Abstract
Even in the absence of hyperglycemia or hyperlipidemia, it has been demonstrated that insulin resistance is an independent risk factor for atherosclerosis. Finding markers of insulin resistance that are associated with markers of atherosclerosis could help identify patients early in their disease course and allow for earlier initiation of preventative treatments. We reviewed available evidence regarding associations between known markers of insulin resistance and known markers of atherosclerosis. Serum triglycerides (TG), triglyceride-glucose index (TyG), and homeostasis model assessment (HOMA) were the insulin resistance markers reviewed. The coronary artery calcium score (CAC), carotid intimal medium thickness (cIMT), and pulse wave velocity (PWV) were reviewed as markers of atherosclerosis. TyG showed the most consistent association with CAC across broad demographic groups, though HOMA showed potential in obese individuals and those without diabetes. The data regarding cIMT and the reviewed insulin resistance markers did not yield any consistent associations, though very elevated TyG did appear to be associated with cIMT among normal weight individuals. Serum triglycerides showed a strong and consistent association with PWV across numerous studies and populations, though TyG index also demonstrated a strong association with PWV in a large systematic review. Of the insulin resistance markers reviewed, the TyG index appears to be most consistently associated with markers of atherosclerosis. TyG can be easily calculated with routine labwork and has the potential to inform decisions regarding early initiation of therapies in patients who would otherwise not be treated. Targeting insulin sensitivity prior to the development of T2DM has the potential to reduce development and progression of atherosclerosis, and patients without T2DM but who have elevated TyG index should be the topic of further research.
Keywords: Atherosclerosis, Coronary artery calcium, Insulin resistance, Triglyceride-Glucose Index
Central illustration.
Alt-text: Unlabelled box
1. Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance, and T2DM has long been linked to an elevated risk of atherosclerosis. At first glance, the association between T2DM and atherosclerosis appears to come down to high blood glucose levels and glucose's role in atherosclerotic plaque development. However, a closer look reveals that insulin resistance may itself precipitate atherosclerosis. Even after correcting for hypertension (HTN), hyperlipidemia (HLD), and blood sugar levels, patients with T2DM still have an elevated risk of cardiovascular disease, which is largely attributable to insulin resistance [1]. The molecular basis of insulin resistance can help explain this association.
When insulin binds to insulin receptors on the cell membrane, insulin receptor substrate (IRS-1/2) undergoes tyrosine phosphorylation, phosphatidylinositol 3-kinase (PI3K) is activated, and glucose transport is subsequently enhanced [1]. Nitric oxide synthase, which controls nitric oxide synthesis, is also activated by insulin signaling [1]. Nitric oxide is a powerful vasodilator and a well-known anti-atherogenic compound [2]. Impaired insulin signaling hinders glucose metabolism, but also induces hypertension and atherogenesis via nitric oxide deficiency [2]. Insulin resistance also simultaneously leads to increased synthesis of the potent vasoconstrictor endothelin-1 and decreased availability of the vasodilator prostacyclin [3]. These dual changes promote further narrowing of blood vessels and are an additional example of how insulin resistance promotes the vasomotor dysfunction that is an important feature of atherosclerotic plaque formation [3].
Increased insulin production by the pancreatic beta cell is another impact of insulin resistance. Insulin at high levels acts as a growth factor that exerts its effects through the Mitogen-activated protein kinase (MAPK) pathway [1]. The MAPK pathway activates inflammatory pathways including Nuclear factor-kB (NF-kB) while simultaneously promoting vascular smooth muscle cell development, proliferation, and differentiation [1]. Despite resistance within the IRS pathway, the MAPK pathway retains normal insulin sensitivity and is hyper-stimulated by elevated plasma insulin [1].
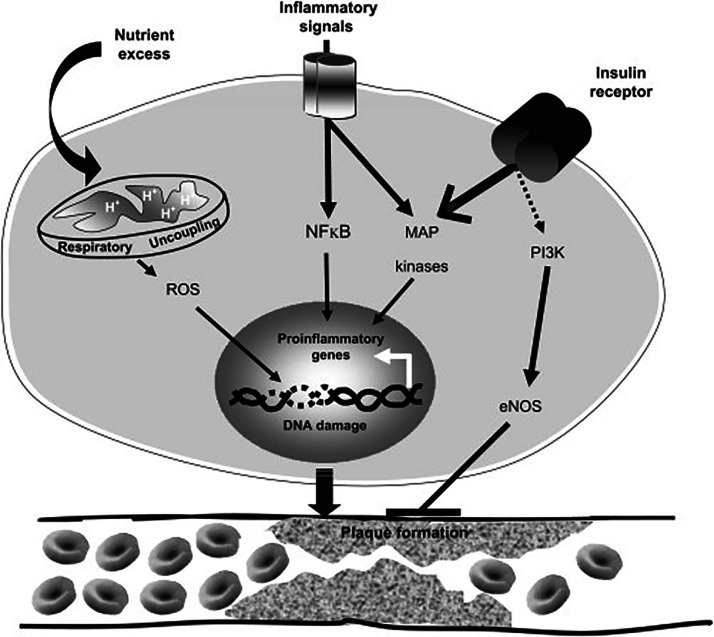
Insulin resistance also leads to formation of smaller and denser LDL particles [3]. These modified LDL particles are more likely to undergo oxidation and can enter the inner layer of arteries with greater ease [3]. Oxidized LDL is a powerful instigator of atherosclerosis due to its ability to attract inflammatory cells and promote endothelial dysfunction [3]. Insulin resistance additionally reduces the function of lipoprotein lipase, which then leads to increased lipoproteins high in triglycerides and further atherosclerotic plaque development [3,4] Fig. 1.
Fig. 1.
Signaling events that promote atherosclerosis in the setting of insulin resistance [4]. eNOS endothelial nitric oxide, NFkB nuclear factor kappa beta, PI3K phosphatidylinositol-3 kinase, ROS reactive oxygen species.
“Reprinted from Endocrinology and Metabolism Clinics, volume 37, issue 3, Razani B, Chakravarthy M, Semenkovich C, Insulin resistance and atherosclerosis, pages 603–621, 2008, with permission from Elsevier.”
When discussing the metabolic effects of insulin resistance, it becomes clear that T2DM's association with atherosclerotic plaque development comes down to more than hyperglycemia. Even in the absence of T2DM, insulin resistance establishes itself as a distinct risk factor for atherosclerosis through its myriad of proatherogenic effects [3]. Insulin resistance may predate the development of T2DM by years or decades and may initiate the process of atherosclerosis years before a diagnosis of T2DM is made. Numerous attempts have been made to measure insulin resistance, but their clinical significance is unclear. This narrative review examines existing literature surrounding serum triglycerides (TG), triglyceride-glucose (TyG) index, and homeostatic model assessment (HOMA) model as markers of insulin resistance. The quantitative insulin sensitivity check index, insulinogenic index and insulin:glucose ratio were also considered as potential markers of insulin resistance but were ultimately excluded given paucity of available evidence comparing these to our chosen markers of atherosclerosis, which are discussed below. Studies involving the euglycemic clamp as a measure of insulin resistance were also excluded given this method cannot be feasibly applied in day-to-day clinical practice. Measurement of serum triglycerides is performed routinely as part of routine health maintenance screening, and it has been theorized that high levels correlate with reduced insulin sensitivity. TyG index is measured with Ln(fasting triglycerides (mg/dL) x (fasting glucose (mg/dL)/2)); a higher score indicates insulin resistance. HOMA is measured with (fasting insulin (micromole/L) x fasting glucose (mg/dL))/405, and higher scores indicate insulin resistance. All three of our chosen insulin resistance markers can be easily calculated in a clinical setting using routine labwork. The measures of atherosclerosis in this review included coronary artery calcium score, carotid intimal media thickness, and pulse wave velocity.
Coronary artery calcium score, or CAC, is used to determine how much calcium is present in a person's coronary arteries. While not all atherosclerotic plaques are calcified, coronary calcium burden is closely associated with atherosclerosis [5]. A strong correlation between CAC and the risk of medium- and long-term incidence of cardiovascular events has been observed [6]. Multidetector computed tomography (CT) without contrast is the modality of choice for determining CAC [6]. It is based on 3 mm thick axial slices within the cardiac area recorded in synchrony with ECG at a specified point in the R-R interval, most frequently in mid to late diastole [6]. Three basic methods—the Agatston method, calcium volume score, and calcium mass score—are used to quantify the CAC [6]. The Agatston method is most commonly used and was utilized in most of the studies reviewed [6]. Using the Agatston method to calculate CAC, patients can be risk stratified regarding their risk of future coronary events [6]. Patients who receive a CAC score of zero are deemed very low risk, scores between 1 and 100 are deemed low risk, scores between 101 and 400 indicate increased risk, and scores >400 indicate increased probability of myocardial ischemia [6].
Carotid intimal medium thickness, or cIMT, can assist in determining one's risk of cardiovascular disease and numerous observational studies have shown a correlation between cIMT and the risk of stroke, myocardial infarction, and death from coronary causes [7]. Carotid plaque is defined as focal arterial wall thickening at least 50 % greater than the surrounding wall or a localized patch of cIMT > 1.5 mm [7]. CIMT is often defined as the combined thickness of the intimal and medial layers of the far wall of the common carotid artery [7]. CIMT measures are utilized to detect artery wall thickening in pre-atherosclerosis and non-occlusive plaque formation in subclinical atherosclerosis, while carotid duplex US is frequently used to identify occlusive carotid plaques in advanced atherosclerosis [7].
A measure of systemic arterial stiffness called brachial-ankle pulse wave velocity (baPWV) is obtained by analyzing the ultrasound waves in the brachial and tibial arteries [8]. The metric is often used in East Asia, and meta-analyses have shown that among high-risk patients, a 1 m/s rise in baPWV is associated with a 12 % greater risk of cardiovascular events [8]. Carotid-femoral pulse wave velocity is another measure of arterial stiffness and is a known marker of atherosclerosis [9]. Thus, arterial stiffness measured via pulse wave velocity presents itself as a useful marker of cardiovascular risk and atherosclerosis.
We reviewed available evidence regarding associations between each insulin resistance marker and known measures of atherosclerosis. Pubmed searches were performed to find articles comparing our chosen insulin resistance markers with our markers of atherosclerosis. Included studies had to involve participants ≥ 18 years of age, be published after 2005 and have directly compared one of the chosen insulin resistance markers with one of the chosen atherosclerosis markers. Paired search terms were used to pull articles comparing each atherosclerosis marker with each marker of insulin resistance. For example, with regards to coronary artery calcium score, searched terms included: [“CAC” AND “serum triglycerides”]; [“CAC” AND “TyG Index”]; [“CAC” AND “HOMA”]. The same search parameters were used with cIMT in place of CAC for that set of studies. BaPWV and PWV were both searched in place of CAC for that set of studies. Full names of each marker were also inserted into the above search parameters to cast a wider search net.
2. Coronary artery calcium score (CAC)
2.1. Association between serum triglycerides and CAC
One study from Taiwan compared serum triglycerides with CAC scores among 3586 adult Taiwanese patients between 2011 and 2016 at a tertiary hospital [10]. They defined prognostically significant coronary calcification (PSCC) as CAC over 100 [10]. Of all the patients studied, 10.2 % had PSCC [10]. Their findings revealed that high serum triglycerides, defined as serum triglycerides ≥ 200 mg/dL, were significantly associated with PSCC with adjusted odds ratio of 1.36 [10]. The odds ratios comparing total cholesterol and LDL to PSCC were not significant [10]. Additionally, the relationship between high triglycerides and PSCC showed no modification by sex [10].
A multi-center population-based prospective cross-sectional study evaluated relationships between various risk factors and incident ASCVD among a population with baseline ASCVD risk scores ≥ 7.5 % and < 20 % [11]. Participants had no history of clinical ASCVD or diabetes at baseline [11]. 1688 participants were recruited with a mean age of 65, 57.8 % of the population were men, and the mean follow-up was 12 years [11]. Incident ASCVD included myocardial infarction, cardiac arrest, death related to coronary heart disease, hemorrhagic stroke, ischemic stroke, or transient ischemic attack (TIA) [11]. There was no relationship among triglycerides and incident ASCVD regardless of baseline CAC [11]. Serum triglycerides were also not found to be significantly correlated with CAC scores [11].
3754 middle-aged people with low to moderate cardiovascular risk participated in an observational, longitudinal, and prospective cohort study to determine the link between subclinical atherosclerosis and hypertriglyceridemia [12]. Participants were between the ages of 40 and 54 and were without previous diagnoses of cardiovascular disease [12]. Cardiovascular risk was assessed using the Systematic Coronary Risk Evaluation (SCORE), and those with risk scores ≥ 5 % were excluded from the study [12]. Serum triglycerides showed no significant association with CAC [12]. However, serum triglycerides ≥ 150 mg/dl were significantly related to prevalence of non-coronary atherosclerosis, which was determined using ultrasound (US) of carotid arteries, infrarenal aorta, iliac arteries, and femoral arteries [12].
2.2. Association between TyG index and CAC
A group of Korean researchers studied the relationship between the triglyceride glucose index and the progression of CAC in adult Koreans [13]. 1175 subjects with previous CAC studies were ultimately included in the retrospective longitudinal study [13]. 71.1 % of the subjects were men [13]. A rise of at least 2.5 units between the square roots of the baseline and follow-up CACs in participants with detectable CAC at baseline or incident CAC in a population devoid of CAC at baseline were both considered to be signs of CAC progression [13]. According to increasing TyG the study population was separated into three tertiles, and it was discovered that the high TyG group had a greater incidence of CAC progression [13]. With an odds ratio of 1.82 (95 % confidence interval: 1.20 - 2.77) comparing the highest and lowest tertiles of TyG, there was a significant link between TyG and CAC progression even after controlling for established cardiovascular risk factors [13].
Another study looked at postmenopausal women who underwent CT angiography with concern for myocardial ischemia [14]. Subjects were first evaluated via ECG and cardiac marker values, and only those not at high risk of active myocardial ischemia underwent CT angiography [14]. The 228 individuals studied had median age 54, median CAC of 79.5, and median TyG index of 9.0 [14]. Subjects were divided in three populations by rising CAC score and were compared with regard to demographics, lab test outcomes, ECG findings, and TyG [14]. They found a positive correlation between TyG and CAC with a correlation coefficient of 0.424 and a p-value of 0.001 [14].
12,326 asymptomatic adults who had undergone at least two prior CAC examinations were included in a sizable Korean investigation [15]. The population included 84.2 % men who were 51.7 years of age on average [15]. A difference of 2.5 between the square roots of the baseline and follow-up CAC was used to identify CAC progression [15]. The overall incidence of CAC progression was 30.6 %, and the average follow-up time was 3.3 years [15]. After controlling for age, male sex, body mass index (BMI), HTN, diabetes, HLD, smoking, and serum creatinine, they discovered that increasing TyG was significantly correlated with an increased risk of CAC progression in persons without heavy CAC at baseline, defined as CAC <100 [15]. This association was not seen in the population with high baseline CAC, defined as CAC >100, after risk factor adjustment [15].
A systematic review evaluated 26 observational studies with 87,307 participants in total to compare TyG index with CAC [16]. Patients were at least 18 years of age and the studies included originated in China, South Korea, Japan and the United States [16]. Mean participant age ranged from 28.7 to 75 years [16]. They found that elevated TyG index was significantly associated with CAC with odds ratio of 1.66 [16].
2.3. Association between HOMA and CAC
HOMA was employed as a measure of insulin resistance in a cohort study that included 2777 people from the Coronary Artery Risk Development In Young Adults (CARDIA) study and looked at the likelihood of developing CAC in middle age [17]. The mean age at follow-up was 50.1, and the study population was comprised of 56.2 % women [17]. They used a 25-year follow-up period [12]. CAC >0 defined CAC incidence [17]. After controlling for factors like BMI, current smoking, serum creatinine (Cr), systolic blood pressure (BP), diastolic BP, LDL, and A1c, they discovered that high HOMA levels were significantly associated with an increased prevalence of CAC in obese people [17]. They also discovered that obese participants with moderate and high HOMA had significantly higher CAC scores than those in the low HOMA group [17]. The associations were not statistically significant in subjects without obesity [17].
A population-based study included 5464 patients between the ages of 45 and 84 without known cardiovascular disease [18]. Mean age of participants was 62, and the population was 53 % women [18]. Any detectable CAC in people without CAC at baseline was considered CAC incidence, and an increase in CAC score in persons with detectable baseline CAC was considered CAC progression [18]. After controlling for age, sex, ethnicity, study site, and years between CAC scans, higher HOMA levels were significantly linked with incident CAC and CAC progression, with a relative risk of 1.78 and 11.5, respectively [18]. However, after controlling for waist circumference, impaired fasting glucose, low HDL, high triglycerides, and HTN, the relationships were no longer significant [18].
A community-based prospective cohort study enrolled 1155 subjects who were 52 % men and 57 years of age on average [19]. Individuals with a CAC of zero were excluded [19]. The CAC volume was determined by adding the plaque volumes within each calcified lesion [19]. By dividing the total volume score by the thickness of the slices, the average area score was created [19]. The Agatston score was then divided by the average area score to provide the CAC density [19]. After correcting for age, sex, race, education, income, birth country, years lived in the United States, and CAC density, HOMA was not significantly associated with CAC density but was significantly associated with CAC volume [19].
A Japanese population-based study included 1006 men with a mean age of 64 to investigate associations between CAC and insulin resistance [20]. After adjusting for age, smoking history, alcohol consumption, LDL, type of CT scan, HTN, use of antihypertensive medications, HLD, use of cholesterol-lowering medications, and BMI, HOMA was significantly associated with prevalence of baseline CAC > 0 in all participants and those without diabetes [20]. The odds ratio for all participants was 1.34 and 1.29 for participants without diabetes [20].
A cross-sectional study involving 105 patients with T2DM and 103 non-diabetic patients investigated the relationship between HOMA and CAC score [21]. Participants ranged in age from 20 to 70 and did not have any prior history of cardiovascular disease [21]. Calculations in the diabetic group were adjusted for age and in the non-diabetic group adjustments were made for age and HLD [21]. In patients without diabetes, HOMA was significantly associated with an elevated risk of CAC ≥10, with an odds ratio of 1.778, using a multivariable binary logistic regression model [21]. No significant association was found among participants with diabetes [21].
In summary, TyG appears to be more consistently associated with CAC in more diverse circumstances than the other markers. Still, HOMA may be a useful marker in obese individuals and those without diabetes Table 1.
Table. 1.
Association between insulin resistance and CAC.
| Ref # | Study Design | Participant Characteristics | Marker of Insulin Resistance | Key Findings |
|---|---|---|---|---|
| [10] | Cross-Sectional | 3586 Taiwanese adults | Serum Triglycerides | Triglycerides ≥ 200 mg/dl significantly associated with CAC > 100 (OR: 1.36) |
| [11] | Prospective Cross-Sectional | 1688 US adults, mean age 65, no history clinical ASCVD or diabetes | Serum Triglycerides | No significant association found between serum triglycerides and incident ASCVD regardless of baseline CAC; Serum triglycerides not significantly correlated with CAC |
| [12] | Prospective Cohort | 3754 middle aged adults, no history of cardiovascular disease, low-moderate risk of developing cardiovascular disease | Serum Triglycerides | Serum triglycerides showed no significant association with CAC |
| [13] | Retrospective Longitudinal | 1175 Korean adults, 71.1 % men | TyG Index | Significant association between higher TyG and CAC progression (OR: 1.82) |
| [14] | Cross-Sectional | 228 postmenopausal women who underwent CT angiography with suspicion of acute coronary syndrome | TyG Index | Significant positive correlation between TyG and CAC (r = 0.424) |
| [15] | Prospective Cohort | 12,326 asymptomatic Korean adults, mean age 51.7, 84.2 % men | TyG Index | Increasing TyG significantly associated with increased risk of CAC progression in those with baseline CAC ≤ 100 |
| [16] | Systematic Review | 87,307 participants across 26 studies. Mean age 28.7 – 75 years. Studies originated in China, South Korea, Japan and the United States. | TyG Index | Elevated TyG significantly associated with CAC (OR: 1.66) |
| [17] | Prospective Cohort | 2777 young adults, 25 year follow up period, mean age at follow up 50.1 | HOMA | High HOMA significantly associated with increased CAC prevalence among obese individuals (OR: 1.82); Higher HOMA significantly associated with higher CAC among obese individuals (OR: 2.08 for high HOMA group); no significant associations seen in subjects without obesity |
| [18] | Population Study | 5464 participants aged 45–84 | HOMA | Higher HOMA significantly associated with incident CAC (relative risk = 1.78) and CAC progression (relative risk =11.5); associations not significant after adjusting for waist circumference, fasting glucose, HDL, triglycerides and HTN |
| [19] | Prospective Cohort Study | 1155 participants, mean age 57, baseline CAC > 0 | HOMA | HOMA not significantly associated with CAC density; HOMA was significantly associated with CAC volume (beta = 0.12) |
| [20] | Population Study | 1006 Japanese adults, mean age 64 | HOMA | Among all participants (OR: 1.34) and among those without diabetes (OR: 1.29) HOMA significantly associated with prevalence of baseline CAC > 0 |
| [21] | Cross-Sectional Study | 105 patients with T2DM, 103 non-diabetic patients, ages 20–70, no history of cardiovascular disease | HOMA | Among participants without diabetes HOMA significantly associated with CAC ≥ 10 (OR: 1.778); no significant association among participants with diabetes |
CAC coronary artery calcium, OR odds ratio, US united states, ASCVD atherosclerotic cardiovascular disease, TyG triglyceride-glucose index, CT computed tomography, HOMA homeostatic model assessment, T2DM type 2 diabetes mellitus.
3. Carotid intimal media thickness (cIMT)
3.1. Association between serum triglycerides and cIMT
1822 Taiwanese young adults without any history of diabetes were included in a study that examined associations between indices of insulin resistance and cIMT [22]. After controlling for age, sex, smoking, drinking, blood pressure, total cholesterol, HDL, and fasting blood sugar, serum triglycerides were compared to cIMT [22]. They found a significant association between cIMT and triglycerides with a beta of 0.057 and p = 0.04 [22]. Subgroup analysis revealed that cIMT was associated with triglycerides in the obese and overweight groups, defined as BMI ≥ 25 kg/m2, but not in normal weight individuals [22].
There were 64 randomized controlled trials with 96,807 patients in experimental groups and 98,681 controls that were part of a systematic review and meta-regression analysis [23]. There was no significant correlation between the pace of cIMT progression and the degree of change in triglyceride levels, according to the primary meta-regression analysis utilizing cIMT as the primary outcome [23].
In another Taiwanese trial, 1520 military personnel between the ages of 18 and 40 who weren't receiving HTN or HLD treatment were included [24]. The mean age was 27.3, and the population was comprised of 88.6 % men [24]. They discovered that triglycerides were significantly linked with cIMT with a beta coefficient of 0.063 after controlling for age, tobacco usage, and alcohol use [24]. The association between cIMT and serum triglycerides was also significant when men were isolated with a beta coefficient of 0.072, but this correlation was not found among women [24]. With additional subgroup analysis, it was discovered that persons with BMI ≥ 25 kg/m2 showed a significant relationship between triglycerides and cIMT, with a beta coefficient of 0.130 [24]. No significant association was found between cIMT and serum triglycerides among individuals with BMI < 25 kg/m2 [24].
The prospective cohort study mentioned in the CAC section also presented some relevant findings regarding serum triglycerides and non-coronary atherosclerosis. They discovered that, with an odds ratio of 1.35, serum triglycerides ≥ 150 mg/dl were significantly related to non-coronary atherosclerosis, as assessed by ultrasonography of the bilateral femoral, bilateral iliac, and carotid arteries [12].
3.2. Association between TyG index and cIMT
1523 ischemic stroke patients with available carotid artery imaging and TyG index data were enrolled in a study from China [25]. Participants' median TyG was 8.69, and their average age was 68.8 [25]. Maximum cIMT was defined as the largest value between the right and left common carotids, whereas mean cIMT was the average value found between the common carotids [25]. A mean cIMT and maximum cIMT value ≥ 1 mm were considered abnormal [25]. Patients were broken into four quartiles based on increasing TyG and were compared using the odds ratio of having abnormal [25]. They discovered that an elevated risk of abnormal mean and maximum cIMT values was significantly associated with the highest quartile TyG index after adjusting for cardiovascular risk factors [25].
An adult population-based study in Shanghai, China, enrolled 5751 men and women over 40 years of age with normal baseline cIMT, defined as cIMT < 0.9 mm [26]. Obesity was defined as BMI ≥ 28 kg/m2 [26]. The maximum cIMT value obtained was used in the analysis [26]. The groups were divided into four quartiles based on mean TyG, and cIMT >0.9 mm at follow-up defined incident cIMT elevation [26]. 4.3 years was the median follow-up period [26]. 722 subjects progressed to elevated cIMT [26]. They discovered a U-shaped relationship between the TyG index and high cIMT, with the first and fourth quartiles of the TyG index showing a significant increase in risk of elevated cIMT [26]. In non-obese people, subgroup analysis showed a similar U-shaped link between TyG and higher cIMT, while the association was not present in obese individuals [26]. After controlling for age, sex, smoking status, alcohol usage, physical activity, HDL, BMI, and glucose-lowering treatment, these relationships were determined [26].
A study out of Ankara City Hospital's Internal Medicine Clinic looked at 185 patients with primary hypertension over 18 years of age [27]. The population was separated into two groups depending on the existence or absence of target organ damage, with 70.8 % of the total population being female [27]. CIMT ≥ 0.9 mm, LV mass index > 95 g/m2 in women, LV mass index > 115 g/m2, urine protein > 150 mg/dl, or microalbumin excretion > 30 mg/dl were all considered indicators of target organ damage [27]. By averaging three measurements for each common carotid artery, the mean cIMT was determined [27]. They found that TyG had a significant positive correlation with risk of cIMT ≥ 0.9 mm with correlation coefficient of 0.434 among adult patients with hypertension [27]. Triglycerides were also positively correlated with an increased risk of elevated cIMT [27].
3.3. Association between HOMA and cIMT
A cross-sectional study of 8028 civil servants aged 35–74 years across Brazil investigated the relationship between cIMT and HOMA [28]. They discovered that a one standard deviation increase in HOMA was significantly associated with cIMT ≥ the 75th percentile with an odds ratio of 1.10 after adjusting for HTN, HLD, low HDL, systolic blood pressure, BMI, abdominal obesity, smoking status, excessive drinking, and family history of early cardiovascular disease [28]. These relationships initially maintained in subgroup analyses of men and women, but after controlling for the aforementioned risk factors they were no longer significant [28].
A longitudinal British study investigated 1779 15-year-olds who were followed over 9 years [29]. HOMA was calculated at ages 15, 17, and 24, while cIMT was measured at ages 17 and 24 [29]. After correcting for sex, age, C-reactive protein, insulin, glucose, total fat mass, systolic blood pressure, diastolic blood pressure, smoking status, sedentary time, and light physical activity, they discovered no connection between HOMA and 7-year cIMT progression [29].
A population-based study included 5810 participants without any history of diabetes and examined the relationship between cIMT and HOMA [30]. In the male, female, white, and Chinese subgroups, they discovered a significant linear relationship between HOMA and cIMT after controlling for age, sex, clinical site, education, smoking status, LDL, and metabolic syndrome [30]. The Hispanic subgroup showed no significant relationship before or after adjustments, whereas the black subgroup showed a significant linear relationship only when controlling for age, sex, clinical site, smoking status, and LDL [30]. No significant association between HOMA and cIMT was found in any of the study groups after adjusting for the non-glucose components of metabolic syndrome, which included the use of antihypertensive medications, HDL, triglycerides, systolic blood pressure, diastolic blood pressure, and waist circumference [30].
Overall, the data reviewed regarding cIMT's relationship to markers of insulin resistance is frequently contradictory and no strong conclusions can be made. Several studies did show significant associations between serum TG and general non-coronary atherosclerosis, but these findings are contradicted by a large meta-analysis which found no association. One potential conclusion is that very elevated TyG may be indicative of cIMT among normal weight individuals Table 2.
Table. 2.
Association between insulin resistance and cIMT.
| Ref # | Study Design | Participant Characteristics | Marker of Insulin Resistance | Key Findings |
|---|---|---|---|---|
| [22] | Cross-Sectional | 1822 Taiwanese young adults, no history of diabetes | Serum Triglycerides | Significant association between cIMT and triglycerides among all participants (beta = 0.057) and among overweight/obese subgroup (beta = 0.127); no significant association normal weight subgroup |
| [23] | Meta-Analysis | 96,807 patients in experimental groups, 98,681 controls | Serum Triglycerides | No significant relationship between degree of change in triglyceride levels and cIMT progression rate |
| [24] | Cross-Sectional | 1520 Taiwanese military members aged 18–40, 88.6 % men | Serum Triglycerides | Significant association between triglycerides and cIMT among all participants (beta = 0.063), male subgroup (beta = 0.072) and overweight/obese subgroup (beta = 0.130); no significant association seen in female subgroup or normal weight subgroup |
| [12] | Prospective Cohort Study | 3754 middle aged adults, no history of cardiovascular disease, low-moderate risk of developing cardiovascular disease | Serum Triglycerides | Serum triglycerides ≥ 150 mg/dl significantly associated with non-coronary atherosclerosis (OR: 1.35) |
| [25] | Cross-Sectional | 1523 Chinese ischemic stroke patients, mean age 68.8 | TyG Index | Significant association between TyG ≥ 9.19 and increased risk of abnormal mean/max cIMT (OR: 1.46) |
| [26] | Population Study | 5751 Chinese men and women over 40 years of age, normal baseline cIMT | TyG Index | Significant association between TyG and elevated cIMT found in highest and lowest quartiles of TyG in total population (OR 1st quartile: 1.29; OR 4th quartile: 1.42) and among non-obese individuals (OR 1st quartile: 1.60; OR 4th quartile: 1.89); no associations seen in middle quartiles of TyG or among obese individuals |
| [27] | Cross-Sectional | 185 patients over 18 years of age with primary HTN, 70.8 % women | TyG Index; Serum Triglycerides | TyG had significant correlation with increased risk of cIMT ≥ 0.9 mm (r = 0.434); Triglycerides had significant correlation with increased risk of cIMT ≥ 0.9 mm (r = 0.361) |
| [28] | Cross-Sectional | 8028 Brazilian civil servants, aged 35–74 | HOMA | 1 standard deviation increase in HOMA significantly associated with cIMT ≥ 75th percentile in total population (OR: 1.10); no significant associations in subgroup analysis after adjustments |
| [29] | Prospective Cohort Study | 1779 British 15-year-olds, followed up at ages 17 and 24 | HOMA | No significant association between HOMA and 7-year cIMT progression after adjustments |
| [30] | Population Study | 5810 adult participants without history of diabetes | HOMA | No significant relationship between HOMA and cIMT seen after adjusting for non-glucose components of metabolic syndrome |
cIMT carotid intimal media thickness, OR odds ratio, TyG triglyceride-glucose index, HTN hypertension, HOMA homeostatic model assessment.
4. Pulse wave velocity (PWV)
4.1. Association between serum triglycerides and PWV
A Japanese cross-sectional study with 11,640 participants examined the association between high triglycerides, characterized as ≥150 mg/dL, and baPWV > 1400 cm/s, which indicated arterial stiffness [31]. High triglycerides were found to be correlated with elevated baPWV [31]. Another cross-sectional study was conducted at Murakami Memorial Hospital in Japan with 909 participants whose baPWV and lipid indices were measured [32]. Triglycerides were positively correlated with baPWV regardless of cardiovascular risks and liver function [32]. A similar Chinese cross-sectional study enrolled 16,733 participants [33]. They defined arterial stiffness as baPWV in the upper quartile and high triglycerides as ≥150 mg/dL [33]. Participants with TG 150 ≥mg/dL had an OR of 2.44 compared to those with TG <150 gm/dL when comparing likelihood of arterial stiffness [33]. Higher TG/HDL-C ratios are associated with increased arterial stiffness, according to a cross-sectional study conducted in Guangdong, China, involving 1137 males who were receiving healthy checkups [34]. 14,071 hypertensive patients in the Chian Stroke Primary Prevention Trial were split into four groups according to their baPWV [35]. Even after correcting for age, sex, BMI, and other cardiovascular factors, they came to the conclusion that greater triglyceride levels were linked to arterial stiffness in Chinese patients with hypertension [35]. At Huashan Hospital, Shanghai, 1133 Chinese participants were recruited at a local health-check program from the downtown district [36]. Serum TG levels were found to have a positive relationship with baPWV values [36]. Serum triglycerides and PWV were found to have a positive correlation across all six cross-sectional investigations.
At Tongji Hospital, Shanghai, 659 healthy males had baseline lipid markers collected and were followed for 4.1 years [37]. PWV was measured with the brachial dash ankle pulse [34]. BaPWV ≥ 1400 cm/s was considered elevated [37]. 448 people in all had an average baPWV at baseline, and 100 of them had an increased baPWV by follow-up [37]. They concluded that elevated baseline TG and TG/HDL-C correlated with increased or persistently elevated baPWV as well as new incidents of baPWV [37]. A similar study conducted in Beijing with 1447 participants and 4.8 years of follow-up showed similar results [38]. In this study, PWV was measured with the carotid-radial and carotid-femoral pulses [38]. Lower triglyceride levels were found to be significantly associated with a reduction in the carotid-femoral PWV, but not the carotid-radial PWV [38]. This association was strengthened in participants older than 65 years [38].
4.2. Association between TyG index and PWV
An investigation conducted by Murakami Memorial Hospital in Japan enrolled 912 participants (592 men and 320 women) between March 2004 and December 2012 [39]. It included baseline demographics, basic lab chemistries, ABI, baPWV, abdominal ultrasonography, and lifestyle details [39]. TyG index was divided into three quartiles with an average of 8.3 ± 0.7 [39]. Model 1 was not adjusted, model 2 adjusted for sex and age, while Model 3 adjusted for sex, age, BMI, SBP, DBP, HDL-C, fatty liver, and eGFR [39]. Model 1 demonstrated that a one unit rise in TyG index conferred an 84 % greater risk of baPWV, Model 2 demonstrated an increased risk of 91 %, and Model 3 demonstrated an increased risk of 57 % [39]. These three models were all statistically significant [39]. Further subgroup analyses demonstrated a statistically significant linear relationship between TyG and baPWV among males, females, BMI <25 kg/m2, age <55 years old, total cholesterol <208 mg/dL, LDL-C < 120.1 mg/dL, eGFR ≥60 mL/min/1.73m2, smoking in the past or not at all, alcohol use of 0–40 g/week, exercise ≤1/week, and absence of fatty liver disease [39].
In a different study, the TyG index and other indicators of vascular damage, such as carotid-femoral pulse wave velocity (cfPWV) and baPWV, were measured in 2830 people [40]. The population was comprised of 44.5 % men and the mean age was 71.56 years [40]. Univariate logistic regression was used [40]. After adjusting for factors such as age, gender, BMI, waist circumference, smoking, hypertension, family history of CVD, diabetes, HDL-C, LDL-C, insulin use, and statin use, the findings revealed that an elevated TyG index was significantly associated with a higher risk of both cfPWV >10 m/s (OR = 1.86) and baPWV >1800 cm/s (OR = 1.39) [40]. Overall, this study concluded that TyG index was statistically significant (p=<0.05) to assess vascular damage [40].
A systematic review was performed that included 7 studies and a total of 30,241 participants to compare TyG index and arterial stiffness measured by baPWV [41]. Mean age across all studies was 55.5 years, 60.6 % of the studied population was male and the studies originated in China, Japan and South Korea [41]. The study revealed that patients in the highest TyG index category had significantly increased odds of arterial stiffness with odds ratio 1.96 [41]. This association was consistent in subgroup analyses involving females and males [41].
4.3. Association between HOMA and PWV
There are many studies regarding HOMA with variable outcomes. One Korean study in postmenopausal women showed a significantly positive incremental correlation between aortic PWV and HOMA values [42]. A small study of 45 healthy adult participants, 29 of them with first-degree relatives of diabetic patients, were randomly recruited from the clinic for lab measurements and cfPWV [43]. Those with first-degree relatives with diabetes had less insulin sensitivity and, therefore, higher HOMA [43]. PWV was found to be 5 % higher in this group than in the control patients [43]. Given the increased prevalence of childhood obesity, studies in children also exist. A community cross-sectional study with 573 children (mean age 10.1 years) was used to assess cfPWV and HOMA along with other cardiovascular measurements [44]. They also concluded that higher HOMA was positively correlated with increased cfPWV [44].
Of the insulin resistance markers reviewed, serum triglycerides consistently showed a positive correlation with arterial stiffness measured via PWV among adult patients of both sexes. While fewer studies were examined regarding PWV and TyG index, TyG was found to correlate with PWV in a large systematic review Table 3.
Table. 3.
Association between insulin resistance and PWV.
| Ref # | Study Design | Participant Characteristics | Marker of Insulin Resistance | Key Findings |
|---|---|---|---|---|
| [31] | Cross-sectional | 11,640 Japanese participants | Serum Triglycerides | in univariate analysis, high TG significantly associated with high baPWV in LDL-C < 79 mg/dL (OR 3.611, p < 0.0001) and 80–119 mg/dL (OR 1.881, p < 0.0001)). In multivariate analysis, there was also significant association in those with LDL-C ≤ 79 (OR 2.558, p = 0.0040) and 80–119 (OR 1.677, p < 0.0001)). |
| [32] | Cross-sectional | 909 Japanese participants, age 24–84, 54.4 % male | Serum Triglycerides | After adjustment, serum TG was the only lipid index correlated positively with baPWV. |
| [33] | Cross-sectional | 16,733 Chinese participants, 61.5 % male | Serum Triglycerides | Those with TG ≥150 mg/dl had an OR of 2.44 (1.61–3.71) compared to those with TG <150 mg/dl. |
| [34] | Cross-sectional | 1137 Chinese men, age 18–44 | Serum Triglycerides | Higher TG/HDL-C ratios are associated with elevated arterial stiffness (1.81 (1.22–2.62, p < 0.001). |
| [35] | Cross-sectional | 14,071 Chinese participants with hypertension | Serum Triglycerides | Higher levels of triglycerides were associated with arterial stiffness in Chinese patients with hypertension after adjusting for age, sex, BMI, and other cardiovascular risks. |
| [36] | Prospective Cohort | 1133 Chinese participants age 50–90, 38 % male | Serum Triglycerides | Serum TG levels were found to have a positive relationship with baPWV values. |
| [37] | Prospective Cohort | 659 healthy Chinese males, average 47.4 ± 10.7 years old, mean follow up 4.4 years | Serum Triglycerides | Baseline serum TG and TG/HDL-C ratio were associated with development of, increases in, and persistently high baPWV. |
| [38] | Prospective Cohort | 1447 Chinese participants, mean age 61.3 years, follow up 4.8 years | Serum Triglycerides | Lower triglyceride levels were significantly associated with decreases in carotid–femoral PWV without significance seen in carotid-radial PWV. |
| [39] | Cross-sectional | 912 Japanese participants, mean age 51.1 ± 9.6 years, 64.9 % male | TyG index | There was a linear relationship seen between TyG and baPWV after adjusting and within subgroup analyses. Within quartiles of TyG index, those in the third quartile had a 1.78 higher risk of baPWV compared to those in the first quartile. |
| [40] | Cross-sectional | 2830 participants, mean age 71.5 ± 6.2 years, 44.5 % male | TyG index | Elevated TyG index was significantly associated with a higher risk of elevated cfPWV >10 m/s and baPWV >1800 cm/s. |
| [41] | Systematic Review | 30,241 participants across 7 studies. Mean age 55.5 years. 60.6 % male. Studies from China, Japan, South Korea. | TyG index | Patients in highest TyG Index category had significantly increased odds of arterial stiffness measured via baPWV (OR: 1.96). Association consistent in male and female subgroups. |
| [42] | Cross-sectional | 455 Korean participants, postmenopausal women ≥50 years old | HOMA | Increases in HOMA were positively correlated with increased measurements of aortic PWV. |
| [43] | Cross-sectional | 45 healthy participants ages 18–42, 29 with first-degree relatives of diabetic patients | HOMA | 5 % more patients in the participants with diabetic first-degree relatives had elevated PWV. |
| [44] | Cross-sectional | 573 children, mean age 10.1 ± 0.3 years, 51 % boys | HOMA | Higher HOMA was associated with increased PWV. |
TG triglycerides, baPWV brachial-ankle pulse wave velocity, OR odds ratio, BMI body mass index, PWV pulse wave velocity, cfPWV carotid-femoral pulse wave velocity, HOMA homeostatic model assessment.
5. Conclusion
Insulin resistance can precipitate atherosclerotic changes in one's vasculature independent of hyperglycemia. Finding markers of insulin resistance that correlate with markers of atherosclerosis could help identify patients early in their disease course and allow for sooner initiation of preventative treatments.
Across the studies reviewed, TyG index showed a consistent positive correlation with CAC, while both serum triglycerides and TyG index showed consistent positive correlations with PWV. CIMT ultimately did not show consistent correlations with any of the insulin resistance markers. Of the insulin resistance markers reviewed, the TyG index appears to be most consistently associated with markers of atherosclerosis. TyG is admittedly not a perfect indicator of insulin resistance, as demonstrated in a recent systematic review which found only moderate-to-low quality evidence of TyG as a useful marker of insulin resistance [45]. However, a cross-sectional study found that the TyG index has high sensitivity and specificity when compared to the impractical euglycemic-clamp test, which is considered the gold standard for measuring insulin resistance [46]. Additionally, TyG's association with markers of atherosclerosis supports its potential clinical merit. The relationship between TyG and incidence of future cardiovascular mortality, myocardial infarction, stroke and T2DM was demonstrated in a recent prospective cohort study of 141,243 individuals across 22 countries with median follow up of 13.2 years [47]. This study found that compared to the lowest tertile of TyG index, individuals in the highest tertile of TyG index had significantly greater incidence of myocardial infarction with hazard ratio 1.24 [47]. Thus, higher TyG index may increase one's risk of atherosclerosis and thereby increase one's likelihood of having a cardiovascular event. It may even increase one's risk of a major cerebrovascular event, as demonstrated in a recent retrospective study that showed a significant positive association between TyG and 90-day mortality rates in patients with cerebrovascular disease [48].
Early identification of patients with elevated TyG index prior to development of clinically significant atherosclerosis or T2DM may allow for early initiation of treatment and decreased rates of progression to clinical disease. TyG can be easily calculated with routine labwork and has the potential to inform decisions regarding early initiation of therapies in patients who would otherwise not be treated. There is work yet to be done as there is no established cutoff point for these insulin resistance markers that can reliably predict atherosclerosis development. Additionally, measurement of fasted triglycerides will yield different results than non-fasted triglycerides given the relatively short half-life of triglycerides [49]. But these limitations can likely be overcome by further elucidating these relationships. Another contemporary review found evidence that the addition of the TyG index to established risk models could improve prediction of adverse cardiac events in patients with acute coronary syndrome [50]. Further research into these insulin resistance markers could yield significant findings regarding the prevention of atherosclerosis and thus the prevention of clinical atherosclerotic disease. Specifically targeting insulin sensitivity prior to the development of T2DM has the potential to reduce development and progression of atherosclerosis, and patients without T2DM but who have elevated TyG index should be the topic of further research.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Drake A. Scott: Writing – original draft, Investigation, Conceptualization. Cynthia Ponir: Writing – original draft, Investigation. Michael D. Shapiro: Writing – review & editing. Parag A. Chevli: Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Michael D. Shapiro reports a relationship with Amgen Inc that includes: consulting or advisory. Michael D. Shapiro reports a relationship with Novartis that includes: consulting or advisory. Michael D. Shapiro reports a relationship with Ionis Pharmaceuticals Inc that includes: consulting or advisory. Michael D. Shapiro reports a relationship with Novo Nordisk Inc that includes: consulting or advisory. Michael D. Shapiro reports a relationship with Regeneron Pharmaceuticals Inc that includes: consulting or advisory. Michael D. Shapiro reports a relationship with Kaneka Corporation that includes: consulting or advisory. Michael D. Shapiro reports a relationship with Aidoc that includes:. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None
References
- 1.Di Pino A., DeFronzo R.A. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–1467. doi: 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poznyak A., Grechko A.V., Poggio P., et al. The diabetes mellitus–atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashraf F., Ghouri K., Someshwar F.N.U., et al. Insulin resistance and coronary artery disease: untangling the web of Endocrine-Cardiac Connections. Cureus. 2023;15(12):e51066. doi: 10.7759/cureus.51066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razani B., Chakravarthy M., Semenkovich C. Insulin resistance and atherosclerosis. Endocrinol. Metabol. Clinics. 2008;37(3):603–621. doi: 10.1016/j.ecl.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohan J., Bhatti K., Tawney A., et al. Coronary Artery Calcification. StatPearls. 2023 [PubMed] [Google Scholar]
- 6.Neves P.O., Andrade J., Moncao H. Coronary artery calcium score: current status. Radiol Bras. 2017;50(3):182–189. doi: 10.1590/0100-3984.2015.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhrzadeh H., Sharifi F., Alizadeh M., et al. Relationship between insulin resistance and subclinical atherosclerosis in individuals with and without type 2 diabetes mellitus. J. Diabetes Metab. Disord. 2015;15:41. doi: 10.1186/s40200-016-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munakata M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr. Hypertens Rev. 2014;10(1):49–57. doi: 10.2174/157340211001141111160957. [DOI] [PubMed] [Google Scholar]
- 9.Valencia-Hernandez C.A., Lindbohm J.V., Shipley M.J., et al. Aortic pulse wave velocity as adjunct risk marker for assessing cardiovascular disease risk: a prospective study. Hypertension. 2022;79(4):836–843. doi: 10.1161/HYPERTENSIONAHA.121.17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J.S., Chiang H.Y., Wang Y.C., et al. Dyslipidemia and coronary artery calcium: from association to development of a risk-prediction nomogram. Nutr. Metab. Cardiovasc Dis. 2022;32(8):1944–1954. doi: 10.1016/j.numecd.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Patel J., Pallazola V.A., Dudum R., et al. Assessment of coronary artery calcium scoring to guide statin therapy allocation according to risk-enhancing factors. JAMA Cardiol. 2021;6(10):1161–1170. doi: 10.1001/jamacardio.2021.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roubin S.R., Rossello X., Oliva B., et al. Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. 2021;77(24):3031–3041. doi: 10.1016/j.jacc.2021.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park K., Ahn C.W., Lee S.B., et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 14.Gurbuz D.C., Varis E. Correlation between coronary artery calcium score and triglyceride-glucose index in post-menopausal women. Cureus. 2023;15(5):e39034. doi: 10.7759/cureus.39034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F., Ling Q., Xie S., et al. Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: a systematic review and exposure-effect meta-analysis. Cardiovasc Diabetol. 2023;22(1):111. doi: 10.1186/s12933-023-01819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won K.B., Park E.J., Han D., et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19:34. doi: 10.1186/s12933-020-01008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke Z., Huang R., Xu X., et al. Long-term high level of insulin resistance is associated with an increased prevalence of coronary artery calcification: the CARDIA study. JAHA. 2023;12 doi: 10.1161/JAHA.122.028985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaha M.J., DeFilippis A.P., Rivera J.J., et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification. Diabetes Care. 2011;34(3):749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rifai M.A., Kanaya A.M., Kandula N.R., et al. Association of coronary artery calcium density and volume with predicted atherosclerotic cardiovascular disease risk and cardiometabolic risk factors in south Asians. Curr Probl Cardiol. 2023;48(4) doi: 10.1016/j.cpcardiol.2022.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazoe M., Hisamatsu T., Miura K., et al. Relationship of insulin resistance to prevalence of and progression of coronary artery calcification beyond metabolic syndrome components. Arterioscler Thromb Vasc. Biol. 2016;36:1703–1708. doi: 10.1161/ATVBAHA.116.307612. [DOI] [PubMed] [Google Scholar]
- 21.Sharma K., Blaha M.J., Blumenthal R.S., et al. Clinical research application of carotid intima-media thickness. Am J Cardiol. 2009;103(9):1316–1320. doi: 10.1016/j.amjcard.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y.P., Hsu Y.C., Tsai K.Z., et al. Insulin resistance indices and carotid intima-media thickness in physically fit adults: CHIEF atherosclerosis study. Endocr. Metab. Immune. Disord. Drug. Targets. 2023;23 doi: 10.2174/1871530323666230324104737. [DOI] [PubMed] [Google Scholar]
- 23.Labreuche J., Deplanque D., Touboul P.J., et al. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9–15. doi: 10.1016/j.atherosclerosis.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Lin G.M., Liu P.Y., Tsai K.Z., et al. Cardiorespiratory fitness and carotid intima-media thickness in physically active young adults: CHIEF atherosclerosis study. J Clin Med. 2022;11(13):3653. doi: 10.3390/jcm11133653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao M., Zhou G., Bao A., et al. Triglyceride-glucose index and common carotid artery intima-media thickness in patients with ischemic stroke. Cardiovasc Diabetol. 2022;21:43. doi: 10.1186/s12933-022-01472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia X., Zhu Y., Qi Y., et al. Association between triglyceride glucose index and carotid intima-media thickness in obese and nonobese adults. J. Diabetes. 2022;14(9):596–605. doi: 10.1111/1753-0407.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inan O., Sahiner E.S., Ates I. The role of triglyceride-glucose index in determining subclinical atherosclerosis in patients with primary hypertension. Eur Rev Med Pharmacol Sci. 2022;26:7125–7134. doi: 10.26355/eurrev_202210_29898. [DOI] [PubMed] [Google Scholar]
- 28.Santos I.S., Bittencourt M.S., Goulart A.C., et al. Insulin resistance is associated with carotid intima-media thickness in non-diabetic subjects. A cross sectional analysis of the ELSA-Brasil cohort baseline. Atherosclerosis. 2017;260:34–40. doi: 10.1016/j.atherosclerosis.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Agbaje A.O., Zachariah J.P., Bamsa O., et al. Cumulative hyperinsulinemia, hyperglycemia and insulin resistance from mid-adolescence through young adulthood with carotid intima-media thickness progression: a 9-year longitudinal study. Eur Heart J. 2022;43:2. [Google Scholar]
- 30.Bertoni A.G., Wong N.D., Shea S., et al. Insulin resistance, metabolic syndrome and subclinical atherosclerosis: the multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2007;30(11):2951–2956. doi: 10.2337/dc07-1042. [DOI] [PubMed] [Google Scholar]
- 31.Kawasoe S., Ide K., Usui T., et al. Assocation of serum triglycerides with arterial stiffness in subjects with low levels of low-density lipoprotein cholesterol. Circ J. 2018;82(18):3052–3057. doi: 10.1253/circj.CJ-18-0607. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Zhi F., Gao B., et al. Association between lipid profiles and arterial stiffness: a secondary analysis based on a cross-sectional study. J Int Med Res. 2020;48(7) doi: 10.1177/0300060520938188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen J., Huang Y., Lu Y., et al. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens Res. 2019;42(8):1223–1230. doi: 10.1038/s41440-019-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen J., Zhong Y., Kuang C., et al. Lipoprotein ratios are better than conventional lipid parameters in predicting arterial stiffness in young men. J Clin Hypertens. 2017;19(8):771–776. doi: 10.1111/jch.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan B., Huang X., Wang J., et al. Association between lipid profiles and arterial stiffness in Chinese patients with hypertension: insights from the CSPPT. Angiology. 2019;70(6):515–522. doi: 10.1177/0003319718823341. [DOI] [PubMed] [Google Scholar]
- 36.Zhao W., Gong W., Wu N., et al. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese. Lipids Health Dis. 2014;13:37. doi: 10.1186/1476-511X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sang Y., Cao M., Wu X., et al. Use of lipid parameters to identify apparently healthy men at high risk of arterial stiffness progression. BMC Cardiovasc Disord. 2021;21:34. doi: 10.1186/s12872-020-01846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Ye P., Cao R., et al. Triglycerides are a predictive factor for arterial stiffness: a community-based 4.8-year prospective study. Lipids Health Dis. 2016;15:97. doi: 10.1186/s12944-016-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X., Gao Z., Huang X., et al. The correlation of atherosclerosis and triglyceride glucose index: a secondary analysis of a national cross-sectional study of Japanese. BMC Cardiovasc Disord. 2022;22(1):250. doi: 10.1186/s12872-022-02685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sajdeya O., Beran A., Mhanna M., et al. Triglyceride glucose index for the prediction of subclinical atherosclerosis and arterial stiffness: a meta-analysis of 37,780 individuals. Curr Probl Cardiol. 2022;47(12) doi: 10.1016/j.cpcardiol.2022.101390. [DOI] [PubMed] [Google Scholar]
- 41.Zhao S., Yu S., Chi C., et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the northern shanghai study. Cardiovasc Diabetol. 2019;18(1):95. doi: 10.1186/s12933-019-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J.S., Nam J.S., Cho M.H., et al. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause. 2010;17(4):779–784. doi: 10.1097/gme.0b013e3181cd3d60. [DOI] [PubMed] [Google Scholar]
- 43.Scuteri A., Tesauro M., Rizza S., et al. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18(5):349–356. doi: 10.1016/j.numecd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Sakuragi S., Abhayaratna K., Gravenmaker J., et al. Influence of adiposity and physical activity on arterial stiffness in healthy children. Hypertension. 2009;53(4):611–616. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 45.Garcia A., Gutierrez R., Adame L., et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. 2020;2020 doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerrero-Romero F., Simental-Mendia L.E., Gonzales-Ortiz M., et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 47.Jaramillo P., Arbelaez D., Bello D., et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Heatlhy Longev. 2023;4(1):e23–e33. doi: 10.1016/S2666-7568(22)00247-1. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y.A., Chen P., Zhao Y.Y., et al. Association between triglyceride glucose index and all-cause mortality in patients with cerebrovascular disease: a retrospective study. Diabetol Metab Syndr. 2024;16:1. doi: 10.1186/s13098-023-01243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keirns B., Sciarrillo C., Koemel N., et al. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J Nutr Sci. 2021;10:e75. doi: 10.1017/jns.2021.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao L.C., Xu J.N., Wang T.T., et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]