Abstract
Infection by Epstein-Barr virus (EBV) generates several types of latency with different profiles of gene expression but with expression of Epstein-Barr nuclear antigen 1 (EBNA-1) in common. The BamHI Q promoter (Qp) is used for the transcription of EBNA-1 mRNA in type I latency, which is an EBV infection state exemplified by Burkitt's lymphoma (BL). However, Qp is inactive in type III latency, and other promoters (C/Wp) are used for transcription of EBNA-1, which raises the question of how usage of these promoters is governed. Interferon (IFN) regulatory factor 7 (IRF-7) was identified first as a negative regulator of Qp. Expression of IRF-7 is associated with EBV type III latency, where Qp is inactive, but not with type I latency, raising the possibility that a viral gene product(s) expressed in type III latency might induce IRF-7 and repress Qp. Here, detailed analysis of the expression of IRF-7 revealed that it is associated with the expression of EBV latent membrane protein 1 (LMP-1) and that LMP-1 stimulates the expression of IRF-7 in type III latency in which Qp is inactive. In contrast, LMP-1 is not expressed in type I latency cells in which Qp is active. LMP-1 represses the constitutive activity of Qp reporter constructs. Mutational analysis of Qp reporter constructs revealed that the Qp IFN-stimulated response element (ISRE) is essential for the repression by LMP-1. Furthermore, LMP-1 reduced EBNA-1 mRNA derived from Qp only in type I cells in which IRF-7 could be induced. Finally, IFN-α, but not IFN-γ, repressed endogenous Qp activity, which is consistent with the ability of IFN-α to induce IRF-7. Thus, IRF-7 may mediate repression of Qp by LMP-1. The induction of IRF-7 by LMP-1 may be relevant to the silencing of Qp in EBV type III latency.
The biologic hallmark of Epstein-Barr virus (EBV) and its usual interaction with B lymphocytes is latency. Several types of latency with distinct patterns of viral gene expression have been described. Type I latency is exemplified by Burkitt's lymphomas (BL) in vivo and earlier passages of cultured cell lines derived from BL tissues. EBV nuclear antigen 1 (EBNA-1), and in some cases latent membrane protein 2A (LMP-2A), is expressed in this form of latency (7, 35, 36, 41, 59). Several reports suggest that a type I-like form of latency exists in healthy carriers of EBV (7, 35, 36, 41, 59). Interestingly, cells in type I latency can escape host immune surveillance because EBNA-1 can interfere with its peptide presentation on major histocompatibility complex class I molecules (29), which might explain the lifelong reservoir of virus in immunocompetent, seropositive persons. Type III latency is represented by lymphoblastoid cell lines established after EBV infection of adult primary B cells in vitro, by some BL lines, and in B-cell lymphomas in immunodeficient states. Nine viral proteins, including six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP) and three integral membrane proteins (LMP-1, LMP-2A, and LMP-2B), are expressed. In addition, in all three forms of latency, EBV-encoded RNAs (EBERs) are expressed (reviewed in references 25 and 43).
EBNA-1 is the sole viral protein needed for the replication of the EBV episome and maintenance of the latent infection state; both events are essential for cell immortalization (reviewed in references 25 and 43). The promoter usage for expression of EBNA-1 differs in different types of latency. In type I latency, the BamHI Q promoter (Qp) is used for the transcription of EBNA-1 mRNA. However, in type III latency, Qp is silent, and the BamHI C and/or BamHI W promoters (C/Wp) are used. The biological consequence of the Qp-to-C/Wp switch and the conversion to type III latency is the expression of the full spectrum of latency genes (reviewed in references 25 and 43), which confers enhanced cell survival, growth, and invasive potential (8, 17, 19, 22, 53, 60, 65).
Since Qp usage not only relates to the survival of the virus in an immunocompetent host but also is associated with several tumors, dissecting the regulation of Qp is essential for understanding the viral program in EBV-associated malignancies. The functional importance of Qp for the EBV life cycle is underscored by the facts that all EBV-positive tumor specimens collected from Africa, North America, and Asia have conserved Qp sequence (58) and that conserved structural and functional cis elements (e.g., an interferon [IFN]-stimulated response element [ISRE] and the Q locus [see Fig. 4A]) also exist in the Old World primate lymphocryptoviruses, which are simian EBVs (47). Both EBNA-1 and host factors are involved in the transcriptional regulation of Qp. The downstream element of Qp, the Q locus (Fig. 4A), contains two binding sites for the EBNA-1 protein, which binds to and acts in an autoregulatory manner to repress Qp transcription (48, 56). However, E2F-1 overcomes EBNA-1-mediated repression of Qp in transient transfection assays, and E2F-1 binds to the Q locus and displaces the binding of EBNA-1 (55), so that the promoter is regulated in a cell cycle-dependent manner (10).
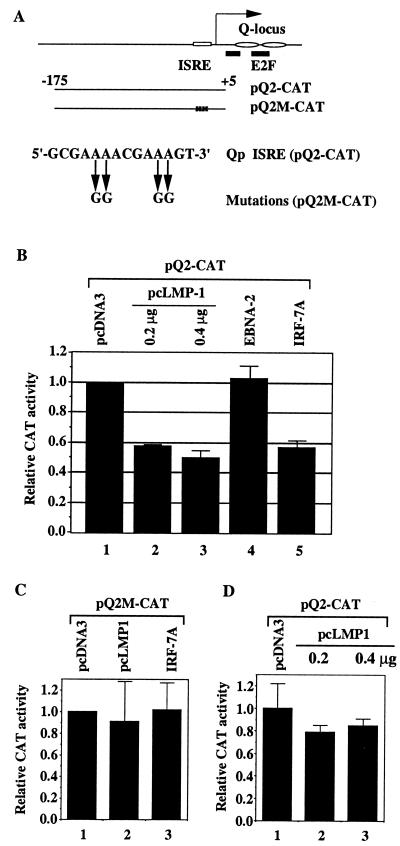
FIG. 4.
Repression of Qp reporter constructs by LMP-1. (A) Schematic diagram of Qp and Qp reporter constructs. Open rectangle, ISRE; solid bars, E2F binding sites; ovals, EBNA-1 sites (Q locus). The RNA start site for Qp is indicated by an arrow (50). The mutations in the Qp ISRE are shown. (B) Repression of Qp by LMP-1 in B cells. DG75 cells were transfected with the reporter construct pQ2-CAT along with the pcDNA-3 vector (column 1), with 0.2 and 0.4 μg of LMP-1 expression plasmid pcLMP-1 (columns 2 and 3, respectively), with an EBNA-2 expression plasmid (column 5), or with pcDNA-IRF-7A (column 6). (C) Mutations in ISRE abolish the repression of Qp by LMP-1 and by IRF-7. DG75 cells were transfected with reporter construct pQ2M-CAT and pcDNA-3 (column 1), pcLMP-1 (column 2), or pcDNA-IRF-7A (column 3). (D) LMP-1 did not repress Qp reporter construct in Akata cells. Akata cells were transfected with the reporter construct pQ2-CAT and with pcDNA-3 vector (column 1) or with 0.2 and 0.4 μg of LMP-1 expression plasmid pcLMP-1 (columns 2 and 3, respectively). CAT assay results were normalized by β-galactosidase activity. CAT activity is expressed relative to the vector control level. Standard deviations are shown.
An ISRE immediately upstream of the transcriptional initiation site has been discovered and appears to be essential for Qp constitutive activity (39, 49, 56, 67). IFN regulatory factors (IRFs), which are a group of transcription factors with multiple functions (reviewed in reference 37), have the potential to bind to the Qp ISRE and to regulate the activity of Qp. Although the major positive regulator of Qp through the ISRE is disputed (39, 49, 68), both IRF-2 and the newly identified IRF-7 have been reported to be negative regulators of Qp (67, 68). IRF-7 is predominately expressed in lymphoid tissues, and four splicing forms (IRF-7A, IRF-7B, IRF-7C, and IRF-7H) have been identified (4, 67). IRF-7A is apparently the major form of IRF-7 in peripheral blood leukocytes (67).
LMP-1 can induce a variety of cellular genes and enhance cell survival, adhesion, and invasive potential (13, 22, 34, 62, 63, 65). LMP-1 expression is also necessary for B-cell transformation by EBV. Here, we report that LMP-1 stimulates the expression of IRF-7. Furthermore, LMP-1 can repress Qp activity. Because IRF-7 is a negative regulatory of Qp and LMP-1 is expressed in type III latency, induction of IRF-7 by LMP-1 may provide an indirect pathway for the silencing of Qp in type III latency.
MATERIALS AND METHODS
Cell culture.
DG75 is an EBV-negative BL cell line (5); BL30 and BL41 are EBV-negative BL lines with EBV-infected counterparts generated by in vitro infection with the P3HR1 strain (BL30-P3HR1 and BL41-P3HR1) or the B95-8 strain (BL30-B95-8 and BL41-B95-8) of EBV (6). Akata, Eli-BL (gift from Alan Rickinson), and Rael (gift from Richard Ambinder) are all EBV-positive type I BL cell lines (27, 45, 57). Jijoye and its derivative P3HR1 are EBV-positive type III BL cell lines (2, 42). The stable cell lines expressing either LMP-1 or EBNA-2 in BJAB or BL41 cells were gifts from Fred Wang (63). All cell lines described were maintained in RPMI 1640 plus 10% fetal bovine serum. Suitable selection drugs were added to the stable cell lines as reported elsewhere (63).
Plasmids and antibodies.
IRF-7A and EBNA-2 expression plasmids and IRF-7 antibody have been described elsewhere (40, 54, 67). pcDNA/CD4 is a human CD4 expression plasmid (gift from Jenny Ting). The β-galactosidase expression plasmid, pCMVβ (6177-1), was purchased from Clontech. pQ1-CAT and pQ2-CAT are described elsewhere (68). The LMP-1 expression plasmid, pcLMP-1, was a gift from Tomakazu Yoshizaki. pQ2M-CAT (−173 to +5) was made by cloning the corresponding PCR fragment with mutations in ISRE sequence into pBS-CAT (14). The IRF-1 (C-20) and IRF-2 (C-19) antibodies were purchased from Santa Cruz Biotechnology, Inc. The EBNA-2 monoclonal antibody, PE-2, and LMP-1 monoclonal antibody, S12, were derived from their respective hybridoma cell lines (31, 66). IFN-stimulated gene 15 (ISG-15) monoclonal antibody (11) was a gift from Ernest Borden.
Western blot analysis with enhanced chemiluminescence.
Separation of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done according to standard methods. After the proteins were transferred to a nitrocellulose or Immobilon membrane, the membrane was blocked with 5% nonfat dry milk in TBST (50 mM Tris [pH 7.5], 200 mM NaCl, 0.05% Tween 20) at room temperature for 10 min. It was then washed briefly with water and incubated with a primary antibody in 5% milk in TBST for 1 to 2 h at room temperature or overnight at 4°C. After being washed with TBST for 10 min three times, the membrane was incubated with the secondary antibody at room temperature for 1 h. It was then washed three times with TBST as before, treated with enhanced chemiluminescence (Amersham) or SuperSignal (Pierce) detection reagents, and exposed to Kodak XAR-5 film.
Transient transfection, CAT assays and isolation of transfected cells.
For DG75 and Akata cells, 107 cells in 0.5 ml of medium with 5 to 10 μg of DNA were used for transfection with the use of a Bio-Rad Gene Pulser (320 V and 925 μF). For Eli-BL cells, 50 μg of DNA was used at 320 V and 975 μF. Two days after transfection, cells were collected for chloramphenicol acetyltransferase (CAT) assay or for isolation of transfected cells. The CAT and β-galactosidase assays were essentially the same as described elsewhere (16). The CAT assay results were analyzed on a Molecular Dynamics PhosphorImager.
For isolation of transfected cells, cells were collected after transfection and enrichment for CD4 positive cells was performed with the use of anti-CD4 antibody conjugated to magnetic beads as recommended by the manufacturer (Dynal, Inc.). The isolated cells were used for the extraction of total RNA.
RNA extraction and RPA.
Total RNA was isolated from cells with the use of RNease total RNA isolation kit (Qiagen). RNase protection assays (RPA) was performed with total RNA with the use of an RNase Protection Kit II (Ambion Inc.). The hybridization temperature was 45°C. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was supplied by U.S. Biochemicals, Inc. The IRF-7 probe was generated with the use of ApaLI-digested pBS-IRF7A as a template and T7 RNA polymerase. The protected region is nucleotides 1671 to 1890 of IRF-7A (67). This probe cannot distinguish various splicing forms of IRF-7. The EBNA-1 probe was described previously (68). Molecular weight markers were made with the use of a γ-32P-labeled DNA marker, (ØX174 DNA/HinfI; Promega).
IFN.
IFN-α-2a was purchased from Hoffman La Roche Inc.; IFN-γ was from Genzyme. For IFN treatments, cells were treated with either IFN-α (500 U/ml) or IFN-γ (500 U/ml) for 48 h and were collected for further analysis. The same amounts of H2O or 1× phosphate-buffered saline were used as mock treatments.
RESULTS
Expression of IRF-7 is associated with LMP-1 protein in type III latency.
We have shown that high levels of IRF-7 and IRF-2 are associated with type III, but not type I, latency (67, 68), which raises the possibility that a viral gene(s) may regulate the expression of IRF-7 or IRF-2. Because only EBNA-1, and possibly LMP-2A, is expressed in type I latency, but all of the latency proteins (EBNA-1, -2, -3A, -3B, and -3C, and LMP-1, -2A, and -2B) are expressed in type III latency, one or more viral genes expressed in type III latency may be responsible for the upregulation of IRF-2 and IRF-7.
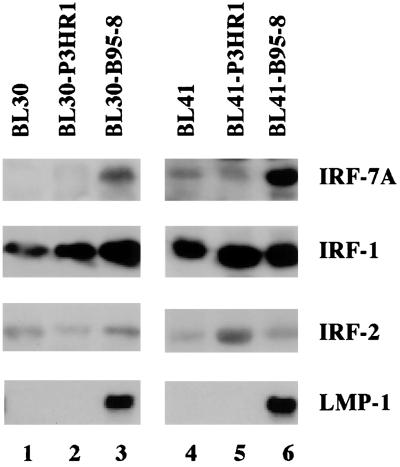
To select a candidate gene(s), paired cell lines infected by EBV P3HR1 virus or the prototype B95-8 strain were examined. BL30-P3HR1 and BL30-B95-8 lines were established by infecting the EBV-negative BL30 line with P3HR1 and B95-8 viruses, respectively. The BL41-P3HR1 and BL41-B95-8 lines were established similarly (6). B95-8 virus has all of the latency genes in its viral genome, whereas the P3HR1 virus genome lacks the EBNA-2 gene and a portion of the EBNA-LP gene (2). The cell lines infected with P3HR1 virus did not express EBNA-2 and had a much lower level of LMP-1, whereas both cell lines infected with B95-8 virus expressed high levels of LMP-1 because EBNA-2 can transactivate the LMP-1 promoter (1, 15, 61, 64) (Fig. 1 and data not shown). The expression of IRFs in these lines was examined by Western blotting with specific antibodies. IRF-7 was shown to be expressed at high levels in the two cell lines infected with B95-8 virus, both of which have a high level of LMP-1 (Fig. 1, lanes 3 and 6). The levels of IRF-1 and IRF-2, on the other hand, did not correlate with the high levels of LMP-1 expression. The IRF-1 level was high in all cell lines tested. These experiments were repeated several times, and consistent results were obtained. Furthermore, the type III Jijoye cell line, which has high-level expression of LMP-1 and is the parental line for P3HR1 cells, has a higher level of IRF-7 than P3HR1 cells, which have a lower LMP-1 level (data not shown).
FIG. 1.
Association between the expression of IRF-7 and LMP-1. Equal amounts of protein lysates from the indicated cell lines were separated by SDS-PAGE (10% gel) and stained with Ponceau S red after transfer of protein to the membrane. Western blotting with IRF-7, IRF-1, IRF-2, and LMP-1 antibodies was performed.
These data strongly suggest that high-level expression of IRF-7 in type III latency is associated with the expression of LMP-1. In contrast, an association of IRF-1 and IRF-2 with EBV protein expression was not apparent.
LMP-1 stimulates the expression of IRF-7 protein.
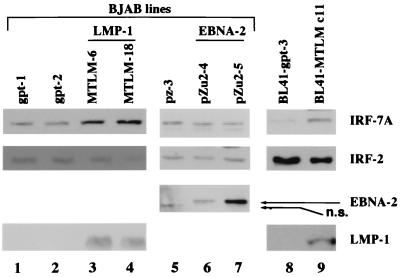
Since there was a consistent association between high levels of LMP-1 in type III latency and IRF-7, whether LMP-1 could directly stimulate IRF-7 was examined in stable cell lines expressing either LMP-1 or EBNA-2 (Fig. 2). MMLM-6 and MMLM-18 are EBV-negative BJAB lines expressing LMP-1 protein constitutively, whereas pZu2-4 and pZu2-5 express EBNA-2 protein. pZ-3, gpt-1, and gpt-2 are vector control lines for the EBNA-2 (pZ-3) and LMP-1 (gpt-1 and gpt-2) lines (63). The expression of EBNA-2 and LMP-1 was confirmed by Western blot analysis with EBNA-2 or LMP-1 antibody. As shown in Fig. 2, expression of IRF-7 is higher in LMP-1-expressing lines (lanes 3 and 4) than it is in the negative control lines (lanes 1 and 2). However, IRF-2 was not affected by LMP-1 expression. Neither the IRF-7 nor the IRF-2 level was stimulated in the EBNA-2-expressing cell lines (lanes 5 to 7).
FIG. 2.
LMP-1 increases levels of endogenous IRF-7 protein. Western blotting with IRF-7, IRF-2, LMP-1, and EBNA-2 antibodies was performed. Lanes 1 to 7, stable cell lines established from the EBV-negative BJAB cell line; lanes 3 and 4, LMP-1 expression lines (MTLM-6 and MTLM-18); lanes 6 and 7, EBNA-2 expression lines (pZu2-4 and pZu2-5); lanes 1 and 2, vector control lines for LMP-1 (gpt-1 and gpt-2); lane 5, vector control line for EBNA-2 (pZ-3); lanes 8 and 9, LMP-1 expression line in BL41 (BL41-MTLM c11; lane 9) and its control cell line (BL41-gpt-3; lane 8). n.s., nonspecific band in lane 5.
To address further whether LMP-1 could stimulate IRF-7, we used BL41-MTLM c11, a BL41 line stably transfected with an LMP-1 expression plasmid (63). As shown in Fig. 2, expression of IRF-7 is also higher in this LMP-1-expressing line than in its control line, BL41-gpt-3 (lanes 8 and 9). These results suggest that LMP-1, but not EBNA-2, can stimulate the expression of IRF-7 protein.
LMP-1 stimulates the expression of IRF-7 mRNA.
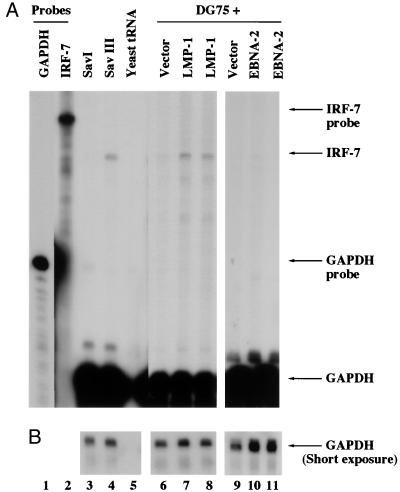
Whether LMP-1 can increase IRF-7 mRNA was examined by RPA with a specific probe (see Materials and Methods for details). In a pair of genetically identical lines, the IRF-7 mRNA level was higher in the type III line (Sav III), where LMP-1 is expressed, than in the type I line (Sav I), where there is no LMP-1 expression (Fig. 3, lanes 3 and 4). The difference in IRF-7 mRNA between the two lines is more than 10-fold (Fig. 3). Also, the level of IRF-7 mRNA is low in Eli-BL, Akata, and Rael cells (type I lines) but higher in Jijoye and CB95 cells (type III lines) (data not shown). Therefore, IRF-7 mRNA expression is also associated with type III latency, in agreement with previous reports (39, 67).
FIG. 3.
LMP-1 increases endogenous IRF-7 mRNA. (A) IRF-7 and GAPDH probes were labeled with [α-32P]UTP and used for RPA. Lanes 1 and 2, undigested IRF-7 and GAPDH probes; lanes 3 and 4, RNAs from Sav I and Sav III cells, respectively; lane 5, yeast tRNA; lanes 6 to 11, RNAs from transfected and concentrated DG75 cells; lanes 7 and 8, an LMP-1 expression plasmid was used for transfection; lanes 10 and 11, EBNA-2 expression plasmid; lanes 6 and 9, control vectors. Specific protection of IRF-7 and GAPDH mRNAs and undigested probes is indicated. (B) Short time exposure for GAPDH-protected areas. The IRF-7 mRNA in Sav III was 10.4-fold ± 1.9-fold higher than in Sav I. The enhancement of IRF-7 mRNA by LMP-1 was 2.4-fold ± 0.4-fold, whereas enhancement by EBNA-2 was 0.63-fold ± 0.2-fold. Data were obtained by normalizing IRF-7 levels to the GAPDH level with the use of a PhosphorImager.
Whether LMP-1 could increase IRF-7 mRNA was examined by transient transfection of LMP-1 and a CD4 expression plasmid into DG75 cells, which is an EBV-negative line, and selecting transfected cells by the use of anti-CD4 antibody-conjugated magnetic beads (see Materials and Methods for details). As shown in Fig. 3, LMP-1 expression causes an increased level of IRF-7 mRNA (lanes 7 and 8) compared with vector alone (lane 6). In contrast, EBNA-2 did not stimulate the expression of IRF-7 mRNA (lanes 9 to 11). Western blot analysis confirmed that IRF-7A protein was greater in amount in LMP-1-transfected cells than in pcDNA3-transfected cells (data not shown). The mRNA data are also consistent with results from Western blot analyses of LMP-1- and EBNA-2-expressing lines (Fig. 2). The data together suggest that LMP-1 stimulates the expression of IRF-7 mRNA.
LMP-1 represses the constitutive activity of Qp reporter constructs.
Because IRF-7 has been shown to be a negative regulator of Qp (67, 68), the stimulation of IRF-7 by LMP-1 predicts that LMP-1 might act indirectly as a negative regulator of Qp. To test this idea, an LMP-1 expression plasmid and a Qp reporter construct were cotransfected into DG75 cells, and CAT activity was assayed 2 days later, with β-galactosidase activity as an internal transfection efficiency control. The pQ2CAT construct was chosen to assay Qp activity because the constitutive activity of pQ2CAT correlates with endogenous Qp activity and is more responsive to IRF-7 (68). As shown in Fig. 4B, LMP-1 repressed activity of the Qp reporter construct (columns 1 to 3). EBNA-2 protein expression did not repress Qp (column 4), which is consistent with the fact that EBNA-2 does not induce IRF-7 (Fig. 2 and 3). Overexpression of IRF-7 repressed Qp as expected (column 5). Furthermore, mutations in the ISRE of Qp (Fig. 4A) prevented the repression by LMP-1 or by IRF-7A (Fig. 4C). It should be noted that ISRE mutation greatly reduced the constitutive activity of Qp as reported previously (39, 49, 56, 67).
It is interesting that we did not detect any stimulation of IRF-7 expression after overexpression of LMP-1 in Akata cells (Fig. 5B). Therefore, whether LMP-1 could repress Qp reporter constructs in these cells was tested. As shown in Fig. 4D, LMP-1 failed to repress Qp in Akata cells. The Akata line is unusual in that some LMP-1-inducible genes cannot be induced in these cells, and anti-human immunoglobulin G can trigger lytic replication of EBV (46, 57). However, LMP-1 could activate NF-κB in this line (data not shown).
FIG. 5.
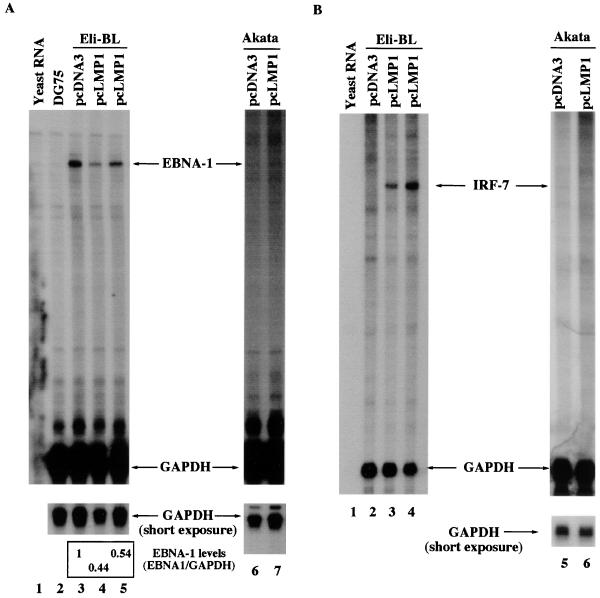
LMP-1 reduces the endogenous EBNA-1 mRNA transcribed from Qp in type I cells. (A) Reduction of endogenous EBNA-1 mRNA in cells transfected with an LMP-1 expression plasmid. RPA was performed with GAPDH and EBNA-1 probes with various RNAs. Lane 1, yeast RNA; lane 2, total RNA from DG75, an EBV-negative cell line; lanes 3 to 5, RNAs from Eli-BL cells transfected with pcDNA3, pcLMP1 (5 μg), and pcLMP1 (10 μg), respectively; lanes 6 and 7, RNAs from Akata cells transfected with pcDNA3 and pcLMP1, respectively. The relative EBNA-1 mRNA levels are shown. Data were analyzed by normalizing EBNA-1 mRNA levels to the GAPDH level with the use of a PhosphorImager. In LMP-1-transfected cells, the average EBNA-1 mRNA level (and standard deviation) relative to pcDNA3-transfected cells from four experiments was 0.52 ± 0.09. One representative experiment is shown. (B) Stimulation of IRF-7 by LMP-1 in Eli-BL cells. RPA was performed with GAPDH and IRF-7 probes with various RNAs. Lane 1, yeast RNA; lanes 2 to 4, RNAs from Eli-BL cells transfected with pcDNA3, pcLMP1 (5 μg), and pcLMP1 (10 μg), respectively; lanes 5 and 6, RNAs from Akata cells transfected with pcDNA3 and pcLMP1 respectively. One representative experiment is shown.
Since high-level expression of LMP-1 has been reported to have a toxic effect on some cells (12, 20), whether the reduction in Qp constitutive activity by LMP-1 was due to toxicity was examined carefully. First, there were no obvious differences in β-galatosidase activity with or without LMP-1. Second, an NF-κB reporter construct was activated with the expression of LMP-1 as expected (data not shown and references 28 and 46). Third, endogenous IRF-7 mRNA was enhanced by LMP-1 in DG75 cells under the same transfection conditions (Fig. 3). Therefore, the data suggest that LMP-1 can repress Qp activity, and the repression is associated with IRF-7 stimulation.
LMP-1 reduces endogenous EBNA-1 mRNA in type I latency cells.
To test if LMP-1 represses endogenous Qp activity, the LMP-1 and CD4 expression plasmids were transfected into Eli-BL, a type I cell line in which Qp is active. The RNA from the transfected cells was isolated, and RPA with an EBNA-1-specific probe was performed. The Eli-BL line was chosen because LMP-1 could stimulate the expression of IRF-7 in this line (Fig. 5B). As shown in Fig. 5A, LMP-1 indeed reduced the level of EBNA-1 mRNA in Eli-BL cells (compare lanes 4 and 5 with lane 3). However, LMP-1 did not induce IRF-7 (Fig. 5B, lane 6) or reduce EBNA-1 mRNA in Akata cells (Fig. 5A, lane 10). The EBNA-1 mRNA level in Akata cells is lower than it is in Eli-BL cells, possibly because the EBV genome is lost in subpopulations of Akata (52), resulting in differences in viral genome copy numbers. The reduced level of EBNA-1 mRNA produced in Eli-BL cells by LMP-1 is most likely due to the repression of Qp activity, because LMP-1 could repress the activity of Qp reporter constructs to a similar extent (Fig. 4B). These data suggest that LMP-1 can repress the endogenous activity of Qp, and the repression is associated with the ability of LMP-1 to induce the expression of IRF-7.
Repression of Qp by IFN is associated with the induction of IRF-7.
Because IFN has been shown to induce the expression of IRF-7 (4, 32, 39), whether IFN can repress endogenous Qp was tested in the type I latency cells, Eli-BL and Rael; in both cell lines, endogenous Qp is active. IFN-α could induce the expression of IRF-7 in Eli-BL cells but not in the Rael cell line (Fig. 6B, lanes 2 and 5). The reason that IFN-α fails to induce IRF-7 in Rael cells is unknown; however, the signal transduction pathway for IFN-α in Rael cells is at least partially functional because the expression of ISG-15 could be greatly induced (Fig. 6C, lanes 2 and 3; reference 11). IFN-γ could not induce IRF-7 in either cell line (Fig. 6B, lanes 3 and 6), which is consistent with a previous report (4). However, IFN-γ could enhance the expression of Tap-2 (transporter associated with antigen processing 2), suggesting that IFN-γ was functional as reported (data not shown; references 30 and 44). Whether IFN represses the endogenous Qp was addressed with the use of RPA with an EBNA-1-specific probe. Figure 6A shows that IFN-α indeed reduced the Qp-derived EBNA-1 mRNA in the Eli-BL cells (lane 4). In contrast, IFN-α failed to repress Qp in the Rael line, in which IFN-α did not induce the expression of IRF-7 (Fig. 6A, lane 7). The reduced level of EBNA-1 mRNA in Eli-BL cells is most likely due to the repression of Qp activity, because IFN-α could repress the activity of Qp reporter constructs (data not shown and reference 39). IFN-γ did not repress Qp in either cell line, as expected (Fig. 6A, lanes 5 and 8). These data suggest that IFN-α can repress the endogenous activity of Qp. This effect is associated with the ability of IFN-α to induce the expression of IRF-7.
FIG. 6.
IFN-α can repress endogenous Qp activity in type I cells. (A) Repression of endogenous Qp by IFN-α in Eli-BL cells. Lanes 1 and 2, undigested GAPDH and EBNA-1 probes, respectively; lanes 3 to 8, RNAs from Eli-BL (lanes 3 to 5) or Rael (lanes 6 to 8) cells were used for RPA; lanes 3 and 6, mock treatment; lanes 4 and 7, IFN-α treatment (500 U/ml, 48 h); lanes 5 and 8, IFN-γ treatment (500 U/ml, 48 h). The reduction of EBNA-1 mRNA by IFN-α was 53%. Data were obtained by normalizing EBNA-1 levels to the GAPDH level with the use of a PhosphorImager. One representative experiment of two independent experiments performed is shown. (B) IFN-α induces the expression of IRF-7 in Eli-BL cells. Protein lysates from Rael (lanes 1 to 3) or Eli-BL (lanes 4 to 6) cells from the same experiment as shown in panel A were separated by SDS-PAGE (8% gel) and stained with Ponceau S red after transfer of protein to the membrane. Lanes 1 and 4, mock treatment; lanes 2 and 5, IFN-α treatment; lanes 3 and 6, IFN-γ treatment. Western blots with IRF-7 and γ-tubulin antibodies were performed. (C) IFN-α induces ISG-15 expression in Rael cells. Equal amounts of protein lysates from Rael with IFN-α treatment (lanes 2 and 3) or mock treatment (lane 1) were separated by SDS-PAGE (12% gel) and stained with Ponceau S red after transfer of protein to the membrane. Western blotting with ISG-15 antibody was performed. ns, nonspecific band.
DISCUSSION
EBV can deregulate B-cell growth through activation of endogenous programs of cellular gene expression. At the same time, these deregulated cellular genes can modulate the program of viral gene expression. The switch from Qp to C/Wp usage, which allows transcription of other EBNA proteins (EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP), produces a phenotypic conversion from type I to type III latency. We previously identified a novel regulator for Qp, IRF-7, which is highly expressed in type III latency. In this work, LMP-1, a key type III latency protein, is identified as an inducer of IRF-7. First, IRF-7 is associated with type III latency, in which LMP-1 is expressed (67). Second, high levels of IRF-7 are associated with LMP-1 expression in EBV-infected BL-30 and BL-41 cell lines (Fig. 1). Third, stable expression of LMP-1 in EBV-negative BJAB as well as BL41 cell lines increases the endogenous expression of IRF-7 protein (Fig. 2). Fourth, transient transfection of LMP-1 into DG75 or Eli-BL cells could increase the endogenous expression of IRF-7 mRNA (Fig. 3 and 5). Fifth, a physiological level of LMP-1 seems also able to stimulate IRF-7 mRNA expression (Fig. 3). More work is needed to dissect the domains of LMP-1 required for induction of IRF-7.
Interestingly, it has been argued that the induction of bcl-2 by LMP-1 may be due to the selection of a cell population with high endogenous expression of bcl-2 (33). Since transient expression of LMP-1 in DG75 or Eli-BL cells could stimulate the expression of IRF-7, LMP-1 is more likely able to enhance IRF-7 expression directly, rather than to select for a cell population with a high level of IRF-7. All of these data suggest that LMP-1 is a factor necessary for the stimulation of the expression of IRF-7 in EBV-infected cells but not sufficient, as in the case of Akata cells. Akata cells may have a mutation(s) in the signal transduction pathway of LMP-1.
The fact that LMP-1 can stimulate the expression of IRF-7, which is a negative regulator of Qp, predicts that LMP-1 may be able to repress Qp through IRF-7. First, there is no LMP-1 expression in type I latency in which Qp is active; however, LMP-1 expression in type III latency is associated with inactivation of Qp and induction of IRF-7. Second, LMP-1 could repress the activity of Qp reporter constructs in DG75 cells and reduce the endogenous EBNA-1 mRNA from Qp in a type I cell line, Eli-BL (Fig. 4 and 5). In both cell lines, IRF-7 could be induced by LMP-1 (Fig. 3 and 5). Third, overexpression of IRF-7 could repress the activity of Qp reporter construct to an extent similar to that exhibited by LMP-1 (Fig. 4B) and marginally reduce the endogenous EBNA-1 mRNA derived from Qp (data not shown). Fourth, in Akata cells LMP-1 could not induce the expression of IRF-7, and LMP-1 failed to repress activity of a Qp reporter construct and to reduce the endogenous EBNA-1 mRNA (Fig. 4B and 5). Fifth, mutations in the ISRE of Qp abolished the repression by LMP-1 or IRF-7 (Fig. 4C). Also, EBNA-2 could not stimulate the expression of IRF-7 in DG75 cells and did not repress Qp activity (Fig. 2 to 4). These data strongly suggest that LMP-1 is a negative regulator of Qp and that the repression may be mediated by IRF-7 through the Qp ISRE. However, since in IRF-7A-transfected cells the average EBNA-1 mRNA level (and standard deviation) relative to pcDNA3-transfected cells from five experiments was 0.68 ± 0.15 (data not shown), an additional factor(s) may cooperate with IRF-7 to repress Qp efficiently (68).
Interestingly, in type II latency, LMP-1 expression and an active Qp can coexist (for a review, see reference 43). One possible explanation is that LMP-1 in type II cells cannot induce IRF-7 and thus fails to repress Qp. Preliminary results suggest that this scenario may be correct (data not shown and reference 68). More work is needed to address the relations among LMP-1, IRF-7, and Qp activity in type II latency cells.
It is apparent that Qp activity is a balanced outcome of positive and negative regulators, with IRFs strongly implicated in its regulation. In type III latency, Qp may be turned off by a combination of LMP-1, IRF-7, IRF-2, and higher levels of EBNA-1 (9, 18, 51, 67, 68). The contribution of each repressor to the inactivation of Qp in type III latency needs to be addressed further. In contrast, in type I latency when Qp is active, low levels or absence of these negative factors plus some unidentified positive regulator(s) presumably cause the activation of Qp. IRF-2 has been proposed as a primary activator of Qp (39, 49), but that conclusion is disputed (68), and the identity of the ISRE-mediated activator(s) is still uncertain.
Finally, IFN-α, but not IFN-γ, has been shown to induce the expression of IRF-7 (Fig. 6B; references 4, 32, and 39). As expected, IFN-α, but not IFN-γ, could repress endogenous Qp activity in type I cells (Fig. 6A). The repression seems to be associated with the ability of IFN to induce IRF-7 as exemplified by Eli-BL cells. In contrast, Qp activity in Rael cells, in which IRF-7 could not be induced by IFN-α, was not repressed by IFN-α (Fig. 6A). Thus, the data overwhelmingly support the idea that IRF-7 is a mediator of Qp repression. Also, it is interesting that primary EBV infection could apparently induce IFN-α (26) and that proliferation of type III latency cells is resistant to IFN (3, 24). These observations together with the data reported here and in a previous report (67) suggest that IFN-α by induction of IRF-7 silences Qp and favors usage of C/Wp and facilitates expression of the EBV oncoproteins. This scenario could function in primary infection.
LMP-1 is essential for the viral transformation process. Interestingly, some IRFs have oncogenic capability (21, 23, 38). Since the cellular function (especially oncogenic potential) of IRF-7 is unknown, it is premature to speculate on the relation between the induction of IRF-7 by LMP-1 and the transformation process in B cells. Whether IRF-7 is involved in the pathogenesis of EBV-associated diseases and malignancies remains to be determined.
The present results expand the role of LMP-1 as a pleiotropic molecule in effecting deregulation of cellular genes. LMP-1 has been shown to induce numerous cellular genes with a variety of functions. LMP-1 is now presented as a stimulator of IRF-7 that, in turn, mediates the repression of the principal type I latency promoter for EBNA-1, Qp, thus favoring the switch to the use of type III latency promoters, C/Wp. The complexity of regulation of EBV's latency states is illustrated by the fact that the principal product transcribed from C/Wp is EBNA-2, which in turn amplifies the transcription of LMP-1. Thus, both viral and IRF pathways may converge to determine latency state.
ACKNOWLEDGMENTS
We thank Fred Wang, Alan Rickinson, Richard Ambinder, and Ernest Borden for providing valuable reagents for this work. We also thank Shannon Kenney and Nancy Raab-Traub for critical reading of the manuscript, Matt Davenport and Val Zacny for editorial help, and Lihong Wu for technical work.
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (AI 42372-01) and from the National Cancer Institute (CA 19014). L.Z. was supported by NIH Individual National Research Service Award 5F 32 CA67433.
REFERENCES
- 1.Abbot S D, Rowe M, Cadwallader K, Ricksten A, Gordon J, Wang F, Rymo L, Rickinson A B. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J Virol. 1990;64:2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adldinger H K, Delius H, Freese U K, Clarke J, Bornkamm G W. A putative transforming gene of Jijoye virus differs from that of Epstein-Barr virus prototypes. Virology. 1985;141:221–234. doi: 10.1016/0042-6822(85)90253-3. [DOI] [PubMed] [Google Scholar]
- 3.Aman P, von Gabain A. An Epstein-Barr virus immortalization associated gene segment interferes specifically with the IFN-induced anti-proliferative response in human B-lymphoid cell lines. EMBO J. 1990;9:147–152. doi: 10.1002/j.1460-2075.1990.tb08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Au W C, Moore P A, LaFleur D W, Tombal B, Pitha P M. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998;273:29210–29217. doi: 10.1074/jbc.273.44.29210. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey J M, Cohen M M, Bentwith Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt-like” malignant lymphoma (line D.G.-75) Int J Cancer. 1977;19:27–33. doi: 10.1002/ijc.2910190105. [DOI] [PubMed] [Google Scholar]
- 6.Calender A, Billaud M, Aubry J P, Banchereau J, Vuillaume M, Lenoir G M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci USA. 1987;84:8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen F, Zou J-Z, DiRenzo L, Winberg G, Hu L-F, Klein E, Klein G, Ernberg I. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP-1. J Virol. 1995;69:3752–3758. doi: 10.1128/jvi.69.6.3752-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J I, Wang F, Mannick J, Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci USA. 1992;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras-Brodin B A, Anvret M, Imreh S, Altiok E, Klein G, Masucci M G. B cell phenotype-dependent expression of the Epstein-Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J Gen Virol. 1991;72:3025–3033. doi: 10.1099/0022-1317-72-12-3025. [DOI] [PubMed] [Google Scholar]
- 10.Davenport M, Pagano J S. Expression of EBNA-1 mRNA is regulated by cell-cycle during Epstein-Barr virus type I latency. J Virol. 1999;73:3154–3161. doi: 10.1128/jvi.73.4.3154-3161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Cunha J, Ramanujam S, Wagner R J, Witt P L, Knight E J, Borden E C. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157:4100–4108. [PubMed] [Google Scholar]
- 12.Floettmann J E, Ward K, Rickinson A B, Rowe M. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology. 1996;223:29–40. doi: 10.1006/viro.1996.0452. [DOI] [PubMed] [Google Scholar]
- 13.Fries K L, Miller W E, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furnari F B, Adams M D, Pagano J S. Regulation of the Epstein-Barr virus DNA polymerase gene. J Virol. 1992;66:2837–2845. doi: 10.1128/jvi.66.5.2837-2845.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh D, Kieff E. cis-acting regulatory elements near the Epstein-Barr virus latent-infection membrane protein transcriptional start site. J Virol. 1990;64:1855–1858. doi: 10.1128/jvi.64.4.1855-1858.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory C D, Dive C, Henderson S, Smith C A, Williams G T, Gordon J, Rickinson A B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 18.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus (EBV)-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 19.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 20.Hammerschmidt W, Sugden B, Baichwal V R. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J Virol. 1989;63:2469–2475. doi: 10.1128/jvi.63.6.2469-2475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993;259:971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- 22.Henderson S, Rowe M, Gregory C, D. C-C, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 23.Iida S, Rao P H, Butler M, Corradini P, Boccadoro M, Klein B, Chaganti R S, Dalla-Favera R. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat Genet. 1997;17:226–230. doi: 10.1038/ng1097-226. [DOI] [PubMed] [Google Scholar]
- 24.Kanda K, Decker T, Aman P, Wahlstrom M, von Gabain A, Kallin B. The EBNA2-related resistance towards alpha interferon (IFN-α) in Burkitt's lymphoma cells effects induction of IFN-induced genes but not the activation of transcription factor ISGF-3. Mol Cell Biol. 1992;12:4930–4936. doi: 10.1128/mcb.12.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 2343–2396. [Google Scholar]
- 26.Kikuta H. IFN production by Epstein-Barr virus in human mononuclear leukocytes and suppression of the virus-induced transformation by the endogenous interferon. Hokkaido Igaku Zasshi. 1986;61:46–57. [PubMed] [Google Scholar]
- 27.Klein G, Dombos L, Gothoskar B. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int J Cancer. 1972;10:44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- 28.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 29.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P M, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 30.Ma W, Lehner P J, Cresswell P, Pober J S, Johnson D R. Interferon-gamma rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. J Biol Chem. 1997;272:16585–16590. doi: 10.1074/jbc.272.26.16585. [DOI] [PubMed] [Google Scholar]
- 31.Mann K P, Staunton D, Thorley-Lawson D. Epstein-Barr-encoded protein found in plasma membranes of transformed cells. J Virol. 1985;55:710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marie I, Durbin J E, Levy D E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin J M, Veis D, Korsmeyer S J, Sugden B. Latent membrane protein of Epstein-Barr virus induces cellular phenotypes independently of expression of Bcl-2. J Virol. 1993;67:5269–5278. doi: 10.1128/jvi.67.9.5269-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller W E, Earp H S, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyashita E M, Yang B, Babcock G J, Thorley-Lawson D A. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyashita E M, Yang B, Lam K M C, Crawford D H, Thorley-Lawson D A. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen H, Hiscott J, Pitha P M. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen H, Mustafa A, Hiscott J, Lin R. Transcription factor IRF-2 exerts its oncogenic phenotype through the DNA binding/transcription repression domain. Oncogene. 1995;11:537–544. [PubMed] [Google Scholar]
- 39.Nonkwelo C, Ruf I K, Sample J. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palombella V J, Maniatis T. Inducible processing of interferon regulatory factor 2. Mol Cell Biol. 1992;12:3325–3336. doi: 10.1128/mcb.12.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu L, Rowe D T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragona G, Ernberg I, Klein G. Induction and biological characterization of the Epstein-Barr virus carried by the Jijoye lymphoma cell line. Virology. 1980;101:553–557. doi: 10.1016/0042-6822(80)90473-0. [DOI] [PubMed] [Google Scholar]
- 43.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 2397–2446. [Google Scholar]
- 44.Rodriguez A M, Mallet V, Lenfant F, Arnaud J, Girr M, Urlinger S, Bensussan A, Le Bouteiller P. Interferon-gamma rescues HLA class Ia cell surface expression in term villous trophoblast cells by inducing synthesis of TAP proteins. Eur J Immunol. 1997;27:45–54. doi: 10.1002/eji.1830270108. [DOI] [PubMed] [Google Scholar]
- 45.Rooney C M, Gregory C D, Rowe M, Finerty S, Edwards C, Rupani H, Rickinson A B. Endemic Burkitt's lymphoma: phenotypic analysis of tumor biopsy cells and of derived tumor cell lines. J Natl Cancer Inst. 1986;77:681–687. doi: 10.1093/jnci/77.3.681. [DOI] [PubMed] [Google Scholar]
- 46.Rowe M, Peng-Pilon M, Huen D S, Hardy R, Croom-Carter D, Lundgren E, Rickinson A B. Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: a B-cell-specific response that is delayed relative to NF-κB activation and to induction of cell surface markers. J Virol. 1994;68:5602–5612. doi: 10.1128/jvi.68.9.5602-5612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruf I K, Moghaddam A, Wang F, Sample J. Mechanisms that regulate Epstein-Barr virus EBNA-1 gene transcription during restricted latency are conserved among lymphocryptoviruses of Old World primates. J Virol. 1999;73:1980–1989. doi: 10.1128/jvi.73.3.1980-1989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sample J, Henson E, Sample C. The Epstein-Barr virus nuclear protein 1 promoter active in type I latency is autoregulated. J Virol. 1992;66:4654–4661. doi: 10.1128/jvi.66.8.4654-4661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer B C, Paulson E, Strominger J L, Speck S H. Constitutive activation of Epstein-Barr virus (EBV) nuclear antigen 1 gene transcription by IRF1 and IRF2 during restricted EBV latency. Mol Cell Biol. 1997;17:873–886. doi: 10.1128/mcb.17.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer B C, Strominger J L, Speck S H. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaefer B C, Strominger J L, Speck S H. Host-cell-determined methylation of specific Epstein-Barr virus promoters regulates the choice between distinct viral latency programs. Mol Cell Biol. 1997;17:364–377. doi: 10.1128/mcb.17.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sung N S, Kenney S, Gutsch D, Pagano J S. EBNA-2 transactivates a lymphoid specific enhancer in the BamHI C promoter of Epstein-Barr virus. J Virol. 1991;65:2164–2169. doi: 10.1128/jvi.65.5.2164-2169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung N S, Wilson J, Davenport M, Sista N D, Pagano J S. Reciprocal regulation of the Epstein-Barr virus BamHI-F promoter by EBNA-1 and an E2F transcription factor. Mol Cell Biol. 1994;14:7144–7152. doi: 10.1128/mcb.14.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung N S, Wilson J, Pagano J S. Characterization of cis-acting elements of the BamHI-F promoter of EBV. In: Tursz T, et al., editors. The Epstein-Barr virus and associated diseases. London, England: INSERM/John Libbey Eurotext Limited; 1993. pp. 239–242. [Google Scholar]
- 57.Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt's lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 58.Tao Q, Robertson K D, Manns A, Hildesheim A, Ambinder R F. The Epstein-Barr virus major latent promoter Qp is constitutively active, hypomethylated, and methylation sensitive. J Virol. 1998;72:7075–7083. doi: 10.1128/jvi.72.9.7075-7083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tierney R, Steven N, Young L, Rickinson A. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7378. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomkinson B, Robertson E, Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsang S F, Wang F, Izumi K M, Kieff E. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J Virol. 1991;65:6765–6771. doi: 10.1128/jvi.65.12.6765-6771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, Leibowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 63.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, Tsang S F, Kurilla M G, Cohen J I, Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990;64:3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshizaki T, Sato H, Furukawa M, Pagano J S. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci USA. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young L, Alfieri C, Hennessy K, Evans H, OHara C, Anderson K C, Ritz J, Shapiro R S, Rickinson A, Kieff E, Cohen J I. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Pagano J S. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III Latency. Mol Cell Biol. 1999;19:3216–3223. doi: 10.1128/mcb.19.4.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]