Abstract
Despite several improvements in outcomes, metastatic prostate cancer remains deadly. Alterations in the homologous recombination repair (HRR) pathway are associated with more aggressive disease. Olaparib and rucaparib, two poly-ADP-ribose polymerase (PARP) inhibitors, have received approval from the authorities of several countries for their anti-tumoral effects in patients with metastatic castration-resistant prostate cancers harboring HRR gene alterations, in particular BRCA2. More recently, it has been hypothesized that new hormonal therapies (NHTs) and PARP inhibitors (PARPi) could have synergistic actions and act independently of HRR deficiency. This review proposes to discuss the advantages and disadvantages of PARPi used as monotherapy or in combination with NHTs and whether there is a need for molecular selection.
Keywords: DNA repair, homologous recombination repair, metastatic prostate cancers, PARP inhibitors
Background
Despite several improvements in outcomes, metastatic castration-resistant prostate cancer (mCRPC) remains the fifth leading cause of cancer death in men worldwide. 1 While the androgen receptor (AR) pathway plays a central role, recent studies have highlighted the significance of DNA repair pathways in tumor growth and progression, particularly the homologous recombination repair (HRR) system.2,3 Furthermore, it seems that germline and somatic HRR-deficient (HRD) prostate cancers are more aggressive and less responsive to taxane-based chemotherapies compared to others.4–7 Approximately one in four patients exhibit HRD due to germline or somatic alterations in HRR genes, mainly BRCA2, ATM, CDK12, and CHEK2. 3
Proteins involved in the HRR system are crucial for repairing double-strand breaks (DSBs), while poly-ADP-ribose polymerases (PARPs) are primarily involved in repairing single-strand breaks (SSBs).8–13 PARP inhibitors (PARPi) prevent PARP action through catalytic or trapping inhibition, inducing SSB accumulation that will eventually be converted into DSB during replication. HRD cells are unable to repair DSBs leading to apoptosis. 14 Based on this concept of synthetic lethality, several studies have assessed the efficacy of PARPi in HRD mCRPC, demonstrating positive outcomes in terms of survival.15,16
On the other hand, preclinical studies have indicated that the AR pathway promotes transcriptional programs of genes involved in DNA repair and that androgen deprivation therapies (ADTs) and new hormonal therapies (NHTs) may induce a BRCAness state independent of the genomic HRR status.17–19 Based on this rationale, three phase III trials evaluating the combination of PARPi and NHT have shown promising results (Table 1).20–22
Table 1.
Summary of phase III trials using PARPis for metastatic prostate cancers.
| Type | Name | Study population | HRR status | Previous NHT (%) | Stratification of HRR status | Standard arm | Intervention | Primary endpoint | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Monotherapy | PROfound15,23
(olaparib) |
mCRPC ⩾ 1 NHT | -BRCA/ATM cohort -Other HRD cohort |
100 | No | NHT | Open label Cross over allowed |
rPFS BRCA/ATM cohort (BICR) | -↑ rPFS 23 and OS 15 BRCA/ATM p < 0.001 and p = 0.02 |
| TRITON-3
16
(rucaparib) |
BRCA/ATM | Yes (BRCA1 versus BRCA2 versus ATM) | Docetaxel or NHT | rPFS (BICR) | -↑ rPFS
16
study population p < 0.001 -↑ rPFS BRCA1/2 16 p < 0.001 - rPFS ATM 16 p not reported |
||||
| Combination with NHT | PROpel20,24
(olaparib/AAP) |
L1 mCRPC | All comers | <1 | No | NHT | Double-blinded No cross over |
rPFS (IA) | - ↑ rPFS
20
p < 0.001 - OS 24 p = 0.054 |
| MAGNITUDE21,25
(niraparib/AAP) |
-HRD cohort -HRP cohort (stopped for futility) |
3 | Yes (BRCA1/2 versus other) | - rPFS BRCA1/2 (BICR) - rPFS HRD cohort (BICR) |
-↑ rPFS
21
and OS
25
BRCA1/2 p < 0.001 and p = 0.024 -↑ rPFS HRD cohort 21 p = 0.028 - rPFS HRP cohort 21 p = 0.66 |
||||
| TALAPRO-2 all comers
22
(talazoparib/enzalutamide) |
All comers | 6 | Yes (HRP versus HRD) | rPFS (BICR) | -↑ rPFS all comers
22
p < 0.001 -↑ rPFS HRD 22 p = 0.0006 -↑ rPFS HRP 22 p = 0.0035 |
||||
| TALAPRO-2 HRD
26
(talazoparib/enzalutamide) |
HRD | 8 | Yes (BRCA1/2 versus other HRD) | rPFS (BICR) | -↑ rPFS
26
p < 0.0001 -↑ rPFS BRCA1/2 26 p < 0.0001 - rPFS other HRD 26 p = 0.06 |
AAP, abiraterone acetate and prednisone; BICR, blinded independent central review; HRD, HRR deficient; HRP, HRR proficient; HRR, homologous recombination repair; IA, investigator-assessed; mCRPC, metastatic castration-resistant prostate cancer; NHT, new hormonal therapy; PARPi, poly-ADP-ribose polymerase; rPFS, radiographic progression-free survival.
This article aims to discuss the advantages and disadvantages of PARPi used as monotherapy or in combination with NHT.
PARPi used as a monotherapy
The PROfound trial was the first to investigate the efficacy of olaparib in men with mCRPC featuring somatic or germline mono or bi-allelic HRR alterations previously treated with at least one NHT.15,23 They were randomly assigned to receive the physician’s choice of NHT or olaparib. The trial met its primary endpoint of median rPFS in cohort A (BRCA1/2 or ATM alterations; 7.4 versus 3.6 months, HR: 0.34, p < 0.001). 23 Despite 84% of the patients in the control group crossing over upon progression, cohort A patients exhibited significantly higher overall survival (OS). 15 No difference between the physician’s choice of NHT and olaparib was observed in cohort B (other HRR alterations). In addition, the olaparib group in cohort A demonstrated a better preserved quality of life. 27 The main limitation of this study lies in its weak control arm, the NHT; the use of docetaxel might have been more appropriate.
Rucaparib was evaluated in the TRITON-3 trial, including mCRPC patients harboring ATM or BRCA1/2 alterations, previously treated with at least one NHT. 16 Patients were randomly assigned to receive either rucaparib or physician’s choice treatment (docetaxel or NHT). The study met its primary endpoint, with a longer rPFS in the experimental group for both the overall and BRCA populations (10.2 versus 6.4 months, p = 0.0003 and 11.2 versus 6.4 months, p < 0.0001, respectively) and a good safety profile. A post hoc analysis confirmed the benefit of rucaparib compared to docetaxel which served as a stronger control arm than in the PROfound trial. 16
Neither of these two trials showed improvements in the ATM population.16,28 Furthermore, in an exploratory gene-by-gene analysis of PROfound, no benefit in terms of rPFS or PSA decline was observed in the analysis of CDK12 and CHEK2 genes, potentially due to a lack of efficacy or limited sample size. 28 Data are currently unavailable for other HRR genes.
These two studies demonstrated the benefits of using PARPi compared to NHT or even docetaxel for pre-treated mCRPC patients with BRCA1/2 alterations. More data are required for other HRR genes. Some questions remain unanswered, such as the impact of germinal or somatic status as well as zygosity, the optimal test to be used (liquid versus solid biopsies, broad versus targeted NGS), and the cost of their use.
To enhance the efficacy of PARPi, overcome primary resistance, and facilitate their use, combinations of PARPi with NHT were evaluated in three phase III studies. These studies are described in the next paragraph.
PARPi used in combination
It has been shown that the AR pathway promotes transcriptional programs of DNA repair genes and that NHT downregulates their transcription. More recently, two studies have shown that the use of ADT or enzalutamide could result in a BRCAness state, leading to PARPi sensitivity.17–19 Moreover, RB1 and BRCA2 are closely localized in chromosome 13q, and codeletions of BRCA2 and RB1 can emerge under selection pressure, being associated with aggressiveness and a poor response to NHT.29,30 Therefore, it is hypothesized that PARPi could suppress early clones resistant to NHT. 31
Based on this rationale and the phase II STUDY-08, 32 PROpel enrolled patients with first-line mCRPC, regardless of their HRR status. 20 Patients were randomly assigned to receive abiraterone in combination with either olaparib or placebo. Approximately 28% of the patients had an HRR alteration, and 10% had a BRCA alteration. The study met its primary endpoint as rPFS was longer with the combination compared to placebo in the overall population (24.8 versus 16.6 months, p < 0.001). This improvement seems to be found in the HRD population and to a lesser extent in the HRR-proficient (HRP) population. 20 Final OS analysis showed a trend toward improvement in the all-comers population (p = 0.054, 48% maturity). However, subgroup analyses suggested that this efficacy was found only in the BRCA and HRD cohorts, not in the non-BRCA and HRP cohorts. 24 As expected, more cases of anemia, fatigue, and nausea were observed in the experimental group. However, no additional cases of cardiac failure were reported, compared to the STUDY-08. 32
The MAGNITUDE trial enrolled patients with mCRPC in first-line treatment. 21 Patients were divided into two pre-specified cohorts: HRD and HRP. Patients of each cohort were randomized to receive abiraterone in combination with either niraparib or placebo. The study met its primary endpoint as rPFS was significantly improved in the combination arm compared to the experimental arm, in both BRCA and HRD populations (19.5 versus 10.9 months, p < 0.001 and 16.7 versus 13.7 months, p = 0.028, respectively). 33 No difference was observed in the subgroup of patients with HRR alterations other than BRCA1/2. OS was improved in the combination arm in the BRCA population (30.4 versus 28.6 months, nominal p = 0.024 adjusted on multivariate analysis). 25 The preplanned futility analysis in the HRP group (233 patients) did not show any benefit of adding niraparib to abiraterone, leading to the close of the study cohort.
Finally, TALAPRO-2 is the latest such trial studying the association of enzalutamide with either talazoparib or placebo, as the first line for mCRPC. 22 Patients were enrolled in two cohorts: the all-comers (cohort 1, 805 patients) and the HRD (cohort 2, 399 patients). The study was positive with a longer rPFS in the combination group of the all-comers population [not-reached (NR) and 21.9 months, p < 0.001]. Subgroup analyses showed an improvement in the HRD (169 patients) and the non-HRD groups (636 patients), HR = 0.45 (0.29–0.69, p = 0.0002), and HR = 0.66 (0.49–0.91, p = 0.009), respectively. The toxicity profile was comparable to previous studies but with more severe anemia and neutropenia. Results from cohort 2 (HRD only) were recently reported, confirming a longer rPFS in the combination group. 26 More interestingly, results from patients with HRD other than BRCA were analyzed (244 patients); however, despite a trend of improvement, the results were not statistically significant (p = 0.10).
These three combination trials met their primary endpoints: rPFS improvement in the all-comers population for PROpel and TALAPRO-2, and the HRD population for MAGNITUDE. Moreover, PROpel and TALAPRO-2 suggested a benefit for rPFS in the HRP population, using subgroup analyses, whereas this was not shown in the MAGNITUDE trial designed with a separate cohort.
Discussion
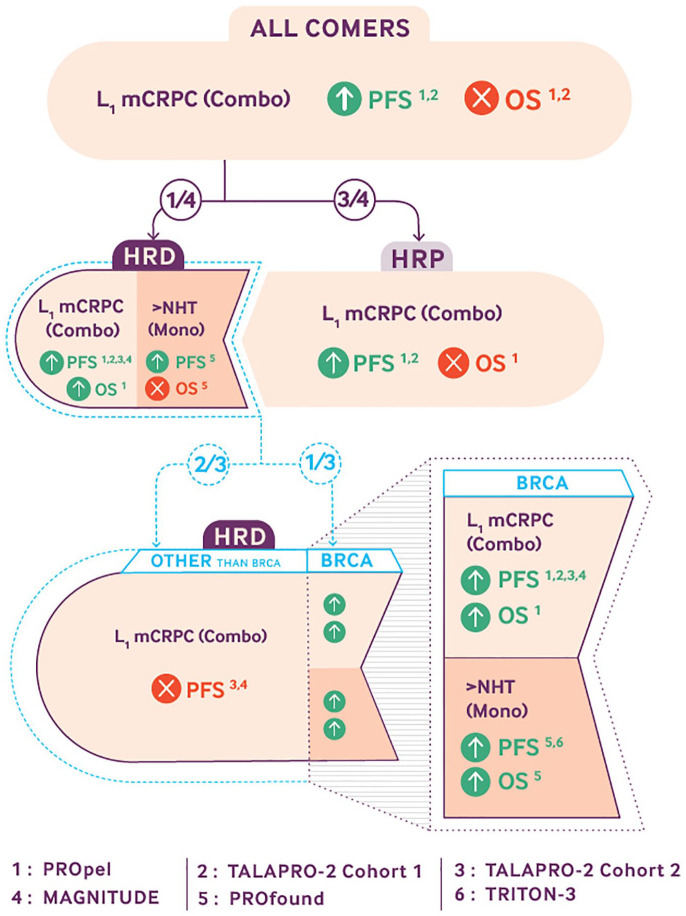
The PROfound and TRITON-3 trials demonstrated convincing results for the use of PARPi as monotherapy in patients with mCRPC and BRCA1/2 alterations after NHT. However, the improvement is debatable for ATM and other HRR genes, and PARPi used as monotherapy is inefficient in HRP patients.15,16,34 To overcome resistance and enhance efficacy, the synergistic action of PARPi and NHT was evaluated in PROpel, MAGNITUDE, and TALAPRO-2 for mCRPC patients as first-line setting (Figure 1).
Figure 1.
Main results provided by the five phase III trials studying PARPis in metastatic prostate cancers. PROpel,20,24 TALAPRO-2 cohort 1, 22 TALAPRO-2 cohort 2, 26 MAGNITUDE,21,25 PROfound,15,23 and TRITON-3. 16
BRCA1/2-altered patients
The efficacy of PARPi, whether used as monotherapy or in combination with NHT, for BRCA2-altered mCRPC, is undeniable. However, it should be stressed that most of studies do not differentiate between BRCA1 and BRCA2 alterations, and since BRCA1 alterations are less common, trials are underpowered to assess efficacy for these later alterations.2,3 Moreover, the question of the optimal sequence is largely unanswered. It is still unclear whether PARPi should be used in combination, even earlier than in the mCRPC setting. Furthermore, it is unclear whether it may increase toxicities without actually providing any survival benefit and whether it is better to administer NHT and PARPi simultaneously or use them sequentially.
PROfound and TRITON-3 taught us that PARPi as monotherapy is more efficient than NHT, and, most importantly, more efficient than docetaxel in the mCRPC setting after at least one prior exposure to NHT.15,16 Moreover, a post hoc analysis suggested a broader effect of olaparib when given before rather than after docetaxel. 35 It could suggest that the earlier PARPi are given, the better the outcomes.
Regarding combinations, only a small proportion of the patients (0.8%, 2%, and 34%) have received PARPi as subsequent therapies in the control arm of PROpel, TALAPRO-2 all comers, and MAGNITUDE, respectively. Therefore, given the results of the PROfound and TRITON-3 trials, there is a bias and they cannot adequately address the question of the optimal sequence, even if an OS improvement is observed.29,25 The phase II BRCAAway trial randomized 60 patients with mCRPC and BRCA1/2 or ATM alterations in three arms: the first received abiraterone, the second Olaparib, and the third a combination of both. 36 Updated data were recently presented, and it was observed that patients in the combination group had greater Prostate-specific antigen (PSA) responses and longer PFS compared to the two other arms suggesting a synergistic or additive action between the two drugs. However, no OS data were reported. 37 Thus, although there is a tendency toward the superiority of the combination, without OS data, the question of the best sequence currently remains unanswered. Ongoing trials investigating such combinations in castration-sensitive settings will provide additional information (NCT04821622 and NCT04497844).
HRR alterations other than BRCA1/2
PARPi monotherapy does not appear to be effective for ATM or CDK12 alterations, and data are lacking for other HRR genes to decipher their predictive implications. 38 Can the combination of PARPi and NHT enhance efficacy and overcome primary resistance?
PROpel is currently the only trial with data on OS in the all HRD population, and it demonstrated an undeniable benefit (HR: 0.50, 95% CI: 0.34–0.73). 24 However, it should be noted that BRCA-altered patients represented approximately 30% of HRD patients, and the OS benefit was even more pronounced in this subgroup (HR: 0.23, 95% CI: 0.12–0.43). Therefore, the question can be raised of whether the majority of the observed benefit in the HRD group should be attributed to BRCA1/2-altered patients. Investigators in both MAGNITUDE and TALAPRO-2 separated BRCA patients from other HRD patients. Unfortunately, with relatively small-sized populations (200 and 244, respectively), they failed to show an improvement. Nevertheless, trends toward improvement were observed when clustering some genes such as those implicated in the HRR-Fanconi pathway (BRIP1, FANCA, and PALB2), or CDK12 cluster.26,39 Therefore, the combination could be of interest for certain HRR alterations, but more data are needed to identify the specific genes of interest.
HRR-proficient patients
The PROpel and TALAPRO-2 trials showed a modest benefit in rPFS for the HRR-proficient population through subgroup analyses, whereas this was not observed in the MAGNITUDE trial which used distinct cohorts. Although the data regarding the non-HRD population in TALAPRO-2 came from a secondary endpoint and a subgroup analysis, they were consistent as patients were stratified. 22 Results from PROpel were less robust as patients were not stratified on their HRR status. Unfortunately, enrollment in the HRP cohort of the MAGNITUDE study was halted after the preplanned futility analysis. These contradictory outcomes are difficult to explain and merit attention considering that the design of the MAGNITUDE trial was the most robust. Moreover, the patient populations were quite similar across the trials. The early discontinuation of enrollment in the HRP cohort of MAGNITUDE may have occurred due to the use of a composite endpoint of time to PSA progression and/or rPFS, rather than solely rPFS. A modest benefit of the combination cannot be precluded with the limited size of the cohort.
Moreover, a recent quantitative meta-analysis of these three trials suggested a rPFS improvement of the combination. However, quantitative meta-analyses involve biases and are less robust than individual patient data meta-analyses. 40 Therefore, given these contradictory outcomes, the combination of PARPi and NHT could be an interesting option for HRP patients and requires monitoring, but more robust data are needed, especially regarding OS and long-term toxicity.
Conclusion
PARPi alone are well tolerated and highly effective for patients with BRCA1/2 alterations; these patients should all receive PARPi at some point in their medical history. However, PARPi efficacy is unclear for other HRR genes, and they should not be used alone for HRP patients. The combination with NHT could overcome resistance and, based on PROpel, MAGNITUDE, and TALAPRO-2 trials, three patient profiles can be delineated:
-For BRCA1/2-altered patients, although an OS improvement was reported in PROpel and MAGNITUDE trials, a large portion of patients did not receive PARPi as a subsequent therapy in their control arms. Therefore, we cannot confirm that the combination is superior to the sequence for the moment.
-For HRD patients with alterations other than BRCA1/2, MAGNITUDE, and TALAPRO-2 showed an improvement trend that was not statistically significant, indicating the need for more data. PROpel did not specifically analyze these cases.
-For HRP patients, subgroup analyses suggest that the combination may provide a modest rPFS benefit in two out of three studies. However, negative results from MAGNITUDE raise questions and rPFS is not a validated surrogate marker of OS for first-line mCRPC in the setting of PARPi therapy.
Overall, the results of these studies raise questions and fuel debates.41,42 Is there a subset of patients in the HRP group harboring another alteration than a canonical HRR mutation, conferring excellent response? Were the HRR alterations well tested, and were there some HRD patients in HRP cohorts? Indeed, approximately one-quarter of the PROpel patients were only evaluated using circulating tumor DNA in the absence of adequate tumor tissue. Are these modest improvements worth the potential increased toxicities?
Furthermore, we must consider the cost-effectiveness of such combinations in an all-comers strategy. Moreover, only a small number of patients in the combination trials received previous NHT, whereas nowadays every patient’s treatment should be intensified with NHT in the castrate-sensitive state. Therefore, without data on the efficacy of the combination of PARPi and NHT in a pre-exposed setting, there is a risk of cross-resistances changing NHT instead of chemotherapy as first-line mCRPC treatment. Faced with all these questions, the majority of experts from the Advanced Prostate Cancer Consensus Conference do not recommend the use of the combination for patients with an HRR alteration other than BRCA or patients without any HRR alteration. A consensus could not be reached regarding the use of the combination in patients with a BRCA alteration. 42
Finally, it is important to emphasize that regardless of future results, it will always be important to undergo a BRCA1/2 screening so both family history and more aggressive cancers are not overlooked.4–6
Acknowledgments
The authors thank Pippa McKelvie-Sebileau for providing medical writing support and Nelly Rivière for the figure.
Footnotes
ORCID iD: Diego Teyssonneau  https://orcid.org/0000-0002-9687-3759
https://orcid.org/0000-0002-9687-3759
Contributor Information
Diego Teyssonneau, Department of Medical Oncology, Institut Bergonié, 229 Cours de l’Argonne, Bordeaux 33000, France.
Charles Dariane, Department of Urology, Hôpital Européen Georges-Pompidou, APHP, Paris University, U1151 Inserm-INEM, Necker, Paris, France.
Eric Barret, Department of Urology, Institut Mutualiste Montsouris, Paris, France.
Jean-Baptiste Beauval, Department of Urology, La Croix du Sud Hôpital, Quint Fonsegrives, France; IUCT-O, Toulouse, France.
Laurent Brureau, Department of Urology, CHU de Pointe-à-Pitre, University of Antilles, University of Rennes, Inserm, EHESP, Irset (Institut de Recherche en Santé, Environnement et Travail) – UMR_S 1085, Pointe-à-Pitre, France.
Gaëlle Fiard, Department of Urology, Grenoble Alpes University Hospital, Université Grenoble Alpes, CNRS, Grenoble INP, TIMC-IMAG, Grenoble, France.
Gaëlle Fromont, Department of Pathology, CHRU Tours, Tours, France.
Gilles Créhange, Department of Radiation Oncology Curie Institute, Paris, France.
Mathieu Gauthé, Department of Nuclear Medicine, Scintep, Grenoble, France.
Alain Ruffion, Service d’Urologie Centre Hospitalier Lyon Sud, Hospices Civils de Lyon; Equipe 2, Centre d’Innovation en Cancérologie de Lyon (EA 3738 CICLY), Faculté de Médecine Lyon Sud, Université Lyon 1, Lyon, France.
Raphaële Renard-Penna, AP-HP, Radiology, Pitie-Salpetriere Hospital, Sorbonne University, Paris, France.
Romain Mathieu, Department of Urology, University of Rennes, Rennes, France; University of Rennes, Inserm, EHESP, Irset (Institut de Recherche en Santé, Environnement et Travail), Rennes, France.
Paul Sargos, Department of Radiotherapy, Institut Bergonié, Bordeaux, Aquitaine, France.
Morgan Rouprêt, AP-HP, Urology, GRC 5 Predictive Onco-Uro, Pitie-Salpetriere Hospital, Sorbonne University, Paris, France.
Guillaume Ploussard, Department of Urology, La Croix du Sud Hôpital, Quint Fonsegrives, France; IUCT-O, Toulouse, France.
Guilhem Roubaud, Department of Medical Oncology, Institut Bergonié, Bordeaux, Aquitaine, France.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Diego Teyssonneau: Conceptualization; Writing – review & editing.
Charles Dariane: Validation.
Eric Barret: Validation.
Jean-Baptiste Beauval: Validation.
Laurent Brureau: Validation.
Gaëlle Fiard: Validation.
Gaëlle Fromont: Validation.
Gilles Créhange: Validation.
Mathieu Gauthé: Validation.
Alain Ruffion: Validation.
Raphaële Renard-Penna: Validation.
Romain Mathieu: Validation.
Paul Sargos: Validation.
Morgan Rouprêt: Validation.
Guillaume Ploussard: Validation.
Guilhem Roubaud: Validation.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abida W, Armenia J, Gopalan A, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol 2017; 1: PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013; 31: 1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol 2019; 37: 490–503. [DOI] [PubMed] [Google Scholar]
- 6. Na R, Zheng SL, Han M, et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur Urol 2017; 71: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olmos D, Lorente D, Alameda D, et al. Presence of somatic/germline homologous recombination repair (HRR) mutations and outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) receiving first-line (1L) treatment stratified by BRCA status. J Clin Oncol 2023; 41(Suppl. 16): 5003–5003. [Google Scholar]
- 8. Stolz A, Ertych N, Bastians H. Tumor suppressor CHK2: regulator of DNA damage response and mediator of chromosomal stability. Clin Cancer Res Off J Am Assoc Cancer Res 2011; 17: 401–405. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, Willers H, Feng Z, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol 2004; 24: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang F, Fan Q, Ren K, et al. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol Cancer Res 2009; 7: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Doty T, Gibson B, et al. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat Struct Mol Biol 2010; 17: 1260–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otto H, Reche PA, Bazan F, et al. In silico characterization of the family of PARP-like poly(ADP-ribosyl)transferases (pARTs). BMC Genomics 2005; 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okano S, Lan L, Caldecott KW, et al. Spatial and temporal cellular responses to single-strand breaks in human cells. Mol Cell Biol 2003; 23: 3974–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teyssonneau D, Margot H, Cabart M, et al. Prostate cancer and PARP inhibitors: progress and challenges. J Hematol Oncol 2021; 14: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med 2020; 383: 2345–2357. [DOI] [PubMed] [Google Scholar]
- 16. Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or physician’s choice in metastatic prostate cancer. N Engl J Med 2023; 388: 719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013; 3: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun 2017; 8: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced ‘BRCAness’ and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal 2017; 10: eaam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid 2022; 1. [DOI] [PubMed] [Google Scholar]
- 21. Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol 2023; 41: 3339–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet 2023; 402: 291–303. [DOI] [PubMed] [Google Scholar]
- 23. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020; 382: 2091–2102. [DOI] [PubMed] [Google Scholar]
- 24. Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol 2023; 24: 1094–1108. [DOI] [PubMed] [Google Scholar]
- 25. Chi KN, Castro E, Attard G, et al. LBA85 niraparib (NIRA) with abiraterone acetate plus prednisone (AAP) as first-line (1L) therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations: three-year update and final analysis (FA) of MAGNITUDE. Ann Oncol 2023; 34: S1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fizazi K, Azad AA, Matsubara N, et al. First-line talazoparib with enzalutamide in HRR-deficient metastatic castration-resistant prostate cancer: the phase 3 TALAPRO-2 trial. Nat Med 2024; 30: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thiery-Vuillemin A, de Bono J, Hussain M, et al. Pain and health-related quality of life with olaparib versus physician’s choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations (PROfound): an open-label, randomised, phase 3 trial. Lancet Oncol 2022; 23: 393–405. [DOI] [PubMed] [Google Scholar]
- 28. De Bono JS, Matsubara N, Penel N, et al. Exploratory gene-by-gene analysis of olaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): PROfound. J Clin Oncol 2021; 39(Suppl. 6): 126–126.33108242 [Google Scholar]
- 29. Chakraborty G, Armenia J, Mazzu YZ, et al. Significance of BRCA2 and RB1 co-loss in aggressive prostate cancer progression. Clin Cancer Res Off J Am Assoc Cancer Res 2020; 26: 2047–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019; 116: 11428–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agarwal N, Zhang T, Efstathiou E, et al. The biology behind combining poly [ADP ribose] polymerase and androgen receptor inhibition for metastatic castration-resistant prostate cancer. Eur J Cancer 2023; 192: 113249. [DOI] [PubMed] [Google Scholar]
- 32. Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2018; 19: 975–986. [DOI] [PubMed] [Google Scholar]
- 33. Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol 2023; 34: 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015; 373: 1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Bono JS, Fizazi K, Saad F, et al. PROfound: Efficacy of olaparib (ola) by prior taxane use in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J Clin Oncol 2020; 38(Suppl. 6): 134–134. [Google Scholar]
- 36. Hussain MHA, Kocherginsky M, Agarwal N, et al. BRCAAWAY: a randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) with DNA repair defects. J Clin Oncol 2022; 40(Suppl. 16): 5018–5018. [Google Scholar]
- 37. Hussain MHA, Kocherginsky M, Agarwal N, et al. BRCAAway: a randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) bearing homologous recombination-repair mutations (HRRm). J Clin Oncol 2024; 42(Suppl. 4): 19–19.37967311 [Google Scholar]
- 38. Teyssonneau D, Thiery-Vuillemin A, Dariane C, et al. PARP inhibitors as monotherapy in daily practice for advanced prostate cancers. J Clin Med 2022; 11: 1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sandhu S, Attard G, Olmos D, et al. Gene-by-gene analysis in the MAGNITUDE study of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J Clin Oncol 2022; 40(Suppl. 16): 5020–5020. [Google Scholar]
- 40. Messina C, Giunta EF, Signori A, et al. Combining PARP inhibitors and androgen receptor signalling inhibitors in metastatic prostate cancer: a quantitative synthesis and meta-analysis. Eur Urol Oncol 2023: S2588-9311(23)00155-4. [DOI] [PubMed] [Google Scholar]
- 41. Beije N, Abida W, Antonarakis ES, et al. PARP inhibitors for prostate cancer: tangled up in PROfound and PROpel (and TALAPRO-2) blues. Eur Urol 2023; 84: 253–256. [DOI] [PubMed] [Google Scholar]
- 42. Gillessen S, Bossi A, Davis ID, et al. Management of patients with advanced prostate cancer – metastatic and/or castration-resistant prostate cancer: report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer 2023; 185: 178–215. [DOI] [PubMed] [Google Scholar]