Abstract
Purpose
To elucidate the clinical characteristics and progression rates of pachychoroid and conventional geographic atrophy (GA).
Design
Retrospective, multicenter, observational study.
Participants
A total of 173 eyes from 173 patients (38 eyes with pachychoroid GA and 135 with conventional GA) from 6 university hospitals in Japan were included. All patients were Japanese, aged ≥50 years and with fundus autofluorescence images having analyzable image quality. A total of 101 eyes (22 with pachychoroid GA and 79 with conventional GA) were included in the follow-up group.
Methods
The studied eyes were classified as having pachychoroid or conventional GA; the former was diagnosed if the eye had features of pachychoroid and no drusen. The GA area was semiautomatically measured on fundus autofluorescence images, and the GA progression rate was calculated for the follow-up group. Multivariable linear regression analysis was used to determine whether the rate of GA progression was associated with GA subtype.
Main Outcome Measures
Clinical characteristics and progression rates of pachychoroid and conventional GA.
Results
The pachychoroid GA group was significantly younger (70.3 vs. 78.7 years; P < 0.001), more male-dominant (89.5 vs. 55.6%; P < 0.001), and had better best-corrected visual acuity (0.15 vs. 0.40 in logarithm of the minimum angle of resolution; P = 0.002), thicker choroid (312.4 vs. 161.6 μm; P < 0.001), higher rate of unifocal GA type (94.7 vs. 49.6%; P < 0.001), and smaller GA area (0.59 vs. 3.76 mm2;P < 0.001) than the conventional GA group. In the follow-up group, the mean GA progression rate (square-root transformation) was significantly lower in the pachychoroid GA group than in the conventional GA group (0.11 vs. 0.27 mm/year; P < 0.001).
Conclusions
Demographic and ocular characteristics differed between GA subtypes. The progression rate of pachychoroid GA, adjusted for age and baseline GA area, was significantly lower than that of conventional GA. Japanese patients with conventional GA showed characteristics and progression rates similar to those in White populations. Some characteristics of GA in Japanese population differ from those in Waucasian populations, which may be due to the inclusion of pachychoroid GA.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Age-related macular degeneration, Geographic atrophy, Japanese, Pachychoroid geographic atrophy, Subtype
Geographic atrophy (GA) is recognized as a leading cause of visual impairment in elderly people in developed countries.1 In recent years, evidence regarding on its prevalence, risk factors, and clinical characteristics in White populations has been growing. Moreover, in these populations, reticular pseudodrusen, banded or diffuse trickling fundus autofluorescence (FAF) pattern, multifocal GA, noncentral GA, and bilateral GA have been reported as markers of rapid progression.2 Ethnic differences have been suggested in the clinical features of GA, especially, its low prevalence in Asian populations.3 However, it remains unclear whether the GA progression rate and risk factors for progression in White populations are also applicable to Asian populations.
Geographic atrophy affects approximately 5 million people worldwide.4 Currently, its prevalence remains low in Asian countries; however, with the rapid aging of the population, effective treatment for GA is becoming an urgent medical need. Numerous previous studies have examined potential treatments for GA, and a series of suitable treatments have been recently approved in the United States.5,6 Nevertheless, previous clinical trials have not considered the impact of GA subtypes, and it has not been discussed whether ethnic differences should be considered in the application of these treatment methods.
Our recent study examined 173 Japanese patients with GA, showing differences between the Japanese and White population samples.7 We revealed that Japanese patients with GA were male dominant and had small lesions, thick choroids, and slow GA progression rates. Moreover, some patients presented with GA without drusen but with features of pachychoroid, known as pachychoroid GA. Although Asian population sometimes presents with pachychoroid GA, which differs from drusen-related conventional GA, the characteristics of these 2 subtypes remain unclear.
In this study, we further examined a Japanese cohort with GA to elucidate whether pachychoroid GA can account for the unique clinical characteristics of Asian GA and clarify whether drusen-related conventional GA has the same features as GA in White populations.
Methods
Ethics Statement
This study adhered to the principles of the Declaration of Helsinki and was approved by the institutional review board and Ethics Committee of Kyoto University Graduate School of Medicine. The requirement for written informed consent was waived because this was a retrospective study and only anonymized data were used for analysis.
Participants
The design of this retrospective multicenter study is described elsewhere.7 Briefly, consecutive patients diagnosed with GA at 6 university hospitals in Japan (Kyoto University Hospital, Tokyo Women’s Medical University Hospital, University of the Ryukyus Hospital, Osaka University Hospital, Yokohama City University Medical Center, and Kansai Medical University Hospital) between January 2009 and December 2021 were enrolled. The inclusion criteria were Japanese ethnicity, age ≥50 years, and definite GA in at least 1 eye. The diagnosis of GA was based on the diagnostic criteria for GA in the Japanese people, described in detail elsewhere.8,9 Briefly, GA diagnosis required the following ocular findings: (1) at least 250 μm in diameter, (2) round/oval/cluster-like or geographic in shape, (3) sharp delineation, (4) hypopigmentation or depigmentation in retinal pigment epithelium, and (5) clear visualization of large and medium choroidal vessels, without any history of inherited diseases, high myopia, chronic central serous chorioretinopathy (CSC), traumatic injury, retinal epithelial tear, history of laser photocoagulation, or macular neovascularization. Macular dystrophies and genetic causes of macular atrophy were excluded based on clinical presentation such as age of onset, symmetry of the right and left eyes and findings of FAF (such as flecks in Stargardt disease). In the cases where inherited diseases were suspected, electroretinography and genetic tests, if possible, were performed. Otherwise, suspicious cases were excluded. Eyes without FAF images and those with poor image quality were excluded. If both eyes met the inclusion criteria, only the right eye was included in the study. Smoking status was confirmed using medical records.
Eyes with FAF images acquired at an interval of more than 6 months were included in the follow-up group. The dates of the first and last FAF assessments were defined as the baseline and final visits, respectively.
Multimodal Imaging Methods
All patients underwent a comprehensive ophthalmologic examination, which included assessments such as best-corrected visual acuity (BCVA), slit-lamp biomicroscopy using a noncontact lens, axial length, FAF, color fundus photography (CFP) (TRC50DX, Topcon, Tokyo, Japan; TRCNW6S, Topcon; TRCNW8F, Topcon), and either spectral-domain (SD) OCT (OCT3000, Carl Zeiss; HRA, Heidelberg Engineering; Avanti, Optovue.) or swept-source (SS) OCT (Atlantis, Topcon; Triton, Topcon). Fundus autofluorescence images were obtained using a confocal scanning laser ophthalmoscope (HRA Heidelberg Engineering) with a 30° × 30° field of view centered on the fovea. The SD/SS-OCT images included horizontal and vertical line scans through the foveal center. Enhanced depth imaging OCT10 scans were also taken.
Image Analysis
Image analysis details are described elswhere.7 Briefly, drusen were diagnosed based on CFP and SD/SS-OCT, and reticular pseudodrusen were diagnosed based on multimodal imaging findings, including CFP, SD/SS-OCT, and FAF. Central macular thickness and subfoveal choroidal thickness (SFCT) were measured manually on SD/SS-OCT images obtained by the vertical and horizontal scans through the foveal center, and the values were averaged. The SFCT was measured using either SS or Enhanced depth imaging -OCT.
The GA location (central or non-central), GA pattern (unifocal or multifocal), and measurements of central macular thickness and SFCT were performed at each research institute. All CFP, FAF, and SD/SS-OCT images were gathered at a single institute (Kyoto University Hospital), and the study eyes were classified as having conventional or pachychoroid GA. Pachychoroid GA was diagnosed when the following conditions were fulfilled: (1) clinical and anatomical features of the pachychoroid phenotype were identified, including reduced fundus tessellation on CFP and dilated outer choroidal vessels on OCT and (2) no drusen were observed (Fig 1).11 The classification of GA was independently performed by 2 retinal specialists (Y.S. and N.UA.). In cases of disagreement, a third retinal specialist (A.Takahashi) was consulted. The areas of GA were measured on FAF images by a single assessor (Y.S.) using the Region Finder software, version 1.10.2.0 (Heidelberg Engineering). Reproducibility was confirmed by another assessor, as previously reported.7
Figure 1.
Multimodal imaging of pachychoroid GA in a 63-year-old man (A–C), conventional GA in a 74-year-old man (D–F) and bilateral pachychoroid GA in an 81-year-old man (G–L). A, D, G, J, Color fundus photography. B, E, H, K, Fundus autofluorescence images. C, I, Enhanced depth imaging OCT. F, L, Spectral domain OCT. Yellow arrows indicate the scan lines of OCT images, that show complete retinal pigment epithelium and outer retinal atrophy. Dilated outer choroidal vessels are seen in pachychoroid GA cases (C, I and L). Subfoveal choroidal thickness is 370 (C), 84 (F), 259 (I), and 287 μm (L), respectively. GA = geographic atrophy.
The GA progression rate was calculated as the difference in the GA area between the baseline and final visit on FAF images, divided by follow-up duration (mm2/year). The square root transformation (SQRT) method12 was used to analyze the GA progression rate to eliminate the dependence of GA progression rate on the baseline GA area (mm/year).
Statistical Analysis
Statistical analysis was performed using JMP software (version 16.2; SAS Institute, Inc). All values are presented as the mean ± standard deviation or counts. BCVA was assessed using a Landolt chart and converted to the logarithm of the minimum angle of resolution (logMAR) for the statistical analysis. Continuous variables were compared using the Mann–Whitney U test. Each n × n table was evaluated using the Fisher exact test. Multivariable linear regression analyses were used to assess the association between the GA subtypes and the rate of GA progression (SQRT). P values of <0.05 were considered statistically significant.
Results
Of 173 study eyes, 38 (22.0%) and 135 (78.0%) were classified as pachychoroid GA and conventional GA, respectively. Table 1 shows the demographic and ocular characteristics of the patients in both groups. The pachychoroid GA group was significantly younger (70.3 vs. 78.7 years, P < 0.001), more male dominant (89.5 vs. 55.6%; P<0.001), and had a higher prevalence of smokers (current or past; 89.7 vs. 52.5%; P < 0.001). Moreover, the pachychoroid GA group demonstrated better BCVA (0.15 vs. 0.40 in logMAR, 20/28 vs. 20/50 in Snellen equivalent; P = 0.002), thicker choroid (312.4 vs. 161.6 μm; P < 0.001), and smaller GA size (0.59 [median: 0.28; range: 0.05–2.81] vs. 3.76 [median: 2.41; range: 0.05–24.88] mm2; P < 0.001) compared with the conventional GA group. None of the eyes in the pachychoroid GA group had drusen or reticular pseudodrusen, whereas 115 (85.2%) eyes had drusen and 73 (54.1%) eyes had reticular pseudodrusen in the conventional GA group. Unifocal GA was more prevalent in the pachychoroid GA group than in the conventional GA group (94.7 vs. 49.6%; P < 0.001). A total of 61 (46.2%) patients in the conventional GA group had bilateral GA, whereas only 1 (2.6%) patient in the pachychoroid GA group did (P < 0.001). Late age-related macular degeneration was observed in the fellow eye of 9 (23.7%) eyes in the pachychoroid GA group and in that of 106 (80.3%) eyes in the conventional GA group (P < 0.001).
Table 1.
Demographic and Ocular Characteristics of Pachychoroid and Conventional GA Groups
| Characteristics | Pachychoroid GA | Conventional GA | P Value |
|---|---|---|---|
| Patients, n | 38 | 135 | |
| Age (median, range), years | 70.3 ± 7.5 (70, 56–86) | 78.7 ± 8.3 (80, 53–97) | <0.001 |
| Sex, n, males (%) | 34 (89.5) | 75 (55.6) | <0.001 |
| Smoking status, n, current or former (%); n = 151 | 26 (89.7) | 64 (52.5) | <0.001 |
| Axial length (median), mm; n = 123 | 23.4 ± 0.9 (23.3) | 23.5 ± 0.9 (23.5) | 0.752 |
| BCVA, logMAR (Snellen equivalent) | 0.15 ± 0.27 (20/28) | 0.40 ± 0.45 (20/50) | 0.002 |
| CMT (median, range), μm | 143.6 ± 63.8 (149.5, 7.0–242.0) | 145.3 ± 74.5 (151, 6.0–617.0) | 0.283∗ |
| SFCT (median, range), μm | 312.4 ± 116.0 (322, 140.5–622.0) | 161.6 ± 74.4 (153, 36.5–414.0) | <0.001† |
| GA area (median, range), mm2 | 0.59 ± 0.76 (0.28, 0.05–2.81) | 3.76 ± 4.26 (2.41, 0.05–24.88) | <0.001 |
| GA area (SQRT) (median, range), mm | 0.66 ± 0.40 (0.52, 0.23–1.68) | 1.66 ± 1.01 (1.55, 0.23–4.99) | <0.001 |
| GA location, central/non-central | 16/22 | 74/61 | 0.199 |
| GA type, unifocal/multifocal | 36/2 | 67/68 | <0.001 |
| Drusen, n, present (%) | 0 (0) | 115 (85.2) | <0.001 |
| Reticular pseudodrusen, n, present (%) | 0 (0) | 73 (54.1) | <0.001 |
| Fellow eye status, GA/neovascular AMD/intermediate AMD/no AMD‡ | 1/8/0/29 | 61/45/22/4 | <0.001 |
AMD = age-related macular degeneration; BCVA = best-corrected visual acuity; CMT = central macular thickness; GA = geographic atrophy; logMAR = logarithm of the minimum angle of resolution; n = number of patients; SFCT = subfoveal choroidal thickness; SQRT = square-root transformation.
Age-adjusted.
Age and sex-adjusted.
Three patients lacked information on their fellow eyes due to phthisis.
Table 2 shows the demographic and ocular characteristics of 22 eyes with pachychoroid GA and 79 eyes with conventional GA in the follow-up group. These characteristics of each group were similar to those of the entire study population. During the follow-up period (mean: 57.0 ± 32.4 [median: 56.5] and 43.2 ± 27.2 [median: 38.0] months, respectively), the mean BCVA in logMAR changed from 0.19 to 0.26 (Snellen equivalent, from 20/31 to 20/36) in the pachychoroid GA group and from 0.39 to 0.55 (Snellen equivalent, from 20/49 to 20/71) in the conventional GA group. The mean GA area increased from 0.65 ± 0.88 (median: 0.26) to 1.91 ± 2.05 (median: 1.10) mm2 in the pachychoroid GA group and from 3.36 ± 3.61 (median: 2.29) to 7.24 ± 7.15 (median: 5.47) mm2 in the conventional GA group (Fig 2). The mean GA progression rate (SQRT) was significantly lower in the pachychoroid GA group than in the conventional GA group (0.11 ± 0.07 [median: 0.09] vs. 0.27 ± 0.18 [median: 0.25] mm/year, P < 0.001). There was no significant difference in the SFCT thinning rate between the 2 groups (6.5 vs. 4.1 μm/year; P = 0.093).
Table 2.
Demographic and Ocular Characteristics at Baseline and the Final Visit in the Follow-Up Group
| Characteristics | Pachychoroid GA |
Conventional GA |
P value∗ |
|||
|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | |
| Patients, n | 22 | 79 | ||||
| Follow-up period (median, range), month | 57.0 ± 32.4 (56.5, 6–123) | 43.2 ± 27.2 (38.0, 6–110) | 0.078 | |||
| Age (median, range), years | 71.2 ± 8.7 (70.5, 56–86) | 76.1 ± 7.6 (76, 63–90) | 78.1 ± 8.2 (79, 53–94) | 81.7 ± 7.9 (83, 58–95) | 0.002 | 0.002 |
| Sex, n, males (%) | 20 (90.9) | 43 (54.4) | 0.002 | |||
| Smoking status, n, current or former (%); n = 90 | 16 (84.2) | 34 (47.9) | 0.005 | |||
| Axial length (median), mm; n = 80 | 23.4 ± 1.0 (23.3) | 23.5 ± 0.9 (23.6) | 0.447 | |||
| BCVA, logMAR (Snellen equivalent) | 0.19 ± 0.30 (20/31) | 0.26 ± 0.31 (20/36) | 0.39 ± 0.46 (20/49) | 0.55 ± 0.53 (20/71) | 0.086 | 0.026 |
| CMT (median, range), μm | 146.5 ± 60.2 (152.5, 17.0–224.0) | 121.1 ± 74.2 (146, 9.0–227.0) | 148.5 ± 84.8 (151, 6.0–617.0) | 116.5 ± 71.4 (105, 6.0–285.0) | 0.072† | 0.021† |
| SFCT (median, range), μm | 325.0 ± 125.2 (324, 140.5–622.0) | 287.7 ± 109.7 (292, 110.5–571.0) | 164.4 ± 78.5 (155, 36.5–414.0) | 150.1 ± 77.5 (143, 15.5–414.0) | <0.001‡ | <0.001‡ |
| CMT thinning (median, range), μm/year | 4.7 ± 8.2 (2.6, −8.0 to 21.8) | 13.6 ± 27.6 (5.1, −12.5 to 162.4) | 0.097 | |||
| SFCT thinning (median, range), μm/year | 6.5 ± 16.1 (5.4, −50.4 to 32.0) | 4.1 ± 15.3 (3.4, −62 to 58.2) | 0.093 | |||
| GA area (median, range), mm2 | 0.65 ± 0.88 (0.26, 0.07–2.81) | 1.91 ± 2.05 (1.10, 0.15–6.75) | 3.36 ± 3.61 (2.29, 0.05–16.16) | 7.24 ± 7.15 (5.47, 0.16–37.10) | <0.001 | <0.001 |
| GA area [SQRT] (median, range), mm | 0.68 ± 0.44 (0.50, 0.26–1.68) | 1.20 ± 0.70 (1.05, 0.38–2.60) | 1.56 ± 0.96 (1.51, 0.23–4.02) | 2.38 ± 1.26 (2.34, 0.40–6.09) | <0.001 | <0.001 |
| GA progression rate (median, range), mm2/year | 0.23 ± 0.25 (0.12, 0.02–1.08) | 1.22 ± 1.13 (0.81, 0.02–4.59) | <0.001 | |||
| GA progression rate [SQRT] (median, range), mm/year | 0.11 ± 0.07 (0.09, 0.02–0.34) | 0.27 ± 0.18 (0.25, 0.01–0.70) | <0.001 | |||
| GA location, central/non-central | 8/14 | 11/11 | 43/36 | 55/24 | 0.154 | 0.127 |
| GA type, unifocal/multifocal | 21/1 | 21/1 | 42/37 | 38/41 | <0.001 | <0.001 |
| Drusen, n, present (%) | 0 (0) | 67 (84.8) | <0.001 | |||
| Reticular pseudodrusen, n, present (%) | 0 (0) | 43 (54.4) | <0.001 | |||
| MNV development, n§ | 1 | 5 | 1.000 | |||
| Fellow eye status, GA/neovascular AMD/intermediate AMD/no AMD; n = 100‖ | 1/6/0/15 | 3/6/0/13 | 30/31/15/2 | 34/33/9/2 | <0.001 | <0.001 |
AMD = age-related macular degeneration; BCVA = best-corrected visual acuity; CMT = central macular thickness; GA = geographic atrophy; logMAR = logarithm of the minimum angle of resolution; MNV = macular neovascularization; n = number of patients; SFCT = subfoveal choroidal thickness; SQRT = square-root transformation.
Pachychoroid GA vs conventional GA.
Age-adjusted.
Age and sex-adjusted.
The subsequent period was not included in the follow-up analysis when macular neovascularization developed during the course of the disease.
In 1 patient, information on the fellow eye was lacking due to phthisis.
Figure 2.
Progression of pachychoroid GA in a 70-year-old man with 73 months’ follow-up (A, C and E), and an 86-year-old man with 42 months’ follow-up (B, D and F), alongside that of conventional GA in an 83-year-old man with 92 months’ follow-up (G, I) and an 87-year-old woman with 73 months’ follow-up (H, J). A, B, G, H, Fundus autofluorescence images. Yellow arrows indicate the scan lines of OCT on panels C, D, I and J. Yellow dotted arrows indicate the scan lines of OCT through the GA lesion on panels E and F. Enhanced depth imaging OCT shows dilated outer choroidal vessels (C, D). Spectral-domain OCT images demonstrate complete outer retinal atrophy (E, F, I and J). In all cases, the GA area increased from baseline to the final visit. The GA progression rate (square-root transformation; SQRT) was 0.07 (A), 0.07 (B), 0.16 (G), and 0.33 mm per year (H), respectively. GA = geographic atrophy.
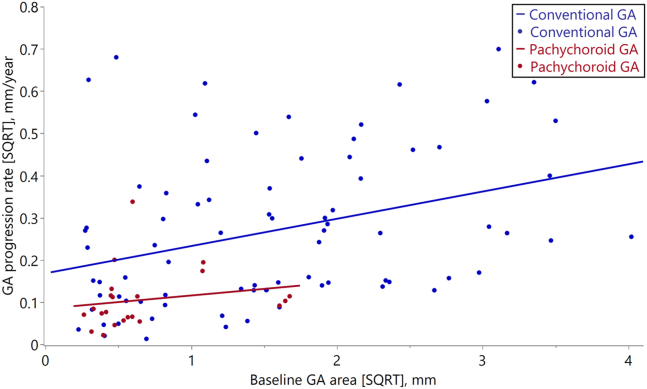
Figure 3 presents a scatter plot showing the association between the baseline GA area (SQRT) and GA progression rate (SQRT) in the follow-up group. The baseline GA area (SQRT) in the conventional GA group showed a relatively wide range, whereas in the pachychoroid GA group, it was small in most cases. Multivariable linear regression analysis adjusted for baseline GA area (SQRT), and age showed that GA progression rate (SQRT) was significantly associated with GA subtypes (P = 0.034).
Figure 3.
Scatter plot showing the association between baseline GA area (SQRT) and GA progression rate (SQRT) in the follow-up group. The red dots show the cases of pachychoroid GA; the blue dots show the cases of conventional GA. The straight lines are the regression lines of the 2 groups (pachychoroid GA group; Y = 0.085 + 0.031 × X, conventional GA group, Y = 0.169 + 0.065 × X). GA = geographic atrophy; sqrt = square-root transformation.
Discussion
In this study, we elucidated the different clinical characteristics of 2 GA subtypes in a Japanese population. Takahashi et al previously investigated pachychoroid GA in relatively younger Asian populations and showed that it is characterized by features such as a thick choroid, choroidal vascular hyperpermeability, small lesions, absence of drusen, and slow progression.11 Within our current Japanese cohort, 38 (22.0%) patients were classified into the pachychoroid GA group. Notably, these patients were significantly younger and predominantly male and had better BCVA, a thicker choroid, a higher prevalence of unifocal-type GA, smaller sized GA lesions, and a slower progression rate than those with conventional GA. The present findings are consistent with those of the previous study.11 In our recent report, the GA progression rate was significantly associated with the baseline GA area, even when using the SQRT method.7 In the present study, multivariable linear regression analysis adjusted for baseline GA area (SQRT), and age showed that the progression rate of pachychoroid GA was significantly lower than that of the conventional GA (regression coefficient, 4.7 × 10−2; 95% confidence interval, 3.7×10−3–9.0 × 10−2; P = 0.034). This is the first study to demonstrate that GA progression rates were significantly different between pachychoroid and conventional GA correcting for the baseline GA area.
The clinical characteristics of GA in Asian populations remain poorly understood. Teo et al compared the clinical characteristics of GA between Asian and non-Asian populations,13 showing a slower GA progression rate (SQRT) in Asian populations than in non-Asian populations (0.2 vs. 0.4 mm/year measured with near-infrared imaging; P < 0.01; 0.1 vs. 0.3 mm/year measured on FAF; P < 0.01). The higher GA progression rate in Asian populations was associated with the presence of drusen and a large baseline GA area. Although the study did not account for GA subtypes (pachychoroid and conventional GA), pachychoroid GA may have been involved in the slow progression rate observed in the Asian cohort. In Asian populations, the pachychoroid is common, partially leading to various conditions such as polypoidal choroidal vasculopathy, pachychoroid neovascularization, or pachychoroid GA.14 The high prevalence of pachychoroid GA could help explain the slow GA progression rate in Asian populations.
In our cohort, the characteristics of conventional GA were more similar to those reported in White populations. This includes age (78.7 vs. 69.7–83.0 years),15,16 bilateral GA rate (46.2 vs. 23.0%–67.4%),17,18 multifocality rate (50.4 vs. 23.0%–77.2%),19,20 and reticular pseudodrusen prevalence (54.1 vs. 36%–38%)21,22 In addition, conventional GA of a size consistent with that defined by clinical trial eligibility criteria showed GA progression rate (SQRT) (0.33 mm/year) similar to that of the sham group in these trials (0.35–0.40 mm/year,5,6 Table 3). Our previous report showed that some characteristics of GA in a Japanese population differ from those in White populations.7 However, except for pachychoroid GA, drusen-related conventional GA in Asian populations presents with characteristics similar to those observed in White populations.
Table 3.
Demographic, Ocular Characteristics and GA Progression Rates of Patients With Conventional GA > 2.5 mm2 at Baseline in the Current Study and Previous Studies With White Samples
| Characteristics | Current Study | Liao et al 20205 | Jaffe et al 20216 |
|---|---|---|---|
| Patients, n, (eyes) | 35 (35) | 81 (81) | 110 (110) |
| Ethnicity | Japanese (100%) | White (100%) | White (97.3%) |
| Follow-up period, months | 42.8 | 12 | 12 |
| Age, years | 76.5 | 78.4 | 78.2 |
| Sex, %, males | 57.1 | 39.5 | 28.2 |
| Baseline GA area eligibility threshold | >2.5 mm2 and <17.5 mm2 | >2.5 mm2 and <17.5 mm2 | >2.5 mm2 and <17.5 mm2 |
| Baseline GA area, mm2 | 6.43 | 8.2 | 7.42 |
| Baseline GA area [SQRT], mm | 2.46 | 2.8 | 2.63 |
| GA progression rate, mm2/year | 1.96 | 2.12 | NA |
| GA progression rate [SQRT], mm/year | 0.33 | 0.35 | 0.40 |
GA = geographic atrophy; n = number of patients; SQRT = square-root transformation.
The pathogenesis of drusen-related GA is considered to be closely related to chronic inflammation via the complement pathway.23,24 Recently, complement C3 and C5 inhibitors have been approved for the treatment of GA.5,6 However, the pathogenesis of pachychoroid GA remains unclear. In a previous study, the patients with pachychoroid GA were found to be less likely to present with a risk allele in ARMS2 A69S compared to those with conventional GA, whereas the frequencies of risk allele in either CFH I62V or CFH Y402H were comparable.11 It is currently unknown whether complement factor inhibitors are effective against pachychoroid GA. Furthermore, given its slow progression rate, including pachychoroid GA in clinical trials may lower the margin to determine treatment efficacy. Conventional drusen-related GA in Asian populations, whose characteristics are comparable to those of GA in White populations, could be included in the clinical trials of complement factor inhibitors or treatment targeting other pathways.
Recent studies have reported on an acquired vitelliform lesion associated with pachychoroid disease, termed pachyvitelliform maculopathy.25,26 Hilely et al reported that 41% of pachyvitelliform maculopathy cases developed macular atrophy and suggested that inner choroidal or choriocapillaris ischemia might cause impairment and dysfunction of the retinal pigment epithelium, leading to retinal pigment epithelium atrophy.25 This proposed pathway could be a contributing factor for the development of pachychoroid GA.
In clinical practice, White patients may present with pachychoroid spectrum diseases, such as polypoidal choroidal vasculopathy or CSC. Although pachychoroid GA can also develop in White populations, the incidence of this disease is likely to differ from that in Asian populations. Consequently, clinical trials investigating treatments for GA may benefit from including GA subtypes in their eligibility criteria.
The current study has several limitations. First, this was a retrospective study, and some data were unavailable. Second, the current diagnostic criteria for pachychoroid GA continue to evolve. Choroidal thickness varies with age and axial length,27 which may need to be considered at the time of diagnosis. In addition, it is often difficult to distinguish between inherited diseases such as Stargardt disease and pachychoroid GA. In this study, patients with macular atrophy for whom inherited diseases could not be ruled out were excluded. Third, we excluded patients with a history of chronic CSC to distinguish focal atrophy due to CSC. However, determining such a history of CSC is often difficult because the condition may present subclinically. Fourth, the baseline GA area in most patients with pachychoroid GA in our cohort was small, and information on large-sized pachychoroid GA was not available. Finally, the current study did not include genetic information. Further long-term observation of a larger number of patients with pachychoroid GA, accounting for genetic information, is required to elucidate the natural course, including the GA progression rate and pathogenesis.
In conclusion, this study provides insight into the clinical characteristics of pachychoroid and conventional drusen-related GA. Asian patients with GA were male dominant and had small lesions, a relatively thick choroid, and a low GA progression rate. The high prevalence of pachychoroid GA may account for its clinical characteristics in Asian populations. Conventional drusen-related GA observed in Asian populations is similar to that observed in White populations. Researchers should consider these differences in GA subtypes when designing studies and considering interventions for GA.
Manuscript no. XOPS-D-23-00283.
Footnotes
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors made the following disclosures:
N. U.-A.: Financial support – Chugai Pharmaceutical; Lecturer – Santen Pharmaceutical, Novartis Pharma, Chugai Pharmaceutical, Bayer Yakuhin: Ayako Takahashi: Lecturer – Santen Pharmaceutical, Novartis Pharma, Bayer Yakuhin, MSD.
M. M.: Financial support – Novartis Pharma, Daiich-Sankyo, KANEKA CORPORATION; Lecturer – Bayer Yakuhin, Kowa Pharmaceutical, Alcon Japan, Santen Pharmaceutical, Novartis Pharma, AMO Japan, Santen Pharmaceutical, Senju Pharmaceutical, Johnson & Johnson K.K., Chugai Pharmaceutical, Japan Ophthalmic Instrument Association, Findex, TOPCON CORPORATION.
Y. M.: Lecturer – Santen Pharmaceutical: Yasunori Miyara: Financial support – Senju Pharmaceutical.
C. H.: Lecturer – Santen Pharmaceutical, Novartis Pharma, Chugai Pharmaceutical, Bayer Yakuhin.
R. M.: Lecturer – Kyowa Kirin, Chugai Pharmaceutical.
H. K.: Financial support – Novartis Pharma, Alcon Japan, Bayer Yakuhin, HOYA, Senju Pharmaceutical, Santen Pharmaceutical, AMO Japan, Otsuka Pharmaceutical, Staar Japan, Pfizer, KOWA Pharmaceutical, Nikon, Chugai Pharmaceutical, TOMEY, Canon; Consultant – Novartis Pharma, Bayer Yakuhin, Chugai Pharmaceutical, Roche, Allergan, Boehringer Ingelheim, Otsuka, Janssen Pharmaceutical; Lecturer – Novartis Pharma, Alcon Japan, Bayer Yakuhin, Senju Pharmaceutical, Santen Pharmaceutical, Chugai Pharmaceutical, Kowa Pharmaceutical, HOYA, AMO Japan, Otsuka Pharmaceutical, Pfizer, Bausch &Lomb, JFC, Canon, Nidek, Topcon, AbbVie GK, TOMEY, Sumitomo Pharma, Chugai Pharmaceutical, Roche, Sanofi, Inami.
Y. Y.: Financial support – Alcon Japan, Sanbio; Consultant – Roche, Boehringer Ingelheim; Lecturer – Chugai Pharmaceutical, Novartis Pharma, Bayer Yakuhin, Santen Pharmaceutical, Novartis India Ltd., Boehringer Ingelheim, Senju Pharmaceutical, Roche; Shares - DeepEyeVision Ltd.
T. I.: Financial support – Nidek, Topcon, Santen Pharmaceutical, Alcon Japan, Novartis Pharma, Senju Pharmaceutical, HOYA, AMO Japan, Pfizer, Otsuka Pharmaceutical; Consultant – Bayer Yakuhin, Novartis Pharma, Chugai Pharmaceutical, Boehringer Ingelheim Japan, Senju Pharmaceutical, Kyowa-kirin, Janssen Pharma; Patent – Topcon; Lecturer – Bayer Yakuhin, Novartis Pharma, Alcon Japan, Santen Pharmaceutical, Senju Pharmaceutical, Topcon, Chugai Pharmaceutical, Canon, Nidek, Otsuka Pharmaceutical, Nikon.
K. T.: Financial support – Santen Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Chugai Pharmaceutical; Consultant – Senju Pharmaceutical, Kyowa-kirin; Lecturer – Santen Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Senju Pharmaceutical, Chugai Pharmaceutical, Alcon Japan, Topcon, Canon, Nidek, Otsuka Pharmaceutical, Nikon.
T. S.: Financial support – Novartis Pharma, Bayer Yakuhin, Senju Pharmaceutical, Santen Pharmaceutical, Alcon Japan, Chugai Pharmaceutical, Bausch &Lomb, Kubota Pharm, Roche; Lecturer – Novartis Pharma, Bayer Yakuhin, Senju Pharmaceutical, Santen Pharmaceutical, Alcon Japan, Chugai Pharmaceutical, Bausch &Lomb, Otsuka Pharm; Grants - Health and Labor Sciences Research Grants for Research on Rare and Intractable Diseases (Grant number 20FC1029).
A. T.: Financial support – Canon, Findex, Santen Pharmaceutical, Sumitomo Pharma, AMO Japan, Senju Pharmaceutical, Wakamoto Pharmaceutical, Alcon Japan, Otsuka Pharmaceutical, Bayer Yakuhin, Rhoto Nitten; Consultant – Senju Pharmaceutical, Boehringer Ingelheim, Bayer Yakuhin, Novartis Pharma, Sumitomo Pharma, Kyowa Kirin, Chugai Pharmaceutical; Lecturer – Bayer Yakuhin, Senju Pharmaceutical, Novartis Pharma, Santen Pharmaceutical, Alcon Japan, AMO Japan, Kowa Company, Canon, Otsuka Pharmaceutical, Wakamoto Pharmaceutical, MSD, Ellex, NIDEK CO LTD, Chugai Pharmaceutical, Johnson & Johnson K.K., Rhoto Pharmaceutical, Nikon Solutions.
This study was supported by Health and Labor Sciences Research Grants for Research on Rare and Intractable Diseases (Grant number JPMH20FC1029 and JPMH23FC1043) and the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (JSPS KAKENHI Grant number JP 21K16895) [to N.U.-A.]). The funding organizations had no role in the design or conduct of this research. The sponsor or funding organization had no role in the design or conduct of the study.
HUMAN SUBJECTS: Human subjects data were included in this study. This study adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee of Kyoto University Graduate School of Medicine. The requirement for written informed consent was waived because this was a retrospective study and only anonymized data were used for analysis.
No animal subjects were included in this study.
Author Contribution:
Conception and design: Sato, Ueda-Arakawa, Takahashi, Miyake, Mori, Koizumi, Maruyama-Inoue, Yanagi, Iida, Takahashi, Sakamoto, Tsujikawa
Analysis and interpretation: Sato, Ueda-Arakawa, Takahashi, Miyara, Hara, Kitajima, Maruko, Kawai, Takahashi
Data collection: Sato, Ueda-Arakawa, Takahashi, Miyake, Mori, Koizumi, Maruyama-Inoue, Yanagi, Iida, Takahashi, Sakamoto, Tsujikawa, Miyara, Hara, Kitajima, Maruko, Kawai, Takahashi
Overall responsibility: Sato, Ueda-Arakawa, Takahashi, Miyake, Mori, Koizumi, Maruyama-Inoue, Yanagi, Iida, Takahashi, Sakamoto, Tsujikawa, Miyara, Hara, Kitajima, Maruko, Kawai, Takahashi
Obtained funding: Ueda-Arakawa
Meeting presentation: None.
References
- 1.Flaxman S.R., Bourne R.R.A., Resnikoff S., et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.Pfau M., von der Emde L., de Sisternes L., et al. Progression of photoreceptor degeneration in geographic atrophy secondary to age-related macular degeneration. JAMA Ophthalmol. 2020;138:1026–1034. doi: 10.1001/jamaophthalmol.2020.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rim T.H., Kawasaki R., Tham Y.C., et al. Prevalence and pattern of geographic atrophy in asia: the Asian eye Epidemiology consortium. Ophthalmology. 2020;127:1371–1381. doi: 10.1016/j.ophtha.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicka A.R., Jarrar Z., Wormald R., et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor Pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 6.J Jaffe G.J., Westby K., Csaky K.G., et al. C5 inhibitor Avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–586. doi: 10.1016/j.ophtha.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y., Ueda-Arakawa N., Takahashi A., et al. Clinical characteristics and progression of geographic atrophy in a Japanese population. Ophthalmol Retina. 2023;7:901–909. doi: 10.1016/j.oret.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K., Shiraga F., Ishida S., et al. [Diagnostic criteria for atrophic age-related macular degeneration] Nippon Ganka Gakkai Zasshi. 2015;119:671–677. [PubMed] [Google Scholar]
- 9.Tsujikawa A., Takahashi K., Obata R., et al. Dry age-related macular degeneration in the Japanese population. Jpn J Ophthalmol. 2022;66:8–13. doi: 10.1007/s10384-021-00892-y. [DOI] [PubMed] [Google Scholar]
- 10.Spaide R.F., Koizumi H., Pozzoni M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi A., Ooto S., Yamashiro K., et al. Pachychoroid geographic atrophy: clinical and genetic characteristics. Ophthalmol Retina. 2018;2:295–305. doi: 10.1016/j.oret.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Feuer W.J., Yehoshua Z., Gregori G., et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of Age-Related Eye Disease study report no. 26. JAMA Ophthalmol. 2013;131:110–111. doi: 10.1001/jamaophthalmol.2013.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teo K.Y.C., Fujimoto S., Sadda S.R., et al. Geographic atrophy phenotypes in subjects of different ethnicity: Asia-pacific ocular imaging society work group report 3. Ophthalmol Retina. 2023;7(7):593–604. doi: 10.1016/j.oret.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Cheung C.M.G., Lee W.K., Koizumi H., et al. Pachychoroid disease. Eye (Lond) 2019;33(1):14–33. doi: 10.1038/s41433-018-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindblad A.S., Lloyd P.C., Clemons T.E., et al. Change in area of geographic atrophy in the Age-Related Eye Disease study AREDS report number 26. Arch Ophthalmol. 2009;127:1168–1174. doi: 10.1001/archophthalmol.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehoshua Z., Rosenfeld P.J., Gregori G., et al. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology. 2011;118:679–686. doi: 10.1016/j.ophtha.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holz F.G., Bindewald-Wittich A., Fleckenstein M., et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Klein R., Meuer S.M., Knudtson M.D., Klein B.E. The rpidemiology of progression of pure geographic atrophy: the Beaver Dam Eye study. Am J Ophthalmol. 2008;146:692–699. doi: 10.1016/j.ajo.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holekamp N., Wykoff C.C., Schmitz-Valckenberg S., et al. Natural history of geographic atrophy secondary to age-related macular degeneration: results from the prospective Proxima A and B clinical trials. Ophthalmology. 2020;127:769–783. doi: 10.1016/j.ophtha.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Agrón E., Domalpally A., Cukras C.A., et al. Reticular pseudodrusen status, ARMS2/HTRA1 genotype, and geographic atrophy enlargement: age-Related Eye Disease study 2 report 32. Ophthalmology. 2023;130:488–500. doi: 10.1016/j.ophtha.2022.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domalpally A., Agrón E., Pak J.W., et al. Prevalence, risk, and genetic association of reticular pseudodrusen in age-related macular degeneration: age-Related Eye Disease study 2 report 21. Ophthalmology. 2019;126:1659–1666. doi: 10.1016/j.ophtha.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrielle P.H., Seydou A., Arnould L., et al. Subretinal drusenoid deposits in the elderly in a population-based study (the Montrachet study) Invest Ophthalmol Vis Sci. 2019;60:4838–4848. doi: 10.1167/iovs.19-27283. [DOI] [PubMed] [Google Scholar]
- 23.Fritsche L.G., Igl W., Bailey J.N., et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yates J.R., Sepp T., Matharu B.K., et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 25.Hilely A., Au A., Lee W.K., et al. Pachyvitelliform maculopathy: an optical coherence tomography analysis of a novel entity. Br J Ophthalmol. 2023;0:1–7. doi: 10.1136/bjo-2022-322553. [DOI] [PubMed] [Google Scholar]
- 26.Iovino C., Ramtohul P., Au A., et al. Vitelliform maculopathy: diverse etiologies originating from one common pathway. Surv Ophthalmol. 2023;68:361–379. doi: 10.1016/j.survophthal.2023.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Wei W.B., Xu L., Jonas J.B., et al. Subfoveal choroidal thickness: the Beijing Eye study. Ophthalmology. 2013;120:175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]