as a guideline evaluation tool to evaluate the draft guideline in terms of validity, reliability, clarity, clinical applicability, clinical flexibility, and documentation. Then, based on their opinions and comments, the guideline was re-examined and some parts were modified. Moreover, to facilitate the use of the guideline, based on the recommendations, an algorithm was developed for the diagnosis and treatment of hypertension.
Update time
The update time for the current guideline was set for the next 3 years.
Results
According to the previous explanations, 36 recommendations and suggestions (18 recommendations and 18 suggestions) were developed. As for COVID-19 subgroup, the systematic review group did not find evidence of differences in the diagnosis and treatment of hypertension in covid-19 patients compared to the general population, so no specific recommendations or suggestions were provided in the field of covid-19. The recommendations and suggestions extracted for the PICO questions related to the updated guideline for the diagnosis, management, and treatment of hypertension along with the relevant documents and evidence are as follows:
Box 1.
Recommendation for diagnosing prehypertension
| 1- It is recommended that the threshold of prehypertension diagnosis be considered as systolic blood pressure (SBP) of 130–139 mm Hg and/or diastolic blood pressure (DBP) of 80–89 mm Hg for BP in the office |
Evidence and rationale
Studies show that prehypertension is an independent risk factor for CVDs [15]. It has been reported that prehypertension, especially in a high range (SBP 130- 139 mmHg and/or DBP 85–89 mmHg), is associated with increased risk of CVDs, coronary heart disease, myocardial infarction (MI), and stroke, and effective control of hypertension can prevent more than 10% of CVD cases.
The overall prevalence of prehypertension in Iran is 31.6% based on the results of a systematic review of 48 studies with 417,349 participants [16]. A meta-analysis performed on 29 clinical trial studies with 127,641 participants demonstrated that the identification of patients with prehypertension and their active treatment compared to placebo had led to a 7% reduction in the risk of the first outcomes of CV events, 14% decrease in stroke, and 10% decline in heart failure (HF) [15].
Evidence-to-decision considerations
An asymptomatic patient may undervalue the importance of diagnosis and treatment of prehypertension unless they are convinced that there is a relationship between immediate side effects and potential long-term health gains [17]. The GUG assessed the resources and costs required for recommendations related to this question to be small. Cost-effectiveness analysis using the Systolic Blood Pressure Intervention Trial (SPRINT) has shown that although the treatment of high-risk/CVD patients with a baseline blood pressure of 130–139 mmHg is affordable, it does not help reduce costs, and the value of an intervention is associated with maintaining its effect for more than 5 years [18]. The effect of diagnosing prehypertension at the mentioned threshold on health inequalities was assessed as unclear because no evidence was found in this field. From the perspective of the panel members, the selected threshold was acceptable to the key beneficiaries and was implementable. In this regard, the results of our FGD with general practitioners demonstrated that they believe that different guidelines provided different numbers for the threshold of diagnosis. Therefore, it is necessary to provide a single diagnostic threshold for diagnosing prehypertension and hypertension in the country.
Box 2.
Recommendation and suggestions for diagnosing hypertension
|
1- It is recommended that the threshold for diagnosing hypertension in adults under 75 years of age without comorbidities should be SBP of ≥ 140 mmHg and/or DBP of ≥ 90 mmHg using office BP measurement 2- It is suggested that the threshold for diagnosing hypertension in adults over 75 years of age should be SBP of ≥ 150 mmHg and/or DBP of ≥ 90 mmHg using office BP measurement 3- It is suggested that the threshold for diagnosing hypertension, in case of Ambulatory Blood Pressure Measurement (ABPM), should be considered SBP of ≥ 130 mmHg and/or DBP of ≥ 80 mmHg in an average whole day (24 h) 4- It is suggested that the threshold for diagnosing hypertension, in case of ABPM, should be considered SBP of ≥ 135 mmHg and/or DBP of ≥ 85 mmHg in the average day time or awake time 5- It is suggested that the threshold for diagnosing hypertension, in case of BP measurement at home, should be considered as SBP of ≥ 135 mmHg and/or DBP of ≥ 85 mmHg |
Evidence and rationale
Despite strong evidence supporting the efficacy of antihypertensive therapy, major disagreements still exist, especially about the appropriate blood pressure threshold to start pharmacotherapy, target blood pressure during treatment, and treatment strategies, and these uncertainties have been reflected in the difference between clinical guidelines for the management of hypertension [10, 11, 19].
Based on the findings of a meta-analysis study of 48 randomized clinical trial studies, involving 344,716 participants with an average age of 65 years, a relative reduction in the risk of major adverse cardiovascular events (MACE) was seen in proportion to the reduction in BP level; accordingly, for every 5 mmHg reduction in SBP, the risk of MACE decreased by 10% [20].
In another meta-analysis of 61 prospective studies, the risk of CVD increased in a log-linear fashion from SBP levels of < 115 mmHg to < 180 mmHg and between DBP levels of < 75 mmHg to < 105 mmHg. Further analysis of the same study showed that a 20 mmHg increase in SBP and a 10 mmHg rise in DBP were associated with a doubling of mortality due to stroke and CVDs [21, 22].
Based on the results of studies, the overall prevalence of hypertension, awareness, treatment, and control in Iranian adult’s population were 20.4% (95% CI 16.5, 24.4; I2 = 99.9%), 49.3% (95% CI 44.8, 53.8; I2 = 98.5%), 44.8% (95% CI 28.3, 61.2; I2 = 99.9%), 37.4% (95% CI 29.0, 45.8; I2 = 99.3%), respectively [23, 24].
Evidence-to-decision considerations
The expected benefits of lowering BP include reducing all course mortality, cardiovascular mortality, stroke, MI, and HF events [21, 22]. Regarding important damages related to the identification and diagnosis of hypertension (e.g., false positives, false negatives, anxiety, and psychological effects) and economic costs (e.g., time and loss of work or insurance), no evidence was found demonstrating that any of these damages are caused by the identification and diagnosis of hypertension; nevertheless, it is noteworthy that the absence of any evidence demonstrating harm does not guarantee that there is no harm [25].
The members of the GUG estimated the resources needed to diagnose blood pressure at the thresholds in the recommendation as medium and the required costs as low. In this regard, the results of American studies show that to improve the equitable control of high blood pressure in all people, health systems must evaluate and address the social needs related to the diagnosis, prevention, and successful control of blood pressure in each patient. Effectively addressing social factors influencing health requires investing in the infrastructure of health systems that are currently under pressure to become more cost-effective and value-oriented. This infrastructure requires a multifaceted approach [26, 27]. In terms of costs, people with hypertension in the United States incur an average of $2,000 more in annual healthcare costs than people without hypertension [28].
According to the members of the GUG, the established threshold for the diagnosis of hypertension was likely to reduce health inequalities. Barriers to accessing hypertension control care in low-income regions include the patient's low health literacy and limited resources [28]. However, it is possible to achieve fair and high rates of hypertension control among low-income and/or racially and ethnically diverse populations. The Million Hearts® Program publishes success stories of healthcare settings for underserved populations that have overcome access, economic, and cultural barriers, resulting in major improvements in hypertension control rates [29].
From the point of view of the panel members, the determined threshold was acceptable to the key beneficiaries and was implementable. The results of a qualitative study on several general practitioners revealed that they believed different guidelines provided different numbers for the diagnosis threshold, and there was a requirement to determine a single diagnostic threshold for the diagnosis of hypertension in the country [30].
Box 3.
Recommendation and suggestion for treatment target of hypertension
|
1- It is recommended that the goal of treatment in adults under 75 years of age with hypertension and without comorbidities and risk factors should be SBP of < 140 mmHg and DBP of < 90 mmHg 2- It is suggested that the goal of treatment in elderly people over 75 years old should be SBP of < 150 mmHg and DBP of < 90 mmHg 3- It is recommended that in patients with CVDs, diabetes mellitus, a history of stroke, and CKD (who have albuminuria of ˃30 mg/g Cr), the target of treatment should be considered SBP of < 130 mmHg and DBP of < 80 mmHg |
Evidence and rationale
In treating hypertension, physicians usually try to reach the target BP in patients. The target blood pressure is the blood pressure value below which there are favorable clinical benefits. The "the lower the better" approach, which has been of interest for many years in the treatment of hypertension, has been challenged over the past decade due to the lack of evidence from randomized trials supporting this approach. For this reason, the standard blood pressure target has been considered less than 140/90 mmHg for the general population of patients with hypertension during the past years.
The results of a systematic review with 38,688 participants with a mean follow-up period of 3.7 years revealed that lower goals did not reduce the overall rate of death and adverse events. This means that the benefits of lower blood pressure targets did not outweigh the harms compared to standard targets. Lower targets may reduce MI and congestive heart failure (CHF) but with low certainty for both outcomes [31].
In a systematic study on 8,221 adults with an average age of 74.8 years, higher BP targets (values less than 150/90 mmHg and less than 160/90 mmHg) were compared with lower targets (values less than 140/90 mmHg). The results of this study demonstrated that treatment for two different target blood pressures over 2 to 4 years failed to make a difference in any of the outcomes of death (from any cause), stroke, and MACE. However, the 95% confidence interval for these outcomes suggested that a lower target of BP might have a greater clinical benefit [32]. The findings of a systematic study with 42,134 participants showed that in people aged 75 years and older, strict SBP targets (120 mmHg and 130 mmHg) were associated with a significant reduction in the risk of all-cause mortality and cardiovascular mortality and heart failure, while higher SBP targets (150 mmHg and 160 mmHg) were linked to a lower risk of HF and stroke [33].
People with hypertension and established CVDs are at particular risk; in this regard, although decreasing blood pressure to a level lower than targets of people with high BP and no CVD may be beneficial and reduce cardiovascular mortality and morbidity, but it can increase the adverse effects. The results of a systematic review of 9,484 participants and a mean follow-up period of 3.7 years indicated that there was no significant difference in lower blood pressure targets for total mortality, cardiovascular deaths, total cardiovascular events, and serious adverse events [34].
Evidence-to-decision consideration
Based on the results of our qualitative survey on patients with hypertension, patients believed the reason for treatment was to increase their life expectancy, prevent disease complications, and the fear of disability [30]; therefore, the goal of appropriate treatment should include the fulfillment of these expectations. The favorable effects of the lower blood pressure goal included a reduction in overall mortality, cardiovascular mortality, and stroke, while the reported adverse effect was an increase in side effects [35].
The members of the GUG evaluated the resources and costs required to achieve the mentioned treatment goals as average. Achieving good blood pressure control in health care in low-income countries is hindered by various obstructions and investing in the effective management of hypertension is often associated with various challenges. That is because dealing with chronic conditions like BP needs suitable policymaking along with a comprehensive and extensive implementation plan and documents that involve beneficiaries, health professionals, and resources to achieve this goal. The direct costs of health care related to the treatment of hypertension as well as risk factors and associated diseases, such as stroke, ischemic heart disease, and CHF, impose a significant financial burden. The results of a study by Howard et al. showed that the intensive management of uncontrolled hypertension was cost-effective compared to usual care [36].
Hypertension treatment leads to the prevention or reduction of mortality and cardiovascular events in the population and thus reduces health inequality. Uncontrolled high blood pressure may be more common in vulnerable populations. However, it is possible to achieve high and fair rates of hypertension control among vulnerable, low-income, and/or racially and ethnically diverse populations. Therefore, improving the treatment and control of hypertension through better treatment with the aim of reducing blood pressure can decrease long-term inequality [29]. In the opinion of the panel members, the determined treatment goals were acceptable and practical to the key beneficiaries.
Box 4.
Recommendation and Suggestion for first line treatment of hypertension
|
1- It is recommended that lifestyle modification be considered the first therapeutic intervention in people with prehypertension, and in individuals with hypertension, both non-pharmacological and pharmacological treatment be considered General recommendations for lifestyle modification include the following: - Body mass index and waist circumference should be measured and it is recommended to keep both within a healthy range - The total daily intake of salt should be less than 5 g, the consumption of salty foods and adding salt during cooking should be limited, and using salt shaker should be avoided - Diet should contain fruits and vegetables, low-fat dairy products, whole grains, fish, poultry, vegetable protein, such as beans and oil nuts that are rich in magnesium, potassium, calcium, and fiber. In the diet of these people, saturated and trans fats, meat, sweets, and soft b drinks should be reduced - The use of potassium, calcium, and magnesium supplements is not recommended for the treatment of hypertension - Adults should perform at least 150–300 min of moderate-intensity aerobic physical activity or at least 75–150 min of vigorous-intensity aerobic physical activity in a week, or an equivalent combination of moderate-intensity and vigorous-intensity activity - Adults should do at least two days a week of moderate- or high-intensity activities that engage all major muscle groups - Any type of tobacco (e.g., cigarettes and hookah) and alcohol should be refrained in any amount, and in case of previous use, it should be stopped 2- It is suggested to consider stress coping methods, such as relaxation and yoga, as an intervention 3- It is suggested that people suffering from prehypertension and hypertension not to be exposed to polluted air 4-It is suggested that people suffering from prehypertension and hypertension and their families be educated on how to measure and monitor blood pressure as well as on adherence to drug treatment and lifestyle modification |
Evidence and rationale
Choosing a healthy lifestyle can prevent or delay the onset of hypertension. Lifestyle modification is the first-line antihypertensive treatment. In addition, changes in lifestyle can increase the effects of antihypertensive treatment [37].
Weight gain and obesity are important risk factors for hypertension. Therefore, keeping weight under control and avoiding obesity, particularly abdominal obesity, are essential for controlling and preventing blood pressure. A ratio of waist circumference to height of less than 0.5 is recommended for most populations [38].
In a meta-analysis study including 25 clinical trials and 4,874 participants, the results showed that for every kilogram of weight loss caused by energy consumption restriction, physical activity, or both, the average SBP and DBP reductions were 1.05 mmHg and 0.92 mmHg, respectively. The effect of weight loss on DBP was significantly greater in the population taking antihypertensive drugs than in the untreated population (5.31 mmHg vs. 2.91 mmHg). This meta-analysis clearly demonstrated that weight loss was essential for both the prevention and treatment of hypertension [39].
There is strong evidence supporting the existence of a link between high salt intake and hypertension.
The results of a meta-analysis study in China showed that salt substitution led to a significant reduction in the average SBP (-5.7 mmHg) and DBP (-2 mmHg). Moreover, teaching to reduce salt consumption in schools led to a decrease in parents' SBP (-2.3 mmHg) [40].
The results of another meta-analysis on 85 RCT with dose–response analysis of sodium reduction showed an approximately linear relationship between sodium intake and reduction in both systolic and diastolic BP across the entire range of dietary sodium exposure. Although this occurred independently of baseline BP, the effect of sodium reduction on level of BP was more pronounced in participants with a higher BP level [41].
Regarding the effect of diet, the results of a meta-analysis of clinical trial studies revealed that adherence to the Mediterranean diet led to a decrease in the average rates of SBP and DBP by 2.35 mg and 1.58 mmHg, respectively [42].
The findings of studies indicate that regular aerobic and resistance exercise may be beneficial for both the prevention and treatment of hypertension. The results of a meta-analysis study including 11 studies on antihypertensive drug users and 25 studies on non-antihypertensive drug users showed that resistance exercise in antihypertensive drug users led to a reduction in SBP (from 1.6 to 2.8 mmHg) and DBP (from 4.6 to 1.6 mmHg). Muscle strength increased significantly in both groups of users and non-users of antihypertensive drugs [43]. The results of a systematic review study also showed that performing tai chi movements, which is originally a Chinese exercise and a combination of deep breathing relaxation techniques and gentle conscious movements, can be recommended as an auxiliary treatment to control hypertension, especially for patients younger than 50 years old [44].
Smoking causes a sharp increase in blood pressure and heart rate. In a clinical trial study in smoking hypertensive patients, by Holter monitoring of blood pressure every 30 min during 24 h, patients' blood pressure and heart rate were evaluated on a day when they smoked and, on another day when they did not. The results showed that the 24-h average blood pressure, daytime blood pressure, and heart rate were significantly higher during the smoking period than in the non-smoking period [45].
Excessive alcohol consumption raises the risk of CVDs, such as cardiomyopathy, hypertension, atrial arrhythmia, or stroke. The findings of a meta-analysis study including 15 clinical trial studies and 2,234 participants revealed that reducing alcohol consumption led to a significant decrease in average SBP by 3.31 mmHg and a drop in average DBP by 2.04 mmHg. A dose–response relationship was observed between the average percent reduction in alcohol consumption and the average blood pressure reduction [46]. On the other hand, the result of another study demonstrated that in spite of consuming less alcohol by patients with hypertension, there was no change in SBP, DBP, and outcomes, such as overall mortality, mortality due to cardiovascular events, and cardiovascular events, compared to the control group [47].
Teaching self-care behaviors is an important approach for blood pressure management and control. The results of a meta-analysis study of 18 clinical trials showed that self-care activities could lead to the management and treatment of hypertension. Such measures can also reduce emergency and outpatient visits by 40% and 17%, respectively [48]. The overall reliability of the presented evidence was evaluated as high.
Evidence-to-decision considerations
According to the findings of a qualitative survey on the values and preferences of hypertensive patients, they preferred lifestyle modification to medication. Additionally, patients considered diet adherence, physical activity, stress control, and the acceptance of the disease to be effective factors in controlling and treating blood pressure, and they requested more education regarding lifestyle modification to manage their blood pressure [49].
The members of the GUG estimated the resources and costs required to implement recommendations related to lifestyle modification to be small. A huge information gap exists in this regard, and Quality cost-effectiveness studies were not available in this area [50].
The recommendations provided by the members of the GUG were acceptable to the key beneficiaries and were implementable. The results of our qualitative survey on general practitioners indicated that regarding treatment, more attention is paid to drug treatment and less to lifestyle, and appropriate referrals in this field (e.g., referral to a nutritionist, sports medicine specialist, and psychologist or healthy lifestyle consultant) are not observed, an issue that needs to be taken into account [30].
Box 5.
Recommendation and Suggestion for Threshold to start Pharmacological treatments
|
1- It is recommended that the threshold for starting pharmacotherapy in people under 75 years of age with hypertension and without comorbidities, be considered as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg 2- It is suggested that the threshold for starting pharmacotherapy in individuals over 75 years of age, should be SBP ≥ 150 mmHg and/or DBP ≥ 90 mmHg 3- It is recommended that in individuals with CVD, CKD (who have albuminuria more than 30 mg/g Cr), and diabetes mellitus, the threshold for starting pharmacotherapy should be SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg 4- It is suggested that in people with a history of stroke, the threshold for starting pharmacotherapy should be SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg |
Evidence and rationale
Based on the results of a meta-analysis of 123 studies with 613,815 participants, every 10-mmHg reduction in SBP significantly decreased the risk of major CVD by 17–23%, coronary artery disease by 12–22%, stroke by 23–33%, HF by 22–33%, and death due to all causes by 9–13% in the studied populations [50].
A meta-analysis study of 48 clinical trials with 344,716 participants showed that a reduction of 5 mmHg in SBP, regardless of previous diagnoses of CVD, and even in normal or high blood pressure values, decreased the risk of MACE by approximately 10% [51]. The overall reliability of the evidence was high, and the results of related studies showed that the predicted benefits of lowering blood pressure (SBP < 140 mmHg in the general population and < 130 mmHg in the high-risk population) included a reduction in mortality, cardiovascular mortality, strokes, MIs, and HF events. The predicted harms were mostly non-serious adverse events, and some were surrogate outcomes, such as increased creatinine [51–59].
Evidence-to-decision considerations
The results of our qualitative study demonstrated that patients' understanding of hypertension was often limited and they were not aware of the existence of treatment options; however, counseling provided opportunities for patients and physicians to reach a common understanding of their treatment choices [60].
From the point of view of the panel members, the resources and costs required to start drug treatment at the mentioned threshold were evaluated as intermediate. A qualitative study's findings revealed that the factors related to health systems, such as the lack of specialists, the cost of medicine, the length of a visit session in the clinic, and the possible weak services provided, were among the major barriers preventing patients from visiting the clinic and starting treatment. In developing countries, weak health systems have been identified as a major obstacle in effectively addressing the growing burden of chronic conditions, such as hypertension [61].
Epidemiological studies have shown that the benefits of treatment (with both lifestyle interventions and medications) clearly outweigh the risks of treatment [62]. Furthermore, modeling studies support the effectiveness and cost-effectiveness of treating younger and low-risk patients [22].
Due to the lack of evidence on the impact of the mentioned threshold on health inequality, this impact was considered unclear by the panel members [63]. From the perspective of the panel members, initiating pharmacotherapy at the mentioned threshold was acceptable to the key beneficiaries and was applicable.
Box 6.
Recommendation and Suggestion for first-line monotherapy of hypertension
|
1- It is recommended for people with hypertension and without comorbidities who need pharmacotherapy, the first-line Medicine should be one of antihypertensive drugs that consists of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), thiazide and thiazide-like agents, and calcium channel blockers (CCBs) 2- It is recommended that in people over 75 years of age with hypertension, the first medicinal treatment should be the use of one of the CCBs or thiazide and thiazide-like agents 3- It is recommended to use one of the medicines in the class of ACEIs/ARBs, CCBs, or thiazide and thiazide-like agents in patients with hypertension and diabetes mellitus (without albuminuria) 4- It is recommended that in patients suffering from hypertension and CKD (who have albuminuria more than 30 mg/g Cr), the first-line therapy should be one of the medications in the medication class of ACEIs or ARB 5- It is recommended that in patients with hypertension and a history of stroke, the first pharmaceutical treatment should be the use of one of the medications in the medication class of ACEIs/ARBs, thiazide and thiazide-like agents, and if not effective or side effects occure, one of the CCB drugs should be used 6- It is recommended to use one of ACEIs or ARBs or beta blockers in patients with hypertension and coronary artery diseases 7- It is suggested to use one of the long-acting CCB drugs in patients with hypertension and coronary artery disease if beta-blockers are not effective or are contraindicated 8- It is recommended that in patients suffering from hypertension and HF, the first-line treatment should be one of the ACEIs or ARBs drugs |
Evidence and rationale
Data from 32 systematic reviews to obtain evidence on the benefits and harms of different medication classes revealed various benefits, including a reduction in mortality and MACE, per 1,000 people undergoing treatment with different medication classes [64].
The results of a systematic review comparing CCBs with other medication classes in blood pressure management, which included the review of 23 clinical trial studies with 153,849 participants having hypertension, showed that there was no difference in death due to any cause between CCBs as the first-line treatment and other antihypertensive medication classes. It is probable that CCBs had increased the incidence of MACE and CHF-related events in comparison to diuretics (moderate- certainty evidence). It was found that compared to beta-blockers, CCBs led to a reduction in the outcomes of MACE, stroke (moderate-certainty evidence), and cardiovascular death (low-certainty evidence). CCBs decrease stroke risk (low certainty) and increase CHF risk when compared to ACEIs. They also reduce the risk of MI (moderate-certainty evidence) and increase the risk of CHF (low-certainty evidence) in comparison to ARBs. Overall, for the treatment of hypertension, there is moderate-certainty evidence indicating that diuretics lead to a reduction in the risk of MACE and CHF more than CCBs. Low-to-moderate-certainty evidence was obtained showing that CCBs were more likely than beta-blockers to reduce MACE, and similar level of evidence suggested that CCBs reduced the risk of stroke compared to ACEIs, and decreased the risk of MI compared to ARBs; however, they increased the possibility of CHF compared to ACEIs and ARBs [65]. A systematic review and network meta-analysis, in which the results of 15 observational studies and 7 clinical trials on 649,790 patients with hypertension were reviewed, showed that in observational studies, treatment with CCBs or ARBs was associated with a lower risk of dementia, in comparison to treatment with other medication classes [66].
In a systematic review of 44 clinical trials with 5,745 participants, aldosterone antagonists had unclear effects on renal failure, mortality, and cardiovascular events, in comparison to placebo or standard care. Aldosterone antagonists, compared to placebo or standard care, may decrease protein excretion, estimated glomerular filtration rate (eGFR), and SBP, and possibly increase the risk of hyperkalemia, acute kidney injury, and gynecomastia [67].
In subgroups, for patients over 65 years of age, the results of studies suggest usefulness of diuretics or CCBs. Diuretics are probably the most effective and CCBs the least effective drugs for HF prevention [68, 69].
Epidemiological studies showed that the benefits of treatment (either with lifestyle interventions or with drugs) clearly outweighed the risks of treatment [70]. Side effects of CCBs, ACEIs/ARBs, and thiazides were rare and usually mild and manageable [71, 72].
Evidence-to-decision considerations
Most patients, healthcare workers, professional associations, and government organizations are fully aware of the importance of drug therapy for hypertension treatment in preventing multi-organ complications. However, asymptomatic patients may consider treatment of little value, unless they have been convinced that there is a relationship between the immediate discomfort/side effects of the drug and potential long-term health gains [17]. In our qualitative study, hypertensive patients preferred the prescription of medicine by a specialist over a general practitioner and believed that taking medicine more often effective in the treatment and would have fewer adverse effects. On the one hand, from health care providers' viewpoint, patient compliance is crucially important because the disease is chronic and the patient has to take the medicine for long time; nevertheless, it is challenging to convince patients that blood pressure medication should be continued even with blood pressure regulation, a fact that is not easily accepted by them [49]. The results of this qualitative study showed that numerous individual and social factors were effective on adherence to treatment, among which is the lack of family support and the need for separate meals to improve treatment as individual factors, and the feeling of security, local facilities, and drugs availability as environmental factors [17].
Regarding the use of resources and costs of treatment, the members of the GUG estimated them to be small due to the low price of antihypertensive drugs in the country and the fact that these drugs are covered by insurance. The results of a study showed that treatment for stage 1 hypertension was cost-effective for all men and women between the ages of 45 and 74 [73].
From the perspective of the panel members, the mentioned pharmaceutical treatments were acceptable to the key beneficiaries and were implementable. Our qualitative study on physician’s findings showed that they believe although there were several medication classes in international guidelines, it was not clear for them which one to follow and what drug needed to be prescribed as the first line of pharmacological treatment. On the other hand, some recommended drugs are not available or are expensive in our country, therefore, the national guidelines need to specify the drugs that are the most efficient and reasonably priced in different steps [30].
Box 7.
Recommendation and Suggestion for first-line combined drug therapy of hypertension
|
1- It is recommended for people with hypertension who prescribed a combination pill of different classes of antihypertensive drugs, as the first-line treatment, a combination pill including ACEIs/ARBs, CCBs, diuretics (thiazide/thiazide like diuretics), to be used to increase compliance and adherence to treatment 2- In patients with hypertension and diabetes mellitus who need drug therapy, a combination therapy of ACEIs/ARBs and CCBs or ARBs/ACEIs and thiazide/thiazide like diuretics is recommended 3- In patients with hypertension and a history of stroke, a combination therapy of ACEIs/ARBs and thiazide/thiazide like diuretics or ACEIs/ARBs and CCBs is suggested 4- In patients with hypertension and coronary artery diseases, a combination therapy should be recommended based on the patient's condition using ACEIs/ARBs and beta blockers or ACEIs/ARBs and CCBs 5- It is recommended that for patients suffering from hypertension and HF without a reduction of ejection fraction, a combination therapy of one of the ACEIs/ARBs drugs and beta blockers be used 6- In hypertensive patients whose DBP is high with or without SBP, it is suggested to adopt a combination therapy using ACEIs/ARBs and CCBs or ACEIs/ARBs and diuretic |
Evidence and rationale
The latest hypertension guidelines have increasingly emphasized the use of combination therapy as the first-line treatment in various patients [10, 11]. Unlike previous guidelines that traditionally recommended a stepped care strategy with initial monotherapy [62], this shift in emphasis on combination therapy as the first-line treatment is reflective of several factors. Most patients with hypertension need to be treated with two or more medications to reach the target blood pressure; however, many of them do not receive such treatment. There are concerns about the risks associated with long-term hypertension control and that the need for multiple clinic visits may negatively affect long-term adherence [74, 75]. These factors have intensified clinical interest in the employment of combination therapy as the primary therapy. Nevertheless, concerns remain about the evidence base to support this strategy, particularly in relation to the risk of adverse events.
The findings of a systematic review including 33 clinical trials with 13,095 participants demonstrated that the use of low-to-standard dose combination therapy compared to standard monotherapy led to the effective control of hypertension without withdrawal from treatment due to adverse effects [76]. Examining 568 patients in a review study, it was found that combination therapy, compared to monotherapy, was associated with a decrease in total mortality (0.8 to 21.72), mortality caused by CVDs, cardiovascular events (0.22 to 4.41), Serious side effects (0.31 to 1.92), and withdrawal due to side effects (0.53 to 1.35) [77].
A systematic review compared single-pill combination therapy containing azilsartan medoxomil and chlorthalidone with the simultaneous administration of two tablets of azilsartan medoxomil and chlorthalidone. The results indicated a greater decrease in SBP in the single-pill combination therapy group (35.1 mmHg) than in the simultaneous administration of two tablets (29.5 mmHg). The findings of another study on 153 individuals with CKD showed that the use of a single-pill combination therapy including azilsartan medoxomil and chlorthalidone, compared to other single- pill combination therapies, led to a more effective reduction of blood pressure and increased adherence to treatment [78].
As a result of a clinical trial involving 125,635 patients, researchers found that two-drug combination therapy in the form of a single tablet or as a separate medication reduced the risk of death by 11–28% and hospitalization due to heart complications by 10–21% compared to single-drug therapy (10–21%) [79].
The overall certainty of the evidence found was moderate. Despite the favorable effects of combination therapy, which include improved adherence and continuation of treatment, and consequently, improved blood pressure control and better clinical outcomes, there have been reports of a few adverse effects, such as the side effects of drugs, especially when drugs are combined in one tablet.
Evidence-to-decision considerations
Our qualitative survey of patients' values and preferences revealed that they preferred drugs with fewer side effects [49]. The results of a systematic review study showed that simplifying the medication regimen of patients led to an increase in their treatment compliance from 6 to 20% [80].
The resources and costs required for the combination therapy were evaluated as moderate by the members of the GUG. In a meta-analysis study, it was found that patients taking single-pill combination therapy had higher total annual healthcare costs than those treated with compounded medications [81]. Although combination therapy is initially associated with a moderate increase in required resources and costs, such as direct drug costs and manufacture and supply chain costs, the net profit of improving blood pressure control and reducing major events related to the hypertensive process outweighs the increased cost because the control of hypertension is probably achieved earlier with combination therapy.
Due to the lack of evidence, the effect of combination therapy on health inequalities was assessed as unclear by the members of the GUG. Moreover, according to them, the mentioned combination therapies were acceptable to the key beneficiaries and were implementable. In a qualitative interview, physicians stated that different times and conditions were provided in the guidelines for prescribing one or more drugs or compounded medications; therefore, there was a need for explicit instruction in this regard. Furthermore, the polypharmacy and different brand names of medications led to confusion in patients and sometimes to their non-compliance with prescribed treatment [49].
Box 8.
Recommendation and Suggestion for visit intervals before and after reaching the treatment goal of hypertension
|
1- It is suggested that at starting the drug therapy or in case of any change in the type or dosage of antihypertensive drugs, the visit sessions should be held on monthly intervals until reaching the treatment goal 2- It is suggested to check the patient every 3 to 6 months after reaching the treatment goal |
Evidence and rationale
The SR group did not find strong recent evidence on the appropriate visit intervals in patients with hypertension before and after reaching the treatment goal. However, there were older studies reported in this field. In a clinical trial study, the follow-up intervals of 3 months and 6 months were compared with each other in patients aged 30 to 74 years with hypertension. The results showed that the average blood pressure, blood pressure control level, patient satisfaction, and treatment adherence did not differ between 3- and 6-month follow-up intervals [82]. Based on the findings of another clinical trial study, follow-ups with shorter intervals would lead to more predicted favorable outcomes, such as better blood pressure control and monitoring of side effects, and perhaps improved adherence, but may be associated with higher costs. Therefore, longer follow-up periods are inevitably expected to cause harm [79]. In large studies such as ACCORD and SPRINT, which showed significant improvement in cardiovascular events with blood pressure control, the initial follow-up period was one month [83, 84]. Undesirable outcomes of holding follow-up sessions with shorter time intervals would increase the treatment burden on patients and the country's health system.
Evidence-to-decision considerations
No evidence was found for patients' values and preferences regarding the proper intervals for patient visits. Re-evaluation of hypertensive patients over 65 years of age or patients who live alone in shorter time intervals, in addition to faster identification of clinical complications, builds confidence in patients and provides them security; however, in younger and especially asymptomatic patients, visits in short intervals may interfere with their family and work responsibilities [85].
There is no evidence for the costs, resources, and cost-effectiveness of reducing or increasing follow- up intervals; nevertheless, it seems that decreasing intervals would be cost-effective in the elderly, and the application of telemedicine methods for follow-up would be helpful as well [86]. The effect of the suggested proposals is unclear on health inequalities.
The members of the GUG also assessed the acceptability of intervals provided by key beneficiaries as unclear., since patients' adherence to treatment was highly important but was not easily accepted by patients, following up with patients was crucial [30]. The GUG members reached a consensus on an appropriate interval that were suggested for visiting patients under pharmacotherapy before and after reaching the treatment goal.
Box 9.
Suggestion for Minimum tests required to initiate and continue treatment of Hypertension
|
1- It is suggested to examine serum electrolytes, creatinine, lipid profile, fasting sugar, urinalysis, and electrocardiogram before starting pharmacotherapy in people with hypertension 2- It is suggested that during treatment, laboratory tests be performed according to the patient's conditions and the appropriate time intervals for these tests to be determined based on the same criterion. In patients who lack comorbidities, tests should be performed annually |
Evidence and rationale
Before starting pharmacotherapy and to achieve the treatment goal, it is necessary to carry out tests to identify cases of secondary hypertension (in case it was overlooked before) and to detect comorbidities like diabetes mellitus, dyslipidemia damage to lower extremities, and possible side effects of treatments (e.g., high uric acid and abnormal electrolytes), as well as to assess the risk of CVDs, cure other risk factors, and choose appropriate antihypertensive drugs [86].
Performing preliminary tests before initiating the treatment may cause a delay in the onset of the treatment and the possible occurrence of cardiovascular events, therefore, treatment should be started earlier and without delay.
Evidence-to-decision considerations
Concerning patients' values and preferences for conducting tests at the beginning and during hypertension treatment, the members of the GUG believed that there was probably no important uncertainty. However, patients believed that some tests were costly, which sometimes prevents the performance of tests [30]. The results of a cohort study showed that the frequency of doing elderly patients' tests who were recently treated with antihypertensive drugs was lower than expected. Accordingly, 59% of patients had not performed any tests before starting antihypertensive therapy, and at a follow-up with a mean of 10 months, less than half of them had tests done, and nearly two- thirds of them had not had their serum electrolytes or renal function tested even once [87].
The resources and costs required to carry out the recommendations were evaluated as average. The cost of conducting tests for a person is relatively negligible compared to the overall costs of lifelong treatment and complications. Nonetheless, performing tests will have a significant impact on the health systems due to the high prevalence of blood pressure in most societies [88]. In countries with fewer resources, the costs of testing may affect its performance. In regions with plenty of resources, laboratory tests are simple to conduct; however, where resources are scarce, having to conduct such tests before treatment onset can hinder treatment and foster inequality [89]. The recommendations presented were acceptable to the beneficiaries and were implementable.
Box 10.
Recommendation and Suggestion for Telehealth strategies to reduce subsequent complications of hypertension
|
1- It is suggested that employees of health care units use Telehealth strategies for BP treatment and control, such as creating electronic health records, training the use of mobile phone applications, and sending text and video messages 2- In order to monitor and record blood pressure, it is suggested that patients use Telehealth strategies, such as mobile phone applications |
Evidence and rationale
Tele Health strategies, such as Telemedicine, eHealth, and mobile-based technologies (e.g., mobile health [m Health]), are new tools that facilitate the process of controlling patients with hypertension [90]. A telemedicine-based intervention can decrease the level of SBP and DBP during the day in populations suffering from hypertension [91]. However, this drop is influenced by various factors, such as behavioral goals, intervention components, implementation methods, and patient participation [90, 92]. Moreover, noticeable outcomes are associated with the role of social networks, social media, and electronic technology as practicable components of lifestyle modification and disease management programs [92].
Although m Health interventions are generally promising in reducing SBP in hypertensive patients, results are inconsistent and it is unclear which combination of telehealth intervention features is most effective; furthermore, there is no sufficient evidence that telehealth, as an independent strategy, can effectively control blood pressure [93–96].
Good blood pressure management is a multifactorial process and requires the participation of patients, families, health care providers and systems. Implementing strong improvement strategies can be successful in identifying and controlling blood pressure levels [97, 98]. On the other hand, studies have shown that for patients with a stable condition, home blood pressure monitoring and electronic communications with the doctor may provide an acceptable alternative to reduce the frequency of visits [82]. Therefore, such strategies as electronic health records and telephone and electronic follow-ups are recommended.
Evidence-to-decision considerations
Examples of eHealth include mobile and wireless technologies, health information technology, remote medicine (telemedicine), health management, and health-related education [99]. Based on the opinion and experience of the members of the GUG, the use of telehealth strategies is necessary to control chronic diseases and they are accessible and easy to use. Examining the values and preferences of the patients also showed that, from the patients' point of view, these technologies are a desirable method for educating and following up with patients [49].
The overall certainty of the evidence found for the positive effect of employing telehealth strategies on blood pressure control was high, and numerous positive effects, such as improving people's health, and boosting the quality of life, have been reported for all types of these strategies, including mHealth, telemedicine, and eHealth [99–101].
Regarding costs and resource consumption, a meta-analysis study showed that effective eHealth interventions should target large populations since they impose limited additional costs. These interventions should involve a lot of participants, and eHealth should be employed as an alternative to routine care [102]. Regarding this, the required costs and resources were considered low by the GUG members.
Due to the lack of sufficient evidence, GUG believed that the effect of these interventions on health inequalities was unclear, and that this intervention was acceptable to the key beneficiaries and was implementable because its infrastructure was available in the country.
In general, the GUG members, taking into account the results of the previous studies and their clinical experiences, extracted significant key points that needed to be considered in the management of patients with hypertension, which are presented in Table 1. Moreover, the algorithm for diagnosis, management, and treatment of hypertension was drawn based on the recommendations and suggestions of the updated guideline, which is illustrated in Fig. 2. This figure contains all the content presented in the recommendations and suggestions section in a summary form.
Table 1.
Key messages to improve hypertension management
Fig. 2.
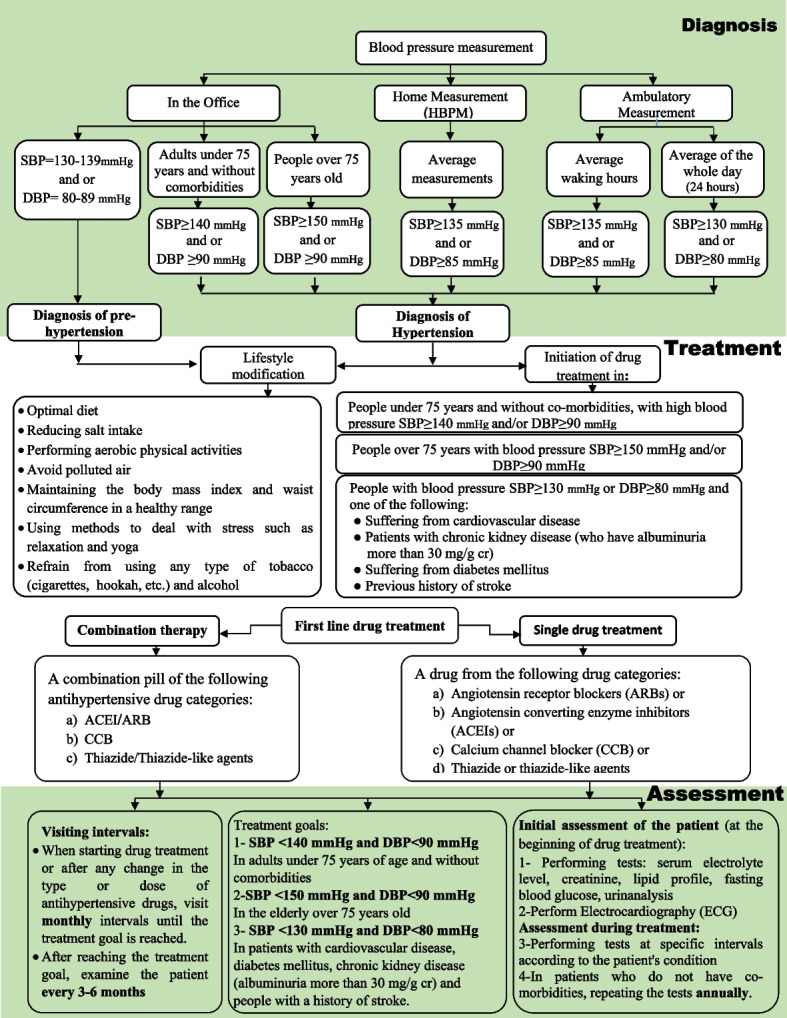
The Flowchart of the Diagnosis and Treatment of Hypertension in Adults (Summary of Guideline Recommendations)
Discussion
Despite significant progress in diagnosis and treatment of hypertension, it is still the most important cause of death from cardiovascular diseases in Iran. In this study, the latest national guideline for hypertension diagnosis, management, and treatment was updated by a group of experts in different but related fields from multiple universities of medical sciences and scientific associations or societies who consist the GUG. They reviewed and updated the scope of previous version of the guideline, including the functional area, target groups, outcomes, and PICO questions, and according to the changes applied in all these sections, they did a comprehensive update.
Participating groups that involved a wide range of health care providers (i.e., specialists, general practitioners, nurses, nutritionist, epidemiologist and health economists) and patients, tried to address the issues in disease management that had not been dealt with in the previous version of the guideline through active participation and interaction, and provide, as much as possible, practical and applicable recommendations and suggestions to resolve them.
In updating of this guideline, an attempt was made to take into account the new developments that have occurred in the management and treatment of hypertension, especially drug treatment and technical interventions. Also, the users of the previous versions of the guideline were only physicians, and other members of the health team were not considered, but the users of the updated version are all PHC health care system employees and patients and their families. In this guideline, a simple and practical treatment algorithm was designed for users. It is also suggested that a mobile application be designed based on guidelines for physicians and health care providers as well as patients and their families in order to apply the recommendations and suggestions of the guideline in the daily management of hypertension.
Conclusion
The authors believe that this guideline, in addition to highly benefitting service providers involved in the diagnosis, treatment, and management of hypertension, will be of great benefit to patients and their families too. It is because this guideline was prepared by employing all standard steps required, using the latest available scientific evidence, and based on the existing infrastructure in the country. Therefore, it is suggested that this guideline be provided to the Ministry of Health to be distributed to all, scientific associations, health centers and followed by the health care providers in the public and private sectors.
Acknowledgements
This project was prepared based on the request of the Vice‑Chancellor for Research of Iran’s Ministry of Health. We appreciate the specialist, faculty members and researchers who contributed to the various stages of project. We also appreciate the physicians and patients for participating in the meetings and expressing their values and preferences, and the support team members and IT experts who cooperated with us in holding the meetings.
Guideline Updating Group (GUG): Mansour Siavash5, Shahrzad Shahidi6, Fariborz Khorvash7, Masoumeh Sadeghi8,2 Hossein Farshidi9,2, Ahmadreza Assareh10,2, Davood Shafie11,2, Masoumeh Jorjani12, Shirinsadat Badri13, Valiollah Hajhashemi14, Ramesh Hoseinkhani15, Mojgan Mortazavi16, Mojdeh Ghabaei17, Somayeh Khanjani18, Elham Hashemi19, Bahar Dehghan20, Majid Davari21, Behzad Fatemi21, Noushin Mohammadifard22, Majid Ghayour Mobarhan23, Maryam Eghbali babadi24, Alireza Ahmadi20, Razieh Hassannejad1, Fereidoun Noohi25,2
Steering Committee: Maryam Kheiri26, Mosa Tabatabaeilotfi27, Sanaz Bakhshandeh27, Azadeh Haghighi27
Systematics Review Group (SRG): Marjan Mansourian28, Ziba Farajzadegan29, Hale Ashraf30, Negar Omidi30, Negah Tavakolifard31, Mahasti Alizade32, Golnaz Vaseghi33
External Review Group: Ebrahim Nematipour34, Samad Ghaffari35,3, Mojgan Sanjari36, Mahmoud Mohammadzade Shabestari37, Maryam Heidarpour5
5. Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
6. Isfahan Kidney Diseases Research Center, Internal Medicine Department, Khorshid Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
7. Isfahan Neurosciences Research Center, Alzahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
8. Cardiac Rehabilitation Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
9. Cardiovascular Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
10. Atherosclerosis Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
11. Heart Failure Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
12. Department of Pharmacology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
13. Department of Clinical Pharmacy and Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
14. Department of Pharmacology and Toxicology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
15. Deputy of Public Health, Isfahan University of Medical Sciences, Isfahan, Iran.
16. Isfahan Kidney Disease Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
17. Iranian Tissue Bank & Preparation Research Centre, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
18. Department of Obstetrics and Gynecology, Perinatologist. Faculty of Medicine. Isfahan University of Medical Sciences, Isfahan, Iran.
19. Metabolic Liver Disease Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
20. Pediatric Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
21. Department of Pharmacoeconomics and Pharmaceutical Administration, Pharmaceutical Management and Economic Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
22. Interventional Cardiology Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
23. Iranian UNESCO Center of Excellence for Human Nutrition, Mashhad University of Medical Sciences, Mashhad, Iran
24. Nursing and Midwifery Care Research Center, Faculty of Nursing and Midwifery, Isfahan University of Medical Sciences, Isfahan, Iran.
25. Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran.
26. Health Department, Ministry of Health and Medical Education, Tehran, Iran.
27. Technology Assessment and Health Standard and Tariff Codification Office, Treatment Department, Ministry of Health and Medical Education, Tehran, Iran.
28. Epidemiology and Biostatistics Department, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran.
29. Community & Preventive Medicine Department, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
30. Cardiac Primary Prevention Research Center (CPPRC), Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
31. Community & Family Medicine Department, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
32. Social Determinants of Health Research Center, Health Management and Safety Promotion Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran.
33. Applied Physiology Research Center, Cardiovascular research institute, Isfahan University of Medical Sciences, Isfahan, Iran
34. Department of Cardiology, School of Medicine, Tehran Heart Center, Tehran University of Medical Sciences, Tehran, Iran.
35. Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
36. Endocrinology and Metabolism Research Center, Institute of Basic and Clinical Physiology Sciences, Kerman University of Medical Sciences, Kerman, Iran.
37. Research Center for Patient Safety, Mashhad University of Medical Sciences, Mashhad, Iran.
Fahimeh Bagheri kholenjani, f_bagherikho@yahoo.com, 0000-0002-4063-891x
Shahla Shahidi, shahidish2003@yahoo.co.in, 0000-0001-5162-5279
Alireza Khosravi, alirezakhosravif@gmail.com, 0000-0003-0736-2090
Asieh Mansouri: mansouri.arn@gmail.com, 0000-0002-8507-6783
Vahid Ashoorion: vashoorion@hrh.ca, 0000-0001-9225-155X
Nizal Sarrafzadegan: nsarrafzadegan@gmail.com, 0000-0002-6828-2169
Mansour Siavash, siavash@med.mui.ac.ir, 0000-0003-0590-6410
Shahrzad Shahidi, shahidi@med.mui.ac.ir, 0000-0002-5442-6424
Fariborz Khorvash, fkhorvash@gmail.com, 0000-0002-3293-8891
Masoumeh Sadeghi, sadeghimasoumeh@gmail.com, 0000-0001-7179-5558
Hossein Farshidi, hfarshidi6@gmail.com, 0000-0002-2144-7798
Ahmadreza Assareh, dr.assareh@gmail.com
Davood Shafie, d.shafie87@gmail.com, 0000-0002-7999-2393
Masoumeh Jorjani, msjorjani@sbmu.ac.ir, 000-0003-4790-4747
Shirinsadat Badri, badri@pharm.mui.ac.ir, 0000-0002-6851-9090
Valiollah Hajhashemi, vhajhashemi@gmail.com, 0000-0001-5783-2324
Ramesh Hosseinkhani, rameshhosseinkhani@ymail.com, 0000-0002-9651-844X
Mojgan Mortazavi, m_mortazavi@med.mui.ac.ir, 000-0002-9636-2553
Mojdeh Ghabaei, mojdeh.ghabaee@gmail.com, 0000-0002-1822-389X
Somayeh Khanjani, somayeh.khanjani@yahoo.com, 0000-0002-2911-2001
Elham Hashemi, hashemielham@ymail.com, 0000-0001-8367-1624
Bahar Dehghan, dr.bahardehghan@gmail.com, 0000-0003-3684-8196
Majid Davari, mim.davari@gmail.com, 0000-0001-7483-0259
Behzad Fatemi, behzad.fatemi63@gmail.com, 0000-0001-7912-7546
Noushin Mohammadifard, noushinmohammadifard@gmail.com, 0000-0003-1776-1060
Majid Ghayour Mobarhan, ghayourm@mums.ac.ir, 0000-0002-1081-6754
Maryam Eghbali babadi, eghbali@nm.mui.ac.ir, 0000-0003-4719-7640
Alireza Ahmadi, ahmadi.cardio@gmail.com, 0000-0002-2462-0162
Razieh.Hassannejad, razieh.hassannejad@gmail.com, 0000-0001-6905-2931
Fereidoun Noohi, fnoohib@yahoo.com
Maryam Kheiri,marysadra@yahoo.com, 0009-0009-3687-378x
Mosa Tabatabaeilotfi, tabatabaei_mt@yahoo.com
Sanaz Bakhshandeh, drsbakhshandeh65@gmail.com
Azadeh Haghighi, Azade_hi@yahoo.com, 0009-0004-9461-9943
Marjan Mansourian, j_mansourian@hlth.mui.ac.ir, 0000-0002-7217-0282
Ziba Farajzadegan, farajzadegan@med.mui.ac.ir, 0000-0001-8548-2843
Hale Ashraf
Negar Omidi, negar.omidi@gmail.com, 0000-0003-2344-1661
Negah Tavakolifard, negahtavakolifard@yahoo.com, 0000-0002-9866-6681
Mahasti Alizade, alizadehm@tbzmed.ac.ir, 0000-0002-9754-2097
Golnaz Vaseghi, golnazvaseghi@yahoo.com, 0000-0003-3040-6135
Ebrahim Nematipour: dr_nematipour@tums.ac.ir
Samad Ghaffari: ghafaris@gmail.com, 0000-0168-0693-8700
Mojgan Sanjari: mjnsanjari@gmail.com, 0000-0002-3402-4401
Mahmoud Mohammadzade Shabestari: shabestarimd@gmail.com
Maryam Heidarpour: heidarpourmaryam110@gmail.com, 0000-0002-4809-4072
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitors
- ARBs
Angiotensin receptor blockers
- ABPM
Ambulatory Blood Pressure Monitoring
- BP
Blood Pressure
- CCBs
Calcium Channel Blockers
- CVD
Cardiovascular Disease
- CKD
Chronic Kidney Disease
- CR
Creatinine
- DM
Diabetes Mellitus
- EF
Ejection Fraction
- ERG
External Review Group
- FGD
Focus Group Discussion
- GUG
Guideline Updating Group
- HBPM
Home Blood Pressure Monitoring
- mg/gCr
Milligram Per Gram Creatinine
- PICO
Population, Interventions, Control, Outcome
- SC
Steering Committee
- SRG
Systematic Review Group
Authors’ contributions
NS, FB, SHSH, AKH, AM and VA designed the project, Coordinated and managed online meetings, Prepared the content of the meetings with guideline updating group and drafted the work. MKH, MT, SB AND AH were in charge of order and financing the plan and supervised the updating process. MM, ZF, HA, NO, NT, MA and GV were in charge of searching for relevant evidence and GRADING them. MS, SHSH, FKH, MS, HF, AA, DSH, MJ, SHB, VH, RH, MM, MGH, SKH, EH, BD, MD, BF, NM, MGH, ME, AA, RH and FN reviewed and interpreted the evidence related to each PICO question, completed the evidence-to-decision table, and updated the recommendations and suggestions based on them. EN, SGH, MS, MMSH and MH evaluated the different stages of updating and also the final version of the guideline. All authors read and approved the final manuscript.
Funding
This study was funded by Vice-Chancellor for Treatment of Iran’s Ministry of Health.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nizal Sarrafzadegan, Email: nsarrafzadegan@gmail.com.
Guideline Updating Group (GUG):
Mansour Siavash, Shahrzad Shahidi, Fariborz Khorvash, Masoumeh Sadeghi, Hossein Farshidi, Ahmadreza Assareh, Davood Shafiei, Masoumeh Jorjani, Shirinsadat Badri, Valiollah Hajhashemi, Ramesh Hoseinkhani, Mojgan Mortazavi, Mojdeh Ghabaei, Somayeh Khanjani, Elham Hashemi, Bahar Dehghan, Majid Davari, Behzad Fatemi, Noushin Mohammadifard, Majid Ghayour Mobarhan, Maryam Eghbali babadi, Alireza Ahmadi, Razieh Hassannejad, and Fereidoun Noohi
Steering Committee:
Maryam Kheiri, Mosa Tabatabaeilotfi, Sanaz Bakhshandeh, and Azadeh Haghighi
Systematics Review Group (SRG):
Marjan Mansourian, Ziba Farajzadegan, Hale Ashraf, Negar Omidi, Negah Tavakolifard, Mahasti Alizade, and Golnaz Vaseghi
External Review Group (ERG):
Ebrahim Nematipour, Samad Ghaffari, Mojgan Sanjari, Mahmoud Mohammadzade Shabestari, and Maryam Heidarpour
References
- 1.Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. doi: 10.1371/journal.pmed.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García LM, Pardo-Hernández H, Sanabria AJ, Alonso-Coello P, Penman K, McFarlane E, et al. Guideline on terminology and definitions of updating clinical guidelines: the updating glossary. J Clin Epidemiol. 2018;95:28–33. doi: 10.1016/j.jclinepi.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg E, Greenfield S, Wolman DM, Mancher M, Graham R. Clinical practice guidelines we can trust. Washington, D.C: National Academies Press; 2011. [PubMed]
- 4.Alderson LJ, Alderson P, Tan T. Median life span of a cohort of National Institute for Health and Care Excellence clinical guidelines was about 60 months. J Clin Epidemiol. 2014;67(1):52–55. doi: 10.1016/j.jclinepi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Martínez García L, Arévalo-Rodríguez I, Solà I, Haynes RB, Vandvik PO, Alonso-Coello P, et al. Strategies for monitoring and updating clinical practice guidelines: a systematic review. Implement Sci. 2012;7:1–10. doi: 10.1186/1748-5908-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Coello P, Martinez Garcia L, Carrasco JM, Solà I, Qureshi S, Burgers JS, et al. The updating of clinical practice guidelines: insights from an international survey. Implement Sci. 2011;6:1–8. doi: 10.1186/1748-5908-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyratzopoulos G, Barnes S, Stegenga H, Peden S, Campbell B. Updating clinical practice recommendations: is it worthwhile and when? Int J Technol Assess Health Care. 2012;28(1):29–35. doi: 10.1017/S0266462311000675. [DOI] [PubMed] [Google Scholar]
- 8.Sarrafzadegan N, Bagherikholenjani F, Noohi F, Alikhasi H, Mohammadifard N, Ghaffari S, et al. Priority setting in cardiovascular research in Iran using standard indigenous methods. J Res Med Sci. 2022;27(1):91. 10.4103/jrms.jrms_343_22. [DOI] [PMC free article] [PubMed]
- 9.Ogedegbe G. Barriers to optimal hypertension control. J Clin Hyperten. 2008;10(8):644–646. doi: 10.1111/j.1751-7176.2008.08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelton P, Carey R, Aronow W. Acc/aha/aapa/abc/acpm/ags/APhA/ASH/ASPC/nma/pcna guideline for the prevention, Detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American heart Association. Task force on clinical practice guidelines//J. Am. Coll. Cardiol.-2017. Пo чки. 2018;7(1):68–74. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 12.Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, et al. Hypertension Canada's 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569–588. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 13.Corr L. NICE guideline: the diagnosis and management of hypertension. Trends Urol Men’s Health. 2019;10(6):10–14. doi: 10.1002/tre.719. [DOI] [Google Scholar]
- 14.Noohi F, Sarrafzadegan N, Khosravi A, Andalib E, Group FRoHBPW The first Iranian recommendations on prevention, evaluation and management of high blood pressure. ARYA Atherosclerosis. 2012;8(3):97. [PMC free article] [PubMed] [Google Scholar]
- 15.Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Lee ET, Devereux RB, Yeh J, Best LG, Fabsitz RR, et al. Prehypertension, diabetes, and cardiovascular disease risk in a population-based sample: the Strong Heart Study. Hypertension. 2006;47(3):410–414. doi: 10.1161/01.HYP.0000205119.19804.08. [DOI] [PubMed] [Google Scholar]
- 17.Shahaj O, Denneny D, Schwappach A, Pearce G, Epiphaniou E, Parke HL, et al. Supporting self-management for people with hypertension: a meta-review of quantitative and qualitative systematic reviews. J Hypertens. 2019;37(2):264–279. doi: 10.1097/HJH.0000000000001867. [DOI] [PubMed] [Google Scholar]
- 18.Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377(8):745–755. doi: 10.1056/NEJMsa1616035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guideline NG136 N. Hypertension in adults: diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2023. (NICE Guideline, No. 136.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK547161/. [PubMed]
- 20.Canoy D, Nazarzadeh M, Copland E, Bidel Z, Rao S, Li Y, et al. How much lowering of blood pressure is required to prevent Cardiovascular Disease in patients with and without previous cardiovascular disease? Curr Cardiol Rep. 2022;24(7):851–860. doi: 10.1007/s11886-022-01706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung M-H, Yi S-W, An SJ, Yi J-J. Age-specific associations between systolic blood pressure and cardiovascular mortality. Heart. 2019;105(14):1070–77. [DOI] [PubMed]
- 22.Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afsargharehbagh R, Rezaie-Keikhaie K, Rafiemanesh H, Balouchi A, Bouya S, Dehghan B. Hypertension and pre-hypertension among Iranian adults population: a meta-analysis of prevalence, awareness, treatment, and control. Curr Hypertens Rep. 2019;21:1–13. doi: 10.1007/s11906-019-0933-z. [DOI] [PubMed] [Google Scholar]
- 24.Shirani S, Kelishadi R, Sarrafzadegan N, Khosravi A, Sadri G, Amani A, et al. Awareness, treatment and control of hypertension, dyslipidaemia and diabetes mellitus in an Iranian population: the IHHP study. EMHJ-East Mediter Health J. 2009;15(6):1455–1463. [PubMed] [Google Scholar]
- 25.Lindsay P, Gorber SC, Joffres M, Birtwhistle R, McKay D, Cloutier L. Recommendations on screening for high blood pressure in Canadian adults. Can Fam Phys. 2013;59(9):927–933. [PMC free article] [PubMed] [Google Scholar]
- 26.Commodore-Mensah Y, Turkson-Ocran R-A, Foti K, Cooper LA, Himmelfarb CD. Associations between social determinants and hypertension, stage 2 hypertension, and controlled blood pressure among men and women in the United States. Am J Hypertens. 2021;34(7):707–717. doi: 10.1093/ajh/hpab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson KW, Kemper AR, Doubeni CA, Tseng C-W, Simon MA, Kubik M, et al. Developing primary care–based recommendations for social determinants of health: methods of the US Preventive Services Task Force. Ann Intern Med. 2020;173(6):461–467. doi: 10.7326/M20-0730. [DOI] [PubMed] [Google Scholar]
- 28.Kirkland EB, Heincelman M, Bishu KG, Schumann SO, Schreiner A, Axon RN, et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003–2014. J Am Heart Assoc. 2018;7(11):e008731. doi: 10.1161/JAHA.118.008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Success Story: FQHC Relies on empowered care teams for underserved populations. Esperanza Health Centers, Chicago, Illinois. 2019.
- 30.Qualitative evaluation report of values, preferences and views of general practitioners regarding the problems of hypertensive patients. 2021.
- 31.Arguedas JA, Leiva V, Wright JM. Blood pressure targets in adults with hypertension. Cochr Database Syst Rev. 2020(12):Art. No.: CD004349. 10.1002/14651858.CD004349.pub3. [DOI] [PMC free article] [PubMed]
- 32.Garrison SR, Kolber MR, Korownyk CS, McCracken RK, Heran BS, Allan GM. Blood pressure targets for hypertension in older adults. Cochrane Database Syst Rev. 2017(8):Art. No.: CD011575. 10.1002/14651858.CD011575.pub2. Accessed 25 Mar 2024. [DOI] [PMC free article] [PubMed]
- 33.Murad MH, Larrea-Mantilla L, Haddad A, Spencer-Bonilla G, Serrano V, Rodriguez-Gutierrez R, et al. Antihypertensive agents in older adults: a systematic review and meta-analysis of randomized clinical trials. J Clin Endocrinol Metab. 2019;104(5):1575–1584. doi: 10.1210/jc.2019-00197. [DOI] [PubMed] [Google Scholar]
- 34.Saiz LC, Gorricho J, Garjón J, Celaya MC, Erviti J, Leache L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Syst Rev. 2022(11):Art.No.: CD010315. 10.1002/14651858.CD010315.pub5. Accessed 25 Mar 2024. [DOI] [PMC free article] [PubMed]
- 35.Group SR A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard K, White S, Salkeld G, McDonald S, Craig JC, Chadban S, et al. Cost-effectiveness of screening and optimal management for diabetes, hypertension, and chronic kidney disease: a modeled analysis. Value Health. 2010;13(2):196–208. doi: 10.1111/j.1524-4733.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- 37.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 38.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 39.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 40.Jin A, Xie W, Wu Y. Effect of salt reduction interventions in lowering blood pressure in Chinese populations: a systematic review and meta-analysis of randomised controlled trials. BMJ open. 2020;10:e032941. 10.1136/bmjopen-2019-032941. [DOI] [PMC free article] [PubMed]
- 41.He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. 10.1136/bmj.f1325. [DOI] [PubMed]
- 42.Kastorini C-M, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 43.Polito MD, Dias JR, Jr, Papst RR. Resistance training to reduce resting blood pressure and increase muscle strength in users and non-users of anti-hypertensive medication: a meta-analysis. Clin Exp Hypertens. 2021;43(5):474–485. doi: 10.1080/10641963.2021.1901111. [DOI] [PubMed] [Google Scholar]
- 44.Zhong D, Li J, Yang H, Li Y, Huang Y, Xiao Q, et al. Tai Chi for essential hypertension: a systematic review of randomized controlled trials. Curr Hypertens Rep. 2020;22:1–12. doi: 10.1007/s11906-020-1031-y. [DOI] [PubMed] [Google Scholar]
- 45.Ohta Y, Kawano Y, Hayashi S, Iwashima Y, Yoshihara F, Nakamura S. Effects of cigarette smoking on ambulatory blood pressure, heart rate, and heart rate variability in treated hypertensive patients. Clin Exp Hypertens. 2016;38(6):510–513. doi: 10.3109/10641963.2016.1148161. [DOI] [PubMed] [Google Scholar]
- 46.Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38(5):1112–1117. doi: 10.1161/hy1101.093424. [DOI] [PubMed] [Google Scholar]
- 47.Acin MT, Rueda J-R, Saiz LC, Mathias VP, Alzueta N, Solà I, et al. Alcohol intake reduction for controlling hypertension. Cochr Database Syst Rev. 2020;2012(9):CD010022. 10.1002/14651858.CD010022. [DOI] [PMC free article] [PubMed]
- 48.Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004;329(7458):145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qualitative evaluation report of values and preferences of HTN patients. Development and Adapting Guidelines Department. 2021.
- 50.Sutton L, Karan A, Mahal A. Evidence for cost-effectiveness of lifestyle primary preventions for cardiovascular disease in the Asia-Pacific Region: a systematic review. Glob Health. 2014;10(1):1–19. doi: 10.1186/s12992-014-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Ramakrishnan R, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. The Lancet. 2021;397(10285):1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 53.Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Wamil M, et al. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398(10305):1053–1064. doi: 10.1016/S0140-6736(21)01921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong X-L, Dong Y, Xu W, Huang Y-Y, Wang H-F, Zhang T-S, et al. Role of blood pressure management in stroke prevention: a systematic review and network meta-analysis of 93 randomized controlled trials. J Stroke. 2021;23(1):1–11. doi: 10.5853/jos.2020.02698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takami Y, Yamamoto K, Arima H, Sakima A. Target blood pressure level for the treatment of elderly hypertensive patients: a systematic review and meta-analysis of randomized trials. Hypertens Res. 2019;42(5):660–668. doi: 10.1038/s41440-019-0227-5. [DOI] [PubMed] [Google Scholar]
- 56.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Djama NM, Lai Y, Li H, Liao W, Liao Y, et al. Cardiovascular outcomes in patients with diabetes when initiating blood pressure lowering at baseline SBP between 130 and 140 mm Hg: a meta-analysis. J Clin Hypertension. 2019;21(2):220–229. doi: 10.1111/jch.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 59.Asayama K, Ohkubo T, Satoh A, Tanaka S, Higashiyama A, Murakami Y, et al. Cardiovascular risk and blood pressure lowering treatment among elderly individuals: Evidence for cardiovascular prevention from observational cohorts in Japan. J Hypertens. 2018;36(2):410–418. doi: 10.1097/HJH.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 60.Johnson R, Turner K, Feder G, Cramer H. Shared decision making in consultations for hypertension: Qualitative study in general practice. Health Expect. 2021;24(3):917–929. doi: 10.1111/hex.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kisigo GA, Mcharo OC, Robert JL, Peck RN, Sundararajan R, Okello ES. Understanding barriers and facilitators to clinic attendance and medication adherence among adults with hypertensive urgency in Tanzania. PLOS Global Public Health. 2022;2(8):e0000919. doi: 10.1371/journal.pgph.0000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 63.Meiqari L, Nguyen TPL, Essink D, Zweekhorst M, Wright P, Scheele F. Access to hypertension care and services in primary health-care settings in Vietnam: a systematic narrative review of existing literature. Global Health Action. 2019;12(1):1610253. doi: 10.1080/16549716.2019.1610253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seeley A, Prynn J, Perera R, Street R, Davis D, Etyang AO. Pharmacotherapy for hypertension in Sub-Saharan Africa: a systematic review and network meta-analysis. BMC Med. 2020;18:1–11. doi: 10.1186/s12916-020-01530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu J, Chen N, Zhou M, Guo J, Zhu C, Zhou J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochr Database Syst Rev. 2021(10):1–80. [DOI] [PMC free article] [PubMed]
- 66.den Brok MG, van Dalen JW, Abdulrahman H, Larson EB, van Middelaar T, van Gool WA, et al. Antihypertensive medication classes and the risk of dementia: a systematic review and network meta-analysis. J Am Med Directors Assoc. 2021;22(7):1386–95. doi: 10.1016/j.jamda.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 67.Chung EYM, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC, Strippoli GFM. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020(10):Art. No.: CD007004. 10.1002/14651858.CD007004.pub4. Accessed 25 Mar 2024. [DOI] [PMC free article] [PubMed]
- 68.Wright JT, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293(13):1595–1608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 69.Sareli P, Radevski IV, Valtchanova ZP, Libhaber E, Candy GP, Den Hond E, et al. Efficacy of different drug classes used to initiate antihypertensive treatment in black subjects: results of a randomized trial in Johannesburg, South Africa. Arch Inter Med. 2001;161(7):965–971. doi: 10.1001/archinte.161.7.965. [DOI] [PubMed] [Google Scholar]
- 70.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. Arterial Hypertension. 2013;17(2):69–168. [Google Scholar]
- 71.Julius S, Kjeldsen SE, Brunner H, Hansson L, Platt F, Ekman S, et al. VALUE trial: Long-term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am J Hypertens. 2003;16(7):544–548. doi: 10.1016/S0895-7061(03)00904-X. [DOI] [PubMed] [Google Scholar]
- 72.Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292(18):2217–2225. doi: 10.1001/jama.292.18.2217. [DOI] [PubMed] [Google Scholar]
- 73.Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, et al. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med. 2015;372(5):447–455. doi: 10.1056/NEJMsa1406751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faria C, Wenzel M, Lee KW, Coderre K, Nichols J, Belletti DA. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267–276. doi: 10.1016/j.jash.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47(3):345–351. doi: 10.1161/01.HYP.0000200702.76436.4b. [DOI] [PubMed] [Google Scholar]
- 76.Salam A, Kanukula R, Atkins E, Wang X, Islam S, Kishore SP, et al. Efficacy and safety of dual combination therapy of blood pressure-lowering drugs as initial treatment for hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37(9):1768–1774. doi: 10.1097/HJH.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 77.Garjón J, Saiz LC, Azparren A, Gaminde I, Ariz MJ, Erviti J. First‐line combination therapy versus first‐line monotherapy for primary hypertension. Cochrane Database Syst Rev. 2020(2):Art. No.:CD010316. 10.1002/14651858.CD010316.pub3. Accessed 25 Mar 2024. [DOI] [PMC free article] [PubMed]
- 78.Kwon A, Kim G-H. Single-pill combination therapy of Azilsartan Medoxomil/Chlorthalidone for treatment of hypertension: a systematic review. Clin Ther. 2020;42(7):1390–1403. doi: 10.1016/j.clinthera.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Rea F, Corrao G, Merlino L, Mancia G. Initial antihypertensive treatment strategies and therapeutic inertia: evidence from a large population-based cohort. Hypertension. 2018;72(4):846–853. doi: 10.1161/HYPERTENSIONAHA.118.11308. [DOI] [PubMed] [Google Scholar]
- 80.Krousel-Wood M, Thomas S, Muntner P, Morisky D. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19(4):357–362. doi: 10.1097/01.hco.0000126978.03828.9e. [DOI] [PubMed] [Google Scholar]
- 81.Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens. 2011;13(12):898–909. doi: 10.1111/j.1751-7176.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Birtwhistle RV, Godwin MS, Delva MD, Casson RI, Lam M, MacDonald SE, et al. Randomised equivalence trial comparing three month and six month follow up of patients with hypertension by family practitioners. BMJ. 2004;328(7433):204. doi: 10.1136/bmj.37967.374063.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cushman WC, Grimm RH, Jr, Cutler JA, Evans GW, Capes S, Corson MA, et al. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12):S44–S55. doi: 10.1016/j.amjcard.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Walker RC, Tong A, Howard K, Palmer SC. Patient expectations and experiences of remote monitoring for chronic diseases: systematic review and thematic synthesis of qualitative studies. Int J Med Informatics. 2019;124:78–85. doi: 10.1016/j.ijmedinf.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Organization WH. Guideline for the pharmacological treatment of hypertension in adults. World Health Organization; 2021. [PubMed]
- 87.Xu W, Goldberg SI, Shubina M, Turchin A. Optimal systolic blood pressure target, time to intensification, and time to follow-up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158. 10.1136/bmj.h158. [DOI] [PMC free article] [PubMed]
- 88.Fragasso G, Maranta F, Montanaro C, Salerno A, Torlasco C, Margonato A. Pathophysiologic therapeutic targets in hypertension: a cardiological point of view. Expert Opin Ther Targets. 2012;16(2):179–193. doi: 10.1517/14728222.2012.655724. [DOI] [PubMed] [Google Scholar]
- 89.Golicki D, Niewada M, Jakubczyk M, Wrona W, Hermanowski T. Self-assessed health status in Poland: EQ-5D findings from the Polish valuation study. Pol Arch Med Wewn. 2010;120(7–8):276–281. [PubMed] [Google Scholar]
- 90.Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, et al. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2015;132(12):1157–1213. doi: 10.1161/CIR.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao W, Lv X, Xu X, Zhang Z, Yan J, Mao G, et al. Telemedicine interventions to reduce blood pressure in a chronic disease population: A meta-analysis. J Telemed Telecare. 2022;28(9):621–631. doi: 10.1177/1357633X20959581. [DOI] [PubMed] [Google Scholar]
- 92.Ma Y, Cheng HY, Cheng L, Sit JW. The effectiveness of electronic health interventions on blood pressure control, self-care behavioural outcomes and psychosocial well-being in patients with hypertension: A systematic review and meta-analysis. Int J Nurs Stud. 2019;92:27–46. doi: 10.1016/j.ijnurstu.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Omboni S, Gazzola T, Carabelli G, Parati G. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013;31(3):455–468. doi: 10.1097/HJH.0b013e32835ca8dd. [DOI] [PubMed] [Google Scholar]
- 94.Verberk WJ, Kessels AG, Thien T. Telecare is a valuable tool for hypertension management, a systematic review and meta-analysis. Blood Press Monit. 2011;16(3):149–155. doi: 10.1097/MBP.0b013e328346e092. [DOI] [PubMed] [Google Scholar]
- 95.Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57(1):29–38. doi: 10.1161/HYPERTENSIONAHA.110.160911. [DOI] [PubMed] [Google Scholar]
- 96.Liu S, Dunford SD, Leung YW, Brooks D, Thomas SG, Eysenbach G, et al. Reducing blood pressure with Internet-based interventions: a meta-analysis. Can J Cardiol. 2013;29(5):613–621. doi: 10.1016/j.cjca.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 97.Walsh JM, McDonald KM, Shojania KG, Sundaram V, Nayak S, Lewis R, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44(7):646–57. [DOI] [PubMed]
- 98.Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension. 2014;63(4):878–885. doi: 10.1161/HYP.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Dyk L. A review of telehealth service implementation frameworks. Int J Environ Res Public Health. 2014;11(2):1279–1298. doi: 10.3390/ijerph110201279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coorey GM, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol. 2018;25(5):505–521. doi: 10.1177/2047487317750913. [DOI] [PubMed] [Google Scholar]
- 101.Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res. 2016;18(5):e97. doi: 10.2196/jmir.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blok S, van der Linden EL, Somsen GA, Tulevski II, Winter MM, Van den Born B-JH. Success factors in high-effect, low-cost eHealth programs for patients with hypertension: a systematic review and meta-analysis. Eur J Prev Cardiol. 2021;28(14):1579–87. doi: 10.1177/2047487320957170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.