Abstract
Overgrowth–intellectual disability (OGID) syndromes are a collection of rare genetic disorders with overlapping clinical profiles. In addition to the cardinal features of general overgrowth (height and/or head circumference at least two standard deviations above the mean) and some degree of intellectual disability, the OGID syndromes are often associated with neurological anomalies including seizures. In an effort to advance research in directions that will generate meaningful treatments for people with OGID syndromes, a new collaborative partnership called the Overgrowth Syndromes Alliance (OSA) formed in 2023. By taking a phenotype-first approach, OSA aims to unite research and patient communities traditionally siloed by genetic disorder. OSA has galvanized OGID patient organizations around shared interests and developed a research roadmap to identify and address our community’s greatest unmet needs. Here, we describe the literature regarding seizures among those with overgrowth syndromes and present the OSA Research Roadmap. This patient-driven guide outlines the milestones essential to reaching the outcome of effective treatments for OGID syndromes and offers resources for reaching those milestones.
Keywords: DNMT3A; Malan syndrome; NFIX; overgrowth; seizures; Tatton-Brown Rahman syndrome, Sotos syndrome, epilepsy
Plain language summary
Working together to speed up treatments for rare genetic syndromes linked to excessive growth and intellectual disability
To address the shared challenges experienced among those affected by overgrowth–intellectual disability (OGID) syndromes, we recently formed the Overgrowth Syndromes Alliance (OSA). The OSA unites patient advocacy organizations that have typically worked independently of one another, in hopes of accelerating our progress toward treatments. Here, we summarize the OGID syndromes represented by the OSA, the prevalence of seizures in these disorders, and efforts by the OSA to tackle the most pressing needs of the overgrowth community. We also present the steps patient organizations can take in pursuit of developing treatments. We hope the work of our alliance can be a template for creating collaborative, patient-led advances in diagnosis, management guidelines, and, eventually, treatment of rare genetic disorders.
Overgrowth–intellectual disability syndromes
Neurodevelopmental disorders (NDDs) represent a spectrum of conditions, with hundreds of known genetic causes and an array of concomitant clinical findings. 1 Some NDDs are exceedingly rare, with an incidence as low as 1:1,000,000. Overgrowth–intellectual disability (OGID) disorders are a group of NDDs, broadly defined as having overgrowth and some degree of intellectual disability, but with a range of other disorder-specific features. Overgrowth is defined clinically as head circumference and/or height greater than two standard deviations above the mean. 2 While each OGID syndrome has a unique array of associated features, significant convergence exists in clinical findings, encompassing abnormalities in brain structure, behavioral and mental health disorders such as autism and anxiety, as well as musculoskeletal conditions such as hypotonia and scoliosis.
Recurrent seizures/epilepsy are reported for most OGID syndromes; for Smith–Kingsmore syndrome, the incidence is as high as 74% (Table 1). Seizures associated with OGID syndromes tend to have variable age of onset, with some in the neonatal period, most in childhood, and a minority of reports in later adulthood. 3 The type of seizure is highly variable across and within OGID syndromes, with focal, absence, and generalized tonic-clonic being most commonly observed. Febrile seizures are reported as well.3–5 Rare genetic syndromes marked by segmental, rather than generalized, overgrowth are also associated with intellectual disability and seizures. For that reason, we have chosen to include segmental overgrowth syndromes in our summary (Table 1).
Table 1.
Seizures in select overgrowth syndromes.
| Disorder name | Gene | Seizure disorder prevalence | Seizure type |
|---|---|---|---|
| OGID syndromes | |||
| Beck–Fahrner syndrome (MIM: 618798) | TET3 (MIM: 613555) | 38% 4 | Generalized tonic-clonic, focal, absence |
| CHD8-related neurodevelopmental disorder (MIM: 615032) | CHD8 (MIM: 610528) | 10–15% 7 | Afebrile, including absence and generalized tonic-clonic |
| Cohen–Gibson syndrome (MIM: 617561) | EED (MIM: 605984) | Case reports8,9 | Absence, generalized tonic-clonic |
| Imagawa–Matsumoto syndrome (MIM: 618786) | SUZ12 (MIM: 606245) | – | – |
| Kosaki syndrome (MIM: 616592) | PDGFRB (MIM: 173410) | – | – |
| Luscan–Lumish syndrome (MIM: 616831) | SETD2 (MIM: 612778) | 33% overall, but 58% with c.5218C>T
10
9% 11 |
Afebrile, generalized tonic-clonic |
| Malan syndrome (MIM: 614753) | NFIX (MIM: 164005) | 27%
12
63% 13 |
Afebrile14,15 |
| NF1-SUZ12 deletion syndrome | NF1 (MIM: 162200) & SUZ12 | – | – |
| Simpson–Golabi–Behmel syndrome (MIM: 312870) | GPC3 (MIM: 300037) | Case report 16 | – |
| Smith–Kingsmore syndrome (MIM: 616638) | MTOR (MIM: 601231) | 40%
17
74% 18 |
Focal, tonic-clonic, tonic, epileptic spasms |
| Sotos syndrome (MIM: 117550) | NSD1 (MIM: 606681) | 25%
19
9–50% 20 |
Absence, generalized tonic-clonic, and focal including temporal lobe 3 |
| Tatton–Brown–Rahman syndrome (MIM: 615879) | DNMT3A (MIM: 602769) | 20% 5 | Afebrile |
| Weaver syndrome (MIM: 277590) | EZH2 (MIM: 601573) | 8% 21 | Afebrile |
| Segmental overgrowth syndromes | |||
| CLOVES syndrome (MIM: 612918) | PIK3CA (MIM: 171834) | 30–40% 22 | Focal and generalized tonic-clonic |
| Macrocephaly-capillary malformation (M-CM) (MIM: 602501) | PIK3CA (MIM: 171834) | 30–40% 22 | – |
Seizure disorder prevalence is from respective GeneReviews articles unless otherwise noted. A dash (–) indicates that a literature search yielded no information.
OGID, overgrowth–intellectual disability.
There are several challenges limiting scientific progress toward treatments for OGID syndromes. Many OGID syndromes have only recently been described (Supplemental Table S1), contributing to the small size of some OGID syndrome patient populations and a limited understanding of the natural history of these disorders. Furthermore, the patient advocacy organizations representing these disorders are proportionately small and sometimes newly formed, often by the families of affected individuals. With limited resources, patient advocacy groups contend with multiple competing priorities, including raising global awareness, fostering a community of support, and initiating and engaging in basic, clinical, and translational research. As is common for rare disease patient advocacy organizations, limited funding and lack of public awareness are top challenges facing OGID syndrome patient advocacy organizations. 6 These barriers continue to slow the path for developing essential research tools (e.g. biosamples, cell lines, animal models, and patient registries) that are important for attracting researchers and industry partners. For many small organizations, these resources are beyond reach, leaving questions about the etiology and natural history of many OGID syndromes unanswered. As a result, individuals with OGID syndromes often receive inadequate care due to a limited understanding of these rare conditions and the lack of well-developed treatment guidelines.
Collaborating to accelerate therapeutic discovery: Overgrowth Syndromes Alliance
OGID syndromes have greatly overlapping phenotypic and pathologic characteristics. 2 Without genomic sequencing, diagnosis can be difficult based on clinical findings alone, given the similarities in physical appearance and medical associations. 23 Advances in the genetic characterization of OGID syndromes have refined diagnoses and led to the establishment of clinical, research, and patient communities centered around the disease-causing gene (Supplemental Table S1). For some segmental overgrowth syndromes, the underlying genetic variants encode proteins that are components of the same downstream pathways, such as the phosphatidylinositol 3-kinase (PI3K)-AKT pathway. 24 Researchers have recently reported additional molecular pathways common to certain OGID syndromes. For instance, neurons from mouse models of Sotos syndrome (NSD1) and Tatton–Brown–Rahman syndrome (TBRS; DNMT3A) display overlapping patterns in aberrant DNA methylation and gene dysregulation, a finding consistent with the critical role of NSD1 and DNMT3A in gene regulation. 25 These results suggest that points of convergence molecularly represent opportunities for targeted therapeutic intervention that could treat multiple syndromes involving overgrowth.
To raise awareness and unify shared interests across overgrowth disorders, two OGID syndrome patient advocacy organizations, Malan Syndrome Foundation 26 and TBRS Community, 27 formed the Overgrowth Syndromes Alliance (OSA) in 2023. The mission of the OSA is to align research on overgrowth syndromes with the priorities of patient communities, as a means of accelerating the path to treatment and cures that will have the greatest benefit to people living with these syndromes.
To date, OSA has achieved several goals:
Develop a collaborative community of patient advocacy organizations
With guidance from the medical and scientific advisory committees of the Malan Syndrome Foundation and TBRS Community, OSA identified 15 syndromes that involve some form of overgrowth and a component of intellectual disability. We sought to identify patient organizations representing these disorders, although not all syndromes have an organization (Supplemental Table S1). We convened a virtual meeting of a subset of patient organizations in April 2023, which yielded valuable information on the capacity and progress of research efforts for various overgrowth syndromes and how the OSA can fill gaps in organizing and advocating for research. Since the initial meeting, OSA has continued to reach out to and connect with additional patient communities.
Produce a patient priorities survey
Based on a general agreement among our patient advocacy partners that patient priorities should be a driving force of OSA, we developed the OSA Patient Priorities Survey, an online, anonymous questionnaire about treatment priorities. The Patient Priorities Survey consists of 12 questions, collecting information on the age of the affected person, the syndrome they have, whether the diagnosis was confirmed with genetic testing, the top priorities for treatment, and interest in participating in a clinical trial. The OSA Patient Priorities Survey is currently available in English and Spanish. An adapted version of the survey is also available to improve accessibility. Individuals of any age with a diagnosis of an overgrowth syndrome are eligible for inclusion in the survey, which is completed by the affected individual or their caregiver. An exemption for this survey was obtained by TBRS Community’s Institutional Review Board at North Star Review Board.
OSA distributed the survey to patient advocacy partners to share with their communities: AESS Sotos Syndrome Spanish Association, A.S.S.I Gulliver Sotos Syndrome Italian, Beck-Fahrner Syndrome Foundation, Child Growth Foundation, CLOVES Syndrome Community, Luscan-Lumish Syndrome, Malan Syndrome Foundation, M-CM Network, Smith-Kingsmore Syndrome Foundation, Sotos Syndrome Support Association, and TBRS Community. The survey has been open since August 2023 and responses will be collected on an ongoing basis.
Organize an OGID syndrome research conference
OSA is currently in the planning phase of a 2024 OGID syndrome research conference to bring together clinicians and scientists who study these disorders. We have established collaborations at an academic institution to organize the conference and are working with our patient advocacy partners to identify experts across OGID syndromes to present at this meeting. The goal is to have broad representation of expertise across disorders, disciplines, and phases of research (basic, clinical, and translational). The topics of focus will be centered on patient priorities identified through the OSA Patient Priorities Survey.
Malan Syndrome Foundation and TBRS Community are well-poised to lead OSA. Both organizations have developed robust, diverse, and collaborative research networks on their respective syndromes and have met major milestones along their research roadmaps (see below). Roughly 300 individuals have been diagnosed with Malan syndrome globally and 285 individuals are represented in the Foundation’s patient contact registry. The Malan Syndrome Foundation, founded in 2018, convenes a biennial family and scientific engagement conference with 150 in-person attendees and has raised more than $300,000 for research. The disorder is caused by autosomal dominant, de novo variants in the NFIX gene, and characteristic features, in addition to OGID, include vision impairment, skeletal anomalies, epilepsy, and anxiety.
Similarly, around 350 people have been diagnosed with TBRS globally, and 100 participants have completed the TBRS Community’s natural history study. The 2023 TBRS Summit gathered around 160 in-person and 50 virtual attendees from 17 countries and included physicians, scientists, and families. Caused by autosomal dominant pathogenic variants in DNMT3A, TBRS is characterized by OGID and frequently involves cardiac defects, autism, hypotonia, kyphoscoliosis, an elevated cancer risk, obesity, and seizures. TBRS Community, formed in 2017, has raised funds to directly support research over the past several years, and researchers have access to a biorepository with more than 40 patient samples in addition to data from the natural history study (see below for details on research resources).
TBRS Community and Malan Syndrome Foundation have forged a supportive, collaborative relationship that is now extended to the patient advocacy partners of the OSA. Through the OSA, our models for success can be readily exported to organizations in earlier developmental phases of their research roadmaps. By identifying points along our roadmaps that intersect, we can develop opportunities for combining efforts, reducing redundancies, and stretching limited resources for therapeutic development on our patient community’s greatest unmet needs.
OGID syndromes and epilepsy
Through the structured communication and collaboration between our patient advocacy organizations outlined above, we have identified many common challenges. One of the most notable challenges shared across OGID syndromes are recurrent seizures. The patient priorities survey responses so far indicate that seizures are among the top priorities for treatment across overgrowth syndromes. The burden of seizures on patients and their families is neither adequately represented in the literature nor aggressively addressed by the OGID syndrome and greater epilepsy research communities.
Some parents/caregivers who suspect seizures report difficulty with obtaining a diagnosis because seizures are not well documented in the literature for many of the OGID syndromes (JK, KC, CD personal communication, December 2023). Delays in diagnosis and seizure treatment could lead to unfavorable patient outcomes. 28 People with generalized overgrowth are often very tall and, in some cases, obese, as observed in TBRS, with additional co-morbidities including cardiovascular abnormalities, which caregivers are concerned puts them at increased risk for injury or serious medical complications during seizures. Other symptoms concerning to caregivers include staring spells, uncontrolled laughing, tremor, stuttering/disfluent speech with facial grimacing, and speech and cognitive regression. It remains unclear if these reported symptoms are related to seizure activity. 29
Almost all of the 15 syndromes we describe are caused by pathogenic variants in either cell signaling kinases or epigenetic regulatory genes (Supplemental Table S1 and Figure 1). The cell signaling kinases occur in the mammalian target of rapamycin (mTOR) pathway. This pathway is critical for regulating genes involved in cell growth, proliferation, and survival, particularly during neurodevelopment. It also has an important role in normal brain function, regulating neurons and neurotransmission across the lifespan.30,31 Disorders associated with changes in the mTOR pathway include other conditions outside of overgrowth syndromes but share epilepsy as a feature. In general, these mTORopathies are more classically associated with seizures, and therefore, seizure concerns may be more readily recognized and addressed in this patient population, compared to those with OGID syndromes caused by epigenetic regulatory genes.
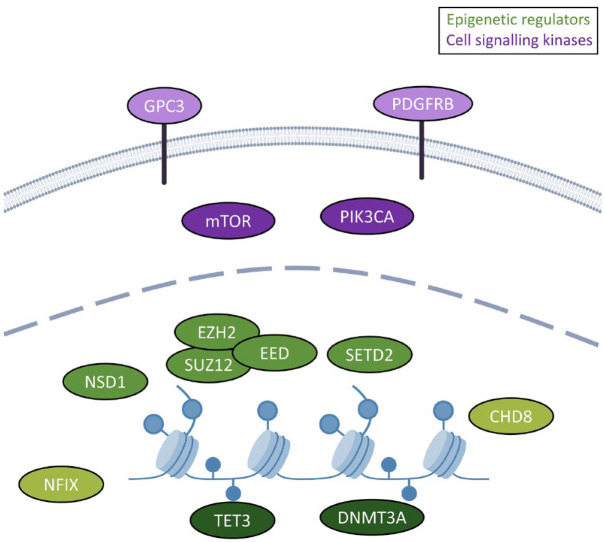
Figure 1.
Genes underlying overgrowth are enriched for roles in epigenetic regulation and cell signaling. The genes representing select conditions encode either epigenetic or transcriptional regulators (9/13; green) or cell signaling/kinases (4/13; purple). The epigenetic regulators can be further divided into DNA methylation regulators (dark green; TET3 and DNMT3A), histone modifiers (medium green; NSD1, EZH2, EED, SUZ12, SETD2) and chromatin remodelers (CHD8) and transcription factors (NFIX; light green). The cell signaling proteins can be divided into cell surface receptors (GPC3 and PDGFRB; light purple) and mTOR pathway genes (mTOR and PIK3CA; medium purple). Note that EZH2, EED, and SUZ12 physically interact, which is reflected in the high degree of clinical overlap of their respective conditions.
Only recently have epigenetic regulators emerged as a family of genes involved in seizures.32–34 Long-lasting changes in gene expression are a major part of the etiology of epilepsy, thought to underlie critical processes including neuronal death, neuroinflammation, ion channel and neurotransmitter receptor abnormalities, and alterations in synaptic plasticity.35,36 Studies have shown that all core epigenetic mechanisms – DNA methylation,37,38 histone modification, 39 and non-coding RNA 40 – are implicated in modulating gene-specific changes in the processes involved in epilepsy. Further, targeting of epigenetic mechanisms is also a key mode of action for some epilepsy drugs. Although it is not commonly used as a targeted treatment in individuals with epilepsy caused by epigenetic regulatory genes, valproic acid is one of the most-prescribed anti-seizure medications, and functions in part by its activity as a histone deacetylase (HDAC) inhibitor. 41 Discovery of this activity has spurred promising research into use of other HDAC inhibitors to treat epilepsy, 39 which may inform future targeted treatments of seizures in OGID syndromes.
Building a patient-centric research roadmap
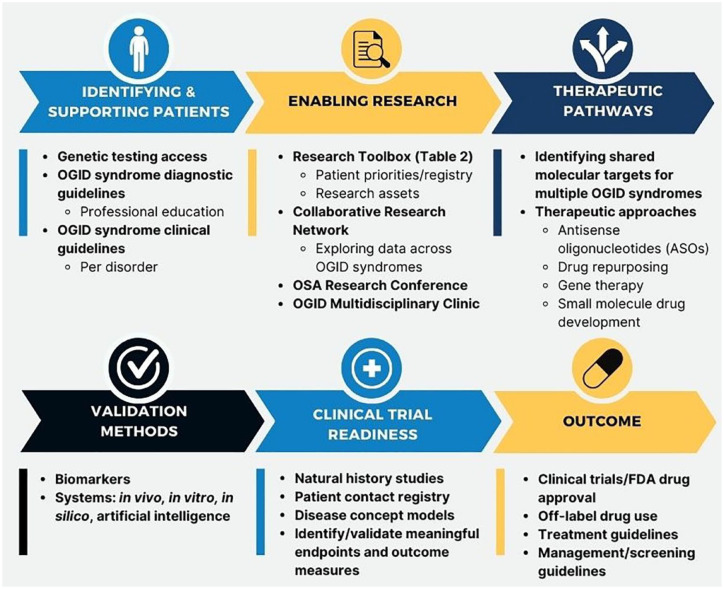
Patient-centered research initiatives are crucial priorities of the OSA, and prior to OSA’s founding, Malan Syndrome Foundation and TBRS Community had designed research roadmaps based on patient priorities. These have informed the development of an OSA Research Roadmap, aimed at furthering research on all OGID syndromes and providing a model for other OGID syndrome patient advocacy organizations (Figure 2). The Research Roadmap displays the steps to be taken toward therapeutic development, including identifying and supporting patients, enabling research, identifying therapeutic pathways and needs in the community, producing validation methods, and preparing for clinical trials. This framework can be utilized for drug development or repurposing, other therapeutic development, clinical or diagnostic guidelines, and OGID syndrome research development. The OSA Patient Priorities Survey will identify top priorities for treatment, and therefore, the roadmap will focus efforts on addressing those greatest unmet needs.
Figure 2.
OSA Research Roadmap. The OSA has developed a comprehensive roadmap for OGID syndromes. This roadmap was created using shared learning from the Malan Syndrome Foundation and TBRS Community. It may also serve as a template for other OGID syndrome patient advocacy organizations.
OGID, overgrowth–intellectual disability; OSA, Overgrowth Syndromes Alliance; Food and Drug Administration (FDA).
OGID syndrome patient advocacy organizations are at many different points in the Research Roadmap, from early patient and researcher identification to clinical trials. Malan Syndrome Foundation and TBRS Community, for example, have developed biorepositories, registries, disease models, and other tools for research and clinical trial readiness (Table 2). Although many of these resources can be applied to any number of priorities, some of them are tailored to seizures and epilepsy, such as the electroencephalogram (EEG)repository in development. While the biorepositories available offer patient samples and controls to researchers at a lower burden on families, the patient registries act as a repository of data on patient experience, symptoms and medical information that often goes beyond what is published in the literature. Many registries also act as natural history studies, providing information over time from patients and families to analyze the progression of the disorder. Biorepository samples acting in conjunction with registry data provide a robust resource for research on the disorder. Universal unique identification codes, such as the Global Unique Identifiers 42 and the Clinical Research ID, 43 can be used to link de-identified biosamples to patient data repositories and other studies.
Table 2.
Malan Syndrome Foundation and TBRS Community research toolbox.
| Research toolbox | Malan Syndrome Foundation | TBRS Community |
|---|---|---|
| Biorepository | • Blood and urine44,a: ○ 9 Patient samples ○ 7 Control samples • Collects CRID |
• Blood45,b: ○ 32 Patient samples ○ 10 Control samples • Collects CRID |
| Cell models | Induced pluripotent stem cell (iPSC)
a
: Del (exon 1 and 2); more in development Fibroblasta: (1) Y48* (2) G105_K106del (3) C110Lfs*17 (4) NFIX exon 2 partial del (5) S199fs*19 |
iPSC
b
: I310N (PWWP domain); more in development Fibroblast: (1) I310N |
| Animal models | ||
| Mouse | NFIX+/− knockout maintained on a C57Bl/6J background
46
Humanized NFIX+/− knockout mouse model maintained on a C57Bl/6J background; in development |
DNMT3A deletion, 47 R882H (R878H in mouse), 48 W297del (W293del in mouse), 49 and P904L (P900L in mouse) 50 |
| Caenorhabditis elegans | nfi-1 gene deletion 51 | Not available (N/A) |
| Patient registry/natural history study | Sanford CoRDS 52 | NORD
53
• Collects CRID (to link data with Biorepository samples) |
| Other data collection initiatives and collaborations | ||
| Ciitizen | Ciitizen is a voluntary program for rare disease patients to collect medical records from all providers in one online location, and patients can choose to share this information with researchers in a de-identified way.
54
Both Malan Syndrome Foundation and TBRS Community are partners with Ciitizen. |
|
| Rare-X | Rare-X provides an international data-sharing platform that enables rare disease research and advancement toward therapeutics. 55 | N/A |
| Simons Searchlight | N/A | Simons Searchlight aims to further the understanding of neurodevelopmental conditions through natural history and data collection. 56 |
| Patient perspectives | OSA Patient Priority Survey | |
| Therapeutic tracks | Drug repurposing (InVivo Biosystems), antisense oligonucleotide (ASO) (Murdoch University) | Drug repurposing, ASO, gene therapy (all with academic partners) |
| Ongoing projects or in development | ||
| EEG repository | Identifying methods and storage | Identifying methods and storage |
| Validated outcome measures and endpoints | In validation: (1) ORCA measure 57 (CDER Pilot Grant Program from US FDA 58 ) (2) Social and cognitive performance measure using webcam collection of gaze and artificial intelligence 59 |
In identification |
| Disease concept model | Ongoing in collaboration with COMBINEDBrain and University of Michigan | Ongoing in collaboration with COMBINEDBrain and Rutgers University |
Patient de-identified data are available upon request by contacting the patient registry and/or data collection program.
Biosamples and cell lines are available through the Malan Syndrome Foundation Biorepository at COMBINEDBrain.
Biosamples and cell lines are available through the TBRS Community Biorepository at COMBINEDBrain.
CRID, Clinical Research ID; ORCA, Observer-Reported Communication Ability; OSA, Overgrowth Syndromes Alliance; TBRS, Tatton–Brown–Rahman syndrome.
Research tools from Malan Syndrome Foundation and TBRS Community can be easily adapted for OGID syndrome patient advocacy organizations in early development, expanding the tools available across disorders. Continued development of an EEG repository and validated outcome measures and endpoints will further support therapeutic progress, guided by the patient priority survey and feedback from the OSA research network.
Future directions
Longitudinal data for the OGID syndromes are lacking; more studies are needed to assess the developmental trajectories and the natural history of individuals with OGID syndromes. Such studies could provide insight into whether epilepsy correlates with the severity of symptoms, regression in speech and cognitive ability, and/or additional clinical sequelae, including neuropsychiatric disorders, or if there are any genotype-phenotype correlations, particularly those related to epilepsy and developmental outcomes. Furthermore, natural history data, both retrospective and prospective, could be leveraged to identify clinical outcome measures and endpoints that include cognitive, communication, and behavioral features that are specific to the OGID syndrome population.
Because there are few studies in the medical literature reporting on the occurrence of seizures in OGID syndromes, many individuals affected by OGID syndromes are not followed by neurology. An OGID syndrome multidisciplinary clinic or Center of Excellence with coordinated care across multiple specialties, including neurology, could contribute to a better understanding of the neurological manifestations associated with these disorders and improve syndrome management.
OGID syndromes, because of their low incidence, are not widely recognized by clinicians. Most OGID syndrome genes are included on the overgrowth-macrocephaly syndrome, 60 congenital hypotonia, 61 congenital heart defects, 62 autism-intellectual disability, 63 and neurodevelopmental multigene panels. 64 However, many of the OGID syndrome genes are not on commercially available genetic epilepsy panels,65,66 despite the common occurrence of seizures in the OGID syndrome population. Future advocacy efforts by the OSA will push for the inclusion of OGID syndrome genes associated with epilepsy on genetic epilepsy panels. Medical providers should consider an underlying genetic cause when treating a patient with seizures who also presents with an OGID clinical phenotype. OGID professional education outreach will be beneficial for improving this type of awareness across medical specialties.
Supplemental Material
Supplemental material, sj-docx-1-trd-10.1177_26330040241254123 for Epilepsy and overgrowth–intellectual disability syndromes: a patient organization perspective on collaborating to accelerate pathways to treatment by Kerry Grens, Kit M. Church, Eric Diehl, Senyene E. Hunter, Katrina Tatton-Brown, Jill Kiernan and Christal G. Delagrammatikas in Therapeutic Advances in Rare Disease
Acknowledgments
The authors gratefully acknowledge the participation of the patient advocacy organizations that have partnered with the OSA, as well as the patient communities who contributed to patient-led research by donating biosamples, patient data, and by participating in research. They also thank the Medical and Scientific Advisory Board members of the Malan Syndrome Foundation and TBRS Community for their expert guidance on OSA programming.
Footnotes
ORCID iD: Christal G. Delagrammatikas  https://orcid.org/0009-0000-0026-8734
https://orcid.org/0009-0000-0026-8734
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kerry Grens, Tatton Brown Rahman Syndrome Community, Stanfordville, NY, USA.
Kit M. Church, Tatton Brown Rahman Syndrome Community, Stanfordville, NY, USA
Eric Diehl, Tatton Brown Rahman Syndrome Community, Stanfordville, NY, USA.
Senyene E. Hunter, Division of Pediatric Neurology, Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Katrina Tatton-Brown, St George’s University Hospitals NHS Foundation Trust, London, UK; St George’s University of London, London, UK.
Jill Kiernan, Tatton Brown Rahman Syndrome Community, Stanfordville, NY, USA.
Christal G. Delagrammatikas, Malan Syndrome Foundation, 15 Wendy Drive, Old Bridge, NJ 08857, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Kerry Grens: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Kit M. Church: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Eric Diehl: Conceptualization; Investigation; Resources; Supervision; Writing – original draft; Writing – review & editing.
Senyene E. Hunter: Conceptualization; Writing – review & editing.
Katrina Tatton-Brown: Conceptualization; Writing – review & editing.
Jill Kiernan: Conceptualization; Project administration; Writing – original draft; Writing – review & editing.
Christal G. Delagrammatikas: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TBRS Community acknowledges support from the Chan Zuckerberg Initiative.
The authors declare that there is no conflict of interest.
Availability of data and materials: Researchers and patient advocacy organizations can request de-identified results from the patient priorities survey by emailing Kerry Grens: kerry@tbrsyndrome.org. Applications to access other research resources from TBRS Community and Malan Syndrome Foundation are indicated in the article.
References
- 1. Leblond CS, Le TL, Malesys S, et al. Operative list of genes associated with autism and neurodevelopmental disorders based on database review. Mol Cell Neurosci 2021; 113: 103623. [DOI] [PubMed] [Google Scholar]
- 2. Edmondson A, Kalish J. Overgrowth syndromes. J Pediatr Genet 2015; 4: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fortin O, Vincelette C, Khan AQ, et al. Seizures in Sotos syndrome: phenotyping in 49 patients. Epilepsia Open 2021; 6: 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fahrner JA. TET3-related Beck-Fahrner syndrome. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK591837/ (2023, accessed 12 December, 2023). [PubMed]
- 5. Ostrowski PJ, Tatton-Brown K. Tatton-Brown-Rahman syndrome. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK581652/ (2022, accessed 12 December, 2023). [PubMed]
- 6. Patterson AM, O’Boyle M, VanNoy GE, et al. Emerging roles and opportunities for rare disease patient advocacy groups. Ther Adv Rare Dis. Epub ahead of print April 2023. DOI: 10.1177/26330040231164425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchel MW, Myers SM, Heidlebaugh AR, et al. CHD8-related neurodevelopmental disorder with overgrowth. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK585456/ (2022, accessed 12 December, 2023).
- 8. Kang YJ, Kim YO. Cohen–Gibson syndrome in a family: the first familial case report. J Genet Med 2021; 18: 70–74. [Google Scholar]
- 9. Cohen ASA, Tuysuz B, Shen Y, et al. A novel mutation in EED associated with overgrowth. J Hum Genet 2015; 60: 339–342. [DOI] [PubMed] [Google Scholar]
- 10. Pappas J, Rabin R. SETD2 Neurodevelopmental disorders. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK575927/ (2022, accessed 12 December, 2023). [PubMed]
- 11. Parra A, Rabin R, Pappas J, et al. Clinical heterogeneity and different phenotypes in patients with SETD2 variants: 18 new patients and review of the literature. Genes (Basel) 2023; 14: 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Priolo M, Schanze D, Tatton-Brown K, et al. Further delineation of Malan syndrome. Hum Mutat 2018; 39: 1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macchiaiolo M, Panfili FM, Vecchio D, et al. A deep phenotyping experience: up to date in management and diagnosis of Malan syndrome in a single center surveillance report. Orphanet J Rare Dis 2022; 17: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabata K, Iida A, Takeshita E, et al. A novel pathogenic NFIX variant in a Malan syndrome patient associated with hindbrain overcrowding. J Neurol Sci 2020; 412: 116758. [DOI] [PubMed] [Google Scholar]
- 15. Oshima T, Hara H, Takeda N, et al. A novel mutation of NFIX causes Sotos-like syndrome (Malan syndrome) complicated with thoracic aortic aneurysm and dissection. Hum Genome Var 2017; 4: 17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young EL, Wishnow R, Nigro MA. Expanding the clinical picture of Simpson-Golabi-Behmel syndrome. Pediatr Neurol 2006; 34: 139–142. [DOI] [PubMed] [Google Scholar]
- 17. Poole RL, Curry PDK, Marcinkute R, et al. Delineating the Smith-Kingsmore syndrome phenotype: investigation of 16 patients with the MTOR c.5395G > A p.(Glu1799Lys) missense variant. Am J Med Genet A 2021; 185: 2445–2454. [DOI] [PubMed] [Google Scholar]
- 18. Gordo G, Tenorio J, Arias P, et al. mTOR mutations in Smith-Kingsmore syndrome: four additional patients and a review. Clin Genet 2018; 93: 762–775. [DOI] [PubMed] [Google Scholar]
- 19. Tatton-Brown K, Cole TRP, Rahman N. Sotos syndrome. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK1479/ (2004, accessed 12 December, 2023).
- 20. Nicita F, Ruggieri M, Polizzi A, et al. Seizures and epilepsy in Sotos syndrome: analysis of 19 Caucasian patients with long-term follow-up. Epilepsia 2012; 53: e102–e105. [DOI] [PubMed] [Google Scholar]
- 21. Tatton-Brown K, Rahman N. EZH2-related overgrowth. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK148820/ (2018, accessed 12 December, 2023).
- 22. Mirzaa G, Conway R, Graham JM, et al. PIK3CA-related overgrowth spectrum. GeneReviews®, https://www.ncbi.nlm.nih.gov/books/NBK153722/ (2023, accessed 12 December, 2023).
- 23. Kamien B, Ronan A, Poke G, et al. A clinical review of generalized overgrowth syndromes in the era of massively parallel sequencing. Mol Syndromol 2018; 9: 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rivière JB, Mirzaa GM, O’Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet 2012; 44: 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamagami N, Wu DY, Clemens AW, et al. NSD1 deposits histone H3 lysine 36 dimethylation to pattern non-CG DNA methylation in neurons. Mol Cell 2023; 83: 1412.e7–1428.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malan Syndrome Foundation. Malan syndrome, https://www.malansyndrome.org/ (2023, accessed 12 December, 2023).
- 27. TBRS Community. Tatton Brown Rahman syndrome, https://tbrsyndrome.org/
- 28. Lewis AK, Taylor NF, Carney PW, et al. What is the effect of delays in access to specialist epilepsy care on patient outcomes? A systematic review and meta-analysis. Epilepsy Behav 2021; 122: 108192. [DOI] [PubMed] [Google Scholar]
- 29. Camfield P, Camfield C. Regression in children with epilepsy. Neurosci Biobehav Rev 2019; 96: 210–218. [DOI] [PubMed] [Google Scholar]
- 30. Lipton JO, Sahin M. The neurology of mTOR. Neuron 2014; 84: 275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 168: 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McTague A, Howell KB, Cross JH, et al. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol 2016; 15: 304–316. [DOI] [PubMed] [Google Scholar]
- 33. Van Loo KMJ, Carvill GL, Becker AJ, et al. Epigenetic genes and epilepsy – emerging mechanisms and clinical applications. Nat Rev Neurol 2022; 18: 530–543. [DOI] [PubMed] [Google Scholar]
- 34. Henshall DC, Kobow K. Epigenetics and epilepsy. Cold Spring Harb Perspect Med 2015; 5: a022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forero DA. Functional genomics of epileptogenesis in animal models and humans. Cell Mol Neurobiol 2021; 41: 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma A. Genome-wide expression analysis in epilepsy: a synthetic review. Curr Top Med Chem 2012; 12: 1008–1032. [DOI] [PubMed] [Google Scholar]
- 37. Kobow K, Jeske I, Hildebrandt M, et al. Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol 2009; 68: 356–364. [DOI] [PubMed] [Google Scholar]
- 38. Belhedi N, Perroud N, Karege F, et al. Increased CPA6 promoter methylation in focal epilepsy and in febrile seizures. Epilepsy Res 2014; 108: 144–148. [DOI] [PubMed] [Google Scholar]
- 39. Citraro R, Leo A, Santoro M, et al. Role of histone deacetylases (HDACs) in epilepsy and epileptogenesis. Curr Pharm Des 2017; 23: 5546–5562. [DOI] [PubMed] [Google Scholar]
- 40. Henshall DC. MicroRNA and epilepsy: profiling, functions and potential clinical applications. Curr Opin Neurol 2014; 27: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Romoli M, Mazzocchetti P, D’Alonzo R, et al. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol 2019; 17: 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson SB, Whitney G, McAuliffe M, et al. Using global unique identifiers to link autism collections. J Am Med Inform Assoc 2010; 17: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nesbitt GC, Murphy PA. CRID – a unique, universal, patient-generated identifier to facilitate collaborative rare disease clinical research. Inform Med Unlocked 2022; 31: 100973. [Google Scholar]
- 44. Combined Brain. https://combinedbrain.org/2024; (2023, accessed 12 December, 2023)
- 45. Kiernan J. TBRS Community Biorepository – Sample Request Form, https://form.jotform.com/233317183492053. (2023, accessed 12 December, 2023).
- 46. Harris L, Dixon C, Cato K, et al. Heterozygosity for nuclear factor one X affects hippocampal-dependent behaviour in mice. PLoS One 2013; 8: e65478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Challen GA, Sun D, Jeong M, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet 2012; 44: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith AM, LaValle TA, Shinawi M, et al. Functional and epigenetic phenotypes of humans and mice with DNMT3A overgrowth syndrome. Nat Commun 2021; 12: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang YH, Chen CW, Sundaramurthy V, et al. Systematic profiling of DNMT3A variants reveals protein instability mediated by the DCAF8 E3 ubiquitin ligase adaptor. Cancer Discov 2022; 12: 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beard DC, Zhang X, Wu DY, et al. Distinct disease mutations in DNMT3A result in a spectrum of behavioral, epigenetic, and transcriptional deficits. Cell Rep 2023; 42: 113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lazakovitch E, Kalb JM, Matsumoto R, et al. nfi-I affects behavior and life-span in C. elegans but is not essential for DNA replication or survival. BMC Dev Biol 2005; 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. CoRDS Researcher Access Request (RAR) Form, https://research.sanfordhealth.org/-/media/research/files/cords/2020-05-18-cords-researcher-access-request-form.pdf (2023, accessed 12 December, 2023).
- 53. TBRS Community Patient Registry: data request form for academic institutions and researchers, https://form.jotform.com/222405379569061 (2023, accessed 12 December, 2023).
- 54. Ciitizen. https://www.ciitizen.com/ (2023, accessed 12 December, 2023).
- 55. RARE-X. https://rare-x.org/ (2023, accessed 12 December, 2023).
- 56. SFARI. Simons Searchlight, https://www.sfari.org/resource/simons-searchlight/ (2023, accessed 12 December, 2023).
- 57. Zigler CK, Lin L, McFatrich M, et al. Validation of the Observer-Reported Communication Ability (ORCA) measure for individuals with Angelman syndrome. Am J Intellect Dev Disabil 2023; 128: 204–218. [DOI] [PubMed] [Google Scholar]
- 58. FDA. CDER pilot grant program: standard core clinical outcome assessments (COAs) and their related endpoints, https://www.fda.gov/drugs/development-approval-process-drugs/cder-pilot-grant-program-standard-core-clinical-outcome-assessments-coas-and-their-related-endpoints (2024, accessed 11 April 2024).
- 59. Frazier TW, Busch RM, Klaas P, et al. Development of webcam-collected and artificial-intelligence-derived social and cognitive performance measures for neurodevelopmental genetic syndromes. Am J Med Genet C Semin Med Genet 2023; 193: e32058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fulgent Genetics. Macrocephaly/Overgrowth Syndrome NGS Panel, https://www.fulgentgenetics.com/Macrocephaly (2023, accessed 12 December, 2023).
- 61. GeneDx. Congenital Hypotonia Xpanded® Panel | Test catalog for genetic & genomic testing, https://providers.genedx.com/tests/detail/congenital-hypotonia-xpanded-panel-1126 (2024, accessed 11 April 2024).
- 62. GeneDx. Xpanded® – Congenital Heart Defects Panel, https://providers.genedx.com/tests/detail/xpanded-congenital-heart-defects-panel-1172 (2024, accessed 11 April 2024).
- 63. GeneDx. Autism/ID Xpanded® Panel, https://providers.genedx.com/tests/detail/autism-id-xpanded-panel-849 (2023, accessed 12 December, 2023).
- 64. Invitae. Invitae Neurodevelopmental Disorders (NDD) Panel, https://www.invitae.com/us/providers/test-catalog/test-728434 (2024, accessed 11 April 2024).
- 65. Invitae. Invitae Epilepsy Panel, https://www.invitae.com/us/providers/test-catalog/test-03401 (2024, accessed 11 April 2024).
- 66. GeneDx. Comprehensive Epilepsy Panel, https://providers.genedx.com/tests/detail/comprehensive-epilepsy-panel-317 (2024, accessed 11 April 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-trd-10.1177_26330040241254123 for Epilepsy and overgrowth–intellectual disability syndromes: a patient organization perspective on collaborating to accelerate pathways to treatment by Kerry Grens, Kit M. Church, Eric Diehl, Senyene E. Hunter, Katrina Tatton-Brown, Jill Kiernan and Christal G. Delagrammatikas in Therapeutic Advances in Rare Disease