Abstract
Background
Osteoarthritis (OA) is the most frequently diagnosed chronic joint disease. CircSEC24A is significantly elevated in OA chondrocytes upon IL-1β stimulation. However, its biological function in OA is still not fully understood.
Methods
The circRNAs-miRNA-mRNA network was predicted by bioinformatics analysis. An in vitro OA chondrocytes model was established by IL-1β stimulation. The expression of circSEC24A, miR-107-5p, CASP3, apoptosis-related molecules and extracellular matrix (ECM) components were detected by Western blot and qRT-PCR. MTT assay and Annexin V/PI staining were employed to monitor cell viability and apoptosis, respectively. The interaction between circSEC24A and miR-107-5p, as well as the binding between miR-107-5p and CASP3 3’ UTR were detected by luciferase reporter and RIP assays. Cytokine secretion was monitored by ELISA assay. The role of circSEC24A was also explored in anterior cruciate ligament transection (ACLT) rat models.
Results
CircSEC24A and CASP3 were increased, but miR-107-5p was decreased in rat OA cartilage tissues and OA chondrocytes. CircSEC24A acted as a sponge of miR-107-5p. Knockdown of circSEC24A promoted chondrocyte proliferation, but suppressed chondrocyte apoptosis, ECM degradation and inflammation via sponging miR-107-5p. CASP3 was identified as a miR-107-5p target gene. MiR-107-5p mimics protected against OA progression via targeting CASP3. Silencing of circSEC24A alleviated OA progression in ACLT model.
Conclusion
CircSEC24A promotes OA progression through miR-107-5p/CASP3 axis.
Keywords: Osteoarthritis, Chondrocyte, circSEC24A, miR-107-5p, CASP3
1. Introduction
Osteoarthritis (OA), a degenerative joint disease, is one of the most common form of arthritis [1]. The major characteristics of OA include articular cartilage degeneration, subchondral bone remodeling disorder and synovial inflammation, leading to pain, stiffness and other symptoms [2]. Due to the increasing aging population worldwide, the prevalence of OA is rapidly rising, resulting in a critical challenge for health care system. A reduction in the number of chondrocytes and extracellular matrix (ECM) degradation are recognized as a hallmark of OA [3]. As the only resident cell type in articular cartilage, chondrocytes play an important role in maintaining ECM. Chondrocyte apoptosis is associated with articular cartilage matrix damage and the progression of OA [4,5]. However, our understanding of the underlying mechanism of OA remains limited.
Circular RNAs (circRNAs) with covalently closed continuous loop structure are recognized as a novel class of RNA transcripts. With the advance of next generation sequencing bioinformatics, numerous differentially expressed circRNAs have been identified during OA progression in recent years [6,7]. Emerging evidence has illustrated that aberrantly expressed circRNAs are implicated in the initiation and progression of OA [6,[8], [9], [10], [11]]. For instance, circRNA.33186 is implicated in OA pathogenesis via sponging miR-127-5p [11]. CircSERPINE2 alleviates ECM degradation and chondrocyte apoptosis by targeting miR-1271-5p/ETS-related gene (ERG) [10]. circSEC24A is significantly elevated in OA chondrocytes upon IL-1β stimulation [8]. However, the biological role of circSEC24A in OA are not been fully understood.
microRNAs (miRNAs), a class of short single-stranded non-coding RNAs, regulate target gene expression via targeting the 3’ untranslated region (UTR) of mRNA [12]. Compelling evidence has emerged in recent years indicating that aberrantly expressed miRNAs are implicated in the pathogenesis of OA [13,14]. Of note, circRNAs have been reported to act as sponges of miRNA or competing endogenous RNAs (ceRNAs) to sequester and suppress miRNA activity [15]. To further explore the function of circSEC24A, bioinformatics analysis was performed to predict circRNAs-miRNA-mRNA network. Among the predicted miRNAs, miR-107-5p attracts remarkable attention due to its important role in OA. miR-107 is significantly downregulated in OA chondrocytes, and contributes to autophagy and apoptosis of OA chondrocytes via targeting TRAF3 [16]. A more recent study has reported that miR-107 protects against knee OA by suppressing caspase-1 [17]. Interestingly, CASP3 has been predicted as a putative target gene of miR-107-5p in this study. As an apoptotic executioner, increased CASP3 is associated with chondrocyte apoptosis in OA [18]. We hypothesized that circSEC24A-miR-107-5p-CASP3 network might be implicated in OA progression.
In this study, we report the expression of circSEC24A, miR-107-5p and CASP3 in rat OA cartilage tissue and OA chondrocytes. The regulatory relationship between the three was demonstrated by the OA cell model. In vivo studies validated the effect of circSEC24A on OA progression. Therefore, revealing the roles of circSEC24A-miR-107-5p-CASP3 network provided a better in-depth understanding of OA progression.
2. Materials and methods
2.1. Chondrocyte culture and in vitro OA model
Human primary chondrocytes were isolated as described [19]. Briefly, human articular cartilages of knees were isolated from the inner part of the medial tibial plateau of OA patients undergoing joint replacement surgery (average age, 40.2 ± 8.7 years). The cartilage slices were digested with 0.25% trypsin for 2 h and incubated with 0.04% collagenase II at 37 °C overnight. Primary chondrocytes were cultured in DMEM/F12 containing 10% FBS (Gibco, Thermo Fisher Scientific, Grand Island, NY, USA). Cells were maintained in a 37 °C and 5% CO2 incubator. Chondrocytes at the 3rd passage were used for subsequent experiments. For in vitro OA model, primary chondrocytes were stimulated with IL-1β recombinant protein (10 ng/ml, Gibco) for 24 h.
2.2. Transfection and lentiviral transduction
The small interfering RNA (siRNA) targeting circSEC24A or scramble siRNA (si-NC) were from GeneChem (Shanghai, China). miRNA mimics control (NC mimics), miR-107-5p mimics, miRNA inhibitor control (NC inhibitor) and miR-107-5p inhibitor were from GenePharma (Suzhou, China). siRNA or miRNA were transfected into chondrocytes using Lipofectamine 2000 reagent (Invitrogen). pLenti-CMV-CASP3 overexpression lentivirus were purchased from GenePharma). The lentiviral particles were mixed with polybrene (5 μg/mL, Sigma-Aldrich) and added into chondrocytes.
2.3. MTT assay
Cell viability was determined by MTT assay. Chondrocytes (1 × 106 cells/ml) were seeded into 96-well plates and treated with IL-1β (10 ng/ml) for 24 h. MTT solution (10 μl; Solarbio, Beijing, China) was added into each well and incubated for 4 h at 37 °C, followed by the addition of Formazan (110 μl/per well). Absorbance was measured at 490 nm wavelength using microplate reader (BioTek, Winooski, VT, USA).
2.4. Flow cytometry
Cell apoptosis was quantified using Dead Cell Apoptosis Kit (Molecular Probes, Thermo Fisher Scientific). Briefly, chondrocytes were collected, rinsed and resuspended in binding buffer. Annexin V-FITC (5 μl) and PI solution (1 μl) were incubated with cells for 15 min. The dual stained chondrocytes were analyzed using flow cytometry (BD Biosciences, San Diego, CA, USA).
2.5. Dual luciferase reporter assay
The wild-type (WT-circSEC24A) or mutated (MUT-circSEC24A) circSEC24A sequence containing the putative binding sites of miR-107-5p was cloned into p-MIR-REPORT vector (Ambion, Thermo Fisher Scientific). 293T cells were co-transfected with WT-circSEC24A/MUT-circSEC24A and miR-107-5p mimics/inhibitor or control using Lipofectamine 2000 transfection reagent. To investigate the interaction between miR-107-5p and CASP3, the wild-type (WT-CASP3) or mutated CASP3 3’ UTR (MUT-CASP3) was cloned into p-MIR-REPORT vector (Ambion). 293T cells were co-transfected with WT-CASP3/MUT-CASP3 and miR-107-5p mimics/inhibitor or control. Luciferase activities were quantified using Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA).
2.6. RNA immunoprecipitation (RIP) assay
RIP assay was conducted using Magna RIP Kit (Millipore, Billerica, MA, USA). Cells were lysed with RIP Lysis Buffer. In brief, normal rabbit IgG (2 μg, 10500C, Invitrogen) or anti-Argonaute 2 (Ago2) antibody (2 μg, ab186733, Abcam, Cambridge, UK) were conjugated with protein A/G beads. This is followed by the incubation with cell lysates at 4 °C overnight. The enriched circSEC24A, miR-107-5p and CASP3 3′UTR were analyzed by qRT-PCR.
2.7. Animal model of OA
Male Sprague-Dawley rats (16-week-old; n = 20 per group) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The experimental protocols for animal study were approved by the Institutional Animal Care and Use Committee of First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China). Anterior cruciate ligament transection (ACLT) model of OA was established as previously described [20]. Briefly, rats were randomly divided into following groups: Sham (n = 5), ACLT (n = 5), ACLT + sh-NC (n = 5) and ACLT + sh-circSEC24A (n = 5). After anesthesia with ketamine and xylazine, the right knees of rats were exposed using the medial parapatellar approach. The patella was dislocated laterally, and the ACL was transected. Rats in Sham group underwent the same surgery without ACL transection. For intra-articular injection of short hairpin RNA (shRNA), ACLT rats received lentiviral sh-NC or sh-circSEC24A (1 × 109 transducing units (TU)/ml) at 1 week postoperatively. The knee joints were harvested at 8 weeks postoperatively.
2.8. Hematoxylin and eosin (H&E) and Safranin-O staining
The articular tissues were dissected, embedded using paraffin and sectioned. For histopathological analysis, the serial sections were dewaxed and stained with H&E solution (C0105S, Beyotime, Haimen, China). Safranin-O staining was conducted as described [21]. The sections were stained with Safranin-O dye (G1067, Solarbio), and scored using Osteoarthritis Research Society International (OARSI) grading system [22].
2.9. TUNEL assay
The sections of articular tissue were subjected to TUNEL assay using a In Situ Cell Death Detection Kit, Fluorescein (Sigma-Aldrich, St Louis, MO, USA). Briefly, the dewaxed and rehydrated sections were incubated with Proteinase K. After rinsing, sections were incubated with TUNEL reaction mixture at 37 °C for 1 h. Sections were mounted in Antifade Mountant with DAPI (Invitrogen). Images were acquired using Zeiss confocal laser scanning microscopy (Carl Zeiss, Jena, Germany).
3. ELISA
Cell media were harvested and centrifuged at 1500 rpm for 10 min, the supernatant were collected for ELISA assay. For serum, the whole blood was kept at room temperature for 20 min and centrifuged at 3000 rpm for 10 min. The levels of TNF-α (88-7340-88, Invitrogen), IL-6 (88-50625-88, Invitrogen) and IL-10 (88-50629-88, Invitrogen) in cell culture supernatant or serum were detected using commercial ELISA kits (Invitrogen) according to the manufacturer's instructions. A450 was measured using microplate reader (BioTek).
3.1. RNA isolation and qRT-PCR
Total RNAs were isolated from tissues and chondrocytes using TRIzol reagent (Invitrogen). Reverse transcription was carried out using SuperScript III RT (Invitrogen). qRT-PCR reaction was performed using Power SYBR Green Master Mix (Applied Biosystems). Data were analyzed using 2−ΔΔCT method. GAPDH was employed as an internal control. For RNase R digestion, 5 μg total RNA was incubated with 15U RNase R (Epicentre biotechnologies, Madison, WI, USA) at 37 °C for 15 min. The levels of circSEC24A and SEC24A were detected by qRT-PCR. Primers were listed in Table 1.
Table 1.
| Pirmer | Sequence 5′-3′ |
|---|---|
| circSEC24 A F | 5′- CATTTGTAACCCATGCAGACCATTG-3′ |
| circSEC24 A R | 5′- GCTCCTGGTTGAAAAGTTGTAGGC-3′ |
| miR-107 F | 5′- CGCCCGTTCAAGTAATTCAGG-3′ |
| miR-107 R | 5′- CGGCCCAGTGTTCAGACTAC-3′ |
| CASP3 F | 5′- GAGGCCGACTTCTTGTATGC-3′ |
| CASP3 R | 5′- CGGTTAACCCGGGTAAGAAT-3′ |
| Col2a1 F | 5′- ATGACAATCTGGCTCCCAAC-3′ |
| Col2a1 R | 5′- CTTCAGGGCAGTGTACGTGA-3′ |
| ACAN F | 5′- ACGTCCTCACACCAGGAAAC-3′ |
| ACAN R | 5′- GAAAGGCATCGTGTTCCATT-3′ |
| ADAMTS5 F | 5′- AACTGGGGGTCCTGGGGGTCCTGG-3′ |
| ADAMTS5 R | 5′- CATTTCTTGCCTCACACTGCTCAT-3′ |
| MMP13 F | 5′- CTTGACCACTCCAAGGACCC-3′ |
| MMP13 R | 5′- CCTCGGAGACTGGTAATGGC-3′ |
| U6 F | 5′-CTCGCTTCGGCAGCACA-3′ |
| U6 R | 5′-AACGCTTCACGAATTTGCGT-3′ |
| GAPDH F | 5′-GAGTCAACGGATTTGGTCGTT-3′ |
| GAPDH R | 5′-TTGATTTTGGAGGGATCTCG-3′ |
3.2. Western blot
Protein lysates were prepared in RIPA lysis buffer (Abcam) with protease inhibitor cocktail (Pierce, Rockford, IL, USA). Protein estimation was conducted using BCA Protein Assay Kit (Pierce). Equal amounts of proteins were separated and transferred onto a nitrocellulose membrane (Genscript, Nanjing, China). After blocking with 5% non-fat milk, the membranes were incubated with primary antibody at 4 °C overnight. The blots were then incubated with secondary antibody (1:2000; CST) for 1 h, and visualized using ECL Substrate (Pierce). Primary antibodies were listed in Table 2.
Table 2.
Antibodies used in this study. Abcam, Cambridge, UK; Cell signaling technologies (CST), Beverly, MA, USA; Santa Cruz Biotechnology, Santa Cruz, CA, USA.
| Antibody | Vendor | Catalog no. | Working dilution |
|---|---|---|---|
| CASP3 | CST | #9662 | 1:1000 |
| Cleaved caspase-3 | CST | #9664 | 1:1000 |
| Bax | CST | #14796 | 1:1000 |
| Bcl-2 | CST | #3498 | 1:1000 |
| Col2a1 | Santa Cruz | sc-52658 | 1:200 |
| ACAN | Santa Cruz | sc-33695 | 1:200 |
| ADAMTS5 | Abcam | ab41037 | 1:250 |
| MMP13 | Abcam | ab84594 | 1:5000 |
| β-actin | CST | #3700 | 1:2000 |
3.3. Statistical analysis
All data were presented as the means ± S.D. For statistical analysis between two groups, Student's t-test was performed. For multiple comparison, one-way analysis of variance (ANOVA) was conducted. Statistical analysis was performed using the Prism software (GraphPad, La Jolla, CA, USA). P < 0.05 was considered statistically significant.
4. Results
4.1. Knockdown of circSEC24A promoted chondrocyte proliferation, but suppressed chondrocyte apoptosis and ECM degradation
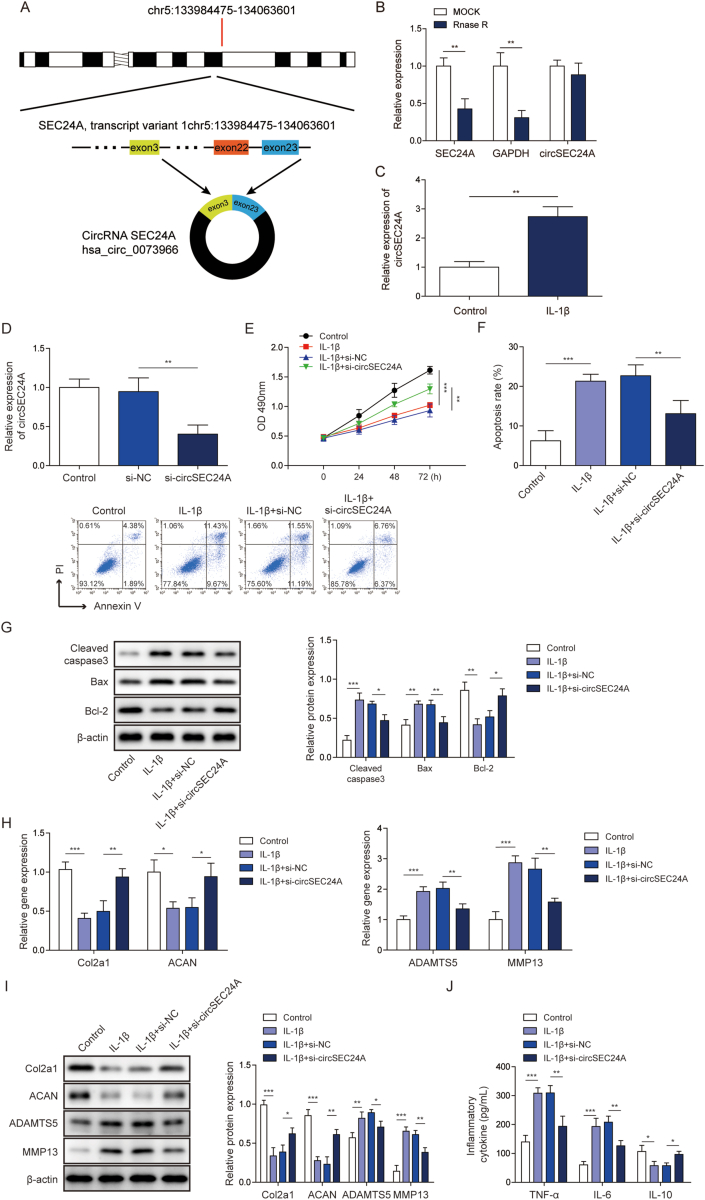
circSEC24A (hsa_circ_0073966) located in chromosome 5 (Fig. 1A). As shown in Fig. 1B, circSEC24A was more stable than SEC24A mRNA in the presence of RNase R. An in vitro OA model was established using IL-1β-stimulated primary chondrocytes. circSEC24A was remarkably increased in IL-1β-induced chondrocytes as detected by qRT-PCR (Fig. 1C). To unravel the biological role of circSEC24A in OA, knockdown experiments were conducted. si-circSEC2A successfully downregulated circSEC24A level in chondrocytes (Fig. 1D). Knockdown of circSEC24A significantly reversed IL-1β-suppressed chondrocyte proliferation compared to si-NC group (Fig. 1E). Moreover, Annexin V/PI staining revealed that silencing of circSEC24A inhibited IL-1β-induced chondrocyte apoptosis (Fig. 1F). In line with this result, knockdown of circSEC24A attenuated IL-1β-mediated upregulation of apoptotic proteins cleaved caspase-3 and Bax, but led to a rebound of anti-apoptotic protein Bcl-2 in comparison with si-NC group (Fig. 1G). To address the effect of circSEC24A on ECM degradation, we next examined the expression of two major ECM components produced by chondrocytes, namely collagen type II alpha 1 (Col2a1) and aggrecan (ACAN) [23]. As expected, IL-1β dramatically decreased the mRNA levels of Col2a1 and ACAN, whereas Col2a1 and ACAN expression were markedly rebound in si-circSEC24A group (Fig. 1H). Previous studies have demonstrated that MMP13 and ADAMTS5 are involved in Col2a1 and ACAN degradation, respectively [24,25]. Subsequent qRT-PCR analysis showed that knockdown of circSEC24A decreased IL-1β-mediated upregulation of MMP13 and ADAMTS5 (Fig. 1H). Similar results were found at the protein level by Western blot (Fig. 1I). Additionally, IL-1β-mediated release of pro-inflammatory cytokines TNF-α, IL-6 and anti-inflammatory cytokine IL-10 were reversed by circSEC24A knockdown (Fig. 1J). Collectively, these findings indicate that knockdown of circSEC24A promotes proliferation of chondrocyte, but suppresses chondrocyte apoptosis and ECM degradation and inflammation.
Fig. 1.
Knockdown of circSEC24A promoted proliferation of chondrocyte, but suppressed chondrocyte apoptosis and ECM degradation.(A) Schematic drawing illustrated the formation of circSEC24A. (B) qRT-PCR analysis of circSEC24A and SEC24A after RNase R treatment. (C) The levels of circSEC24A in primary chondrocytes were determined by qRT-PCR. (D) The level of circSEC24A was determined by qRT-PCR. (E) Cell viability was monitored by MTT assay. (F) Cell apoptosis was assessed by Annexin V/PI staining followed by flow cytometry. (G) The protein levels of cleaved caspase 3, Bax and Bcl-2 were determined by Western blot. (H) The mRNA levels of Col2a1, ACAN, MMP13 and ADAMTS5 were determined by qRT-PCR. (I) The protein levels of Col2a1, ACAN, MMP13 and ADAMTS5 were determined by Western blot. (J) The secreted TNF-α, IL-6 and IL-10 levels were measured by ELISA assays. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
4.2. Knockdown of circSEC24A alleviated OA progression in vivo
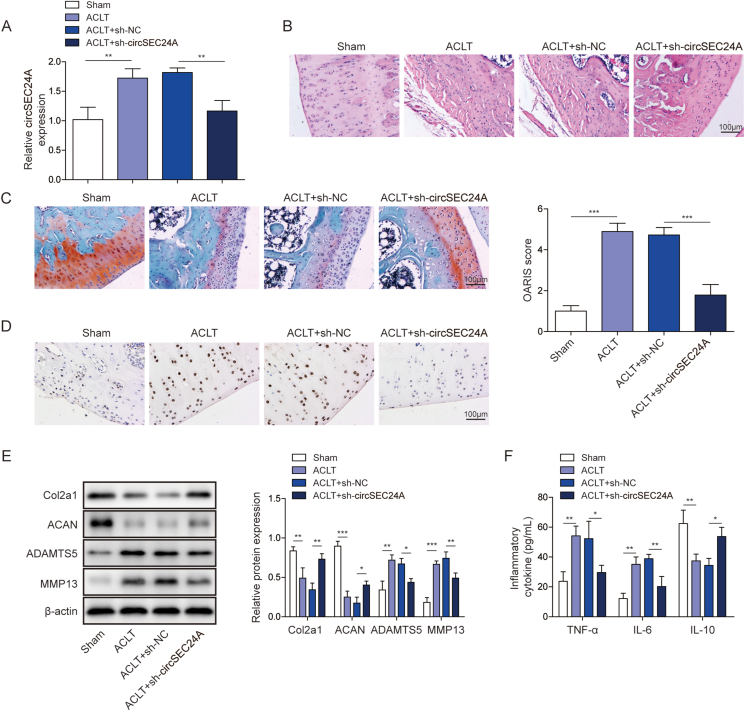
In order to further validate the in vitro findings, we next studied the effects of sh-circSEC24A on the progression of OA in ACLT model. Intra-articular injection with sh-circSEC24A was performed at 1 week postoperatively. At 8 weeks following ACLT, articular cartilage tissues were harvested from the right knees of rats and subjected to qRT-PCR analysis. Intra-articular injection of sh-circSEC24A successfully downregulated circSEC24A level in vivo (Fig. 2A). Compared with the smooth and flat surface of cartilage in Sham group, ACLT rats exhibited rough surface and cracks of cartilage, whereas ACLT-mediated membrane hyperplasia was significantly alleviated by sh-circSEC24A (Fig. 2B). The loss of cartilage matrix was detected using Safranin-O staining and OARSI grading system [22,26]. Consistently, ACLT-induced matrix loss was slowed down by sh-circSEC24A in which the OARSI score of sh-circSEC24A group was much lower than that of sh-NC group (Fig. 2C). TUNEL assays further revealed that ACLT-induced chondrocyte apoptosis was rescued by sh-circSEC24A (Fig. 2D). Moreover, the in vivo studies also demonstrated that silencing of circSEC24A reversed ACLT-induced changes on Col2a1, ACAN, ADAMTS5 and MMP13 protein levels (Fig. 2E). Consistently, knockdown of circSEC24A attenuated ACLT-induced serum levels of TNF-α and IL-6, but led to a rebound of IL-10 in serum (Fig. 2F). These data indicate that knockdown of circSEC24A alleviates OA progression in vivo.
Fig. 2.
Knockdown of circSEC24A alleviated OA progression in vivo.(A) The level of circSEC24A in rat cartilage tissues was determined by qRT-PCR. (B) The histopathological changes of articular cartilage tissues were assessed by H&E staining. Scale bar, 100 μm. (C) Cartilage degeneration was assessed by Safranin-O staining. Scale bar, 100 μm. (D) The in situ apoptosis was detected by TUNEL assay. Scale bar, 100 μm. (E) The protein levels of Col2a1, ACAN, MMP13 and ADAMTS5 in rat cartilage tissues were determined by Western blot. (F) The serum levels of TNF-α, IL-6 and IL-10 were measured by ELISA assay. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
4.3. circSEC24A acted as a sponge of miR-107-5p
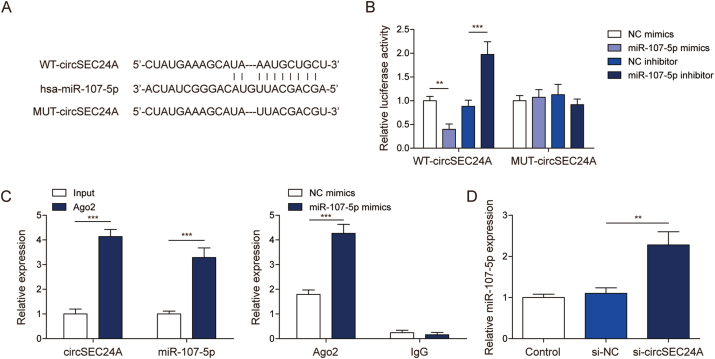
The potential binding sites between circSEC24A and miR-107-5p were predicted by Starbase (https://rnasysu.com/encori/index.php)(Fig. 3A). To verify the direct interaction between circSEC24A and miR-107-5p, wild-type (WT) and mutated (MUT) circSEC24A luciferase reporter constructs were generated. Luciferase assay revealed that co-transfection of WT-circSEC24A and miR-107-5p mimics caused a significant reduction of luciferase activity, whereas co-transfection of WT-circSEC24A and miR-107-5p inhibitor exerted an opposite effect compared to corresponding controls. In contrast, MUT-circSEC24A abrogated these effects on luciferase activity (Fig. 3B). RIP assay further revealed that antibody against Ago2 successfully enriched circSEC24A and miR-107-5p, compared with normal rabbit IgG. miR-107-5p mimics also increased Ago2-enriched circSEC24A as detected by RIP assay (Fig. 3C), indicating the direct association between circSEC24A and miR-107-5p. Moreover, knockdown of circSEC24A resulted in a dramatic induction of miR-107-5p in chondrocytes (Fig. 3D). These data suggest that circSEC24A acts as a miR-107-5p sponge, and suppresses its expression in chondrocytes.
Fig. 3.
circSEC24A acted as a miR-107-5p sponge.(A) The potential binding sites between circSEC24A and miR-107-5p. (B) Relative luciferase activity was assessed by dual luciferase reporter assay. (C) The direct interaction between circSEC24A and miR-107-5p was evaluated by RIP assay. Normal rabbit IgG served as a negative control. (D) The level of miR-107-5p was determined by qRT-PCR. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
4.4. Knockdown of circSEC24A slowed OA progression via sponging miR-107-5p in vitro
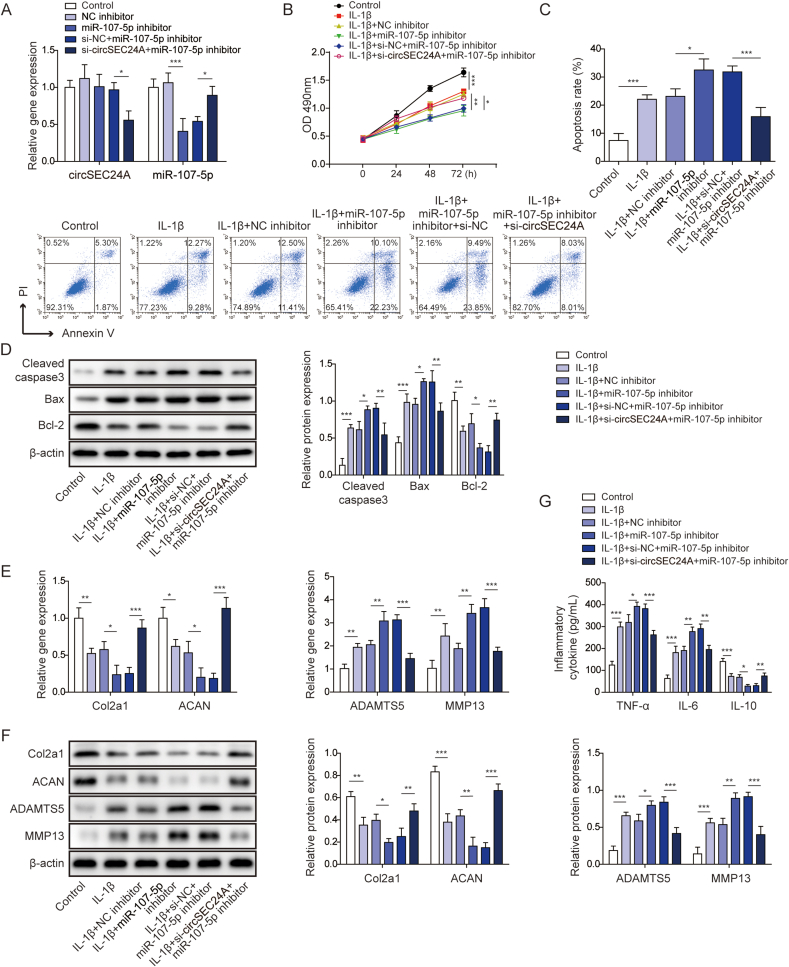
In unravel the ceRNA function of circSEC24A in chondrocytes, a series of functional experiments were performed using the in vitro OA model. As shown in Fig. 4A, miR-107-5p inhibitor-mediated reduction of miR-107-5p was rescued by si-circSEC24A. In IL-1β-stimulated chondrocytes, miR-107-5p inhibitor remarkably potentiated IL-1β-inhibited cell proliferation, whereas co-transfection of si-circSEC24A and miR-107-5p inhibitor reversed the inhibition of cell growth (Fig. 4B). In addition, IL-1β-induced chondrocyte apoptosis was exacerbated by miR-107-5p inhibitor, whereas silencing of circSEC24A decreased miR-107-5p inhibitor-induced apoptotic rate as detected by flow cytometry (Fig. 4C). This finding was further supported by the Western blot analysis of apoptosis-related proteins. miR-107-5p inhibitor potentiated IL-1β-induced upregulation of cleaved caspase 3 and Bax, as well as downregulation of Bcl-2. Knockdown of circSEC24A counteracted the effect of miR-107-5p inhibitor on the expression of these molecules (Fig. 4D). Furthermore, we also found that miR-107-5p inhibitor potentiated the effects of IL-1β on Col2a1, ACAN, MMP13 and ADAMTS5 mRNA and protein levels, whereas si-circSEC24A counteracted the miR-107-5p inhibitor-mediated changes of Col2a1, ACAN, MMP13 and ADAMTS5 in chondrocytes (Fig. 4E and F). Consistently, IL-1β-mediated release of TNF-α, IL-6 and IL-10 were potentiated by miR-107-5p inhibitor, while circSEC24A silencing reversed these effects (Fig. 4G). These results indicate that knockdown of circSEC24A alleviates OA progression via sponging miR-107-5p in vitro.
Fig. 4.
Knockdown of circSEC24A slowed OA progression via sponging miR-107-5p in vitro. (A) The levels of circSEC24A and miR-107-5p were determined by qRT-PCR. (B) Cell viability was monitored by MTT assay. (C) Cell apoptosis was assessed by Annexin V/PI staining followed by flow cytometry. (D) The protein levels of cleaved caspase 3, Bax and Bcl-2 were determined by Western blot. (E) The mRNA levels of Col2a1, ACAN, MMP13 and ADAMTS5 were determined by qRT-PCR. (F) The protein levels of Col2a1, ACAN, MMP13 and ADAMTS5 were determined by Western blot. (G) The secreted TNF-α, IL-6 and IL-10 levels were measured by ELISA assays. ∗P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
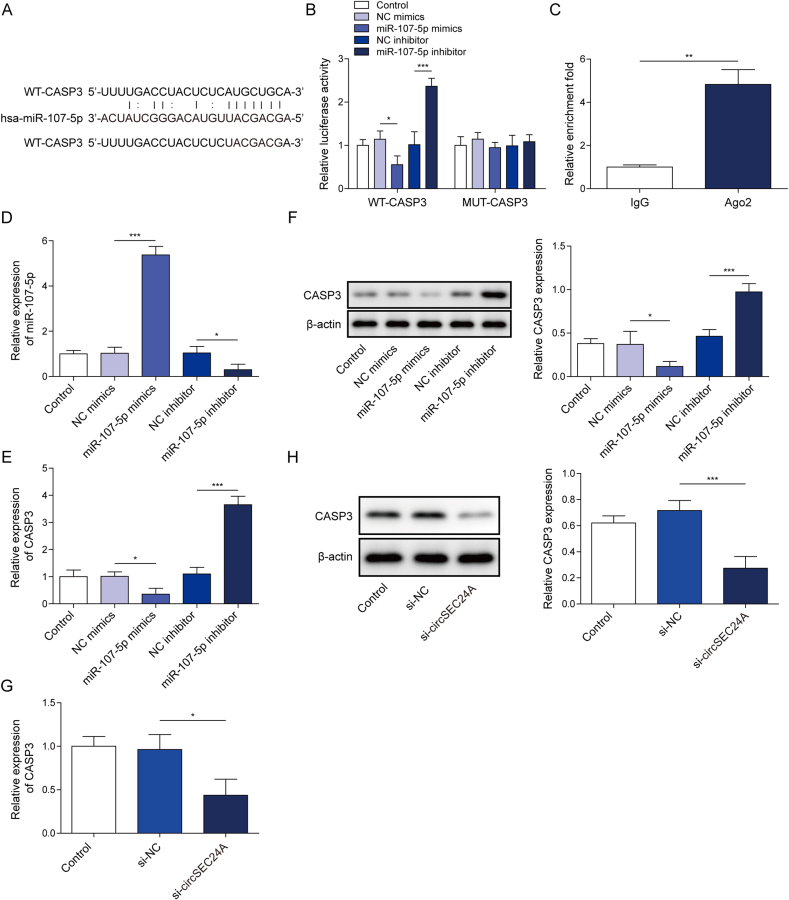
4.5. CASP3 was a miR-107-5p target gene
CASP3 was predicted as a potential target of miR-107-5p using bioinformatics database, including Starbase and miRDB. The putative miR-107-5p binding sites on 3′ UTR of CASP3 were shown in Fig. 5A. Luciferase assay revealed that luciferase activity was markedly lowered when 293T cells were co-transfected with WT-CASP3 and miR-107-5p mimics, whereas mutation of putative binding sites restored it (Fig. 5B). By contrast, luciferase activity was significantly increased by co-transfection of WT-CASP3 and miR-107-5p inhibitor, while MUT-CASP3 abolished this effect (Fig. 5B). Additionally, RIP assay showed that the direct interaction between miR-107-5p and CASP3 3′UTR (Fig. 5C). We next validated that miR-107-5p-mediated regulation of CASP3 by gain- and loss-of function experiments. As expected, miR-107-5p mimics and miR-107-5p inhibitor increased and decreased miR-107-5p expression, respectively (Fig. 5D). miR-107-5p mimics downregulated CASP3, whereas miR-107-5p inhibitor upregulated CASP3 at both mRNA and protein levels (Fig. 5E and F). Interestingly, we further validated the ceRNA function of circSEC24A in which silencing of circSEC24A remarkably downregulated CASP3 mRNA and protein levels (Fig. 5G and H). These data indicating that circSEC24A sponges miR-107-5p, thereby positively regulating the expression of its direct target CASP3. And the circSEC24A/miR-107-5p/CASP3 ceRNA network in chondrocytes was identified.
Fig. 5.
CASP3 was a miR-107-5p target gene.(A) The potential binding sites between miR-107-5p and CASP3 3′ UTR. (B) Relative luciferase activity was assessed by dual luciferase reporter assay. (C) The direct interaction between miR-107-5p and CASP3 3′UTR was evaluated by RIP assay. Normal rabbit IgG served as a negative control. (D) The level of miR-107-5p was determined by qRT-PCR. (E, G) The mRNA level of CASP3 was determined by qRT-PCR. (F, H) The protein level of CASP3 was determined by Western blot. ∗P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
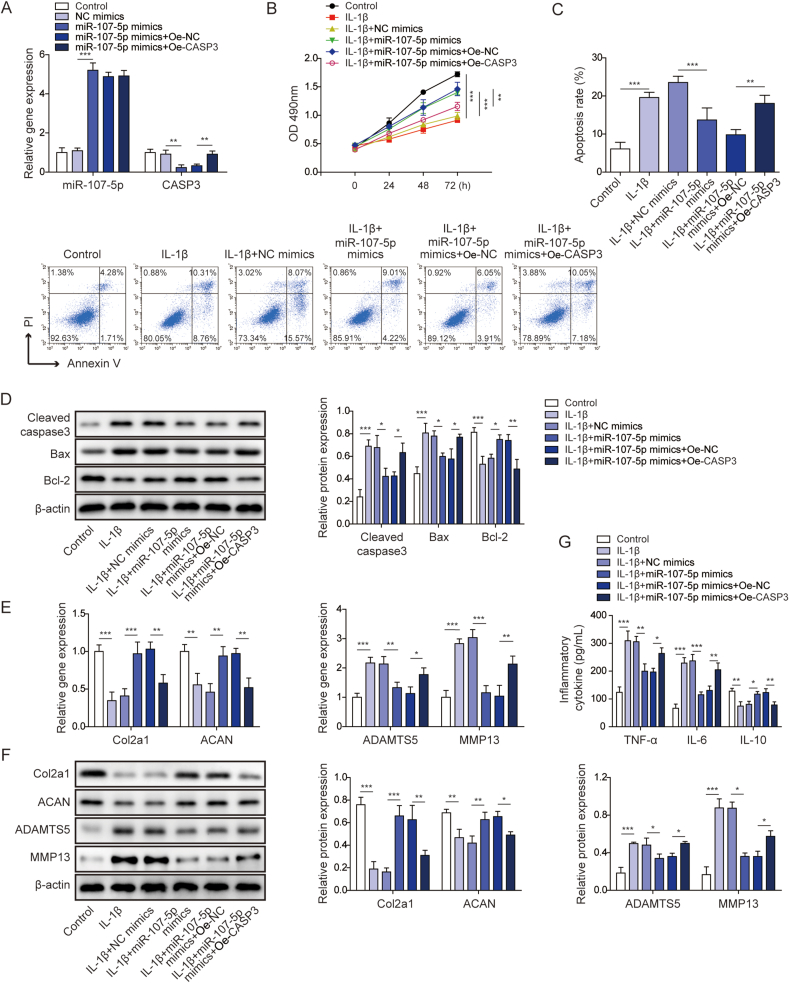
4.6. miR-107-5p mimics promoted proliferation of chondrocyte, but suppressed chondrocyte apoptosis and ECM degradation via targeting CASP3
We next studied the biological roles of miR-107-5p and CASP3 in the in vitro OA model. As presented in Fig. 6A, qRT-PCR confirmed that miR-107-5p mimics upregulated miR-107-5p level, and overexpression of CASP3 rescued miR-107-5p-mediated downregulation of CASP3. In accordance with previous results, miR-107-5p mimics alleviated IL-1β-suppressed cell growth, while overexpression of CASP3 attenuated the effects of miR-107-5p mimics on chondrocyte proliferation (Fig. 6B). In contrast to miR-107-5p inhibitor, miR-107-5p mimics partially abrogated IL-1β-induced chondrocyte apoptosis, whereas the apoptotic rate was significantly rebound in chondrocytes co-transfected with miR-107-5p mimics and OE-CASP3 (Fig. 6C). Consistent with flow cytometry data, Western blot also illustrated that miR-107-5p mimics decreased IL-1β-mediated upregulation of cleaved caspase-3 and Bax, but increased IL-1β-induced downregulation of Bcl-2. The effects of miR-107-5p mimics on the expression of apoptosis-related proteins were counteracted by OE-CASP3 (Fig. 6D). Similarly, miR-107-5p mimics abolished IL-1β-induced changes of Col2a1, ACAN, MMP13 and ADAMTS5, while the effects of miR-107-5p mimics were reversed by CASP3 overexpression at both mRNA and protein levels (Fig. 6E and F). As anticipated, IL-1β induced the secreted levels of pro-inflammatory cytokines TNF-αand IL-6, but reduced the level of anti-inflammatory cytokine IL-10. miR-107-5p mimics attenuated IL-1β-mediated changes of these cytokines, while OE-CASP3 further abrogated the anti-inflammatory effects of miR-107-5p on cytokine secretion (Fig. 6G). Collectively, these data suggest that miR-107-5p promotes proliferation of chondrocyte, but suppresses chondrocyte apoptosis and ECM degradation via targeting CASP3.
Fig. 6.
miR-107-5p mimics promoted proliferation of chondrocyte, but suppressed chondrocyte apoptosis and ECM degradation via targeting CASP3.(A) The levels of miR-107-5p and CASP3 were determined by qRT-PCR. (B) Cell viability was monitored by MTT assay. (C) Cell apoptosis was assessed by Annexin V/PI staining followed by flow cytometry. (D) The protein levels of cleaved caspase 3, Bax and Bcl-2 were determined by Western blot. (E) The mRNA levels of Col2a1, ACAN, MMP13 and ADAMTS5 were determined by qRT-PCR. (F) The protein levels of Col2a1, ACAN, MMP13 and ADAMTS5 were determined by Western blot. (G) The secreted levels of TNF-α, IL-6 and IL-10 were measured by ELISA assay. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
5. Discussion
OA is a degenerative joint disease with increasing prevalence [2]. Given the primary role of pain in OA clinical syndromes, current OA treatments have focused on alleviating pain using pharmacological and non-pharmacological strategies [27,28]. To develop novel therapeutic treatments for OA, it is urgent to unravel the molecular mechanism underlying OA progression.
circRNAs was used to regarded as the splicing by products without biological function [29].However, growing evidence indicates that circRNAs are involved in diverse biological processes at the transcriptional, post-transcriptional and translational levels [9,30]. As a miRNA sponge, circRNA binds miRNA through a miRNA response element (MRE), thereby regulating target gene expression [31]. circSEC24A arises from the exon 14–18 of the SEC24 Homolog A (SEC24A) gene [32]. A recent study has reported that circSEC24A is higher expressed in colorectal cancer, and plays an oncogenic role by modulating miR-488-3p/TMEM106B axis [32]. More importantly, it has been illustrated that circSEC24A is upregulated by IL-1β in human chondrocytes time-dependently, and circSEC24A promotes ECM degradation via miR-26a/NAMPT axis [8]. In accordance with previous research, our findings showed that circSEC24A was remarkably upregulated in rat OA cartilage tissues and IL-1β-stimulated primary chondrocytes. Besides ECM degradation, we also found that circSEC24A promoted OA progression by regulating proliferation and apoptosis of chondrocyte in vitro and in vivo. It is well-established that chondrocytes are the only cell type responsible for ECM synthesis and maintenance in articular cartilage [2,4,33]. Therefore, circSEC24A-mediated chondrocyte loss by apoptosis might induce an imbalance in the deposition and degradation of ECM in articular cartilage.
As circRNAs might have sponge activity to multiple miRNAs, the potential circRNAs-miRNA-mRNA networks were predicted by bioinformatics analysis. In addition to the circSEC24A-miR-26a-NAMPT axis [8], a novel circSEC24A-miR-107-5p-CASP3 axis was identified in OA model. Luciferase assay validated the direct interaction between circSEC24A and miR-107-5p. MiR-107-5p was significantly reduced in rat OA cartilage tissues and IL-1β-stimulated primary chondrocytes, suggesting that miR-107-5p is negatively correlated with circSEC24A in rat OA cartilage tissues and chondrocytes. These observations were consistent with previous studies which reported the decreased expression of miR-107-5p in human OA cartilage tissues. Moreover, functional experiments revealed that miR-107-5p mimics promoted chondrocyte proliferation, but suppressed chondrocyte apoptosis and ECM degradation in this study, indicating that miR-107-5p protects against OA progression. It is worth noting that miR-107 modulates cartilage matrix degradation via suppressing caspase-1, c-caspase-1, GSDMD-N and TLR4 [17]. However, the upstream signaling of miR-107-5p in OA remains elusive. To the best of our knowledge, we first identified circSEC24A as an upstream molecule of miR-107-5p in OA.
CASP3 protein (capspase-3) is a well-known mediator of cell apoptosis. Once cleaved and activated by initiator caspases (−8 and −9), the executioner capspase-3 can activate apoptotic pathway [34]. Previous study has illustrated that capspase-3 is markedly increased in OA cartilage tissues, and its expression is positively associated with degree of ECM damage [33]. Consistently, increased capspase-3 expression was found in rat OA cartilage tissues and IL-1β-stimulated primary chondrocytes in the current study. Western blot further revealed the activation of capspase-3 by detecting cleaved caspase-3. Our results also illustrate that capspase-3 is a miR-107-5p target gene, and miR-107-5p exerted the protective effects in OA via targeting caspase-3. These findings provide novel insights into the pathogenesis of OA.
In conclusion, this study identified the circSEC24A-miR-107-5p-CASP3 network in chondrocytes. circSEC24A was significantly elevated in OA tissues and chondrocytes. Silencing of circSEC24A protected against OA progression by promoting chondrocyte proliferation, suppressing chondrocyte apoptosis and ECM degradation in vitro and in vivo. In addition, gain- and loss-of function experiments demonstrated that circSEC24A exerted its effects via sponge miR-107-5p, thereby modulating its direct target CASP3 in chondrocytes. The novel circSEC24A-miR-107-5p-CASP3 axis has been established in OA, and it may serve as a promising therapeutic target for OA treatment.
Ethics approval and consent to participate
The experimental protocols for animal study were approved by the Institutional Animal Care and Use Committee of First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China).
Consent for publication
N/A.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01D21).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., et al. Osteoarthritis Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.van der Kraan P.M., van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20(3):223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Hwang H.S., Kim H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlier E., Relic B., Deroyer C., Malaise O., Neuville S., Collee J., et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q., Zhang X., Hu X., Dai L., Fu X., Zhang J., et al. Circular RNA related to the chondrocyte ECM regulates MMP13 expression by functioning as a MiR-136 ‘sponge’ in human cartilage degradation. Sci Rep. 2016;6 doi: 10.1038/srep22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z., Du D., Chen A., Zhu L. Circular RNA expression profile of articular chondrocytes in an IL-1beta-induced mouse model of osteoarthritis. Gene. 2018;644:20–26. doi: 10.1016/j.gene.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y., Zhang Y., Zhang Y., Wang J.J. CircRNA hsa_circ_0005105 upregulates NAMPT expression and promotes chondrocyte extracellular matrix degradation by sponging miR-26a. Cell Biol Int. 2017;41(12):1283–1289. doi: 10.1002/cbin.10761. [DOI] [PubMed] [Google Scholar]
- 9.Li H.Z., Lin Z., Xu X.H., Lin N., Lu H.D. The potential roles of circRNAs in osteoarthritis: a coming journey to find a treasure. Biosci Rep. 2018;38(5) doi: 10.1042/BSR20180542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen S., Wu Y., Chen J., Xie Z., Huang K., Wang G., et al. CircSERPINE2 protects against osteoarthritis by targeting miR-1271 and ETS-related gene. Ann Rheum Dis. 2019;78(6):826–836. doi: 10.1136/annrheumdis-2018-214786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z.B., Huang G.X., Fu Q., Han B., Lu J.J., Chen A.M., et al. CircRNA.33186 contributes to the pathogenesis of osteoarthritis by sponging miR-127-5p. Mol Ther. 2019;27(3):531–541. doi: 10.1016/j.ymthe.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C., Chen W.P., Wang X.H. MicroRNA in osteoarthritis. J Int Med Res. 2011;39(1):1–9. doi: 10.1177/147323001103900101. [DOI] [PubMed] [Google Scholar]
- 14.Panagopoulos P.K., Lambrou G.I. The involvement of microRNAs in osteoarthritis and recent developments: a narrative review. Mediterr J Rheumatol. 2018;29(2):67–79. doi: 10.31138/mjr.29.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Li H., Wang L. MicroRNA-107 regulates autophagy and apoptosis of osteoarthritis chondrocytes by targeting TRAF3. Int Immunopharm. 2019;71:181–187. doi: 10.1016/j.intimp.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Qian J., Fu P., Li S., Li X., Chen Y., Lin Z. MiR-107 affects cartilage matrix degradation in the pathogenesis of knee osteoarthritis by regulating caspase-1. J Orthop Surg Res. 2021;16(1) doi: 10.1186/s13018-020-02121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharif M., Whitehouse A., Sharman P., Perry M., Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50(2):507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 19.Yin X., Wang J.Q., Yan S.Y. Reduced miR26a and miR26b expression contributes to the pathogenesis of osteoarthritis via the promotion of p65 translocation. Mol Med Rep. 2017;15(2):551–558. doi: 10.3892/mmr.2016.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng L.H., Ko J.Y., Wang C.J., Sun Y.C., Wang F.S. Dkk-1 promotes angiogenic responses and cartilage matrix proteinase secretion in synovial fibroblasts from osteoarthritic joints. Arthritis Rheum. 2012;64(10):3267–3277. doi: 10.1002/art.34602. [DOI] [PubMed] [Google Scholar]
- 21.Lian W.S., Ko J.Y., Wu R.W., Sun Y.C., Chen Y.S., Wu S.L., et al. MicroRNA-128a represses chondrocyte autophagy and exacerbates knee osteoarthritis by disrupting Atg12. Cell Death Dis. 2018;9(9) doi: 10.1038/s41419-018-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Martel-Pelletier J., Boileau C., Pelletier J.P., Roughley P.J. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Pelletier J.P., Boileau C., Boily M., Brunet J., Mineau F., Geng C., et al. The protective effect of licofelone on experimental osteoarthritis is correlated with the downregulation of gene expression and protein synthesis of several major cartilage catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis Res Ther. 2005;7(5):R1091–R1102. doi: 10.1186/ar1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song R.H., Tortorella M.D., Malfait A.M., Alston J.T., Yang Z., Arner E.C., et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56(2):575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 26.Gerwin N., Bendele A.M., Glasson S., Carlson C.S. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–S34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y., Goh E.L., Wang D., Ma S. Novel treatments for osteoarthritis: an update. Open Access Rheumatol. 2018;10:135–140. doi: 10.2147/OARRR.S176666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghouri A., Conaghan P.G. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis. 2019;11 doi: 10.1177/1759720X19864492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cocquerelle C., Mascrez B., Hetuin D., Bailleul B. Mis-splicing yields circular RNA molecules. Faseb J. 1993;7(1):155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 30.Lasda E., Parker R. Circular RNAs: diversity of form and function. RNA. 2014;20(12):1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra A., Pfeifer K., Park K.S. Circular RNAs and competing endogenous RNA (ceRNA) networks. Transl Cancer Res. 2018;7(Suppl 5):S624–S628. doi: 10.21037/tcr.2018.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu G., Peng W., Cao Y. CircSEC24A promotes colorectal cancer progression by regulating miR-488-3p/TMEM106B axis. World J Surg Oncol. 2020 Version 1, PPR124649. [Google Scholar]
- 33.Thomas C.M., Fuller C.J., Whittles C.E., Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 34.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harbor Perspect Biol. 2015;7(4) doi: 10.1101/cshperspect.a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.