Abstract
Benign prostatic hyperplasia (BPH) is a common chronic urologic condition affecting approximately 50% of men above the age of 60. As per European Association of Urology Guidelines, BPH can be treated according to a stepwise approach starting from a conservative management, a pharmacologic approach, and finally surgery. Both medical and surgical therapies have side effects, impacting on ejaculation and sexual function and patients with multiple comorbidities might not be considered surgically suitable candidates. Prostatic stents offer a minimally invasive procedures in an out-patient setting, possibly under local anaesthesia. Utilized since the 1980s, the past stents encompassed permanent (epithelializing) or temporary (non-epithelializing) devices, like the Uro-Lume (American Medical Systems, Minnetonka, MN, USA) and the Memokath, or Memotherm (Engineers & Doctors A/S, Denmark), and the biodegradable stents made of self-reinforced poly-L-lactide or braided poly lactic-co-glycolic acid. Previous stents however showed a quite high rate of complications among which pain, incontinence, infections, stent migration or blockage, and incomplete degradation that might lead to premature removal of stent. The stents currently available on the market instead are the temporary device Allium Triangular Prostatic Urethral Stent (Allium Urological Solutions, Caesarea, Israel) and the temporary stent SPANNER (AbbeyMoor Medical, Inc., Parkers Prairie, MN, USA), which might be used in case of bladder outflow obstruction, post-operatively, or for acute urinary retention. Studies showed encouraging results, in terms of effectiveness and safety improving patients’ quality of life and International Prostate Symptom Score, but longer-term studies are needed to identify the most suitable patients who might benefit from their use. Newer stents and nitinol devices are currently investigated, and we are waiting for the results of the ongoing clinical trials.
Keywords: benign prostatic hyperplasia, LUTS, MIST, prostate, prostatic stent
Introduction
Benign prostatic hyperplasia (BPH) is a common chronic urologic condition affecting ageing men. Approximately 50% of men above the age of 60 have bladder outflow obstruction to some degree, resulting in lower urinary symptoms (LUTS) which greatly impacts their quality of life (QoL). 1 According to European Association of Urology (EAU) guidelines, LUTS should be treated with a stepwise approach. 2 First, a conservative management with behavioural and dietary modifications should be considered, as 79% of LUTS remain clinically stable at 5 years. 2 Then, a pharmacologic approach can be considered, which might involve single or combination therapies according to the prostate volume and patients’ symptoms. 2 Surgery is considered mandatory in case of urinary retention, recurrent infections, bladder stones, recurrent macrohematuria, renal insufficiency or overflow incontinence. 2 However, both medical and surgical therapies have side effects and usually impact ejaculation and sexual function. Additionally, patients with multiple comorbidities are usually not considered surgically suitable candidates, and this is especially important considering the general trend of an ageing population. 3 In this scenario, prostatic stents may play a pivotal role, offering minimally invasive procedures in an out-patient setting possibly under local anaesthesia. 3
Utilized since the 1980s, stents are tubes placed temporarily or permanently in the prostatic urethra to compress prostatic tissue and overcome the bladder outlet obstruction (BOO). Stents can be inserted on an outpatient basis, under regional or topical anaesthesia. 3 They offer rapid relief but necessitate the presence of a functional detrusor. Stents can be of different materials and shapes or can be categorized as permanent (epithelializing) or temporary (non-epithelializing). Materials that inhibit epithelial ingrowth make their removal easier. Temporary stents may be either biostable or biodegradable. On the other hand, permanent stents are biocompatible, promoting epithelialization.
Our aim was to perform a narrative review of the current literature and to give an overview on the recent advancements in prostatic stents for managing BPH.
Methods
A systematic search of literature was performed on PubMed, Scopus, Web of Science, Clinical trial.gov, and Cochrane Library database were searched systematically for English-language articles published up to October 2023. The search term used included ‘BPH’, ‘benign prostatic hyperplasia’, ‘benign prostatic hypertrophy’, ‘prostate hypertrophy’, ‘stent’, ‘prostatic stent’, ‘prostate expander’, ‘Urocross’, ‘Prostaplant’, ‘Zenflow’, ‘Optilume’, ‘Proverum’, ‘XFLO’, ‘Provee’ and ‘Butterfly’. Boolean operators (AND, OR) were used to refine the search. Supplementary studies were identified by examining the references of systematic reviews and literature reviews. Inclusion criteria were (1) all age groups, (2) involve patients with BPH treated with a prostatic stent, (3) all available stents and (4) be available in English. Exclusion criteria encompassed BPH treatments different from prostatic stents, other intraprostatic or urethral devices different from prostatic stents, ex vivo or animal studies on prostatic stents, preclinical studies, and those classified as editorials, commentaries, literature reviews, guidelines or systematic reviews.
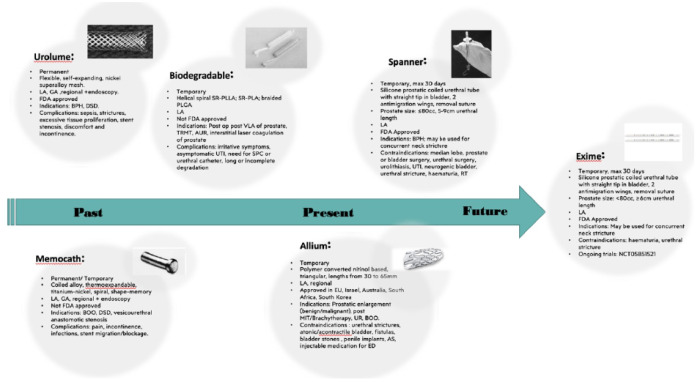
Two investigators (C.C. and V.A.) independently screened all titles and abstracts from the literature overview to identify the eligible studies, and then evaluated the full-text manuscripts to determine the final selected articles. Any discrepancies were resolved by consultation with a senior author (B.S.). The stents were comprehensively summarized and presented in Figure 1.
Figure 1.
Past, present, and future prostatic stents their characteristics.
AS, artificial sphincter; BOO, bladder outler obstruction; BPH, benign hyperplasia; DSD, detrusor-sphincter dyssynergia; ED, erectile dysfunction; GA, general anaesthesia; IA, local anaesthesia; MIT, minimally invasive therapy; PLGA, poly(lactic-coglycolic acid); RT, radiotherapy; SPC, suprapubic catheter; SR-PLA, self-reinforced poly-DL-lactic acid; SR-PLIA self-reinforced poly-L-lactide; UR, urinary retention; UTI, urinary tract infection.
Past stents – metallic and biodegradable stents
One of the first available stents has been the Uro-Lume (American Medical Systems, Minnetonka, MN, USA), an epithelializing permanent stent first developed in the beginning of the 90s (Table 1). It has the configuration of a woven, self-expanding tubular mesh made of fine superalloy wire. The stent is placed by a special delivery system which allows direct endoscopic visualization of the device during placement. It is a self-expanding mesh cylinder that can be endoscopically implanted under a general or local anaesthesia.4,5 Usually, the epithelium covers the stent entirely. 6 It was firstly developed to treat urethral stricture disease and detrusor-sphincter dyssynergia due to spinal cord injury.7,8 Several long-term studies reported on stent migration and post-operative complications, like sepsis, stricture, excessive tissue proliferation, stent stenosis, discomfort, and incontinence, complications which were in contrast with the initial enthusiasm.7,9,10 The stent has therefore been reviewed in 2007, 11 and more recent reports indicate that it was a reasonable minimally invasive treatment option, but still with 36% of ingrowth, needing a reasonable rate of need for subsequent interventions. 12 For these reasons, this stent might be used in fragile patients, who are not suitable for other surgical procedures. Another modification of Urolume is the Gianturco stent, a self-expanding device made of stainless steel but with larger spacing between the interstices and lesser shortening with increasing diameter. 13
Table 1.
Prostatic stents, nitinol devices, and their characteristics.
| Stent | Industry | Past, present, trial | Temporary or permanent | Description | Prostate characteristics | Anaesthetic | Indications | Contraindications and/or complications | Approvals |
|---|---|---|---|---|---|---|---|---|---|
| UroLume | Allium Urological Solutions | Past | Permanent | Flexible self-expanding device; braided mesh cylinder made from nickel superalloy wire mesh | NA | Local, regional, or general Endoscopically placed |
Bulbar urethral strictures BPH DSD |
Complications: sepsis, stricture, excessive tissue proliferation, stent stenosis, discomfort, and incontinence | FDA |
| Memocath/Memokath | Engineers & Doctors A/S | Past | Permanent or temporary | Alloy coiled thermo-expandable titanium- nickel spiral; shape-memory | NA | Local, regional, or general Endoscopically placed |
BOO DSD as a rendezvous procedure Vesicourethral anastomotic stenosis post prostatectomy |
Complications: pain, incontinence, infections, and stent migration or blockage | No |
| Biodegradable/dissolvable | NA | Past | Temporary | Helical-spiral shaped self-reinforced poly-l-lactic, poly-l-glycolic (SR-PLLA), braided PLGA copolymers | NA | Local | Post-op after visual laser ablation of the prostate, transurethral microwave therapy, interstitial laser coagulation of the prostate, AUR | Complications: irritative symptoms, asymptomatic urinary infection, long or incomplete degradation, sometimes need of SPC or transurethral catheter | No |
| Allium | Allium Urological Solutions | Present | Temporary | Polymer- covered nitinol-based product, triangular in shape, available in various lengths from 30 to 65 mm | NA | Local or regional | Prostatic enlargement (benign or malignant), after MIT based or thermal tissue damage of the prostate, Brachytherapy, voiding difficulties, urinary retention | Urethral strictures Anticoagulation treatment Atonic/acontractile bladder Fistulas Bladder stones In case of injectable medications for erectile dysfunction Penile implants Artificial sphincters |
Approved in European Union, Israel, Australia, South Africa, and South Korea |
| Spanner | AbbeyMoor Medical | Present | Temporary, max for 30 days | Silisone elastomer composed of a proximal baloon, a stent, and a distal anchor. Available in 20Fr or 22Fr, from 4 to 9 cm | Prostate size ⩽80cc 5–9 cm urethral length |
Local anaesthesia | BPH; may be used for concurrent neck stricture | Contraindications: median lobe, prostate or bladder surgery, urethral surgery, urolithiasis, UTI, neurogenic bladder, urethral stricture, haematuria, RT | FDA |
| iTiND | Medi-Tate | Present | Temporary | Three nitinol struts, an anti-migration anchor- ing leaflet and a polyester retrieval suture | Prostate size <70cc | Local, General, Regional | BOO, for patients wishing to maintain ejaculation | Contraindications: previous prostate surgery, median lobe Complications: irritative symptoms |
FDA |
| Exime | ROCAMED | Future | Temporary, max for 30 days | Silicone prostatic coiled urethral tube with straight tip to the bladder and two anti-migration wings and removal suture | Prostate size ⩽80cc ⩾6 cm urethral length |
Local | AUR After minimally invasive surgeries (MIS) for prostate not causing oedema |
UTI, Haematuria with clots, Sphincteric failures, Urethral stenosis, Bladder stones PVR > 150 ml Bladder apex-neck > 7cm After treatment with agents causing prostate oedema (HIFU, brachytherapy, radiotherapy) |
No Ongoing trials: NCT05851521 |
| Optilume BPH Catheter System | Urotronic | Future | Temporary | Balloon mechanical + paclitaxel | Prostate size 20–80cc 3.2–5.5 cm urethral length |
Local | BPH for glands up to 80 cc Might be used for concurrent neck stricture |
Contraindications: AS, previous prostate surgery, median lobe, prostate/bladder cancer, UTI, RT/pelvic trauma, neurogenic bladder, incontinence, urethral stricture, high bladder neck | No Ongoing trials: NCT04131907 |
| ZenFlow Spring | Zenflow | Future | Permanent or Temporary | Nitinol implant devices | Prostate size < 80cc 2.5–4.5 cm urethral length |
Local | BOO | Contraindications: previous prostate surgery, median lobe, urethral strictures, UTI, RT | No Ongoing trials: NCT04309695, NCT03595735, NCT03577236, NCT02786290 |
| Urocross Expander System | Prodeon Medical | Future | Temporary | Nitinol implant devices | Prostate size < 80cc | Local | BOO | Contraindications: previous prostate surgery, median lobe, urethral strictures, high bladder neck, neurogenic bladder, bladder stones, AUR, UTI, RT, pelvic surgery | No Ongoing trails: NCT05400980, NCT03758222 |
| ProVee | ProVerum | Future | Temporary | Nitinol implant devices | Prostate size 30–80cc ⩾4 cm urethral length |
Local | BOO | Contraindications: median lobe, urethral strictures, incompetent external sphincter, neurogenic bladder, haematuria, UTI | No Ongoing trails: NCT03972371, NCT05186740 |
| Butterfly device | Butterfly | Future | Temporary | Nitinol implant shaped like a butterfly | Prostate size 30–90cc ⩾2.5–4.5 cm urethral length |
Local | BOO | Contraindications: median lobe, urethral strictures, high bladder neck. previous prostate surgery, UTI, SUI, prostate cancer, CPPS, bladder cancer, neurogenic bladder, bladder stones | No Ongoing trials: NCT05341661, NCT03912558, NCT05330520 |
AS, artificial sphincter; AUR, acute urinary retention; BOO, bladder outlet obstruction; BPH, benign prostate hyperplasia; CPPS, chronic pelvic pain syndrome; DSD, detrusor-sphincter dyssynergia; MIT, minimally invasive therapy; NA, not applicable; PLGA, poly(lactic-coglycolic acid); PVR, post voiding residual; RT, radiotherapy; SPC, suprapubic catheter; SR-PLLA, self-reinforced poly-L-lactide; SUI, stress urinary incontinence; UTI, urinary tract infection.
Overall, several studies investigated the complications of permanent stents. In a study involving 47 patients, the stent was extracted from 14 out of 36 patients over a two-year period. Primary reason for removal included stent migration and obstruction of the stent lumen due to epithelial hyperplasia. 14 In another study, during the 12 months follow-up of 96 patients fitted with Urolume, the rates of urinary tract infection (UTI) were 16%, and stent encrustation was observed in 7% of cases. Of note, the encrustation risk was higher when urothelium didn’t cover the implanted stent completely.8,15
The Memokath, or Memotherm, is a temporary (Engineers & Doctors A/S, Denmark) NiTinol (nickel–titanium) alloy coiled stent designed to prevent urothelial ingrowth. It has a thermosensitive shape memory, and the stent is endoscopically placed under general or local anaesthesia. 16 Using warmed irrigant (50°C), upon which the stent expands to 34Ch or 44Ch, anchoring the stent in place. 17 A cooled irrigant (approximately at 5–10°C) is used to easily remove the stent, and removal could be up to 9 years from implantation and is generally atraumatic for a non-encrusted stent.17,18 Due to its characteristics, Memokath stent can be used for BOO symptoms for more fragile patients, with good results, when compared to Transurethral Resection of Prostate (TURP) in terms of International Prostate Symptom Score (IPSS) improvement in 12-months, with scores improving from 6.8 ± 2.9 to 6.1 ± 3.1. 19 It is also used for vesicourethral anastomotic stenosis post radical prostatectomy, with success rates up to 93% in some series, providing superior patency results, when compared to other techniques such as bladder neck incision and Mitomycin C injection. 20 In selected patients with prior failed transurethral sphincterotomy or as rendezvous procedure in those suitable for reconstructive surgery, it has been used to temporarily reduce the bladder outlet resistance by treating detrusor sphincter dyssynergia of neurogenic bladder dysfunction associated with spinal cord injury. 17 Of note, the main complications of Memokath stent are pain, incontinence, infections, and stent migration or blockage that might lead to premature removal of stent.11,17,21–23
Improvements and progress in prostatic stent technology have led to the creation of biodegradable and polyurethane stents. Biodegradable stents utilize materials such as polylactic acid, polyglycolic acid, and copolymers of lactide and glycolide, which naturally break down and eliminate the need for removal. The first biodegradable stent was introduced in 1993 by Kemppainen et al., 24 which consisted of a self-reinforced poly-L-lactide, constructed as a helical spiral. 24 Biodegradable stents might be considered temporary stents, as their aim is to offer sufficient support to the urethra, keeping the lumen open both during and after the healing process, while being capable of gradual absorption by the body. Therefore, the material’s rigidity should be tailored according to the body’s characteristics, and its degradation products must be metabolically compatible. Because of their features, biodegradable stents were used as temporary stents in preventing postoperative urinary retention following visual laser ablation of the prostate and transurethral microwave therapy.25,26 Laaksovirta et al. published results on two cohorts of patients who had a biodegradable stent placed for the oedema and necrosis induced after an interstitial laser coagulation of the prostate. In their series, patients started to void on the first postoperative day, assisting to increase both the maximum and average flow rates, and to decrease the post voiding residual (PVR) urine volume.25,26 In 4–6 months, most stents were degraded, but parts of the stent were found at the bottom of the bladder in two patients.25,26 In one series half of the patients complained of post-operative irritative symptoms, and 10% had an asymptomatic urinary infection postoperatively. 26 Pétas et al. 27 conducted a comparative study to evaluate the efficacy and safety of a biodegradable self-reinforced poly-DL-lactic acid (SR-PLA) spiral stent versus the suprapubic catheter after visual laser ablation of the prostate. In their randomized study, when compared to those with suprapubic catheter (SPC), patients with the prostatic stent voided earlier (median 1 day versus 6 days). Degradation time was longer than six months, and infection rate increased at the time of SPC. Both groups had a significant improvement of symptom scores, mean Qmax and PVR at 6 months. 27 Kotsar et al. 28 conducted a pilot study combining dutasteride and a braided poly lactic-co-glycolic acid (PLGA) urethral stent in the treatment of acute urinary retention (AUR). The braided design aimed to prevent stent migration, potentially treating AUR safely. Ten patients were treated with an indwelling braided prostatic stent inserted via an insertion device and were then treated with dutasteride. Five patients voided with a low PVR (<150 ml) by 1 and 3 months. Some patients however required either supra-pubic or urethral catheter insertion. 28
Present stents – the Allium TPS and the SPANNER
The need for newer stents was almost inevitable since although the past stents were effective in improving symptoms, they had a high rate of complications. The main advantage of the new stents are that they are made of a different material, nitinol, and much less material is used, so that they have a lower likelihood to become encrusted, and their design is such that they less likely migrate or move.
The Allium Triangular Prostatic Urethral Stent (TPS) (Allium Urological Solutions, Caesarea, Israel) is a temporary device that might provide long-term reversible solution up to 3 years, intended for transurethral insertion. It comprises a nitinol-built coiled super-elastic structure covered with a co-polymer to prevent encrustations, composed of a main trans-prostatic body, a triangular sphincteric segment, a trans-sphincteric segment, and a triangular anchoring segment. 29 It is inserted endoscopically with the aid of its specific inserter under local anaesthesia, and it is released to allow its self-expansion. The large calibre triangular cross-section gives the stent the ability to exert varying degrees of radial force depending on prostate anatomy, with higher forces in the main body to maintain urine passage, and lower forces in the area near the external sphincter to prevent sphincteric dysfunction and urinary retention or incontinence. 29 Allium TPS is available in various lengths, ranging from 30 to 65 mm, and these serve to minimize the likelihood of stent migration. Patients should be evaluated in terms of PVR, DRE, prostate ultrasound, uroflowmetry, PSA, urethrography and urinalysis. Allium TPS has been approved for prostatic enlargement (benign or malignant), for use after minimally invasive treatments (MIT) based or thermal tissue damage of the prostate (microwave, RF thermotherapy, laser coagulation surgery, cryotherapy), or interstitial irradiation (brachytherapy) for prostate cancer, which might cause post-procedural temporary oedema and severe voiding difficulties or urinary retention. 29 Yildiz et al. 30 reported on stent insertion in 51 patients for BPO who were unwilling or unfit for surgery. At a last follow-up at 12 months, IPSS decreased from 26.4 to 7.7, the mean peak flow increased from 5.5 to 16.0 ml/s. They did not see any major complications such as stent migration or encrustation, but just transient pain that eventually resolved, and patients reported an overall improvement in QoL. Failure rate was 3.9% (n = 2) at 12 months. 30 The main limitations of their study were that prostates larger than 100cc were excluded, presumably those with an obstructing median lobe were as well. Additionally, the total number of patients at 12-months follow-up was not reported. In another study, Pizzo et al. 31 reported on seven high-risk surgical candidates with BPH, who encountered no postoperative migration or haematuria following stent insertion. Although some discomfort and episodes of urge incontinence were reported, the mean Qmax flow index showed an increase from 8.4 to 13 ml/s among all patients. 31 To the best of our knowledge, no other studies report on long-term functional results for patients treated with the Allium TPS.
The Spanner (The SPANNER, AbbeyMoor Medical, Inc., Parkers Prairie, MN, USA) is an FDA-approved temporary silicone elastomer prostatic stent. 32 It is a temporary stent inserted into the urethra at the neck of the bladder. The Spanner is composed of a proximal balloon that is seated in the bladder neck, and a stent that extends from the bladder neck to just above the external sphincter. It has a tethering device (suture material) that transverses the external sphincter to allow normal sphincteric function providing continence and held by a distal anchor in the bulbar urethra, just below the sphincter to prevent device movement and migration into the bladder. 33 It is usually placed under topical anaesthesia in an outpatient setting without cystoscopic visualization, and candidates must possess an intact detrusor reflex contraction and pelvic floor relaxation for optimal results. 5 A study involving 30 men demonstrated a 42% enhancement in the mean Qmax, a 64% decrease in PVR, and a 68% decrease in IPSS following Spanner implantation, with a remarkable lack of migration on radiological confirmation at up to 12 weeks follow-up (0%). Notably, patients with the Spanner in place reported increased sexual activity and erections without significant pain. 34 In another observational study involving 43 men deemed unsuitable for surgery, the stent was replaced every 3 months. However, an overall 63% of the patients experienced an unsatisfactory outcome due to immediate or delayed urinary retention or elective stent removal caused by severe symptoms. The authors concluded that this stent is primarily indicated for short-term use. 35 In another multicentre randomized controlled trial, Shore et al. reported an 86% patient satisfaction rate and better QoL with the Spanner stent when compared to the standard Foley catheter following transurethral microwave thermotherapy for chronic obstruction due to BPH, also greater improvements from the baseline in PVR, uroflowmetry and IPSS at an 8-week follow-up. 36 Cambio et al. demonstrated the safety and effectiveness of the Spanner stent in catheter-dependent patients with chronic urinary retention due to BPH with good bladder contractility, who were deemed unfit for surgery, leading to an extension of FDA approval for this population. At completed trial after three cycles of 30 days, 73.8% of patients maintained a PVR ⩽ 150 ml, and no device-related serious adverse events (AEs) were reported, while most AEs were asymptomatic bacteriuria (23.4%), pain (9.4%) and urinary urgency (7.5%). 37
Future directions: clinical trials and stents under investigations
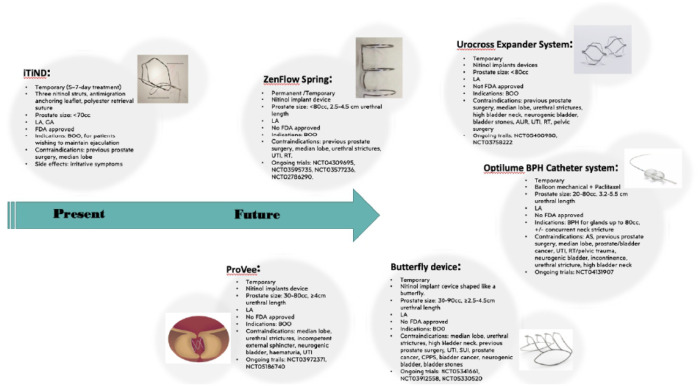
Recently, great awareness has been risen on prostatic stents for their great potential. There are several prostatic stent and urethral devices that are currently under final phase investigation and are coming into the market, with promising efficacy and improved safety profiles (Figure 2).
Figure 2.
Present and future prostatic catheters and nitinol devices and their characteristics. All of them are contraindicated in case of allergic reaction to any of the device components, or in case of inability to stop the anticoagulation therapy.
AS, artificial sphincter; AUR, acute urinary retention; BOO, bladder outlet obstruction; CPPS, chronic pelvic pain syndrome; GA, general anaesthesia; LA, local anaesthesia; RT, radiotherapy; SUI, stress urinary incontinence; UTI, urinary tract infection.
The Exime temporary prostatic stent (ROCAMED, Signes, France) is a prostatic stent made from silicone, proposed for temporary use (1 month lifespan) to restore urinary voiding in males with AUR, as an alternative to indwelling urethral catheter and clean intermittent catheterization (CISC) in case of trial without catheter (TWOC) failure. 38 It is composed of a straight tip towards the bladder with no balloon (to decrease trigonal stimulation), a prostatic coiled urethral tube in silicone which prevents lumen kinking, two anti-migration wings one on each side of the sphincter kept together by a monofilament non resorbable thread, a straight bulbar part in silicone that prevents upwards migration, and of a safety and removal suture. 39 The Exime is inserted in an office-based setting, under local anaesthetic without the need for cystoscopy, sonography, or fluoroscopy guidance. 40 In a prospective cohort involving 61 patients with a mean prostate volume of 67 ml (ranging from 30 to 120 ml), 90% of the sample achieved spontaneous urination immediately after the procedure. 41 Cindolo et al. published their experience on patients who underwent REZUM procedure for BPH with a mean operative IPSS of 23 and a mean prostate volume of 61 ml, including those with a median lobe, followed by Exime insertion and found that patients experienced a reduction in postoperative discomfort, with no reported complications or AUR, and removal within 8 h. 42 More recently, Baboudjian et al. 38 published the first data evaluating the Exime device versus the indwelling urethral catheter (IUC). Patients included had a prostate volume up to 120 ml (median volume 49 ml) and an intravesical prostatic protrusion of <5 mm on ultrasound and failed an attempt of TWOC after AUR. The device was then removed after 1 month. Patients treated with the Exime had a median PVR of 45 ml, and when compared to the IUC had lower rated of bladder spasms (p < 0.001), urine leakage (p = 0.02), UTI (p < 0.001), and pain (p < 0.001). Patients generally preferred the Exime device instead of the IUC (84%), and at 1 month it was still retained in 18 patients, representing a success rate of 72% (three obstructions, three migrations). 38
The Optilume BPH Catheter System (Urotronic, Inc., Minneapolis, MN, USA) is an innovative minimally invasive surgical therapy (MIST) that combines the balloon mechanical dilation with the administration of paclitaxel to sustain luminal patency during the healing process. Mechanical dilation with Optilume BPH achieves an anterior commissurotomy, effectively separating the lateral lobes of the prostate. This is the first BPH MIST providing a combination of drug and device therapy, which has been studied for glands ranging from 30 to 80 cc and might be used in the context of concurrent treatment of bladder neck strictures and BPH 43 (NCT04131907). Other clinical trials are conducted on nitinol implant devices such as the ZenFlow Spring (Zenflow, South San Francisco, CA, USA) designed to be permanent but can be removed creating internal tension which helps the device incorporate into the wall of urethra and currently investigated in the contest of the ZEST CAN, a pivotal randomized control trial projected to be concluded by 2026 aimed at assessing the safety, cost-effectiveness, and overall performance of this ZenFlow Spring (NCT04309695, NCT03595735, NCT03577236, NCT02786290); the Urocross Expander System (Prodeon Medical, Inc. (PMI), Sunnyvale, CA, USA) designed to expand and reshape the prostatic urethra through mechanical tissue contraction (NCT05400980, NCT03758222); the ProVee (ProVerum, Dublin, Ireland) device is a ‘stent-like’ nitinol expander which is currently tested in a prospective trial named the ProVIDE study that is presently in the recruitment stage and is anticipated to conclude in 2028 (NCT03972371, NCT05186740); the Butterfly device (Butterfly, Medical LTD, Yokneam, Yilit, Israel) a metallic retractor of prostatic lobes implanted under local anaesthesia, which showed promising results in terms of improvement of mean IPSS and QoL, 44 but for which further studies are warranted and ongoing (NCT05341661, NCT03912558, NCT05330520).
Complications and contraindications
Complications associated with prostatic stents, although varying in frequency and severity, warrant careful consideration. Among the commonly reported complications are stent migration, encrustation, and UTIs.7,9–12,15,17,21–23 Stent migration, where the device shifts from its intended position within the prostatic urethra, can lead to inadequate symptom relief or urinary obstruction. Encrustation, characterized by the build-up of mineral deposits on the stent surface, may result in decreased efficacy and discomfort.4,15 Other potential complications include stent-related irritation, haematuria, and urinary retention. 28 UTIs represent a significant concern, as the presence of a foreign body such as a stent can serve as a nidus for bacterial colonization and subsequent infection. Jung et al. 45 reported of a patient who developed Fournier’s disease after he had undergone insertion of a thermo-expandable urethral stent (Memokath 028). While the direct association between prostatic stents and Fournier’s disease is not well-established, it’s important to note that cases of Fournier’s disease associated specifically with prostatic stents are exceedingly rare and typically occur in the setting of other predisposing factors such as immunosuppression, diabetes mellitus, or prior genitourinary procedures.
Downsizing of the most recent stents coupled with some anchoring mechanisms, has offered several advantages, including decreased risk of stent migration, encrustation and irritation. A smaller stent profile enhances patient comfort and minimizes urinary tract obstruction. However, downsizing must be balanced with ensuring adequate stent stability and efficacy in relieving obstructive symptoms associated with BPH. For these reasons, further downsizing may necessitate advancements in stent design and materials to maintain structural integrity and durability. While advancements in stent design and material composition aim to mitigate these risks, careful patient selection, appropriate sizing, and regular monitoring remain crucial in minimizing complications and optimizing outcomes.
Potential contraindications for intraprostatic stents include meatal or urethral strictures, UTIs, bladder stones, neurogenic bladder dysfunction, a substantial median prostatic lobe, a prostatic urethra shorter than 2 cm, and the existence of bladder neck contracture. 46 As per industry advice, the Allium TPS is not indicated either for the definitive treatment of prostate disease or for complications of prostatic disease or urethral strictures, and its use is contraindicated in case of anticoagulation treatment, atonic/acontractile bladder, fistulas, bladder stones, patients who use injectable medications for erectile dysfunction, with penile implants or artificial sphincters. 29 The Exime catheter instead is contraindicated in case of UTIs, haematuria with clots, sphincteric failures, urethral stenosis, bladder stones, PVR > 150 ml or bladder apex-neck >7 cm, or after treatment with agents causing prostate oedema (High intensity focused ultrasound (HIFU), brachytherapy, radiotherapy). 39
Nitinol devices and drug coated catheters
The iTIND (Temporary Implantable Nitinol Device) is the second-generation version of the temporary nitinol implantable device (TIND). 47 The device is placed within the prostate gland, where it exerts gentle pressure to reshape and remodel the tissue, thus reducing urethral obstruction and improving urinary flow. Porpiglia et al. 48 reported the initial clinical outcomes of the first-generation mechanical device in a cohort of 32 patients in 2015. After 1 year, the IPSS decreased by 45%, while the maximum urinary flow rate (Qmax) increased by 67%. Early complications included prostate abscess, UTI, transient urinary incontinence due to device displacement, and urinary retention, each occurring once. 48 Follow-up over 3 years revealed no further AEs, but three cases required re-intervention within 24 months. Ultimately, the IPSS improved by 19%, and the Qmax increased by 41%. 49 In a multicentre study (MT-02), the second-generation device was assessed for managing bothersome LUTS due to Benign Prostate Enlargement. With 81 patients enrolled and follow-ups at 1, 3, 6 and 12 months post-procedure across various international sites, including Italy, the United Kingdom, Switzerland, Belgium and Hong Kong, the study revealed promising results. 50 Patients, with a mean age of 65 years and meeting specific inclusion criteria, experienced significant improvements in both maximum urinary flow rate (Qmax) and IPSS. Complications were minimal, and all surgeries were successful, leading to same-day discharge. However, subsequent surgeries were required in a few cases, primarily associated with prominent median lobes, highlighting their impact on treatment outcomes. 50 In the most recent analysis of the same MT-02 with over 48 months of follow-up, iTind® (iTind, 17 Hauman st., Hadera, Israel) demonstrated sustained symptom reduction and enhanced QoL, lasting over 48 months post-treatment. No complications were reported beyond 36 months of follow-up, indicating long-term safety. The surgical re-treatment rate after 36 months was minimal, at 4%, suggesting enduring efficacy. 51
UVENTA stents are cutting-edge medical devices designed to address urethral strictures, a common condition characterized by the narrowing of the urethra, which can lead to difficulties in urination and potential complications. These stents are crafted from biocompatible materials and are meticulously engineered to provide optimal support and patency to the urethra while minimizing discomfort for the patient. Sedigh et al. 52 conducted a recent retrospective analysis of bulbar urethral stenting procedures following direct vision internal urethrotomy across seven centers. Patients either declined urethroplasty or were deemed unfit for surgery. The stents remained in place for a minimum of 6 months before removal, unless early complications necessitated earlier intervention. Overall, 49% had complications, with the most common being discomfort (23.8%), stress incontinence (17.5%), and stent dislocation (9.8%). Approximately 85% of these AEs were classified as Clavien-Dindo grade <3. The overall success rate, measured at a median follow-up of 38.2 months, stood at 76.9%. Notably, the success rate was significantly lower if the stent was removed before 6 months (53.3% versus 79.7%; p = 0.026). 52
Robust and Optilume are both innovative medical devices utilized in the treatment of urinary conditions, particularly urethral strictures. Robust, a urethral stent manufactured by Pnn Medical, is designed to provide structural support to the urethra, effectively maintaining its patency and facilitating urinary flow. This device is crafted from biocompatible materials and offers a minimally invasive alternative to traditional surgical interventions, leading to reduced patient discomfort and faster recovery times. On the other hand, Optilume, developed by Urotronic Inc., is a drug-coated balloon catheter designed to treat urethral strictures by delivering localized drug therapy directly to the affected area. By combining balloon dilation with drug delivery, Optilume aims to improve long-term outcomes and reduce the likelihood of stricture recurrence. 43 Both Robust and Optilume represent significant advancements in the field of urology, offering promising treatment options for patients suffering from urethral strictures.
Limitations
Although we provided an overview on the available prostatic stents and devices that might be used or have been used for BPH, we recognize that our work has some limitations. First, this is a narrative review, and therefore suffers from its inherent biases. Second, there was variability in how functional and sexual outcomes were reported across different studies, making comparisons between stents challenging. Third, we were not able to include a cost-analysis. In fact, companies typically engage in direct communication with surgeons or patients to discuss pricing details. This personalized approach allows for a thorough assessment of individual needs, consideration of insurance coverage, and discussion of any potential financial assistance options. More research will also need to be done with other MIST such as prostate artery embolization or Urolift.53,54
Conclusion
The advancement of prostatic stent technology for BPH has been steadily progressing over several years. Early iterations of these devices demonstrated adverse outcomes such as migration, encrustation, and complications, leading to stent failure. However, recent data with temporary or permanent stents have shown a much higher success rate. Although prostatic stents seem to be promising in terms of effectiveness and safety improving patients’ QoL and IPSS, their effectiveness relies on intact voluntary or reflex detrusor contraction and associated pelvic floor relaxation, making patients with detrusor abnormalities potentially unsuitable candidates for these stents. While we are looking forward to the results of the current ongoing trials in exploring the implications of prostatic stents, more long-term data are needed to further titrate and identify patients most suitable and likely to benefit most for these prostatic stents.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872241255262 for Prostatic stents: a narrative review of current evidence by Clara Cerrato, Vaki Antoniou, Shriya Napolean Fernandes, Sanjeev Madaan and Bhaskar Kumar Somani in Therapeutic Advances in Urology
Acknowledgments
None.
Footnotes
ORCID iDs: Clara Cerrato  https://orcid.org/0000-0002-5013-7617
https://orcid.org/0000-0002-5013-7617
Sanjeev Madaan  https://orcid.org/0000-0003-4220-5613
https://orcid.org/0000-0003-4220-5613
Bhaskar Kumar Somani  https://orcid.org/0000-0002-6248-6478
https://orcid.org/0000-0002-6248-6478
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Clara Cerrato, University Hospital Southampton NHS Trust, Southampton, UK.
Vaki Antoniou, University Hospital Southampton NHS Trust, Southampton, UK.
Shriya Napolean Fernandes, University Hospital Southampton NHS Trust, Southampton, UK.
Sanjeev Madaan, Darent Valley Hospital (DVH), Dartford, UK.
Bhaskar Kumar Somani, University Hospital Southampton NHS Trust, Tremona Road, Southampton, Hampshire, SO16 6YD, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Clara Cerrato: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Vaki Antoniou: Data curation; Formal analysis; Investigation.
Shriya Napolean Fernandes: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Sanjeev Madaan: Supervision; Writing – review & editing.
Bhaskar Kumar Somani: Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The Editor in Chief and Associate Editor of Therapeutic Advances in Urology are authors of this paper. Therefore, the review process was managed by alternative members of the Editorial Board and the submitting Editors had no involvement in the decision-making process.
Availability of data and materials: Not applicable.
References
- 1. Panser LA, Chute CG, Guess HA, et al. The natural history of prostatism: the effects of non-response bias. Int J Epidemiol 1994; 23: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 2. Cornu J, Gucci M, Hashim H, et al. 2023 EAU Guidelines on Non-Neurogenic Male LUTS, https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-Neurogenic-Male-LUTS-2023.pdf (accessed November 2023).
- 3. Ebbing J, Bachmann A. Anesthesia-free procedures for benign prostate obstruction: worth it? Curr Opin Urol 2015; 25: 32–39. [DOI] [PubMed] [Google Scholar]
- 4. Milroy E, Allen A. Long-term results of urolume urethral stent for recurrent urethral strictures. J Urol 1996; 155: 904–908. [PubMed] [Google Scholar]
- 5. Nguyen ALV, Verma I, Ferreira R, et al. A scoping review of office-based prostatic stents: past, present, and future of true minimally invasive treatment of benign prostatic hyperplasia. World J Urol 2023; 41: 2925–2932. [DOI] [PubMed] [Google Scholar]
- 6. Oesterling JE, Kaplan SA, Epstein HB, et al. The North American experience with the UroLume endoprosthesis as a treatment for benign prostatic hyperplasia: long-term results. The North American UroLume Study Group. Urology 1994; 44: 353–362. [DOI] [PubMed] [Google Scholar]
- 7. Wilson TS, Lemack GE, Dmochowski RR. UroLume stents: lessons learned. Journal of Urology 2002; 167: 2477–2480. [PubMed] [Google Scholar]
- 8. Milroy E, Chapple CR. The urolume stent in the management of benign prostatic hyperplasia. J Urol 1993; 150: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 9. De Vocht TF, Van Venrooij GEPM, Boon TA. Self-expanding stent insertion for urethral strictures: a 10-year follow-up. BJU Int 2003; 91: 627–630. [DOI] [PubMed] [Google Scholar]
- 10. Frankiewicz M, Karolina M, Marcin M. Diagnosis and management of urolume urethral stent complications using ultrasonography and magnetic resonance imaging. Urology 2020; 144: e4–e5. [DOI] [PubMed] [Google Scholar]
- 11. Armitage JN, Cathcart PJ, Rashidian A, et al. Epithelializing stent for benign prostatic hyperplasia: a systematic review of the literature. Journal of Urology 2007; 177: 1619–1624. [DOI] [PubMed] [Google Scholar]
- 12. McNamara ER, Webster GD, Peterson AC. The urolume stent revisited: the duke experience. Urology 2013; 82: 933–936. [DOI] [PubMed] [Google Scholar]
- 13. Guazzoni G, Montorsi F, Coulange C, et al. A modified prostatic urolume wallstent for healthy patients with symptomatic benign prostatic hyperplasia: a European multicenter study. Urology 1994; 44: 364–370. [DOI] [PubMed] [Google Scholar]
- 14. Bajoria S, Agarwal SA, White R, et al. Experience with the second generation Urolume prostatic stent. Br J Urol 1995; 75: 325–327. [DOI] [PubMed] [Google Scholar]
- 15. Williams G, Coulange C, Milroy EJG, et al. The urolume, a permanently implanted prostatic stent for patients at high risk for surgery; results from 5 collaborative centres. Br J Urol 1993; 72: 335–340. [DOI] [PubMed] [Google Scholar]
- 16. Eaton Turner E, Jenks M, McCool R, et al. The Memokath-051 stent for the treatment of ureteric obstruction: a NICE medical technology guidance. Appl Health Econ Health Policy 2018; 16: 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta SS, Tophill PR. Memokath® stents for the treatment of detrusor sphincter dyssynergia (DSD) in men with spinal cord injury: the princess royal spinal injuries unit 10-year experience. Spinal Cord 2006; 44: 1–6. [DOI] [PubMed] [Google Scholar]
- 18. Forster LR, Watson L, Breeze CE, et al. The fate of ureteral memokath stent(s) in a high-volume referral center: an independent long-term outcomes review. J Endourol 2021; 35: 180–186. [DOI] [PubMed] [Google Scholar]
- 19. Song PH, Kim YU, Choi JY, et al. MP3-13 Efficacy of thermos-expandable intra-prostatic stent (MEMOKATHTM028) as an alternative approach for benign prostatic hyperplasia patients with significant comorbidities: comparison with transurethral resection of the prostate. J Urol 2015; 193: e23. [Google Scholar]
- 20. Nathan A, Mazzon G, Pavan N, et al. Management of intractable bladder neck strictures following radical prostatectomy using the Memokath®045 stent. J Robot Surg 2020; 14: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uguzova S, Beisland C, Honoré A, et al. Refractory bladder neck contracture (BNC) after radical prostatectomy: prevalence, impact and management challenges. Res Rep Urol 2023; 15: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bozkurt IH, Yalcinkaya F, Sertcelik MN, et al. A good alternative to indwelling catheter owing to benign prostate hyperplasia in elderly: memotherm prostatic stent. Urology 2013; 82: 1004–1007. [DOI] [PubMed] [Google Scholar]
- 23. Martov AG, Al Plekhanova O, Ergakov DV, et al. Thermoexpandable urethral nickel-titanium stent memokath for managing urethral bulbar stricture after failed urethroplasty. J Endourol Case Rep 2020; 6: 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kemppainen E, Talja M, Riihelä M, et al. A bioresorbable urethral stent - an experimental study. Urol Res 1993; 21: 235–238. [DOI] [PubMed] [Google Scholar]
- 25. Laaksovirta S, Talja M, Välimaa T, et al. Expansion and bioabsorption of the self-reinforced lactic and glycolic acid copolymer prostatic spiral stent. J Urol 2001; 166: 919–922. [PubMed] [Google Scholar]
- 26. Laaksovirta S, Isotalo T, Talja M, et al. Interstitial laser coagulation and biodegradable self-expandable, self-reinforced poly-L-lactic and poly-L-glycolic copolymer spiral stent in the treatment of benign prostatic enlargement. J Endourol 2002; 16: 311–315. [DOI] [PubMed] [Google Scholar]
- 27. Pétas A, Talja M, Tammela TLJ, et al. The biodegradable self-reinforced poiy-DL-lactic acid spiral stent compared with a suprapubic catheter in the treatment of post-operative urinary retention after visual laser ablation of the prostate. Br J Urol 1997; 80: 439–443. [DOI] [PubMed] [Google Scholar]
- 28. Kotsar A, Isotalo T, Juuti H, et al. Biodegradable braided poly(lactic-co-glycolic acid) urethral stent combined with dutasteride in the treatment of acute urinary retention due to benign prostatic enlargement: a pilot study. BJU Int 2009; 103: 626–629. [DOI] [PubMed] [Google Scholar]
- 29. Allium Ltd. Allium triangular prostatic urethral stent system (TPS) instructions for use, https://aprmedtech.com/wp-content/uploads/2017/05/IFU_TPS.pdf (accessed November 2023).
- 30. Yildiz G, Bahouth Z, Halachmi S, et al. AlliumTM TPS - a new prostatic stent for the treatment of patients with benign prostatic obstruction: the first report. J Endourol 2016; 30: 319–322. [DOI] [PubMed] [Google Scholar]
- 31. Pizzo M, Carbone A, Pastore A. Experience with allium stent (Triangular Prostatic Stent) for treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia. Neurourol Urodyn 2016; 35: S21–S22. [Google Scholar]
- 32. FDA. U. S. Food & Drug Administration, https://www.Fda.Gov/Medical-Devices/Recently-Approved-Devices/Spanner-Temporary-Prostatic-Stent-P060010s013 (accessed November 2023).
- 33. SRS Medical. The SPANNER® Stent, https://www.srsmedical.com/products/downloads/Spanner-Physician/Physicians-IFU-for-The-Spanner-and-Surveyor.pdf (accessed November 2023).
- 34. Corica AP, Larson BT, Sagaz A, et al. A novel temporary prostatic stent for the relief of prostatic urethral obstruction. BJU Int 2004; 93: 346–348. [DOI] [PubMed] [Google Scholar]
- 35. Grimsley SJS, Khan MH, Lennox E, et al. Experience with the spanner prostatic stent in patients unfit for surgery: an observational study. J Endourol 2007; 21: 1093–1096. [DOI] [PubMed] [Google Scholar]
- 36. Shore ND, Dineen MK, Saslawsky MJ, et al. A temporary intraurethral prostatic stent relieves prostatic obstruction following transurethral microwave thermotherapy. J Urolog 2007; 177: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 37. Cambio AJ, Roach RM, Arnold P, et al. Extended use of the Spanner® temporary prostatic stent in catheter-dependent men with comorbidities. Adv Urol 2022; 2022: 7367851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baboudjian M, Berchiche W, Fourmarier M. Exime temporary prostatic stent: a new alternative to indwelling urethral catheters. Eur Urol Open Sci 2023; 56: 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. EXIME ROCAMED. Temporary prostatic stent, https://www.rocamed.com/products/prostate/exime/ (accessed November 2023).
- 40. Charbonnel C, Neuville P, Paparel P, et al. Feasibility of EXIME® temporary prosthesis placement and removal in men with acute or chronic urinary retention after failure or inability to selfcatheterize. Prog Urol 2022; 32: 717–725. [DOI] [PubMed] [Google Scholar]
- 41. Amara N, Youssef T, Al Massa J, et al. MP01-01 EXIME® urethral stent implantation (EUSI) as initial treatment for patients presenting with acute urinary retention (AUR) for the first time as a result of BPH. J Urol 2022; 207: e5. [Google Scholar]
- 42. Cindolo L, Ferrari R, Rabito S, et al. Rezum procedure with Exime® stent: a step forward to micro-invasiveness. Minerva Urol Nephrol 2021; 73: 273–275. [DOI] [PubMed] [Google Scholar]
- 43. Elterman DS, Coutinho K, Hagedorn JC. How i do it: the optilume drug-coated balloon for urethral strictures. Can J Urol 2020; 27: 10322–10328. [PubMed] [Google Scholar]
- 44. Katz R, Abu Ahmed M, Safadi A, et al. MP01-02 The “BUTTERFLY” transurethral retraction device for BPH – over 1 year follow up. J Urol 2022; 207: e5. [Google Scholar]
- 45. Jung HC, Kim YU. Fournier’s gangrene after insertion of thermo-expandable prostatic stent for benign prostatic hyperplasia: a case report. World J Clin Cases 2023; 11: 6498–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lam JS, Volpe MA, Kaplan SA. Use of prostatic stents for the treatment of benign prostatic hyperplasia in high-risk patients. Curr Urol Rep 2001; 2: 277–284. [DOI] [PubMed] [Google Scholar]
- 47. Balakrishnan D, Jones P, Somani BK. ITIND: the second-generation temporary implantable nitinol device for minimally invasive treatment of benign prostatic hyperplasia. Ther Adv Urol 2020; 12: 1756287220934355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Porpiglia F, Fiori C, Bertolo R, et al. Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up. BJU Int 2015; 116: 278–287. [DOI] [PubMed] [Google Scholar]
- 49. Porpiglia F, Fiori C, Bertolo R, et al. 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int 2018; 122: 106–112. [DOI] [PubMed] [Google Scholar]
- 50. Kadner G, Valerio M, Giannakis I, et al. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World J Urol 2020; 38: 3235–3244. [DOI] [PubMed] [Google Scholar]
- 51. Amparore D, De Cillis S, Schylman C, et al. Temporary implantable nitinol device for benign prostatic hyperplasia-related lower urinary tract symptoms: over 48-month results. Minerva Urol Nephrol 2023;75: 743–751. [DOI] [PubMed] [Google Scholar]
- 52. Sedigh O, Dalmasso E, Gobbo A, et al. Feasibility and outcomes of temporary bulbar urethral stent placement after internal urethrotomy in the largest multicenter series. Eur Urol 2023; 84: 313–320. [DOI] [PubMed] [Google Scholar]
- 53. Maclean D, Harris M, Drake T, et al. Factors predicting a good symptomatic outcome after prostate artery embolization (PAE). Cardiovasc Interv Radiol 2018; 41: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 54. Jones P, Rajkumar GN, Rai BP, et al. Medium-term outcomes of Urolift (minimum 12 months follow-up): evidence from a systematic review. Urology 2016; 97: 20–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872241255262 for Prostatic stents: a narrative review of current evidence by Clara Cerrato, Vaki Antoniou, Shriya Napolean Fernandes, Sanjeev Madaan and Bhaskar Kumar Somani in Therapeutic Advances in Urology