Abstract
The human microbiome, particularly the gut microbiome, has emerged as a central determinant of health and disease. Dysbiosis, an imbalance in the microbial composition of the gut, is associated with a variety of metabolic and other diseases, highlighting the potential for microbiota-targeted treatments. Fecal microbiota transplantation has received considerable attention as a promising therapy to modulate the gut microbiome and restore microbial homeostasis. However, challenges remain, including standardization, safety, and long-term efficacy. This review summarizes current knowledge on fecal microbiota transplantation and describes the next generation therapies targeting microbiome. This review looked at the mechanistic understanding of fecal microbiota transplantation and alternative strategies, elucidating their potential role in improving dysbiosis-associated metabolic disorders, such as obesity, and type 2 diabetes and others. Additionally, this review discussed the growing application of therapies targeting the gut microbiome. Insights from clinical trials, preclinical studies, and emerging technologies provide a comprehensive overview of the evolving landscape of microbiome-based interventions. Through a critical assessment of current advances and prospects, this review aims to highlight the therapeutic potential of targeting gut microbiome and pave the way for innovative approaches in precision medicine and personalized treatments.
Keywords: Fecal microbiota transplantation, gut microbiota, metabolic disorders, microbiota-targeted therapies, dysbiosis
Introduction
The human gut microbiota, a diverse ecosystem of microorganisms, influences host physiology, metabolism, and immune function.1,2 Dysbiosis, or disruptions in microbial composition and function, is associated with various diseases. 2 Metabolic disorders like obesity, diabetes, and metabolic syndrome are a major global health issue, causing illness, death, and high healthcare expenses. 3 Emerging evidence indicates that imbalanced gut microbiota may also play a role in causing these conditions. 4 Therefore, there is a growing interest in microbiota-targeted therapies to modulate microbial communities and restore homeostasis in metabolic disorders.5–9 The microbes in the digestive tract are called the human gastrointestinal microbiota. They outnumber human cells by 10 times, with an average ratio of 1:3 microbiota to human cells. 10 FMT is a potential intervention to reshape the gut microbiota and treat metabolic dysfunction and autoimmune disease.11,12 It involves transferring fecal microbial communities from a healthy donor to restore diversity and function. Initially used mainly for recurrent Clostridiodes difficile infection, FMT has now also been applied to other conditions, including metabolic disorders. 13
Despite promising results several studies, 13 challenges remain, including standardization of protocols, safety concerns, and variability in long-term effectiveness.14–17 Additionally, the mechanisms underlying the therapeutic effects of FMT and the optimal selection of donors and recipient are areas of ongoing investigation.15,18 In addition to FMT, a variety of next-generation therapies targeting microbiome has emerged, including prebiotics, probiotics, postbiotics, antibiotics, and microbial therapies. 19 These approaches aim to modulate the gut microbiota through various mechanisms, including promoting the growth of beneficial bacteria, inhibiting pathogens, and modulating host-microbiota interactions. This review aims to provide a comprehensive overview of the current landscape of FMT and next-generation microbiota-targeted therapies to target physiological dysregulation in metabolic disorders and beyond. Through a critical appraisal of the existing evidence, mechanistic insights, and clinical applications, we seek to elucidate the therapeutic potential of these interventions and identify future directions for research and future clinical application in the growing field of microbiome-based therapies.
Pathophysiological justification for fecal microbiota transplantation
Gut microbiota encompasses the set of microorganisms that colonize the gastrointestinal tract with mutual relationships that are key for host homeostasis. 20 In the first year of life, the gut microbiome is developed and is impacted by a variety of internal and environmental variables, including nutrition and antibiotic use. 21 Low diversity and relative dominance of Proteobacteria and Actinobacteria define the gut microbiota of neonates. 22 The adult microbiota, which is characterized by Firmicutes and Bacteroidetes dominance, then causes the microbiota to become more varied. 23 The host may benefit from the metabolites that the microbiota creates, including those that have anti-inflammatory and antioxidant activity, regulate intestinal barrier function, and provide vitamins and energy. 24 Human intestine commensal bacteria play a role in the growth and maintenance of gut sensory, motor, and immunologic functions. 25
Fecal microbiota transplantation has been used in several clinical investigations where metabolic syndrome like obesity, 26 and non-alcoholic fatty liver disease 27 have been present. According to previous study, several clinical investigations using FMT have been carried out in the context of metabolic syndrome, obesity, and non-alcoholic fatty liver disease in both rats and humans. Patients in those studies had lower levels of gut microbial diversity, and after receiving FMT from lean, healthy donors, the gut microbial diversity levels were increased. 28
There’s a growing recognition of the complex relationship between a host and its microbiota across different species. 29 Recent progress in identifying and isolating specific members of the gut microbiota, advancements in gnotobiology and a deeper understanding of host genetics have collectively facilitated research in this field. 30 This has enabled researchers to delve deeper into the dynamics of this relationship and its implications for health and disease. The gut microbiota is indispensable for the host’s overall health, influencing various aspects of host biology. It actively participates in the development and differentiation of the intestinal epithelium and the immune system, 31 crucial for maintaining tissue homeostasis and defending against pathogen invasion.32,33 Additionally, it plays a pivotal role in metabolizing indigestible polysaccharides, providing essential nutrients and energy sources to the host.34–36 Furthermore, certain members of the gut microbiota contribute to the production of vital vitamins necessary for various physiological processes.37,38 This intricate symbiotic relationship underscores the importance of a balanced and diverse gut microbiota in promoting optimal health and wellbeing.
The gut microbiota actively regulates numerous metabolic pathways in the host, fostering intricate metabolic, signaling, and immune-inflammatory axes that connect the gut, liver, muscle, and brain. 39 A thorough comprehension of these axes is essential for refining therapeutic approaches aimed at modulating the gut microbiota to address diseases and enhance overall health. Organ morphogenesis, intestinal vascularization, tissue homeostasis, carcinogenesis, bone mass, and behavior are just a few of the physiological aspects of the host that the gut microbiota influences. 20 It also expands nutrient sources, produces essential vitamins, and carries out xenobiotic metabolism. 37
Fecal transplantation procedure and selection
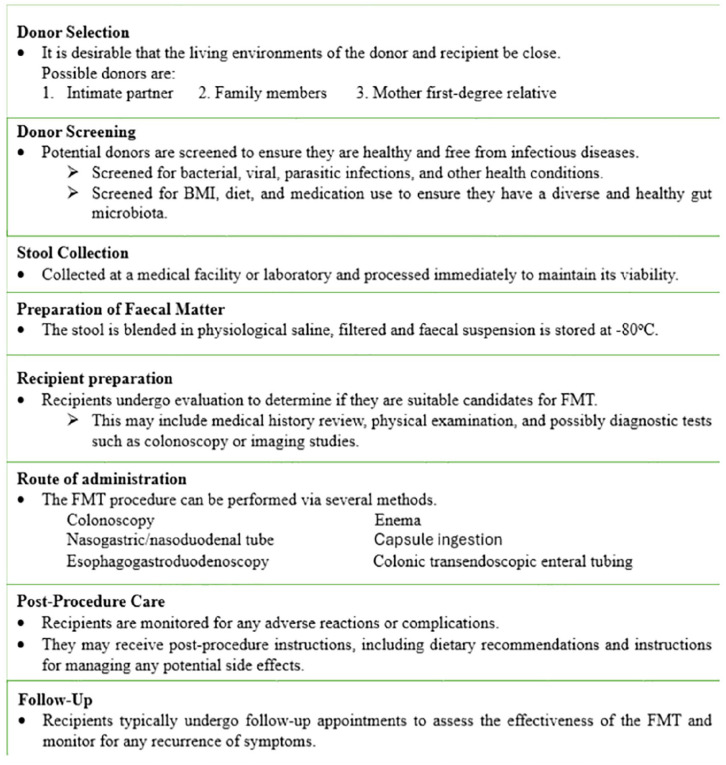
fecal transplantation has emerged as a promising therapeutic option for restoring gut microbiota balance and treating certain gastrointestinal conditions, with ongoing research focused on optimizing the procedure and selecting appropriate candidates for treatment. Even though the preparation of stool sample was varied, it is feasible to reestablish a healthy intestinal microbiome by transferring fecal material from a healthy donor to the patient to increase the intestinal microbial variety. 40 Fecal transplantation was first carried out on people in 1958, and it has been done on animals for more than a century. 41 The selection of donors for FMT is crucial and typically involves screening for infectious diseases and medical conditions to ensure the safety of the procedure. 42 Donors are often selected based on strict criteria to minimize the risk of transmitting pathogens or other adverse effects to the recipient. 43 Whether the donor is a close friend or family member, a first-degree relative, or even a stranger, it makes no difference. Clinicians must choose a donor for fecal transplantation who is free of infectious pathogens that could be transferred to the recipient. If a potential donor has been exposed to hepatitis B, hepatitis C, or HIV viruses within the past year or has a confirmed diagnosis of one of these conditions, they are disqualified from participating. 44 The donation criteria also disallow anyone with a tattoo or body piercing, as well as those who engage in risky sexual activity or use illegal substances 44 (Figure 1).
Figure 1.
The flow of fecal microbiota transplantation.
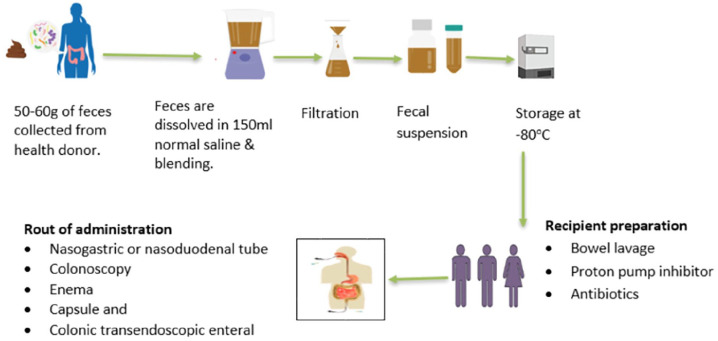
Following donor selection, a fecal sample from the donor is suspended in milk, tap or bottled water, or no bacteriostatic saline solution, however the latter is thought to be less likely to impact the microbiota of donor stool. Then, the donor feces are homogenized, either by hand agitation and shaking or by means of a machine blender. The mixture is strained through a steel strainer or gauze after being suspended with the diluent to get rid of the bigger particles41,45 (Figure 2). A fecal suspension can be delivered via nasogastric or nasoduodenal tube, colonoscopy, enema, or capsule and colonic transendoscopic enteral tubing. 46 After FMT procedure the receipt should be followed for any adverse effect, complications, and for the effectiveness of the procedure (Figure 1).
Figure 2.
The schematic diagram of the stool collection and fecal microbiota preparation.
Composition of fecal microbiota
There hasn’t been much research done on the makeup of human feces. However, studies that have looked at composition have revealed variable findings, possibly due to various factors including genetics, diet, age, environment, and medication use. 24 Adult feces typically contain 25% solid substance and 75% water. 47 Organic material, of which 25%–54% are microbial cells, makes up the great majority of solid matter. 48 Fecal microbiota primarily composed of bacteria, but also including viruses, fungi, and archaea. 49 The fecal microbiota plays crucial roles in digestion, immune function, metabolism, and even neurological processes. 50 Bacterial species within the fecal microbiota belong to various phyla, with Firmicutes and Bacteroidetes typically dominating in healthy individuals. Firmicutes are adept at breaking down complex carbohydrates, while Bacteroidetes contribute to the degradation of dietary fiber. Other phyla such as Actinobacteria and Proteobacteria are also present, even though in smaller proportions. 50 Within these phyla, there exists a vast array of genera and species, each with its own unique functions and interactions. For instance, members of the genera Bifidobacterium and Lactobacillus are renowned for their probiotic properties, promoting gut health and aiding in the synthesis of vitamins. 51 Conversely, certain taxa like Clostridioides difficile can become pathogenic under dysbiotic conditions, causing infections and gastrointestinal disorders. 52 Apart from bacteria, the fecal microbiota also harbors viruses (mainly bacteriophages), fungi (such as Candida and Saccharomyces), and archaea (including Methanobrevibacter smithii).49,50 These microbial constituents contribute to the overall balance and functionality of the gut ecosystem.
Fecal microbiota transplantation and its clinical application
Fecal bacteriotherapy, also known as fecal microbiota transplantation (FMT), is a revolutionary therapeutic approach that involves the transfer of fecal microbiota from a healthy donor to a recipient with a dysbiotic gut microbiome. This procedure aims to restore microbial balance and functionality in the gastrointestinal tract, offering a promising treatment for various gastrointestinal and systemic disorders. 46 FMT has shown remarkable efficacy in the management of recurrent Clostridiodes difficile infection, with success rates exceeding 90%, surpassing those of conventional antibiotic therapy. 53 Now a day, the most frequent indication for fecal microbiota transplantation is recurrent Clostridiodes difficile infection; however, fecal transplantation is also being tested as a treatment for other gastrointestinal diseases as well as some non-gastrointestinal conditions, such as Parkinson’s disease, fibromyalgia, chronic fatigue syndrome, multiple sclerosis, obesity, insulin resistance, metabolic syndrome, autism, and more, though these should be further evaluated in clinical trials. 54
Clostridium difficile infection
Even though Clostridiodes difficile is a normal component of the intestinal microbial environment, pre-exposure to broad-spectrum antibiotics can increase the risk of infection because they upset the gut flora’s balance, which Clostridiodes difficile needs to survive. 55 The speed and accuracy of the prescribed therapy will determine how quickly and effectively the infection progresses, but even in the presence of an effective treatment, there is a chance that the infection will return. 56 In the past few decades, FMT has received considerable attention because of a convincing clinical trial of treatment of recurrent Clostridiodes difficile infection. The first randomized controlled trial of FMT for 43 patients with recurrent Clostridiodes difficile infection compared FMT administered via nasoduodenal tube after 4–5 days of oral vancomycin with 14 days of continued vancomycin alone and with 14 days of vancomycin plus bowel lavage. 57
Numerous research has examined the fecal microbiota of donors and patients with Clostridiodes difficile infection recurrence, and they have demonstrated that the Clostridiodes difficile infection is linked to less changes in the diversity and composition of the fecal microbiota. When compared to samples from post-FMT patients with recurrent Clostridiodes difficile infection and healthy donor samples, the number of members of the Streptococcaceae, Enterococcaceae, or Enterobacteriaceae was raised, while the number of butyrate-producing (Lachnospiraceae and Ruminococcaceae) species was decreased. 58 According to the review article, 59 85% of recurrent Clostridium difficile infection and 55% of new Clostridiodes difficile infection were successfully treated with FMT, whereas medical therapy had success rates ranging from 30% to 80%. Recently, for individuals with recurring Clostridiodes difficile infection, RBX2660, a live biotherapeutic agent, presents a very promising therapy alternative. RBX2660 helps patients achieve clinically significant improvements by reestablishing a healthy gut microbiota. 60 In parallel, researchers are investigating the potential of adjunctive therapies, including bacteriophages, 61 Bacteriocin, 62 Probiotics, 62 antimicrobial peptides,63–65 and immunomodulators, 66 to enhance the efficacy of FMT or next-generation microbial therapies for Clostridiodes difficile infection. These combination therapies target various aspects of Clostridiodes difficile infection pathogenesis, such as bacterial virulence, host immune response, and microbiota restoration, to provide more comprehensive and durable treatment outcomes.
Irritable bowel syndrome
Fecal microbiota transplantation has emerged as a promising avenue for the treatment of irritable bowel syndrome (IBS), a common gastrointestinal disorder characterized by abdominal pain, bloating, and changes in bowel habits. 67 While the exact etiology of IBS remains unclear, growing evidence suggests that dysbiosis, an imbalance in the gut microbiota composition, plays a significant role in its pathogenesis. 68 It is thought that the intestinal microbiota, immune system, and brain-gut axis interact intricately in the etiology of irritable bowel syndrome, which is complicated and poorly understood. 69 FMT involves the transfer of fecal microbiota from a healthy donor to an IBS patient, with the aim of restoring microbial balance. Initial clinical studies have shown encouraging results, with some patients experiencing significant improvements in their gastrointestinal symptoms and overall quality of life following FMT.67,68
Furthermore, ongoing research is exploring next-generation therapies that build upon the principles of FMT to enhance efficacy and safety. These include targeted microbial interventions, such as the administration of specific microbial strains or microbial consortia tailored to address the dysbiosis observed in IBS patients.70,71 Additionally, advances in microbiome science have led to the development of personalized approaches, where fecal microbiota from a healthy donor is selected based on the recipient's unique microbial profile, maximizing compatibility and therapeutic benefits. 72 Moreover, innovative delivery methods, such as oral capsules or microbial biofilms, are being investigated to optimize the delivery and retention of microbial therapeutics in the gastrointestinal tract.73,74 These next-generation therapies hold great promise in reshaping the treatment landscape for IBS, offering patients more effective and personalized interventions that target the underlying mechanisms driving their symptoms. 75 As research in this field continues to advance, the integration of FMT and next-generation therapies into clinical practice has the potential to revolutionize the management of IBS and improve outcomes for millions of individuals worldwide.
Carcinoma of the colon
It is now thought that dysbiosis and the pro-carcinogenic qualities of bacteria (genotoxicity, inflammation, and oxidative stress) may be connected to the development of colorectal cancer. Some bacterial species, including Bacteroides fragilis, Streptococcus bovis, Clostridium septicum, Helicobacter pylori, Enterococcus faecalis, Escherichia coli, and Fusobacterium spp., have been found and are suspected of contributing to the development of colorectal cancer. 76 The complicated interaction between tumor cells, non-neoplastic cells (stromal cells), and a great deal of microbes results in colorectal cancer. In recent decades, more focus has been placed on the role of microbial infection in carcinogenesis in addition to known risk factors (fat-rich diets, obesity, population, and living in a developed country) and uncontrolled cellular proliferation. Microbes are suspected to be responsible for 20% of cancers, particularly colorectal cancer. 77 Fecal transplantation procedures could take the place of colorectal cancer associated dysbiosis and restore eubiosis in chronic disease, assisting in lowering the activation of inflammatory, proliferative, and pro-carcinogenic pathways as well as microbiota-induced genotoxicity. Future transplantation studies will be a crucial next step in this line of study, even if fecal transplantation has not been well investigated in colorectal cancer. 78
Crohn’s disease and ulcerative colitis
Crohn’s disease and ulcerative colitis, collectively known as inflammatory bowel diseases (IBD), chronic inflammatory conditions of the intestines, and the etiology of these conditions involves dysbiosis, which causes the mucosal immune system to become activated, causing chronic inflammation and the emergence of mucosal lesions. While the exact causes of IBD remain elusive, dysbiosis of the gut microbiota is believed to play a significant role in disease pathogenesis. 10 Fecal microbiota transplantation (FMT) has emerged as a promising therapeutic option for IBD, particularly for patients who fail to respond to conventional treatments.
Initial clinical studies have shown that FMT can induce remission and improve symptoms in a subset of patients with IBD.79–81 However, the efficacy of FMT varies widely among individuals, highlighting the need for optimized protocols and personalized approaches. Next-generation therapies for IBD are now being explored to enhance the therapeutic potential of FMT and address the limitations associated with donor variability, safety concerns, and unpredictable outcomes. One approach involves the development of microbial-based therapeutics, such as defined microbial consortia or engineered probiotics, tailored to target specific dysbiotic patterns associated with Crohn's disease and ulcerative colitis.82,83 Furthermore, advancements in delivery methods, such as encapsulation or targeted delivery systems, are being investigated to improve the efficacy and safety of microbial-based interventions for IBD.84,85 By enhancing the delivery and retention of therapeutic microbes in the gut, these innovative approaches may improve treatment outcomes and reduce the need for repeated administrations.
In addition to microbial-based therapies, other next-generation treatments for IBD include microbiome-modulating agents, 86 immunomodulators, 87 and targeted biologics 88 that aim to modulate the host immune response and restore intestinal homeostasis. These novel therapies offer promising avenues for precision medicine approaches in the management of Crohn’s disease and ulcerative colitis, with the potential to improve patient outcomes and quality of life.
Neuropsychiatric diseases
Today, it’s thought that changes to the intestinal microbiome during the first few months of life may be the cause of serious neuropsychiatric conditions that manifest as adulthood approaches, including schizophrenia, autism, behavioral or cognitive disorders, depression, anxiety, Alzheimer’s disease, multiple sclerosis, chronic fatigue syndrome, and schizophrenia. 89 There is an increasing focus on the impact of intestinal bacteria on human health, and recent data points to a potential function for the microbiota, gut, and brain axis in neuropsychiatric diseases. There has been a lot of research in this area during the past few years. Although the paths for this relationship are not completely understood, they include metabolic, humoral, immunological, and neurological pathways. 90 It was established that the endotoxin lipopolysaccharide would have an impact on how the central nervous system was modulated. Additionally, it generates inflammatory cytokines, which alter the physiological activity of the brain. 91
Recently, a child with chronic illness and epilepsy who had been receiving sodium valproate medication up to the transplant was described as the first patient to use fecal transplantation to achieve remission of digestive symptoms. After 20 weeks, effectiveness in preventing seizures was seen without the use of anti-epileptic medications. 89
Obesity and metabolic syndrome
Obesity and metabolic syndrome are multifactorial conditions influenced by genetic, environmental, and lifestyle factors. Emerging research suggests that alterations in the gut microbiota composition and function may contribute to the development of these conditions. Intestinal microbiota is one the primary cause of obesity,92–94 modifying nutrient absorption and energy management in addition to food consumption,95,96 which is crucial to the physiology of obesity. Obesity is a significant contributor to the risk of developing diabetes, hypertension, and the metabolic syndrome.97,98 The etiology of obesity and related disorders is therefore greatly influenced by the gut microbiota.
Fecal microbiota transplantation (FMT) has been proposed as a new way to change the gut microbiota that may result in beneficial metabolic changes, though the evidence for FMT effectiveness in the treatment of obesity.98–102 Now, bariatric surgery is the only treatment option for morbid obesity that sustains significant weight loss. 103 Next-generation therapies for obesity and metabolic syndrome may involve more targeted approaches, such as precision microbiome modulation. 104 This could include the development of microbial-based interventions, such as engineered probiotics or prebiotics designed to specifically target metabolic pathways or modulate the gut microbiota composition in a more controlled manner. 105 Next-generation therapies that leverage advances in microbiome science may offer more targeted and effective approaches for managing these conditions in the future.
Glucose intolerance
Chronic hyperglycemia and changes to the metabolisms of carbohydrates, lipids, and proteins are the hallmarks of the metabolic illness known as diabetes mellitus (DM), which is on the rise throughout the world. Polymorphonuclear leukocytes, T lymphocytes, and the immune system's reaction to antigen exposure are all altered in diabetic patients, along with bactericidal activity, bactericidal response, and polymorphonuclear leukocyte function. 106 Numerous potential long-term consequences are associated with both type 1 and type 2 diabetes mellitus, and they are often inversely correlated with the degree and persistence of hyperglycemia. 107
Studies have shown that individuals with glucose intolerance often exhibit dysbiosis, characterized by alterations in the gut microbiota composition and decreased microbial diversity. 108 It has been found that there is a correlation between the ratio of Bacteroidetes to Firmicutes and plasma glucose levels in type 2 diabetic and obese patients, suggesting that altering the microbial composition may offer a fresh method for preventing and treating obesity and type 2 diabetes. 109 Preclinical studies in animal models and some small-scale human trials have demonstrated improvements in glucose metabolism following FMT.110–112 These improvements are thought to be mediated by changes in the gut microbiota that positively influence metabolic function.
Precision microbiome modulation represents a cutting-edge approach to targeting specific microbial taxa or metabolic pathways associated with glucose intolerance. Engineered probiotics or prebiotics can be designed to selectively alter the gut microbiota, aiming to enhance glucose metabolism. 113 Additionally, the development of microbiome-targeted drugs holds promise for directly targeting microbial metabolites or signaling pathways involved in glucose regulation. 114 By leveraging advances in microbiome research, including metagenomics and metabolomics, personalized interventions tailored to an individual’s unique gut microbiota profile could revolutionize the management of glucose intolerance. These innovative strategies offer the potential for more targeted and effective interventions.
Illnesses resulting from allergies
Allergy-related conditions such as food allergies, asthma, and eczema have become more common in recent years. According to recent research, gut microbial alterations have a significant impact on the immunological processes that may result in the emergence of allergy illnesses. These are susceptible to modulation by a variety of environmental factors, including nutrition, antibiotic use, and early-life microbial exposures. 115 Immunological processes that might result in the emergence of allergy disorders are significantly influenced by changes in gut microbiota. The potential of the microflora to affect the immune response has led to new therapeutic modalities that utilize these variations in microbiota for the treatment and prophylaxis of allergy. By transplanting a complex population of bacteria that is more stable and has a larger capacity to colonize, FMT appears to hold promise for restoring immunological homeostasis.116,117
Adverse effect of fecal microbiome transplantation
Fecal microbiota transplantation (FMT) is generally considered a safe and effective treatment for certain conditions, but there are potential adverse effects associated with the procedure. 118 One significant risk is the transmission of infections from the donor to the recipient, despite strict donor screening protocols. These infections can include bacterial, viral, and parasitic agents.118,119 Additionally, recipients may experience gastrointestinal symptoms such as abdominal pain, bloating, diarrhea, or constipation following FMT, although these symptoms are typically mild and transient.120,121 In rare cases, allergic reactions to components of the fecal material or medications used during the procedure may occur, ranging from mild itching to severe anaphylaxis.119,122 There is also a risk of unintended changes or imbalances in the recipient’s gut microbiota, potentially leading to new or exacerbated gastrointestinal symptoms.119,123 Furthermore, since FMT is still a relatively novel procedure, there may be unknown long-term risks associated with it. These could include the transmission of chronic diseases from the donor to the recipient or triggering immunological reactions in the recipient. Thus, careful consideration of the potential risks and benefits, along with thorough donor screening and close monitoring of recipients, is essential in the use of FMT as a therapeutic intervention.
Conclusions and recommendation
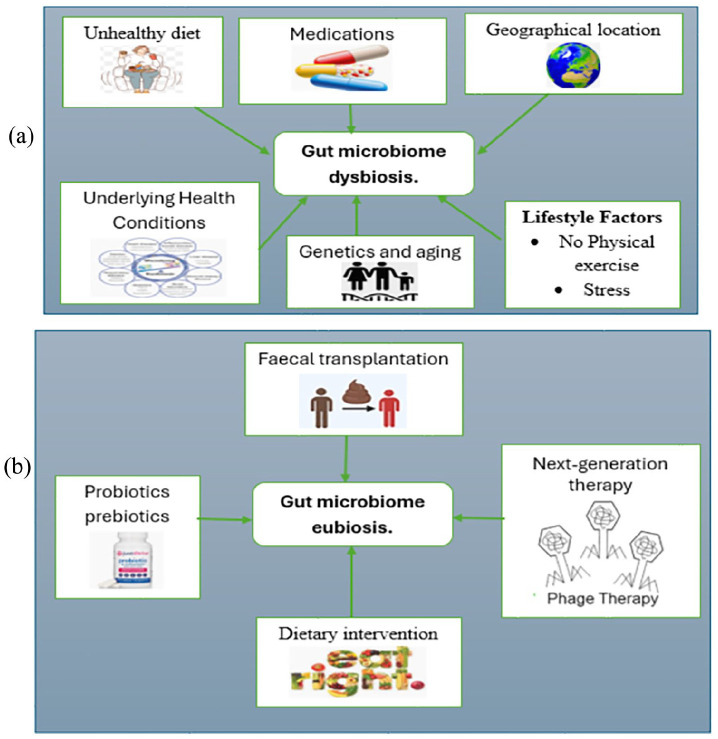
Dysbiosis, the imbalance of gut flora, is influenced by various factors including diet, lifestyle, medications, and underlying health conditions (Figure 3(a)). To counter dysbiosis and restore a healthy gut composition (eubiosis), microbiota modulation techniques are being explored (Figure 3(b)). By targeting specific microbial communities or metabolic pathways, these techniques hold promise for precisely and effectively restoring gut microbiota balance, offering potential advancements in therapeutic. In conclusion, fecal microbiota transplantation (FMT) and next-generation therapies represent exciting avenues for targeting dysbiosis in metabolic disorders and other health conditions. As our understanding of the gut microbiome continues to evolve, so does the potential for innovative interventions aimed at restoring microbial balance and promoting health. FMT, with its demonstrated efficacy in treating conditions like recurrent Clostridioides difficile infection, serves as a cornerstone in this field, while emerging therapies such as personalized microbiome modulation and synthetic microbial consortia offer promise for more tailored and precise interventions. Regulatory considerations, ongoing research efforts, and public awareness initiatives will be instrumental in realizing the full therapeutic potential of these approaches. With continued advancements in microbiome science and therapeutic innovation, the future holds great promise for harnessing the power of the gut microbiota to improve health and well-being across diverse patient populations.
Figure 3.
(a) Factors influencing dysbiosis, that is, alteration of gut flora and (b) microbiota modulation techniques inducing eubiosis, that is, restoration of altered gut composition.
Future perspective of fecal microbiota transplantation
The future of fecal microbiota transplantation is promising, with ongoing research to further elucidate its therapeutic potential and expand its clinical applications across a wide range of health conditions. By altering the human gut flora, fecal microbiota transplantation could benefit HIV-infected patients. 124 Phage therapy may be able to eradicate aggressive bacteria from a diseased gut and promote the growth of commensal bacteria. Future research in the field of gut microbiota modulation should focus on several key areas to advance our understanding and enhance therapeutic strategies.
Refinement of Treatment Protocols: Continued research and clinical trials will likely lead to the refinement of FMT protocols, including optimal donor selection criteria, preparation methods, and delivery techniques. This could improve the safety, efficacy, and reproducibility of FMT procedures.
Personalized Medicine Approaches: Advances in microbiome sequencing technologies and computational analysis techniques may enable personalized FMT treatments designed to the unique microbial profiles of individual patients. This could involve matching donors and recipients based on specific microbial signatures or using synthetic microbiota formulations designed to address specific dysbiosis patterns.
Microbiome Modulation Therapies: Beyond whole stool-based FMT, future therapies may involve targeted manipulation of the gut microbiome using defined microbial consortia, microbial metabolites, or microbial-derived products. These approaches could offer more precise and controlled interventions with potentially fewer risks compared to traditional FMT.
Regulatory Considerations: As FMT becomes more widely used and novel applications emerge, regulatory frameworks may evolve to ensure the safety, quality, and ethical standards of fecal microbiota transplantation. This includes establishing guidelines for donor screening, standardized protocols for FMT procedures, and oversight of FMT-related research and clinical practice.
Integration with Other Therapies: FMT may be integrated with other therapeutic modalities, such as antibiotics, probiotics, prebiotics, dietary interventions, and immunomodulatory agents, to optimize treatment outcomes and address underlying disease mechanisms comprehensively. Combining FMT with complementary therapies could enhance its efficacy and reduce the risk of disease recurrence.
Public Awareness and Acceptance: Continued education and outreach efforts aimed at healthcare providers, patients, and the public will be crucial to raise awareness about FMT, dispel misconceptions, and foster acceptance of this innovative treatment modality. Increased awareness may also facilitate greater participation in donor recruitment efforts and clinical trials.
Acknowledgments
We extend our gratitude to all the authors who have contributed their work and data to this topic.
Footnotes
Author contributions: All authors contributed significantly to the paper’s conception and design, data collection, and writing the paper; agreed to submit the manuscript to the current journal; granted final approval of the version to be published.
Availability of data and materials: Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Not applicable.
Informed consent: Not applicable.
Consent for publication: Not applicable.
ORCID iDs: Zenawork Sahle  https://orcid.org/0000-0002-9296-993X
https://orcid.org/0000-0002-9296-993X
Demissew Shenkute Gebreyes  https://orcid.org/0000-0003-2245-2609
https://orcid.org/0000-0003-2245-2609
Tsegahun Asfaw Abebe  https://orcid.org/0000-0002-7410-5484
https://orcid.org/0000-0002-7410-5484
References
- 1. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J 2017; 474(11): 1823–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bull MJ, Plummer NT. Part 1: the human gut microbiome in health and disease. Integr Med (Encinitas) 2014; 13(6): 17–22. [PMC free article] [PubMed] [Google Scholar]
- 3. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020; 126(11): 1477–1500. [DOI] [PubMed] [Google Scholar]
- 4. Sitkin SI, Tkachenko EI, Vakhitov TY. Metabolic dysbiosis of the gut microbiota and its biomarkers. Eksp Klin Gastroenterol 2016; 12(12): 6–29. [PubMed] [Google Scholar]
- 5. Crudele L, Gadaleta RM, Cariello M, et al. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine 2023; 97: 104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strati F, Lattanzi G, Amoroso C, et al. Microbiota-targeted therapies in inflammation resolution. Semin Immunol 2022; 59: 101599. [DOI] [PubMed] [Google Scholar]
- 7. Mutalub YB, Abdulwahab M, Mohammed A, et al. Gut microbiota modulation as a novel therapeutic strategy in cardiometabolic diseases. Foods 2022; 11(17): 2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olofsson LE, Bäckhed F. The metabolic role and therapeutic potential of the microbiome. Endocr Rev 2022; 43(5): 907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allegretti JR, Mullish BH, Kelly C, et al. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019; 394(10196): 420–431. [DOI] [PubMed] [Google Scholar]
- 10. Burke KE, Lamont JT. Fecal transplantation for recurrent C lostridium difficile infection in older adults: a review. J Am Geriatr Soc 2013; 61(8): 1394–1398. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Luo X, Tian L, et al. The gut microbiome dysbiosis and regulation by fecal microbiota transplantation: umbrella review. Front Microbiol 2023; 14: 1286429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belvoncikova P, Maronek M, Gardlik R. Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int J Mol Sci 2022; 23(18): 10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ademe M. Benefits of fecal microbiota transplantation: a comprehensive review. J Infect Develop Countries 2020; 14(10): 1074–1080. [DOI] [PubMed] [Google Scholar]
- 14. Sandhu A, Chopra T. Fecal microbiota transplantation for recurrent Clostridioides difficile, safety, and pitfalls. Therap Adv Gastroenterol 2021; 14: 17562848211053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep 2018; 20: 14. [DOI] [PubMed] [Google Scholar]
- 16. Osman M, Budree S, Kelly CR, et al. Effectiveness, and safety of fecal microbiota transplantation for Clostridioides difficile infection: results from a 5344-patient cohort study. Gastroenterology 2022; 163(1): 319–322. [DOI] [PubMed] [Google Scholar]
- 17. Kellermayer R. Fecal microbiota transplantation: great potential with many challenges. Transl Gastroenterol Hepatol 2019; 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bowman KA, Broussard EK, Surawicz CM. Fecal microbiota transplantation: current clinical efficacy and future prospects. Clin Exp Gastroenterol 2015; 8: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hyland N, Stanton C. (eds.) The gut-brain axis: dietary, probiotic, and prebiotic interventions on the microbiota. Amsterdam, The Netherlands: Elsevier, 2023. [Google Scholar]
- 20. Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013; 11(4): 227–238. [DOI] [PubMed] [Google Scholar]
- 21. Arrieta MC, Stiemsma LT, Amenyogbe N, et al. The intestinal microbiome in early life: health and disease. Front Immunol 2014; 5: 105813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015; 33(9): 496–503. [DOI] [PubMed] [Google Scholar]
- 23. Mariat D, Firmesse O, Levenez F, et al. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 2009; 9: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019; 7(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol 2015; 21(29): 8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marotz CA, Zarrinpar A. Focus: microbiome: treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J Biol Med 2016; 89(3): 383. [PMC free article] [PubMed] [Google Scholar]
- 27. Xue L, Deng Z, Luo W, et al. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol 2022; 12: 759306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghorbani Y. Fecal microbiota transplant from healthy lean donors to individuals with obesity: effect on insulin resistance, metabolic parameters, appetite and metabolites. Doctoral Dissertation, University of Toronto, Canada, 2023. [Google Scholar]
- 29. Schluter J, Foster KR. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol 2012; 10(11): e1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elzinga J, van der Oost J, de Vos WM, et al. The use of defined microbial communities to model host-microbe interactions in the human gut. Microbiol Mol Biol Rev 2019; 83(2): 10–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res 2020; 30(6): 492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pickard JM, Zeng MY, Caruso R, et al. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 2017; 279(1): 70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung H, Kasper DL. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr Opin Immunol 2010; 22(4): 455–460. [DOI] [PubMed] [Google Scholar]
- 34. Song Q, Wang Y, Huang L, et al. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res Int 2021; 140: 109858. [DOI] [PubMed] [Google Scholar]
- 35. Cockburn DW, Koropatkin NM. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J Mol Biol 2016; 428(16): 3230–3252. [DOI] [PubMed] [Google Scholar]
- 36. Hijova E. Gut bacterial metabolites of indigestible polysaccharides in intestinal fermentation as mediators of public health. Bratisl Lek Listy 2019; 120(11): 807–812. [DOI] [PubMed] [Google Scholar]
- 37. Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Euro J Nutr 2018; 57: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol 2013; 28: 9–17. [DOI] [PubMed] [Google Scholar]
- 39. Ahlawat S, Asha Sharma KK. Gut–organ axis: a microbial outreach and networking. Lett Appl Microbiol 2021; 72(6): 636–668. [DOI] [PubMed] [Google Scholar]
- 40. Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2012; 9(2): 88–96. [DOI] [PubMed] [Google Scholar]
- 41. Brandt LJ. Fecal transplantation for the treatment of clostridium difficile infection. Gastroenterol Hepatol (N Y) 2012; 8(3): 191–194. [PMC free article] [PubMed] [Google Scholar]
- 42. Bibbò S, Settanni CR, Porcari S, et al. Fecal microbiota transplantation: screening and selection to choose the optimal donor. J Clin Med 2020; 9(6): 1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nicco C, Paule A, Konturek P, et al. From donor to patient: collection, preparation and cryopreservation of fecal samples for fecal microbiota transplantation. Diseases 2020; 8(2): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent clostridium difficile infection. Am J Gastroenterol 2012; 107(5): 761–767. [DOI] [PubMed] [Google Scholar]
- 45. Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 2015; 149(1): 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi HH, Cho Y-S. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc 2016; 49(3): 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rose C, Parker A, Jefferson B, et al. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol 2015; 45: 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bojanova DP, Bordenstein SR. Fecal transplants: what is being transferred? PLoS Biol 2016; 14(7): e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matijašić M, Meštrović T, Paljetak HČ, et al. Gut microbiota beyond bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int J Mol Sci 2020; 21(8): 2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khaledi M, Poureslamfar B, Alsaab HO, et al. The role of gut microbiota in human metabolism and inflammatory diseases: a focus on elderly individuals. Ann Microbiol 2024; 74(1): 1. [Google Scholar]
- 51. Kleerebezem M, Vaughan EE. Probiotic and gut lactobacilli and bifidobacteria: molecular approaches to study diversity and activity. Ann Rev Microbiol 2009; 63: 269–290. [DOI] [PubMed] [Google Scholar]
- 52. Rodríguez C, Romero E, Garrido-Sanchez L, et al. Microbiota insights in Clostridium difficile infection and inflammatory bowel disease. Gut Microbes 2020; 12(1): 1725220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol 2012; 5(6): 403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Filip M, Tzaneva V, Dumitrascu DL. Fecal transplantation: digestive and extradigestive clinical applications. Clujul Med 2018; 91(3): 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Patangia DV, Anthony Ryan C, Dempsey E, et al. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022; 11(1): e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mattila E, Uusitalo–Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142(3): 490–496. [DOI] [PubMed] [Google Scholar]
- 57. Vyas D, Aekka A, Vyas A. Fecal transplant policy and legislation. World J Gastroenterol 2015; 21(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Song Y, Garg S, Girotra M, et al. Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS One 2013; 8: e81330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drekonja D, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for clostridium difficile infection: a systematic review. Ann Intern Med 2015; 162: 630. [DOI] [PubMed] [Google Scholar]
- 60. Chopra T. A profile of the live biotherapeutic product RBX2660 and its role in preventing recurrent Clostridioides difficile infection. Exp Rev Anti Infect Therap 2023; 21(3): 243–253. [DOI] [PubMed] [Google Scholar]
- 61. Zuo T, Wong SH, Lam K, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018; 67(4): 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rea MC, Alemayehu D, Ross RP, et al. Gut solutions to a gut problem: bacteriocins, probiotics and bacteriophage for control of Clostridium difficile infection. J Med Microbiol 2013; 62(9): 1369–1378. [DOI] [PubMed] [Google Scholar]
- 63. Nuding S, Frasch T, Schaller M, et al. Synergistic effects of antimicrobial peptides and antibiotics against Clostridium difficile. Antimicrob Agents Chemother 2014; 58(10): 5719–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu B, Shaoyong W, Wang L, et al. Gut-targeted nanoparticles deliver specifically targeted antimicrobial peptides against Clostridium perfringens infections. Sci Adv 2023; 9(39): eadf8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hing TC, Ho S, Shih DQ, et al. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut 2013; 62(9): 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin Gastroenterol Hepatol 2009; 7(9): 981–987. [DOI] [PubMed] [Google Scholar]
- 67. Leylabadlo HE, Heravi FS, Soltani E, et al. The role of gut microbiota in the treatment of irritable bowel syndrome. Rev Res Med Microbiol 2022; 33(1): e89–e104. [Google Scholar]
- 68. Huang HL, Chen HT, Luo QL, et al. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J Dig Dis 2019; 20(8): 401–408. [DOI] [PubMed] [Google Scholar]
- 69. Iacob T, Ţăţulescu DF, Dumitraşcu D. Therapy of the postinfectious irritable bowel syndrome: an update. Clujul Med 2017; 90(2): 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petrof EO, Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology 2014; 146(6): 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gupta M, Kapoor B, Gulati M. Bacterial consortia-The latest arsenal to inflammatory bowel disease bacteriotherapy. Med Microecol 2024; 31: 100107. [Google Scholar]
- 72. Kingsley MJ, Abreu MT. A personalized approach to managing inflammatory bowel disease. Gastroenterol Hepatol 2016; 12(5): 308. [PMC free article] [PubMed] [Google Scholar]
- 73. Liu J, Li X, Zhang X, et al. Gut lumen-targeted oral delivery system for bioactive agents to regulate gut microbiome. J Future Foods 2022; 2(4): 307–325. [Google Scholar]
- 74. Woo CW, Tso P, Yiu JH. Commensal gut microbiota-based strategies for oral delivery of therapeutic proteins. Trends Pharmacol Sci 2022; 43(12): 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lacy BE, Chey WD, Lembo AJ. New and emerging treatment options for irritable bowel syndrome. Gastroenterol Hepatol 2015; 11(4 Suppl 2): 1. [PMC free article] [PubMed] [Google Scholar]
- 76. Gagnière J, Raisch J, Veziant J, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol 2016; 22(2): 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zur Hausen H. The search for infectious causes of human cancers: where and why. Virology 2009; 392(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 78. Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013; 13(11): 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019; 321(2): 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflam Bowel Dis 2015; 21(3): 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Greenberg A, Aroniadis O, Shelton C, et al. Long-term follow-up study of fecal microbiota transplantation (FMT) for inflammatory bowel disease (IBD): 1791. Am J Gastroenterol 2013; 108: S540. [Google Scholar]
- 82. Barra M, Danino T, Garrido D. Engineered probiotics for detection and treatment of inflammatory intestinal diseases. Front Bioeng Biotechnol 2020; 8: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oka A, Sartor RB. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Digest Dis Sci 2020; 65(3): 757–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hua S, Marks E, Schneider JJ, et al. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine 2015; 11(5): 1117–1132. [DOI] [PubMed] [Google Scholar]
- 85. Zhang S, Langer R, Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today 2017; 16: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alshehri D, Saadah O, Mosli M, et al. Dysbiosis of gut microbiota in inflammatory bowel disease: current therapies and potential for microbiota-modulating therapeutic approaches. Bosn J Basic Med Sci 2021; 21(3): 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aberra FN, Lichtenstein GR. Monitoring of immunomodulators in inflammatory bowel disease. Aliment Pharmacol Therap 2005; 21(4): 307–319. [DOI] [PubMed] [Google Scholar]
- 88. Chan HC, Ng SC. Emerging biologics in inflammatory bowel disease. J Gastroenterol 2017; 52: 141–150. [DOI] [PubMed] [Google Scholar]
- 89. He Z, Cui B-T, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: the first report. World J Gastroenterol 2017; 23(19): 3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wrona D. Neural–immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol 2006; 172(1–2): 38–58. [DOI] [PubMed] [Google Scholar]
- 91. Kalyan M, Tousif AH, Sonali S, et al. Role of endogenous lipopolysaccharides in neurological disorders. Cells 2022; 11(24): 4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol 2013; 11(9): 639–647. [DOI] [PubMed] [Google Scholar]
- 93. Gérard P. Gut microbiota and obesity. Cell Mol Life Sci 2016; 73(1): 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu BN, Liu XT, Liang ZH, et al. Gut microbiota in obesity. World J Gastroenterol 2021; 27(25): 3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Garcia G, Sunil TS, Hinojosa P. The fast food and obesity link: consumption patterns and severity of obesity. Obes Surg 2012; 22: 810–818. [DOI] [PubMed] [Google Scholar]
- 96. Machado PP, Steele EM, Levy RB, et al. Ultra-processed food consumption and obesity in the Australian adult population. Nutr Diabetes 2020; 10(1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 2013; 7(5): e330–e341. [DOI] [PubMed] [Google Scholar]
- 98. Nguyen NT, Magno CP, Lane KT, et al. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am College Surg 2008; 207(6): 928–934. [DOI] [PubMed] [Google Scholar]
- 99. Yu EW, Gao L, Stastka P, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 2020; 17(3): e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mocanu V, Zhang Z, Deehan EC, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med 2021; 27(7): 1272–1279. [DOI] [PubMed] [Google Scholar]
- 101. Ng SC, Xu Z, Mak JW, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut 2022; 71(4): 716–723. [DOI] [PubMed] [Google Scholar]
- 102. Allegretti JR, Kassam Z, Mullish BH, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol 2020; 18(4): 855–863. [DOI] [PubMed] [Google Scholar]
- 103. Lahtinen P, Juuti A, Luostarinen M, et al. Effectiveness of fecal microbiota transplantation for weight loss in patients with obesity undergoing bariatric surgery: a randomized clinical trial. JAMA Network Open 2022; 5(12): e2247226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vallianou NG, Kounatidis D, Tsilingiris D, et al. The role of next-generation probiotics in obesity and obesity-associated disorders: current knowledge and future perspectives. Int J Mol Sci 2023; 24(7): 6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Green M, Arora K, Prakash S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci 2020; 21(8): 2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Daryabor G, Atashzar MR, Kabelitz D, et al. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol 2020; 11: 546198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mauri-Obradors E, Estrugo-Devesa A, Jané-Salas E, et al. Oral manifestations of diabetes mellitus. A systematic review. Med Oral Patol Oral Cir Bucal 2017; 22(5): e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao M, Liao D, Zhao J. Diabetes-induced mechanophysiological changes in the small intestine and colon. World J Diabetes 2017; 8(6): 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Larsen N, Vogensen FK, Van Den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010; 5(2): e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen L, Guo L, Feng S, et al. Fecal microbiota transplantation ameliorates type 2 diabetes via metabolic remodeling of the gut microbiota in db/db mice. BMJ Open Diabetes Res Care 2023; 11(3): e003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wu L, Li MQ, Xie YT, et al. Washed microbiota transplantation improves patients with high blood glucose in South China. Front Endocrinol 2022; 13: 985636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 2017; 26(4): 611–619. [DOI] [PubMed] [Google Scholar]
- 113. Ji J, Jin W, Liu SJ, et al. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023; 4(6): e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brown JM, Hazen SL. Targeting of microbe-derived metabolites to improve human health: the next frontier for drug discovery. J Biol Chem 2017; 292(21): 8560–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chung KF. Airway microbial dysbiosis in asthmatic patients: a target for prevention and treatment? J Aller Clin Immunol 2017; 139(4): 1071–1081. [DOI] [PubMed] [Google Scholar]
- 116. Maksimova O, Gervazieva V, Zverev V. Intestine microbiota and allergic diseases. J Microbiol Epidemiol Immunobiol 2014; 91(3): 49–60. [PubMed] [Google Scholar]
- 117. Reynolds LA, Finlay BB. A case for antibiotic perturbation of the microbiota leading to allergy development. Exp Rev Clin Immunol 2013; 9(11): 1019–1030. [DOI] [PubMed] [Google Scholar]
- 118. Park SY, Seo GS. Fecal microbiota transplantation: is it safe? Clin Endosc 2021; 54(2): 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang JW, Kuo CH, Kuo FC, et al. Fecal microbiota transplantation: review and update. J Formosan Med Assoc 2019; 118: S23–S31. [DOI] [PubMed] [Google Scholar]
- 120. Bénard MV, de Bruijn CM, Fenneman AC, et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS One 2022; 17(10): e0276323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Woodworth MH, Carpentieri C, Sitchenko KL, et al. Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes 2017; 8(3): 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Liu SX, Li YH, Dai WK, et al. Fecal microbiota transplantation induces remission of infantile allergic colitis through gut microbiota re-establishment. World J Gastroenterol 2017; 23(48): 8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rapoport EA, Baig M, Puli SR. Adverse events in fecal microbiota transplantation: a systematic review and meta-analysis. Ann Gastroenterol 2022; 35(2): 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Serrano-Villar S, Talavera-Rodríguez A, Gosalbes MJ, et al. Fecal microbiota transplantation in HIV: a pilot placebo-controlled study. Nat Commun 2021; 12(1): 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]