Abstract
Background
Oral candidiasis (OC) is a prevalent opportunistic infection in patients with human immunodeficiency virus (HIV) infection. The increasing resistance to antifungal agents in HIV-positive individuals suffering from OC raised concerns. Thus, this study aimed to investigate the prevalence of drug-resistant OC in HIV-positive patients.
Methods
Pubmed, Web of Science, Scopus, and Embase databases were systematically searched for eligible articles up to November 30, 2023. Studies reporting resistance to antifungal agents in Candida species isolated from HIV-positive patients with OC were included. Baseline characteristics, clinical features, isolated Candida species, and antifungal resistance were independently extracted by two reviewers. The pooled prevalence with a 95% confidence interval (CI) was calculated using the random effect model or fixed effect model.
Results
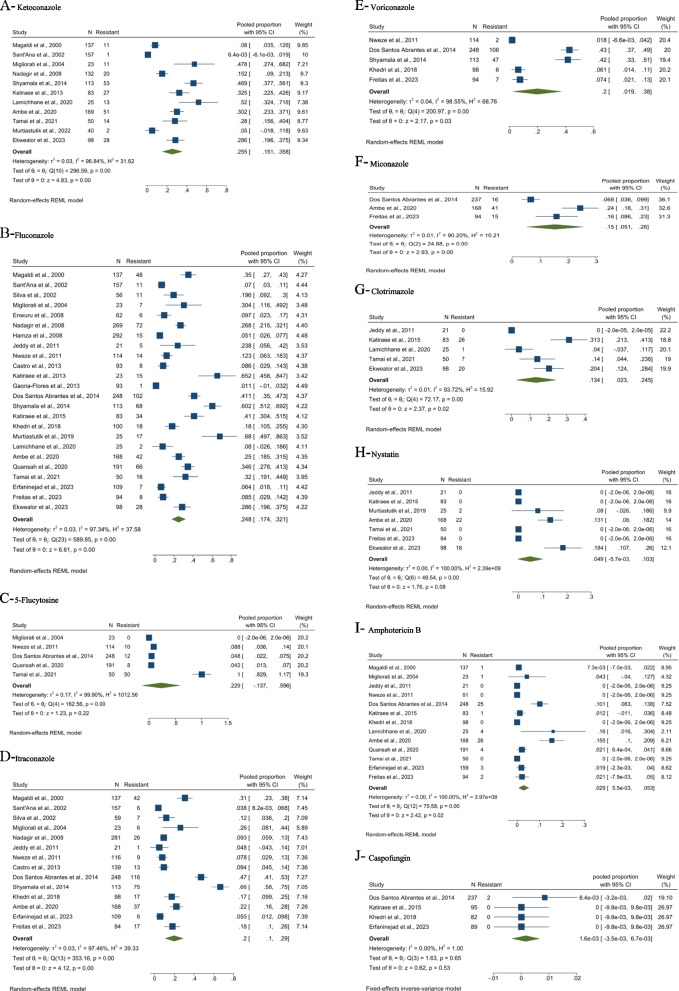
Out of the 1942 records, 25 studies consisting of 2564 Candida species entered the meta-analysis. The pooled prevalence of resistance to the antifungal agents was as follows: ketoconazole (25.5%, 95% CI: 15.1–35.8%), fluconazole (24.8%, 95% CI: 17.4–32.1%), 5-Flucytosine (22.9%, 95% CI: -13.7-59.6%), itraconazole (20.0%, 95% CI: 10.0–26.0%), voriconazole (20.0%, 95% CI: 1.9–38.0%), miconazole (15.0%, 95% CI: 5.1–26.0%), clotrimazole (13.4%, 95% CI: 2.3–24.5%), nystatin (4.9%, 95% CI: -0.05-10.3%), amphotericin B (2.9%, 95% CI: 0.5–5.3%), and caspofungin (0.1%, 95% CI: -0.3-0.6%). Furthermore, there were high heterogeneities among almost all included studies regarding the resistance to different antifungal agents (I2 > 50.00%, P < 0.01), except for caspofungin (I2 = 0.00%, P = 0.65).
Conclusions
Our research revealed that a significant number of Candida species found in HIV-positive patients with OC were resistant to azoles and 5-fluocytosine. However, most of the isolates were susceptible to nystatin, amphotericin B, and caspofungin. This suggests that initial treatments for OC, such as azoles, may not be effective. In such cases, healthcare providers may need to consider prescribing alternative treatments like polyenes and caspofungin.
Registration
The study protocol was registered in the International Prospective Register of Systematic Reviews as PROSPERO (Number: CRD42024497963).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09442-6.
Keywords: HIV, Candida, Drug Resistance, Opportunistic infections, Oral candidiasis
Background
Oral candidiasis (OC) is an infection of the mucous membrane of the mouth caused by Candida species [1]. Although Candida spp. are commensal fungi, they can invade the oral mucosa in certain conditions [2]. Candida albicans is the most common etiologic factor for OC. However, the importance of non-albicans Candida species (e.g., C. glabrata, C. tropicalis, C. krusei, C. dubliniensis, C. parapsilosis, C. guilliermondi, and C. kefyr) is increasing over time [3, 4]. Poor oral hygiene, smoking, age extremes (infants and elderly), excessive consumption of antifungal agents, malnutrition, and immunodeficiency are predisposing factors for OC [1, 2].
The impaired cellular immunity in people living with the human immunodeficiency virus (HIV) imposes a substantial threat of opportunistic infections [5–7]. OC has emerged as both a first clue for diagnosing acquired immunodeficiency syndrome (AIDS) and an indicator of its severity [8, 9]. OC is the most leading and recurring opportunistic infection in HIV-positive patients with a prevalence ranging from 0.9 to 83.0% [10]. It can manifest in diverse clinical forms in people living with HIV, including pseudomembranous (thrush), erythematous, atrophic, hyperplastic, and angular cheilitis [1, 10, 11].
Unlike other immunocompromised patients, for those with HIV infection, no antifungal prophylaxis for OC is recommended. Whereas the first line treatment for OC in HIV-positive patients is fluconazole [12, 13]. Overall, the resistance pattern to antifungal agents in HIV/AIDS individuals undergoing OC is changing, leading to increasingly serious medical concerns [14–16]. Recurrent infections necessitate the extensive consumption of antifungal agents by those living with HIV/AIDS. Thus, they are at increased risk of drug resistance [17, 18]. On the other hand, the incidence of OC caused by non-albicans candida spp. in HIV-positive individuals is increasing [19–21]. Surprisingly, these species have a considerable resistance rate to common antifungal agents. These pathogens can cause invasive infections and result in morbidity and mortality owing to the existence of antifungal resistance, limited drug options, and lack of prophylactic measures [10, 22, 23]. Hence, investigating the antifungal resistance profile of Candida species responsible for OC in HIV-infected individuals is critical. It would assist clinicians in selecting the most effective antifungal, preventing impending systemic infections, and directing further research toward innovative alternative treatments [1, 10]. This study aimed to explore the prevalence of drug-resistant oral candidiasis in HIV-positive patients.
Methods
The study complies with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement [24]. It was registered in the International Prospective Register of Systematic Reviews as PROSPERO (Protocol number: CRD42024497963).
Eligibility criteria
We included English-language observational studies reporting drug resistance to fluconazole, itraconazole, amphotericin B, ketoconazole, 5-flucytosine, nystatin, clotrimazole, caspofungin, miconazole, or voriconazole in Candida species isolated from HIV-positive adults suffering from OC. In this study, only publications that reported antifungal resistance in each Candida species separately were included. Case reports and case series, review articles, clinical trials, animal studies, commentaries, letters to the editor, guidelines, and conference papers were excluded.
Search strategy and information sources
PubMed/Medline, Embase, Scopus, and Web of Science were systematically searched for eligible articles published from January 2000 to November 30, 2023. The search strategy was as follows: ((((((((((((candida) OR (candidosis)) OR (candidoses)) OR (candidiasis)) OR (candidiases)) OR (thrush)) OR (moniliasis)) OR (moniliases)) OR (oral candidiasis)) OR (oral candidosis)) AND (((((((oral cavity) OR (oral)) OR (mouth)) OR (palate)) OR (palates)) OR (tongue)) OR (buccal cavity))) AND ((((HIV) OR (human immunodeficiency virus)) OR (AIDS)) OR (acquired immunodeficiency syndrome))) AND (((((drug resistance) OR (antifungal drug resistance)) OR (drug-resistant)) OR (resistance)) OR (resistant)). In addition, all the references in the selected publications were manually searched to identify further studies.
Study selection
The records found by searching databases were merged, and the duplicates were removed using EndNote X6 software (Thomson Reuters, New York, NY, USA). The records were screened in two rounds. Initially, they were independently screened by two reviewers regarding the title and abstract (MFT and ZG). Then, the full texts of those that passed the initial screening were independently assessed for eligibility by the same reviewers (MFT and ZG). Disagreements were resolved by the principal investigators (ST and AK).
Data extraction
The following variables were independently extracted from the selected studies by two reviewers (HN and NT): first author name, publication year, country where the study was performed, number of patients, number of isolates, age and sex distribution, current highly active antiretroviral therapy (HAART), CD4 count, history of OC, history of antifungal medication, clinical manifestations of OC, isolated Candida species, method of investigating drug resistance, and antifungal resistance pattern. Disagreements were resolved by the principal investigators (ST and AK).
Statistical analysis
Data were analyzed using STATA software (version 17, IC; Stata Corporation, College Station, TX, USA). The weight of each study in the pooled proportion was the inverse of its variance. The pooled proportion with 95% CI was calculated using the random effect model with restricted maximum likelihood (REML) method or the fixed-effect model. The I2 criteria, with a cut-point of 50%, were considered to assess between-study heterogeneity. Publication bias was evaluated by Egger’s test. In this study, the P-value < 0.05 was considered statistically significant.
Quality assessment
The checklist provided by the Joanna Briggs Institute (JBI) was used to perform quality assessment [25].
Results
Study selection
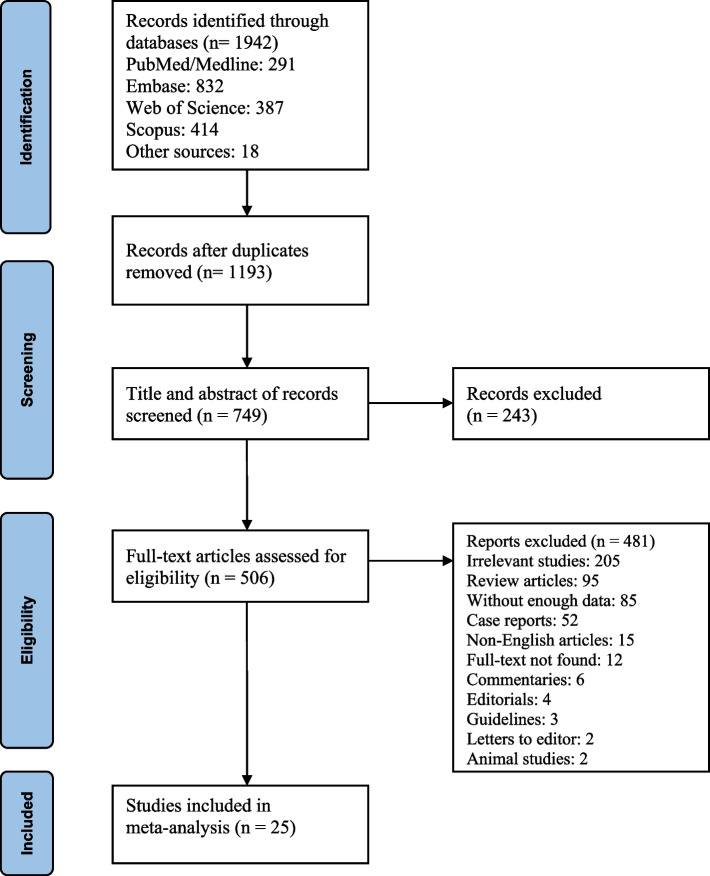
Of the 1942 records obtained from an electronic database search, 1193 duplicates were removed. Screening titles and abstracts resulted in the exclusion of 243 records. After assessing the full-text of the remaining records, 25 studies were included for quantitative synthesis and meta-analysis. Figure 1 illustrates the flow chart of study selection for inclusion in the meta-analysis.
Fig. 1.
Flow chart of study selection for inclusion in the meta-analysis
Study characteristics
The detailed characteristics of the included studies are presented in Table 1. The included studies consisted of 2564 Candida species isolated from HIV-positive patients with OC. Baseline characteristics and clinical features of the patients are summarized in Table S1. Overall, 48.7% and 36.6% of the patients had a history of OC and antifungal medication, respectively. Pseudomembranous candidiasis (91.6%) was the most common clinical manifestation, while erythematous candidiasis (14.6%), hyperplastic candidiasis (3.9%), angular cheilitis (3.6%), and atrophic candidiasis (1.0%) were less common. Table S2 illustrates the frequency of Candida species in different studies. The most frequent species was C. albicans (n = 1798), followed by C. glabrata (n = 230), C. tropicalis (n = 186), C. krusei (n = 98), C.dubliniensis (n = 87), C. parapsilosis (n = 69), C. gulliniermondii (n = 36), C. kefyr (n = 27), and C. famata (n = 17).
Table 1.
Characteristics of the included studies
| First author | Country | Year of publication | Number of patients | Number of isolates | Antifungal susceptibility method | Quality of studies |
|---|---|---|---|---|---|---|
| Magaldi et al. [26] | Venezuela | 2000 | 108 | 137 | Disk diffusion | High |
| Sant’Ana et al. [27] | Brazil | 2002 | 130 | 142 | Broth microdilution | High |
| Silva et al. [28] | Brazil | 2002 | 59 | 59 | Broth microdilution | Moderate |
| Migliorati et al. [29] | Brazil | 2004 | 19 | 23 | Disk diffusion | High |
| Enwuru et al. [30] | Nigeria | 2008 | 73 | 74 | Broth microdilution | High |
| Nadagir et al. [31] | India | 2008 | 132 | 132 | Broth microdilution | High |
| Hamza et al. [32] | Tanzania | 2008 | 292 | 296 | Broth microdilution | High |
| Jeddy et al. [33] | India | 2011 | 21 | 21 | Disk diffusion | Moderate |
| Nweze et al. [20] | Nigeria | 2011 | 120 | 120 | Broth microdilution | High |
| Castro et al. [34] | Columbia | 2013 | 71 | 93 | E-test | High |
| Katiraee et al. [35] | 2013 | 23 | 23 | Disk diffusion | High | |
| Gaona-Flores et al. [36] | Mexico | 2013 | 91 | 91 | Broth microdilution | High |
| Dos Santos Abrantes et al. [37] | South Africa and Cameroon | 2014 | 254 | 254 | Broth microdilution | High |
| Shyamala et al. [38] | India | 2014 | 118 | 121 | Disk diffusion | Moderate |
| Katiraee et al. [39] | Iran | 2015 | NS | 83 | Disk diffusion | High |
| Khedri et al. [17] | Iran | 2018 | 89 | 89 | Broth microdilution | High |
| Murtiastutik et al. [40] | Indonesia | 2019 | 25 | 25 | Disk diffusion | High |
| Lamichhane et al. [41] | Nepal | 2020 | 25 | 25 | Disk diffusion | High |
| Ambe et al. [42] | Cameroon | 2020 | 162 | 171 | Disk diffusion | High |
| Quansah et al. [43] | Ghana | 2020 | 194 | 194 | E-test | High |
| Tamai et al. [44] | Iran | 2021 | 50 | 50 | Disk diffusion | High |
| Murtiastutik et al. [45] | Indonesia | 2022 | 23 | 40 | Disk diffusion | High |
| Erfaninejad et al. [46] | Iran | 2023 | 94 | 109 | Broth microdilution | High |
| Freitas et al. [47] | Brazil | 2023 | 92 | 94 | Broth microdilution | High |
| Ekwealor et al. [48] | Nigeria | 2023 | 98 | 98 | Disk diffusion | High |
NS not specified
Antifungal resistance patterns of different Candida species
Table 2 depicts the antifungal resistance patterns of different Candida species. C. famata (42.9%), C. kefyr (40.0%), and C.dubliniensis (18.2%) were mostly resistant to ketoconazole. C. krusei (61.1%) and C. parapsilosis (30.9%) were mostly resistant to fluconazole. The remaining species were mostly resistant to other azoles as follows: C. gulliniermondii to itraconazole (48.5%), C. tropicalis to miconazole (45.5%), C. glabrata to clotrimazole (43.9%), and C. albicans to voriconazole (29.7%). Furthermore, the most sensitive antifungal agent in almost all species was caspofungin.
Table 2.
Antifungal resistance patterns of Candida species
| Antifungal agents | Number of studies | Number of isolates | Sensitive | Susceptible dose-dependent | Resistant |
|---|---|---|---|---|---|
| C. albicans | |||||
| Fluconazole | 24 | 1775 | 1329(74.9) | 97(5.4) | 349(19.7) |
| Itraconazole | 14 | 1187 | 849(71.5) | 113(9.5) | 225(19.0) |
| Amphotericin B | 13 | 931 | 888(95.4) | 12(1.3) | 31(3.3) |
| Ketoconazole | 10 | 691 | 521(75.4) | 59(8.5) | 111(16.1) |
| 5-Flucytosine | 5 | 453 | 383(84.5) | 1(0.2) | 69(15.2) |
| Nystatin | 7 | 388 | 367(94.6) | 3(0.8) | 18(4.6) |
| Clotrimazole | 5 | 215 | 167(77.7) | 16(7.4) | 32(14.9) |
| Caspofungin | 4 | 351 | 339(96.6) | 12(3.4) | 0(0.0) |
| Miconazole | 3 | 381 | 348(91.3) | 4(1.0) | 29(7.6) |
| Voriconazole | 5 | 391 | 266(68.0) | 9(2.3) | 116(29.7) |
| C. glabrata | |||||
| Fluconazole | 16 | 225 | 110(48.9) | 49(21.8) | 66(29.3) |
| Itraconazole | 11 | 165 | 66(40.0) | 48(29.1) | 51(30.9) |
| Amphotericin B | 9 | 154 | 135(87.7) | 4(2.6) | 15(9.7) |
| Ketoconazole | 8 | 102 | 44(43.1) | 20(19.6) | 38(37.3) |
| 5-Flucytosine | 3 | 50 | 49(98.0) | 0(0.0) | 1(2.0) |
| Nystatin | 4 | 77 | 60(77.9) | 4(5.2) | 13(16.9) |
| Clotrimazole | 2 | 41 | 19(46.3) | 4(9.8) | 18(43.9) |
| Caspofungin | 4 | 94 | 78(82.9) | 15(16.0) | 1(1.1) |
| Miconazole | 3 | 72 | 37(51.4) | 10(13.9) | 25(34.7) |
| Voriconazole | 4 | 61 | 55(90.2) | 0(0.0) | 6(9.8) |
| C. dubliniensis | |||||
| Fluconazole | 9 | 87 | 69(79.3) | 7(8.1) | 11(12.6) |
| Itraconazole | 6 | 59 | 40(67.8) | 14(23.7) | 5(8.5) |
| Amphotericin B | 6 | 63 | 60(95.2) | 0(0.0) | 3(4.8) |
| Ketoconazole | 1 | 22 | 12(54.5) | 6(27.3) | 4(18.2) |
| 5-Flucytosine | 3 | 25 | 24(96.0) | 0(0.0) | 1(4.0) |
| Nystatin | 1 | 2 | 2(100.0) | 0(0.0) | 0(0.0) |
| Caspofungin | 2 | 36 | 36(100.0) | 0(0.0) | 0(0.0) |
| Miconazole | 1 | 2 | 2(100.0) | 0(0.0) | 0(0.0) |
| Voriconazole | 4 | 38 | 35(92.1) | 2(5.3) | 1(2.6) |
| C. tropicalis | |||||
| Fluconazole | 17 | 180 | 119(66.1) | 13(7.2) | 48(26.7) |
| Itraconazole | 12 | 125 | 80(64.0) | 18(14.4) | 27(21.6) |
| Amphotericin B | 9 | 100 | 91(91.0) | 3(3.0) | 6(6.0) |
| Ketoconazole | 7 | 79 | 41(51.9) | 8(10.1) | 30(38.0) |
| 5-Flucytosine | 4 | 47 | 43(91.5) | 0(0.0) | 4(8.5) |
| Nystatin | 3 | 33 | 26(78.8) | 1(3.0) | 6(18.2) |
| Clotrimazole | 1 | 12 | 7(58.3) | 2(16.7) | 3(25.0) |
| Caspofungin | 3 | 24 | 22(91.7) | 2(8.3) | 0(0.0) |
| Miconazole | 3 | 22 | 11(50.0) | 1(4.5) | 10(45.5) |
| Voriconazole | 5 | 75 | 56(74.7) | 3(4.0) | 16(21.3) |
| C. krusei | |||||
| Fluconazole | 15 | 90 | 27(30.0) | 8(8.9) | 55(61.1) |
| Itraconazole | 11 | 64 | 26(40.6) | 10(15.6) | 28(43.8) |
| Amphotericin B | 7 | 41 | 27(65.9) | 5(12.2) | 9(21.9) |
| Ketoconazole | 7 | 57 | 27(47.4) | 7(12.2) | 23(40.4) |
| 5-Flucytosine | 2 | 11 | 6(54.5) | 2(18.2) | 3(27.3) |
| Nystatin | 4 | 34 | 23(67.6) | 6(17.6) | 5 (14.8) |
| Clotrimazole | 2 | 9 | 4(44.4) | 4(44.4) | 1(11.2) |
| Caspofungin | 2 | 6 | 6(100.0) | 0(0.0) | 0(0.0) |
| Miconazole | 3 | 27 | 19(70.4) | 1(3.7) | 7(25.9) |
| Voriconazole | 3 | 18 | 14(77.8) | 0(0.0) | 4(22.2) |
| C. parapsilosis | |||||
| Fluconazole | 12 | 68 | 44(64.7) | 3(4.4) | 21(30.9) |
| Itraconazole | 7 | 48 | 35(72.9) | 0(0.0) | 13(27.1) |
| Amphotericin B | 6 | 42 | 41(97.6) | 1(2.4) | 0(0.0) |
| Ketoconazole | 5 | 31 | 22(71.0) | 0(0.0) | 9(29.0) |
| 5-Flucytosine | 2 | 29 | 27(93.1) | 0(0.0) | 2(6.9) |
| Nystatin | 2 | 6 | 6(100.0) | 0(0.0) | 0(0.0) |
| Caspofungin | 1 | 2 | 2(100.0) | 0(0.0) | 0(0.0) |
| Miconazole | 2 | 6 | 5(83.3) | 0(0.0) | 1(16.7) |
| Voriconazole | 3 | 36 | 27(75.0) | 1(2.8) | 8(22.2) |
| C. kefyr | |||||
| Fluconazole | 6 | 27 | 21(77.8) | 0(0.0) | 6(22.2) |
| Itraconazole | 4 | 22 | 12(54.5) | 3(13.7) | 7(31.8) |
| Amphotericin B | 3 | 12 | 11(91.7) | 0(0.0) | 1(8.3) |
| Ketoconazole | 1 | 10 | 6(60.0) | 0(0.0) | 4(40.0) |
| Nystatin | 1 | 1 | 1(100.0) | 0(0.0) | 0(0.0) |
| Caspofungin | 2 | 11 | 11(100.0) | 0(0.0) | 0(0.0) |
| Miconazole | 1 | 1 | 1(100.0) | 0(0.0) | 0(0.0) |
| Voriconazole | 3 | 18 | 12(66.7) | 0(0.0) | 6(33.3) |
| C. gulliniermondii | |||||
| Fluconazole | 4 | 36 | 22(61.1) | 1(2.8) | 13(36.1) |
| Itraconazole | 2 | 33 | 17(51.5) | 0(0.0) | 16(48.5) |
| Amphotericin B | 1 | 11 | 11(100.0) | 0(0.0) | 0(0.0) |
| Ketoconazole | 2 | 24 | 14(58.3) | 1(4.2) | 9(37.5) |
| 5-Flucytosine | 1 | 11 | 11(100.0) | 0(0.0) | 0(0.0) |
| Voriconazole | 2 | 33 | 23(69.7) | 0(0.0) | 10(30.3) |
| C. famata | |||||
| Fluconazole | 4 | 17 | 11(64.8) | 3(17.6) | 3(17.6) |
| Itraconazole | 3 | 14 | 5(35.7) | 4(28.6) | 5(35.7) |
| Amphotericin B | 2 | 11 | 9(81.8) | 0(0.0) | 2(18.2) |
| Ketoconazole | 2 | 7 | 1(14.2) | 3(42.9) | 3(42.9) |
| 5-Flucytosine | 1 | 4 | 4(100.0) | 0(0.0) | 0(0.0) |
| Caspofungin | 1 | 7 | 7(100.0) | 0(0.0) | 0(0.0) |
| Voriconazole | 1 | 3 | 2(66.7) | 0(0.0) | 1(33.3) |
Values are expressed as frequency (%)
Meta-analysis of resistance to antifungal agents
Figure 2(A-J) demonstrates forest plots of the proportion of anti-fungal resistant OC in HIV-positive patients. The pooled prevalence of resistance to the antifungal agents was as follows: ketoconazole (25.5%, 95% CI: 15.1–35.8%), fluconazole (24.8%, 95% CI: 17.4–32.1%), 5-Flucytosine (22.9%, 95% CI: -13.7-59.6%), itraconazole (20.0%, 95% CI: 10.0–26.0%), voriconazole (20.0%, 95% CI: 1.9–38.0%), miconazole (15.0%, 95% CI: 5.1–26.0%), clotrimazole (13.4%, 95% CI: 2.3–24.5%), nystatin (4.9%, 95% CI: -0.05-10.3%), amphotericin B (2.9%, 95% CI: 0.5–5.3%), and caspofungin (0.1%, 95% CI: -0.3-0.6%).
Fig. 2.
Forest plots of the proportion of anti-fungal resistant oral candidiasis in HIV-positive patients
Furthermore, there were high heterogeneities among almost all included studies regarding resistance to different antifungal agents: ketoconazole (I2 = 96.84%, P < 0.01), fluconazole (I2 = 97.34%, P < 0.01), 5-Flucytosine (I2 = 99.90%, P < 0.01), itraconazole (I2 = 97.46%, P < 0.01), voriconazole (I2 = 98.55, P < 0.01), miconazole (I2 = 90.20%, P < 0.01), clotrimazole (I2 = 93.72%, P < 0.01), nystatin (I2 = 100%, P < 0.01), and amphotericin B (I2 = 100%, P < 0.01). However, studies reporting resistance patterns of caspofungin had no heterogeneity (I2 = 0.00%, P = 0.65).
Publication bias
Egger’s test revealed that included studies reporting resistance patterns of ketoconazole (P = 0.32), fluconazole (P = 0.15), 5-Flucytosine (P = 0.11), itraconazole (P = 0.21), voriconazole (P = 0.14), miconazole (0.20), clotrimazole (0.06), and caspofungin (P = 0.20) had no publication bias. Nevertheless, studies reporting resistance patterns of nystatin (P < 0.01) and amphotericin B (P = 0.01) suffered from publication bias.
Discussion
In the current systematic review and meta-analysis, we aimed to determine the prevalence of drug-resistant oral candidiasis in HIV-positive patients. Our findings indicated that the pooled prevalence of resistance to azoles and 5-flucytosine was relatively high, ranging between 13.4% and 25.5%. However, over 95% of the isolates were sensitive to nystatin, amphotericin B, and caspofungin. This meta-analysis is the first study to comprehensively report resistance rate to several antifungal agents in HIV-positive patients with OC. Our findings will help clinicians by providing them with knowledge about resistance rates to various antifungal agents, ultimately leading to more effective therapeutic options, reduced treatment failure, and fewer recurrent cases.
There are different classes of antifungal agents available for the treatment of OC, each of which targets a specific cellular component of the fungi. Azoles (e.g., ketoconazole, fluconazole, itraconazole, voriconazole, miconazole, and clotrimazole) inhibit the biosynthesis of ergosterol in the endoplasmic reticulum. Polyenes (e.g., amphotericin B and nystatin) disrupt the membrane structure and function of the fungi by targeting ergosterol in the cell membrane. Pyrimidine analogues (e.g., 5-flucytosine) are converted in the fungi cell to 5-fluorouracil, which inhibits DNA synthesis. And echinocandins (e.g., caspofungin) target fungal cell walls by inhibiting the enzyme β [1, 3]-D-glucan synthase [49, 50].
Our findings revealed that many Candida isolates were resistant to azoles, ranging from 13.4% (clotrimazole) to 25.5% (ketoconazole). Nevertheless, many Candida isolates were still sensitive to the second-line therapeutic options, such as nystatin (95.1%), amphotericin B (97.1%), and caspofungin (99.9%). Despite the disparities observed in previous studies regarding the prevalence of azole resistance in OC, it is unanimously acknowledged that a significant proportion of Candida isolates exhibit resistance to various azoles. They reported the prevalence of azole resistance in Candida isolates across the following spectrums: ketoconazole (0.0 [51]-47.8% [39]), fluconazole (4.6 [51]-56.7% [39]), itraconazole (5.4 [46]-66.0% [38]), voriconazole (1.7 [20]-43.0% [37]), clotrimazole (0.0 [33]-38.3% [39]), and miconazole (6.8 [37]-24.0% [42]). The high prevalence of azole resistance may be attributed to cross-resistance to fluconazole, which is routinely administered to HIV-positive patients with clinical manifestations of OC without testing for antifungal sensitivity. Thus, the increased proportion of resistant Candida spp. may be caused by prolonged or constant azole administration [47]. The following mechanisms can be employed to make azoles resistant: alteration of the target enzyme (cytochrome P-450 lanosterol 14 α-demethylase) mediated by the ERG11 gene; and failure of azoles to accumulate inside the fungi, followed by enhanced drug efflux mediated by Multidrug resistance (MDR) and Candida drug resistance (CDR) genes [52].
According to the meta-analysis, the pooled prevalence of resistance to 5-fluocytosine was estimated to be 22%. In this regard, 4 out of the 5 studies included in the meta-analysis exhibited a prevalence of 5-fluocytosine resistance close to zero, while only one study from Iran found it at 100%, which skewed the pooled prevalence. Except for the aforementioned article, it can be concluded that most isolates were sensitive to 5-fluocytosine. As reported by Alves et al., flucytosine was more effective against C. albicans than Candida non-albicans species. Thus, clinicians must consider this matter, when prescribing 5-flucytosine to treat OC [53]. The resistance to this drug is attributed to mutations in the cytosine permease and cytosine deaminase enzymes in Candida species [54].
Based on the literature, the minority of Candida isolates was resistant to polyenes with the following ranges: amphotericin B (0.0 [44]-16.0% [41]) and nystatin (0.0 [44]-18.4% [48]). According to a World Health Organization (WHO) recommendation in 2014, topical therapy with nystatin suspension would be an alternative to oral fluconazole for treating HIV-positive patients suffering from OC [55, 56]. Although amphotericin B is not the first-line therapeutic option for OC, it may be recommended for patients with fluconazole-refractory OC [17]. The emergence of isolates with polyene resistance raises concerns regarding OC treatment. The resistance to polyenes is achieved by the modification of enzymes involved in ergosterol biosynthesis through ERG2 and ERG3 gene alteration and by the generation of deviate reactive oxygen species (ROS) through overactivated catalase [49].
Unanimously, the prevalence of caspofungin resistance was around zero in the four studies included in the meta-analysis. Caspofungin is an exclusively intravenous antifungal drug. Since most Candida isolates are still sensitive to caspofungin, it can be considered as a therapeutic option for refractory or recurrent OC [55].
Furthermore, the prevalence of almost all antifungal agents had high levels of heterogeneity between publications. These heterogeneities may be attributed to temporal variations or differences in the history of antifungal agent administration, drug resistance testing methods, and Candida species causing OC [20, 57]. We discussed each of the factors that contribute to the heterogeneities in the following paragraphs.
Osaigbovo et al. reported that 88.9% and 72.8% of resistant isolates were obtained from HIV-positive patients who had utilized fluconazole and had a history of OC, respectively [57]. Recurrent OC and prolonged exposure to antifungal agents resulted in increased resistance of Candida spp. to azoles and treatment failures [58]. The overexpression of drug efflux pumps by fungi in response to inappropriate use of an individual azole leads to emerging resistance to multiple agents belonging to the azole family. It could explain the increased resistance to azole antifungal agents [4, 59]. Clinicians can consider fluconazole-resistant Candida species as the cause of oral candidiasis in cases of treatment failure or recurrent OC and switch the treatment to alternative therapeutic options [57].
The studies included in our meta-analysis investigated the resistance patterns of Candida species using different antifungal susceptibility testing methods (e.g., disk diffusion, broth microdilution, and E-test). These methods are slightly different in detecting susceptibility to antifungal agents, which may lead to heterogeneity [60, 61].
Different Candida species have variations in their resistance to a particular antifungal agent [62]. As we found in the systematic review, non-Candida albicans species are more resistant to antifungal agents compared with C. albicans, which is explainable based on the genetic characteristics of different species [10, 22, 23]. For example, C. krusei possesses an inherent resistance to fluconazole, while C. glabrata and C. famata species can acquire resistance to fluconazole after the first exposure [19, 32, 50]. Moreover, the co-infection of two or more different Candida species may contribute to the development of antifungal resistance in previously sensitive ones, resulting in refractory or recurrent OC [17]. These cases of OC present clinicians with challenges that require further laboratory investigations and the prescribing alternative antifungal agents [63].
Our study had some limitations. Although reviewing multiple databases with appropriate queries, some relevant articles might be unintentionally missed. We included publications written in English, which could lead to a language bias. The limited number of published articles on resistance to a certain antifungal drug might contribute to publication bias.
Conclusions
Our research revealed that a significant number of Candida species found in HIV-positive patients with OC were resistant to azoles and 5-fluocytosine. However, most of the isolates were susceptible to nystatin, amphotericin B, and caspofungin. This suggests that initial treatments for OC, such as azoles, may not be effective. In such cases, healthcare providers may need to consider prescribing alternative treatments like polyenes and caspofungin.
Supplementary Information
Acknowledgements
The authors appreciate the Infectious Diseases and Tropical Medicine Research Center at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors’ contributions
AK conceptualized the study, analyzed the data, interpreted the data, and critically edited the manuscript. HN extracted the data and wrote the primary draft of the manuscript. NT extracted the data and wrote the primary draft of the manuscript. MFT collected the data and wrote the primary draft of the manuscript. GA searched the literature and wrote the primary draft of the manuscript. ZG collected the data and wrote the primary draft of the manuscript. ST supervised the study, interpreted the data, and critically edited the manuscript. All authors read and approved the final manuscript.
Funding
The authors received no funding for this research project.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: a Disease of Opportunity. J Fungi (Basel Switzerland). 2020;6(1):15. doi: 10.3390/jof6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mardani M, Abolghasemi S, Darvishnia D, Lotfali E, Ghasemi R, Rabiei MM, et al. Oral candidiasis in hematological malignancy patients: identification and antifungal susceptibility patterns of isolates. Jundishapur J Microbiol. 2020;13(8):e103290.
- 3.Deepa A, Nair BJ, Sivakumar T, Joseph AP. Uncommon opportunistic fungal infections of oral cavity: a review. J oral Maxillofacial Pathology: JOMFP. 2014;18(2):235–43. doi: 10.4103/0973-029X.140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mushi MF, Bader O, Taverne-Ghadwal L, Bii C, Groß U, Mshana SE. Oral candidiasis among African human immunodeficiency virus-infected individuals: 10 years of systematic review and meta-analysis from Sub-saharan Africa. J oral Microbiol. 2017;9(1):1317579. doi: 10.1080/20002297.2017.1317579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramaniam M, Pandhare J, Dash C. Immune Control of HIV. Journal of life sciences (Westlake Village. Calif) 2019;1(1):4–37. [PMC free article] [PubMed] [Google Scholar]
- 6.Gondivkar S, Sarode SC, Gadbail AR, Yuwanati M, Sarode GS, Gondivkar RS, et al. Oro-facial opportunistic infections and related pathologies in HIV patients: a comprehensive review. Dis Mon. 2021;67(9):101170. doi: 10.1016/j.disamonth.2021.101170. [DOI] [PubMed] [Google Scholar]
- 7.Ramadian EE, Pradono SA, Wimardhani YS. Successful treatment of persistent oral ulcers in patients with HIV/AIDS. J Int Dent Med Res. 2016;9:398–402. [Google Scholar]
- 8.Malele Kolisa Y, Yengopal V, Shumba K, Igumbor J. The burden of oral conditions among adolescents living with HIV at a clinic in Johannesburg, South Africa. PLoS ONE. 2019;14(10):e0222568. doi: 10.1371/journal.pone.0222568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taverne-Ghadwal L, Kuhns M, Buhl T, Schulze MH, Mbaitolum WJ, Kersch L, et al. Epidemiology and prevalence of oral candidiasis in HIV patients from Chad in the Post-HAART era. Front Microbiol. 2022;13:844069. doi: 10.3389/fmicb.2022.844069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patil S, Majumdar B, Sarode SC, Sarode GS, Awan KH. Oropharyngeal Candidosis in HIV-Infected Patients-An update. Front Microbiol. 2018;9:980. doi: 10.3389/fmicb.2018.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellstein JW, Marek CL. Candidiasis: Red and White manifestations in the oral cavity. Head Neck Pathol. 2019;13(1):25–32. doi: 10.1007/s12105-019-01004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice Guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lortholary O, Petrikkos G, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infection: Official Publication Eur Soc Clin Microbiol Infect Dis. 2012;18(Suppl 7):68–77. doi: 10.1111/1469-0691.12042. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller MA, Carvalhaes CG, DeVries S, Rhomberg PR, Castanheira M. Impact of COVID-19 on the antifungal susceptibility profiles of isolates collected in a global surveillance program that monitors invasive fungal infections. Med Mycol. 2022;60(5):myac028. [DOI] [PMC free article] [PubMed]
- 15.Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY Antifungal Surveillance Program: results for Candida Species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–94. doi: 10.1093/ofid/ofy358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–92. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 17.Khedri S, Santos ALS, Roudbary M, Hadighi R, Falahati M, Farahyar S, et al. Iranian HIV/AIDS patients with oropharyngeal candidiasis: identification, prevalence and antifungal susceptibility of Candida species. Lett Appl Microbiol. 2018;67(4):392–9. doi: 10.1111/lam.13052. [DOI] [PubMed] [Google Scholar]
- 18.Shivaswamy U, Sumana MN. Antifungal Resistance of Candida Species isolated from HIV patients in a Tertiary Care Hospital, Mysuru, Karnataka. Indian J Dermatology. 2020;65(5):423–5. doi: 10.4103/ijd.IJD_385_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junqueira JC, Vilela SF, Rossoni RD, Barbosa JO, Costa AC, Rasteiro VM, et al. Oral colonization by yeasts in HIV-positive patients in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2012;54(1):17–24. doi: 10.1590/S0036-46652012000100004. [DOI] [PubMed] [Google Scholar]
- 20.Nweze EI, Ogbonnaya UL. Oral Candida isolates among HIV-infected subjects in Nigeria. J Microbiol Immunol Infect = Wei Mian Yu Gan ran Za Zhi. 2011;44(3):172–7. doi: 10.1016/j.jmii.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Lei L, Tan D, Jiang L, Zeng X, Dan H, et al. Oropharyngeal Candida colonization in human immunodeficiency virus infected patients. APMIS: acta pathologica, microbiologica. et Immunol Scand. 2013;121(5):375–402. doi: 10.1111/apm.12006. [DOI] [PubMed] [Google Scholar]
- 22.Lu SY. Oral candidosis: pathophysiology and best practice for diagnosis, classification, and successful management. J Fungi (Basel Switzerland). 2021;7(7):555. [DOI] [PMC free article] [PubMed]
- 23.Forsberg K, Woodworth K, Walters M, Berkow EL, Jackson B, Chiller T, et al. Candida Auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol. 2019;57(1):1–12. doi: 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London England) 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evid Implement. 2015;13(3):147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 26.Magaldi S, Mata S, Hartung C, Verde G, Deibis L, Roldán Y, et al. In vitro susceptibility of 137 Candida sp. isolates from HIV positive patients to several antifungal drugs. Mycopathologia. 2001;149(2):63–8. doi: 10.1023/A:1007237711099. [DOI] [PubMed] [Google Scholar]
- 27.Sant’Ana Pde L, Milan EP, Martinez R, Queiroz-Telles F, Ferreira MS, Alcântara AP, et al. Multicenter Brazilian study of oral Candida species isolated from AIDS patients. Mem Inst Oswaldo Cruz. 2002;97(2):253–7. doi: 10.1590/S0074-02762002000200019. [DOI] [PubMed] [Google Scholar]
- 28.Silva MRR, Costa MR, Miranda AT, Fernandes OF, Costa CR, Paula CRd Evaluation of Etest and macrodilution broth method for antifungal susceptibility testing of Candida Sp strains isolated from oral cavities of AIDS patients. Rev Inst Med Trop Sao Paulo. 2002;44:121–5. doi: 10.1590/S0036-46652002000300002. [DOI] [PubMed] [Google Scholar]
- 29.Migliorati CA, Birman EG, Cury AE. Oropharyngeal candidiasis in HIV-infected patients under treatment with protease inhibitors. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2004;98(3):301–10. doi: 10.1016/j.tripleo.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Enwuru C, Ogunledun A, Idika N, Enwuru N, Ogbonna E, Aniedobe M, et al. Fluconazole resistant opportunistic oro-pharyngeal Candida and non-candida yeast-like isolates from HIV infected patients attending ARV clinics in Lagos, Nigeria. Afr Health Sci. 2008;8(3):142–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Nadagir SD, Chunchanur SK, Halesh LH, Yasmeen K, Chandrasekhar MR, Patil BS. Significance of isolation and drug susceptibility testing of non-candida albicans species causing oropharyngeal candidiasis in HIV patients. Southeast Asian J Trop Med Public Health. 2008;39(3):492–5. [PubMed] [Google Scholar]
- 32.Hamza OJ, Matee MI, Moshi MJ, Simon EN, Mugusi F, Mikx FH, et al. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 2008;8:1–9. doi: 10.1186/1471-2180-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeddy N, Ranganathan K, Devi U, Joshua E. A study of antifungal drug sensitivity of Candida isolated from human immunodeficiency virus infected patients in Chennai, South India. J oral Maxillofacial Pathology: JOMFP. 2011;15(2):182–6. doi: 10.4103/0973-029X.84490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro L, Álvarez MI, Martínez E. Pseudomembranous candidiasis in HIV/AIDS patients in Cali. Colombia Mycopathologia. 2013;175(1–2):91–8. doi: 10.1007/s11046-012-9593-0. [DOI] [PubMed] [Google Scholar]
- 35.Katiraee F, Khalaj V, Khosravi AR, Hajiabdolbaghi M. Sequences type analysis of Candida albicans isolates from Iranian human immunodeficiency virus infected patients with oral candidiasis. Acta Medica Iranica. 2014;52(3):187–91. [PubMed] [Google Scholar]
- 36.Gaona-Flores V, Guzmán R, Tovar R, Martínez E, Arrieta M. In vitro sensitivity to Fluconazole through Vitek II Systems, of strains of Candida Spp. Patients with Oropharyngeal Candidiasis and HIV/AIDS. J AIDS Clin Res. 2013;4(230):2. [Google Scholar]
- 37.Dos Santos Abrantes PM, McArthur CP, Africa CW. Multi-drug resistant oral Candida species isolated from HIV-positive patients in South Africa and Cameroon. Diagn Microbiol Infect Dis. 2014;79(2):222–7. doi: 10.1016/j.diagmicrobio.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Shyamala R, Parandekar P. Identification and in vitro azole resistance of Candida species isolated from oropharyngeal candidiasis in human immunodeficiency virus infected patients. Int J Curr Microbiol Appl Sci. 2014;3:816–22. [Google Scholar]
- 39.Katiraee F, Teifoori F, Soltani M. Emergence of azole-resistant Candida species in AIDS patients with oropharyngeal candidiasis in Iran. Curr Med Mycol. 2015;1(3):11–6. doi: 10.18869/acadpub.cmm.1.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murtiastutik D, Maharani CS, Listiawan MY. Nystatin profile on Candida species in HIV/AIDS patients with oral candidiasis: a phenomenology study. J pure Appl Microbiol. 2019;13(4):183–5. doi: 10.22207/JPAM.13.4.12. [DOI] [Google Scholar]
- 41.Lamichhane K, Adhikari N, Bastola A, Devkota L, Bhandari P, Dhungel B, Auckland, et al. Biofilm-Producing Candida Species Causing Oropharyngeal Candidiasis in HIV Patients Attending Sukraraj Tropical and Infectious Diseases Hospital in Kathmandu, Nepal. HIV/AIDS (Auckland, NZ) 2020;12:211–20. doi: 10.2147/HIV.S255698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambe NF, Longdoh NA, Tebid P, Bobga TP, Nkfusai CN, Ngwa SB, et al. The prevalence, risk factors and antifungal sensitivity pattern of oral candidiasis in HIV/AIDS patients in Kumba District Hospital, South West Region, Cameroon. Pan Afr Med J. 2020;36:23. doi: 10.11604/pamj.2020.36.23.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quansah HA, Opintan JA. Distribution and susceptibility profile of Candida isolates from HIV patients with oropharyngeal candidiasis. Health Sci Investigations J. 2020;1(1):43–9. doi: 10.46829/hsijournal.2020.6.1.1.43-49. [DOI] [Google Scholar]
- 44.Tamai IA, Pakbin B, Fasaei BN. Genetic diversity and antifungal susceptibility of Candida albicans isolates from Iranian HIV-infected patients with oral candidiasis. BMC Res Notes. 2021;14(1):93. doi: 10.1186/s13104-021-05498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murtiastutik D, Listiawan MY, Bintanjoyo L, Hidayati AN, Widyantari S, Sari M, Ketoconazole A re-emerging choice for oral candidiasis in patients with human immunodeficiency virus infection/acquired Immunodeficiency Syndrome. Res J Pharm Technol. 2022;15(3):1071–6. doi: 10.52711/0974-360X.2022.00179. [DOI] [Google Scholar]
- 46.Erfaninejad M, Zarei Mahmoudabadi A, Maraghi E, Hashemzadeh M, Fatahinia M. Low level of antifungal resistance in Candida species recovered from Iranian HIV-associated oral infection. Lett Appl Microbiol. 2023;76(3):ovad029. [DOI] [PubMed]
- 47.Freitas VAQ, Santos AS, Zara A, Costa CR, Godoy CSM, Soares RBA, et al. Distribution and antifungal susceptibility profiles of Candida species isolated from people living with HIV/AIDS in a public hospital in Goiânia, GO, Brazil. Brazilian J Microbiology: [publication Brazilian Soc Microbiology] 2023;54(1):125–33. doi: 10.1007/s42770-022-00851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekwealor C, Nweke C, Anaukwu C, Anakwenze V, Ogbukagu C, Mba A. Prevalence and antifungal susceptibility pattern of oral candidiasis among HIV-infected patients in a Mission Hospital, Southeast Nigeria. Afr J Clin Experimental Microbiol. 2023;24(3):289–98. doi: 10.4314/ajcem.v24i3.9. [DOI] [Google Scholar]
- 49.Anuța V, Talianu MT, Dinu-Pîrvu CE, Ghica MV, Prisada RM, Albu Kaya MG, et al. Molecular mapping of antifungal mechanisms accessing biomaterials and new agents to target oral candidiasis. Int J Mol Sci. 2022;23(14):7520. [DOI] [PMC free article] [PubMed]
- 50.Patil S, Rao RS, Majumdar B, Anil S. Clinical appearance of oral Candida infection and therapeutic strategies. Front Microbiol. 2015;6:1391. doi: 10.3389/fmicb.2015.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maninder J, Usha A. Isolation, characterization and antifungal susceptibility pattern of Candida species causing oropharyngeal candidiasis in HIV positive patients. J Commun Dis. 2008;40(3):177–81. [PubMed] [Google Scholar]
- 52.Salari S, Khosravi AR, Mousavi SA, Nikbakht-Brojeni GH. Mechanisms of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV-infected patients with oropharyngeal candidiasis. J De Mycol Medicale. 2016;26(1):35–41. doi: 10.1016/j.mycmed.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Alves SH, Da Matta DA, Azevedo AC, Loreto ES, Boff E, Santurio JM, et al. In vitro activities of new and conventional antimycotics against fluconazole-susceptible and non-susceptible Brazilian Candida spp. isolates. Mycoses. 2006;49(3):220–5. doi: 10.1111/j.1439-0507.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- 54.Delma FZ, Al-Hatmi AMS, Brüggemann RJM, Melchers WJG, de Hoog S, Verweij PE, et al. Molecular mechanisms of 5-Fluorocytosine resistance in yeasts and Filamentous fungi. J Fungi (Basel Switzerland). 2021;7(11):909. doi: 10.3390/jof7110909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quindós G, Gil-Alonso S, Marcos-Arias C, Sevillano E, Mateo E, Jauregizar N, et al. Therapeutic tools for oral candidiasis: current and new antifungal drugs. Med oral Patologia oral y Cir Bucal. 2019;24(2):e172–80. doi: 10.4317/medoral.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO Guidelines Approved by the Guidelines Review Committee . Guidelines on the Treatment of Skin and Oral HIV-Associated Conditions in Children and Adults. Geneva: World Health Organization Copyright © World Health Organization 2014; 2014. [PubMed] [Google Scholar]
- 57.Osaigbovo II, Lofor PV, Oladele RO. Fluconazole Resistance among oral Candida isolates from People Living with HIV/AIDS in a Nigerian Tertiary Hospital. J Fungi (Basel Switzerland). 2017;3(4):69. doi: 10.3390/jof3040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moges B, Bitew A, Shewaamare A. Spectrum and the In Vitro Antifungal susceptibility pattern of yeast isolates in Ethiopian HIV patients with Oropharyngeal Candidiasis. Int J Microbiol. 2016;2016:3037817. doi: 10.1155/2016/3037817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albertson GD, Niimi M, Cannon RD, Jenkinson HF. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40(12):2835–41. doi: 10.1128/AAC.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaur R, Dhakad MS, Goyal R, Haque A, Mukhopadhyay G. Identification and Antifungal Susceptibility Testing of Candida Species: a comparison of Vitek-2 System with Conventional and Molecular methods. J Global Infect Dis. 2016;8(4):139–46. doi: 10.4103/0974-777X.192969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar D, Bhattacharyya S, Gupta P, Banerjee G, Singh M. Comparative analysis of Disc Diffusion and E-test with Broth micro-dilution for susceptibility testing of clinical Candida isolates against amphotericin B, Fluconazole, Voriconazole and Caspofungin. J Clin Diagn Research: JCDR. 2015;9(11):Dc01–4. doi: 10.7860/JCDR/2015/14119.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terças AL, Marques SG, Moffa EB, Alves MB, de Azevedo CM, Siqueira WL, et al. Antifungal Drug Susceptibility of Candida Species isolated from HIV-Positive patients recruited at a Public Hospital in São Luís, Maranhão, Brazil. Front Microbiol. 2017;8:298. doi: 10.3389/fmicb.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson GR, 3rd, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, et al. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):488–95. doi: 10.1016/j.tripleo.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.