Abstract
We performed cardiac resynchronization therapy by means of conduction system pacing in a heart transplant patient suffering from heart failure with reduced ejection fraction and atrial fibrillation with conduction disturbance (bifascicular block and QRS >160 ms). ECG monitoring showed paroxysmal atrioventricular block. Biventricular pacing was not feasible due to the absence of a suitable coronary sinus branch for pacing. His bundle pacing was performed, and an implantable cardioverter-defibrillator was implanted due to severe left ventricular dysfunction. Cardiac allograft vasculopathy was excluded. During follow-up, the patient's left ventricular function improved, and symptoms alleviated with a high percentage of ventricular stimulation.
Keywords: Cardiac pacing, Conduction system pacing, Implantable cardioverter-defibrillator, Heart transplantation, Heart failure
Highlights
-
•
Heart Transplantation patients often develop conduction disturbance and require pacemaker implantation.
-
•
Conduction System pacing in Heart Transplantation is an unexplored field that brings novel challenges.
-
•
Cardiac Resynchronization Therapy with Conduction System Pacing can improve ejection fraction in heart transplant recipients.
-
•
The implantable cardioverter-defibrillator use can be crucial in selected Heart Transplant patients.
1. Introduction
Atrioventricular block (AVB) is a common cause of late permanent pacemaker (PM) implantation in heart transplant (HTx) recipients. The causes for permanent or complete AVB are unclear, but increased donor age and longer operative time (including total ischemia time and reperfusion time) have been associated with an increased risk of AVB [1].
Patients presenting with both AVB and reduced left ventricular ejection fraction can benefit from cardiac resynchronization therapy, and in cases where coronary sinus venography reveals an unsuitable coronary vein for left ventricular lead deployment, Conduction System Pacing (CSP) should be considered [2]. To our knowledge, no cases of cardiac resynchronization therapy (CRT) using CSP have been described in HTx recipients with left ventricular dysfunction.
In this report, we present a His bundle pacing strategy in a 65-year-old patient who underwent heart transplantation 20 years ago. The patient presented with conduction system disease, atrial fibrillation and severe ventricular dysfunction and inadequate anatomy for biventricular pacing.
2. Case report
A 65-year-old male patient who underwent HTx 20 years ago and chronic rejection was admitted to the intensive care unit for acute decompensated heart failure during paroxysmal atrial fibrillation (AF) with a high ventricular response. 3 years earlier he was diagnosed of a moderate rejection at endocardial biopsy (Grade 2R), treated successfully with high dose corticosteroids and recovery of normal ejection fraction. He also suffered of chronic rejection with positive HLA II DQ with high MFI levels requiring cycles of plasmapheresis. The patient presented to our department with heart failure and a severely reduced ejection fraction of 25 %, due to a diffuse hypokinesia and acute renal failure.
Pharmacological cardioversion was performed, and a 12-lead ECG in sinus rhythm revealed right bundle branch block (RBBB) and left anterior fascicular block (LAFB) with a normal PR interval (Fig. 1A). The QRS duration was 168 ms, as shown in Fig. 1B. Despite being in sinus rhythm, the patient was classified as NYHA class III. While the patient was at rest and didn't complain any symptom, continuous ECG monitoring revealed paroxysmal complete AVB.
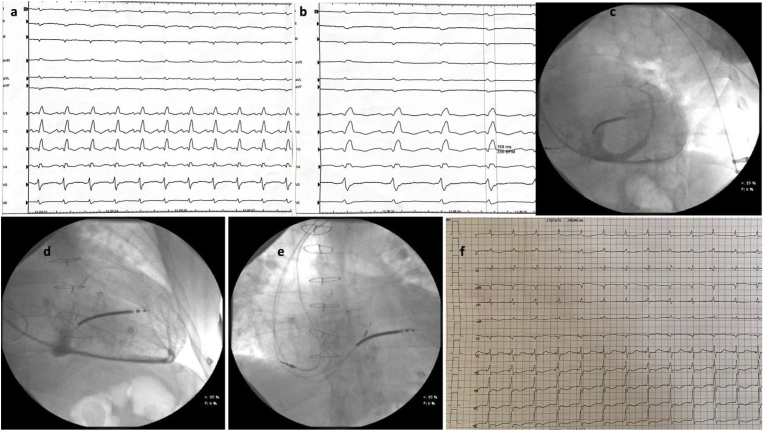
Fig. 1.
A: basal ECG; B: ECG at 100mm/s and QRS duration; C: using right anterior oblique fluoroscopy projection the coronary sinus was contrasted; D: Left anterior oblique fluoroscopy projection that show an apical posterior vein and a small lateral vein; E: HIS position of the 3830 lead with an anterior-posterior view; F: final ECG.
To exclude allograft vasculopathy, coronary angiography was performed, which showed no signs of the condition. Additionally, an endomyocardial biopsy was conducted to investigate the cause of ventricular dysfunction, revealing no evidence of acute cellular rejection (Grade 0 of International Society for Heart & Lung Transplantation scale). Cardiac Magnetic Resonance was not performed because of a low glomerular filtration rate (<30 ml/min), that contraindicated gadolinium administration.
Considering the low ejection fraction and the need for pacing due to AVB with a wide QRS, the patient was eligible for cardiac resynchronization therapy with an implantable defibrillator and biventricular pacing (CRT-D) according to ESC guidelines [2].
Due to the patient's previous cardiac surgery, venography of the left venous system was performed, confirming left subclavian vein patency. The wires and catheter were advanced through axillary vein access obtained with three different punctures. Active fixation leads were used for both the right ventricle and right atrium. Contrast injection in the coronary sinus revealed no branches of adequate caliber for biventricular pacing (Fig. 1C and D), leading to the dismissal of the coronary sinus as an option. Consequently, the decision was made to proceed with CSP, with the initial focus on His bundle pacing (HBP).
HBP was performed using the Select Secure pacing lead (Model 3830, 69 cm, Medtronic) delivered through a fixed curve sheath (Model C315 HIS, Medtronic). His region was identified with endocardial mapping until a distal His bundle signal was obtained, even though with low sensing values. HV time was normal: 45 ms.
During the pace testing, it was observed that the His bundle was captured, resulting in a narrow QRS and correction of the conduction disturbance, while simultaneously screwing in the lead.
After the fixation, at high pacing output the ECG showed nonselective His bundle capture (QRS width 110 ms), and myocardial capture (150ms) at low voltage. Mapping of the left bundle area was also performed, but despite achievement of a narrow QRS (120 ms), this area showed high capture thresholds probably due to tissue disease of the transplanted heart (possible causes will be discussed in the next paragraph).
Due to the better QRS duration, we preferred to perform a CRT using His pacing and to allocate the lead in the previous place due to better QRS duration. A rapid rotation of the lead was performed, and a deep penetration was achieved. The lead advancement was performed in LAO 40°. Final QRS duration was 105 ms (Fig. 1F). The X-ray lead placement in distal His is shown in Fig. 1E.
High-output to low-output pacing was then recorded. The threshold obtained for non-selective capture was 2V × 1 ms with a QRS duration of 105 ms (Fig. 1F), while lower output showed myocardial capture with wider QRS. The amplitude was programmed to 3V × 1 ms. A calculation of battery longevity was 4 years. After assessing the lead stability, the delivery system was retracted gently while advancing the lead with push and pull technique, reducing lead tension and the risk of dislodgement. No complications occurred during and after the implantation. The patient was dismissed with optimal medical therapy.
After 6 months follow up, the patient showed an improved ejection fraction from 25 to 40 % and a reduction of NYHA class from III to I.
3. Discussion
The improved survival rates for heart transplant recipients over time present cardiologists with new challenges in managing various complications such as chronic rejection, coronary allograft vasculopathy, graft dysfunction, and arrhythmias in the context of longer life expectancy.
Conduction system disease is common among HTx patients and can cause conduction disturbances [3]. Factors contributing to histological alterations and altered conduction tissue characteristics must be considered during PM implantation as they can lead to higher capture thresholds, worse sensing, and impedance parameters. These factors include chronic rejection, coronary allograft vasculopathy, fibrosis, and may also be affected by scarring because of persistent inflammatory phenomena, as well as bathmotropy alterations due to heart denervation [4].
Cardiac resynchronization is an established therapy for symptomatic heart failure with reduced ejection fraction and QRS duration >130 ms. The traditional technique involves implantation of pacing leads in both the right ventricle and the lateral wall of the left ventricle through the coronary sinus [7]. Current clinical trials evaluating the benefits of CRT and implantable cardioverter-defibrillator (ICD) therapies did not include HTx recipients, and the indications for CRT and ICD implantation are currently the same as for non-HTx patients.
CSP is an emerging method for effective cardiac resynchronization, and techniques for CSP implantation have been recently described [6]. However, the indications for CSP are still under debate, as the majority of the literature on CRT primarily focuses on biventricular pacing, with limited experience reported on CSP [5]. These new techniques allow for stimulation of the conduction system along the His bundle or the left bundle branch area, providing a more physiological activation of the ventricular mass.
Current indications suggest considering CSP only in cases of unsuccessful biventricular pacing. Large registries have demonstrated a steep learning curve, safety, and feasibility of CSP, which may lead to new indications for its use. However, there is a lack of consistent data on the usefulness of CRT in HTx recipients, especially in the presence of right bundle branch block (RBBB), which is common and represents an additional element of graft dysfunction [8]. Some reports suggest that patients who develop heart failure after HTx and require frequent pacing could benefit from CRT [7,9].
In this report, we present a case of cardiac resynchronization therapy using CSP in a patient with left ventricular dysfunction, atrial fibrillation, RBBB, LAFB and paroxysmal AVB.
AVB in HTx patients tends to be a late manifestation of conduction system disease, while sinus node dysfunction typically occurs in the early postoperative period. AVB is often paroxysmal and sometimes underdiagnosed due to non-specific clinical manifestations.
Furthermore, patients who receive pacemakers for AVB in HTx, may require high percentage of ventricular pacing [10], making CSP potentially effective in preventing pacing-induced ventricular dysfunction. Additionally, ventricular dysfunction may play a role in determining CRT indication and response.
A right ventricle myocardial biopsy was performed during the stay to rule out acute cellular rejection. Despite the biopsy results being negative, it is plausible that the major cause of graft dysfunction was chronic rejection. This is particularly attributed to antibody-mediated humoral rejection against donor major histocompatibility complex antigens (HLA-I and II), which can lead to changes in capillary endothelium. Such changes can result in myocardial damage without any macroscopic evidence of vasculopathy.
In this scenario, conduction system disease could be an epiphenomenon of graft dysfunction, which in this case led to a wide QRS (>160 ms). The wide QRS, in conjunction with atrial fibrillation, could potentially exacerbate the deterioration of graft function. In this complex situation, a multidisciplinary approach involving medical therapy and cardiac resynchronization therapy was employed, leading to the recovery of left ventricular ejection fraction.
In the limited reports on CRT implantation in HTx patients and ICD implantation, rejection has been a major cause for implantation, but more data is required to address a clear benefit in terms of heart failure recurrence or mortality. In our case report a HBP approach to cardiac resynchronization therapy allowed to achieve a narrow QRS complex. After the acute phase, the patient recovered the ability to practise daily life activities and left ventricular ejection fraction increased to 40 %.
4. Conclusion
In this case, we demonstrate the feasibility of CSP in a heart transplant patient experiencing graft dysfunction due to multiple factors. These include high-rate atrial fibrillation, low ejection fraction, and conduction disturbance with paroxysmal AVB.
The indications for Cardiac Resynchronization Therapy in heart transplantation are not standardized, and the clinical benefits are unclear due to a lack of data and multiple confounding factors such as chronic rejection.
Pacing the conduction system in heart transplant patients presents unique challenges, such as the risk of encountering high threshold areas due to allograft rejection. Despite these complications, CRT implantation and HBP procedures are technically feasible. The results, as demonstrated in this case, are comparable with those of non-transplanted hearts.
Ethical statement
Written informed consent was obtained from the patient.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Nothing to declare.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Wellmann P., Herrmann F.E., Hagl C., Juchem G. A single center study of 1,179 heart transplant patients-factors affecting pacemaker implantation. Pacing Clin Electrophysiol. 2017;40(3):247–254. doi: 10.1111/pace.13021. [DOI] [PubMed] [Google Scholar]
- 2.Glikson M., Nielsen J.C., Kronborg M.B., et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy [published correction appears in Europace. 2022 Mar 07;:] Europace. 2021;24(1):71–164. doi: 10.1093/europace/euab232. 2022. [DOI] [PubMed] [Google Scholar]
- 3.DeFilippis, Ersilia M., et al. Cardiac implantable electronic devices following heart transplantation. JACC Clin Electrophysiol. 2020;6(8):1028–1042. doi: 10.1016/j.jacep.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Bharati S., Billingham M., Lev M. The conduction system in transplanted hearts. Chest. 1992;102(4):1182–1188. doi: 10.1378/chest.102.4.1182. [DOI] [PubMed] [Google Scholar]
- 5.Boczar K., Ząbek A., Ulman M., Lelakowski J., Małecka B. His bundle pacing in a patient after heart transplant and complete atrioventricular block. Kardiol Pol. 2021;79(1):81–82. doi: 10.33963/KP.15681. [DOI] [PubMed] [Google Scholar]
- 6.Burri H., Jastrzebski M., Cano Ó., et al. EHRA clinical consensus statement on conduction system pacing implantation: executive summary. Endorsed by the asia-pacific heart rhythm society (APHRS), Canadian heart rhythm society (CHRS) and Latin-American heart rhythm society (LAHRS) Europace. 2023;25(4):1237–1248. doi: 10.1093/europace/euad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDowell D.L., Hauptman P.J. Implantable defibrillators and cardiac resynchronization therapy in heart transplant recipients: results of a national survey. J Heart Lung Transplant. 2009;28(8):847–850. doi: 10.1016/j.healun.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Marcus G.M., Hoang K.L., Hunt S.A., Chun S.H., Lee B.K. Prevalence, patterns of development, and prognosis of right bundle branch block in heart transplant recipients. Am J Cardiol. 2006;98(9):1288–1290. doi: 10.1016/j.amjcard.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Tu S.J., Wong C.X., Stokes M.B., Pitman B.M., Sanders P., Lau D.H. Cardiac resynchronization therapy in an orthotopic heart transplant recipient. J Cardiol Cases. 2022;27(2):80–83. doi: 10.1016/j.jccase.2022.10.011. Published 2022 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghavan C., Maloney J.D., Nitta J., et al. Long-term follow-up of heart transplant recipients requiring permanent pacemakers. J Heart Lung Transplant. 1995;14(6 Pt 1):1081–1089. [PubMed] [Google Scholar]