Summary
Background
Acute rheumatic fever is infrequently diagnosed in sub-Saharan African countries despite the high prevalence of rheumatic heart disease. We aimed to determine the incidence of acute rheumatic fever in northern and western Uganda.
Methods
For our prospective epidemiological study, we established acute rheumatic fever clinics at two regional hospitals in the north (Lira district) and west (Mbarara district) of Uganda and instituted a comprehensive acute rheumatic fever health messaging campaign. Communities and health-care workers were encouraged to refer children aged 3–17 years, with suspected acute rheumatic fever, for a definitive diagnosis using the Jones Criteria. Children were referred if they presented with any of the following: (1) history of fever within the past 48 h in combination with any joint complaint, (2) suspicion of acute rheumatic carditis, or (3) suspicion of chorea. We excluded children with a confirmed alternative diagnosis. We estimated incidence rates among children aged 5–14 years and characterised clinical features of definite and possible acute rheumatic fever cases.
Findings
Data were collected between Jan 17, 2018, and Dec 30, 2018, in Lira district and between June 5, 2019, and Feb 28, 2020, in Mbarara district. Of 1075 children referred for evaluation, 410 (38%) met the inclusion criteria; of these, 90 (22%) had definite acute rheumatic fever, 82 (20·0%) had possible acute rheumatic fever, and 24 (6%) had rheumatic heart disease without evidence of acute rheumatic fever. Additionally, 108 (26%) children had confirmed alternative diagnoses and 106 (26%) had an unknown alternative diagnosis. We estimated the incidence of definite acute rheumatic fever among children aged 5–14 years as 25 cases (95% CI 13·7–30·3) per 100 000 person-years in Lira district (north) and 13 cases (7·1–21·0) per 100 000 person-years in Mbarara district (west).
Interpretation
To the best of our knowledge, this is the first population-based study to estimate the incidence of acute rheumatic fever in sub-Saharan Africa. Given the known rheumatic heart disease burden, it is likely that only a proportion of children with acute rheumatic fever were diagnosed. These data dispel the long-held hypothesis that the condition does not exist in sub-Saharan Africa and compel investment in improving prevention, recognition, and diagnosis of acute rheumatic fever.
Funding
American Heart Association Children’s Strategically Focused Research Network Grant, THRiVE-2, General Electric, and Cincinnati Children’s Heart Institute Research Core.
Introduction
The 2018 World Health Assembly endorsed a global resolution on rheumatic heart disease that called for renewed efforts to prevent acute rheumatic fever. Among the resolution’s priorities, enhanced surveillance for acute rheumatic fever was identified as an urgent need.1 Although echocardiography-based screening studies have improved our understanding of the prevalence of rheumatic heart disease in African countries over the past decade,2–4 no studies have been published on the incidence of acute rheumatic fever during this period.5,6 The absence of published data has been supported anecdotally by the perception that acute rheumatic fever is rarely seen in health-care settings in sub-Saharan Africa. However, this opinion is inconsistent with known high prevalence rates of rheumatic heart disease.6 Accurate profiling of acute rheumatic fever epidemiology and clinical features in Africa will inform strategies to improve diagnosis (and thus, implementation of secondary prevention), control programmes, and planning for primary prevention approaches including future vaccines. By contrast, should acute rheumatic fever be rare in Africa, this would open new avenues for research to explore novel mechanisms of developing rheumatic heart disease that have not been previously described.
Identification of acute rheumatic fever in sub-Saharan African countries is often fraught with diagnostic challenges. Diagnosis, which requires a combination of clinical, laboratory, electrocardiography, and echocardiography data, is rare in comparison to rheumatic heart disease diagnosis,7 which only requires echocardiography data.6 Community and health worker awareness of acute rheumatic fever is low, and the diagnostics required to confirm the condition are frequently absent outside of tertiary referral hospitals.8–11 As a result, only a small fraction of children with acute rheumatic fever receive a diagnosis, and case ascertainment is biased towards those with symptoms severe enough to require sub-specialty care.4 Furthermore, most medical records are paper-based and are kept by patients rather than being captured and stored electronically, making surveillance programmes based on medical records nearly impossible.
In 2017, to overcome these challenges, we piloted a district-wide acute rheumatic fever surveillance programme within Lira district in northern Uganda. Our pilot programme included health education, health worker training, and establishment of a clinic equipped with laboratory and imaging resources needed to diagnose acute rheumatic fever at the district level. Pilot study data were collected for 6 months12 and informed the design of the present study, the main aim of which was to estimate the incidence of acute rheumatic fever among children aged 5–14 years using a community-based approach over a period of 12 months. Our secondary aim was to describe the clinical characteristics of children presenting with definite and possible acute rheumatic fever.
Methods
Study design and participants
We prospectively recruited children with suspected acute rheumatic fever to participate in this population-based study. Uganda is an equatorial, sub-Saharan country, home to approximately 44 million people, of whom more than half are younger than age 18 years.13 The country has four ethnically and geographically distinct regions, including the northern, central, eastern, and western regions. For our primary objective of acute rheumatic fever incidence, we collected data from sites in two of these regions: Lira district (northern) and Mbarara district (western). The two districts in which our study was based were chosen because they were geographically distinct and had established research infrastructure as host sites of the National Rheumatic Heart Disease Registry.
We set up a single acute rheumatic fever clinic at the respective regional referral hospitals in each of these two districts and did community sensitisation (mass media education about the signs and symptoms of the condition) and health worker education as previously described.12 The methods for case referral and enrolment in our district sites (Lira and Mbarara) have previously been described in detail.12 In brief, front-line providers, educators, or community members were encouraged to refer any child between the ages of 3 and 17 years who presented with any of the following: (1) history of fever within the past 48 h in combination with any joint complaint, (2) suspicion of acute rheumatic carditis, or (3) suspicion of chorea. Research staff accepted phone referrals (toll-free number) and walk-in evaluations. Children were excluded from participation if they had a confirmed alternative diagnosis (ie, malaria, sickle cell crisis, or typhoid fever) that accounted for their signs and symptoms. Children aged 8 years or older signed written informed assent and the parent or guardian of all participants signed written informed consent, before clinical evaluation. This study was approved by the Makerere University School of Medicine Research and Ethics Committee (2017–042), Children’s National Hospital (#9096), and the Uganda National Council for Science and Technology, and used the STROBE criteria for reporting epidemiological studies. Details of a pilot study have been previously described.12
Procedures
Components of data collection at the acute rheumatic fever clinics have been previously described.12 In brief, extensive history of present illness, past medical history, and family history information were obtained on enrolment. A complete physical examination, with particular attention to the features of acute rheumatic fever listed in the Jones Criteria (panel),7 was undertaken by trained study staff. Point-of-care testing for HIV (Determine, Abbott, Chicago, IL, USA), malaria (CareStart, Apacor, Berkshire, England), and group A streptococcus (ThermoFisher Scientific rapid antigen detection kit, Waltham, MA, USA) was done. Blood samples were sent out for complete blood count, erythrocyte sedimentation rate, C-reactive protein, malaria blood smear, antistreptolysin O titres, and antideoxyribonuclease B titres, and were determined by standard methodology.12 Participants gave a pharyngeal swab for group A streptococcus culture and a nasopharyngeal swab for influenza PCR. Cardiac testing included 12-lead electrocardiograms (ECG) and limited echocardiograms (GE Vivid I/Q, Chicago IL, USA) focused on the left-sided heart valves, left ventricular systolic function, and pericardium (to assess for effusion). If chorea was suspected, a comprehensive neurological examination was undertaken and recorded on video. If skin findings were noted, these were photographed. ECG, echocardiogram, skin photographs, and neurological videos were shared via telemedicine connections with cardiologists, dermatologists, and neurologists, as appropriate, in the USA.12 Point-of-care results were shared with the referring provider or hospital outpatient clinic who guided immediate care. Participants were asked to return to the clinic after 7 days to receive final testing results and treatment recommendations.
Panel: 2015 Jones Criteria for the diagnosis of acute rheumatic fever in moderate-risk and high-risk populations*.
Acute rheumatic fever diagnosis requires evidence of recent streptococcal infection and either two major criteria or one major and two minor criteria.
Major criteria
Carditis (clinical or subclinical)†
Monoarthritis, polyarthritis, or polyarthralgia
Chorea
Erythema marginatum
Subcutaneous nodules
Minor criteria
Prolonged PR interval on electrocardiograms‡
Monoarthralgia
Fever (≥38·0°C)
Peak erythrocyte sedimentation rate ≥30 mm/h or C-reactive protein ≥3·0 mg/dL
Moderate-risk or high-risk is defined as acute rheumatic fever incidence >2 cases per 100 000 school-aged children or rheumatic heart disease prevalence of >1 case per 1000 population year.
Seen on echocardiography without auscultatory findings.
Accounting for age variability and only if carditis is not counted as a major criterion.
The 2015 Jones Criteria7 for moderate-risk and high-risk populations (panel) were used to adjudicate each study participant’s clinical information and categorise them as definite acute rheumatic fever, possible acute rheumatic fever, known alternative diagnosis, or unknown alternative diagnosis. Definite acute rheumatic fever diagnosis was made strictly according to the Jones Criteria. Possible acute rheumatic fever diagnosis was defined as evidence of past streptococcal infection and partial fulfilment of the Jones Criteria, having one major and only one, instead of two, minor criteria. Children were classified as having a known alternative diagnosis if they had laboratory confirmation of an alternative diagnosis. All others were classified as unknown alternative diagnosis. Since very few patients had a previous history of acute rheumatic fever or rheumatic heart disease, we did not attempt to further classify acute rheumatic fever into new or recurrent categories. We used the 80% upper limit of normal as determining cutoffs for elevated antistreptolysin O (≥389 IU/mL) and antideoxyribo nuclease B (≥586 IU/mL) titres.14
We sought to generate representative estimates of age-specific incidence of acute rheumatic fever in two different districts that reflected relatively low socio economic status (north) and relatively middle socioeconomic status (western) populations in the country. Our study design aimed for a census sample of participants (ie, to recruit all children with new acute rheumatic fever over the study periods in the two districts), although we acknowledge the difficulties in achieving a true census sample in this context. In Lira district, we estimated that there were 123 229 children between the ages of 5 years and 14 years at risk and in Mbarara district, we estimated that there were 107 050 children between the ages of 5 years and 14 years at risk.
The primary outcome of the study was to estimate acute rheumatic fever incidence in a rheumatic heart disease endemic country in sub-Saharan Africa, for children aged 5–14 years, using a community-based approach within two districts that were representative of the variation in socioeconomic status across Uganda. Our secondary outcome was to describe the clinical characteristics of children presenting with definite and possible acute rheumatic fever within the two named districts in Uganda.
Statistical analysis
Data were entered by local study staff into the Research Electronic Data Capture (REDCap) database hosted at the Children’s National Hospital.15 We used descriptive statistics to analyse the baseline characteristics, stratified by clinical enrolment location. Categorical data are presented as absolute values and percentage, and continuous data as median and interquartile range.
We calculated the presenting incidence of acute rheumatic fever separately for the Lira and Mbarara districts and provided 95% CIs by use of Poisson distribution. All children with definite or possible acute rheumatic fever who identified Lira or Mbarara as their primary residence and were between the ages of 5 years and 14 years at diagnosis were included in the numerator. Incidence calculations were limited to the ages of 5–14 years to allow for comparison to the commonly reported age bracket from other international sites. The last country-wide census, done by the Ugandan Bureau of Statistics in 2014,16 was used to determine the total number of children aged 5–14 years in Lira and Mbarara districts. Any participant who identified another district as their primary residence was not included in the incidence calculation. All incidence data are reported with 95% CIs. To account for the possibility of missing data, we planned to do multiple imputations in cases for which more than 10% of the data were missing.
Statistical analysis was done with MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Data were collected over 12 months in Lira district (Jan 17–Dec 30, 2018) and over 9 months (June 5, 2019–Feb 28, 2020) in Mbarara district. We had planned to collect data over 12 months in Mbarara district, but we closed enrolment early because research activities were suspended in March, 2020, owing to the COVID-19 pandemic.
Over the duration of the enrolment periods, 1075 children presented for evaluation (545 from Lira and 530 from Mbarara). Of these, 410 children (38%) met the inclusion criteria and were evaluated for acute rheumatic fever: 264 (64%) in Lira district and 146 (36%) in Mbarara district. The majority were enrolled with fever and joint pain as the presenting symptoms (322 [79%] of 410), with a minority presenting with suspicion of rheumatic carditis (81 [20%] of 410), or suspicion of chorea (6 [1%] of 410). 206 (50%) of the patients evaluated were female and 204 (50%) were male. Very few patients reported a past diagnosis of acute rheumatic fever (4 [1%] of 410) or rheumatic heart disease (4 [1%] of 410). Additional information on the demographics and clinical history of the cohort can be found in table 1.
Table 1:
Demographics and clinical history of children presenting with suspected acute rheumatic fever, by enrolment site
| Lira district (n=264) | Mbarara district (n=146) | Total (n=410) | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Sex | |||
| Male | 127 (48%) | 77 (53%) | 204 (50%) |
| Female | 137 (52%) | 69 (47%) | 206 (50%) |
| Age, years | 10·0 (6·8–13·0) | 10·0 (6·0–12·0) | 10·0 (6·5–12·6) |
| Housing, semi-permanent | 141 (53%) | 65 (45%) | 206 (50%) |
| Number of people in household | 6·0 (5·0–8·0) | 5·5 (4·0–7·0) | 5·8 (4·8–7·8) |
| Number of children younger than age 15 years in household | 3·0 (2·0–4·0) | 3·0 (2·0–4·0) | 3·0 (2·0–4·0) |
| Attendance to any type of school | 224 (85%) | 131 (90%) | 355 (87%) |
| Attendance to boarding school | 34 (13%) | 21 (14%) | 55 (13%) |
| Clinical history | |||
| Sore throat in past 4 weeks | 71 (27%) | 67 (46%) | 138 (34%) |
| Skin infection in past 4 weeks | 30 (11%) | 20 (14%) | 50 (12%) |
| Previous history of acute rheumatic fever | 1 (<1%) | 3 (2%) | 4 (1%) |
| Previous history of rheumatic heart disease | 1 (<1%) | 3 (2%) | 4 (1%) |
| HIV-positive status | 5 (2%) | 1 (1%) | 6 (1%) |
| Family member with acute rheumatic fever or rheumatic heart disease | 11 (4%) | 6 (4%) | 17 (4%) |
Data are n (%), or median (IQR).
Of 410 children evaluated for suspected acute rheumatic fever, 90 (22%) were diagnosed with definite acute rheumatic fever (60 [23%] of the 264 that met the inclusion criteria in Lira, and 30 [21%] of the 146 that met the inclusion criteria in Mbarara) and 82 (20%) with possible acute rheumatic fever (58 [22%] of 264 in Lira, and 24 [16%] of 146 in Mbarara). Another 24 (6%) had rheumatic heart disease without evidence of acute rheumatic fever reoccurrence (14 [5%] of 264 in Lira, and ten [7%] of 146 in Mbarara), 108 (26%) had confirmed alternative diagnoses, and 106 (26%) had an unknown alternative diagnosis. Of the 24 children diagnosed with rheumatic heart disease, only four (17%) were previously aware of their diagnosis. Nearly half of the children diagnosed with acute rheumatic fever were excluded from incidence calculations on the basis of age or district of residence (figure 1 and figure 2).
Figure 1: Study profile for participants in Lira and Mbarara districts.
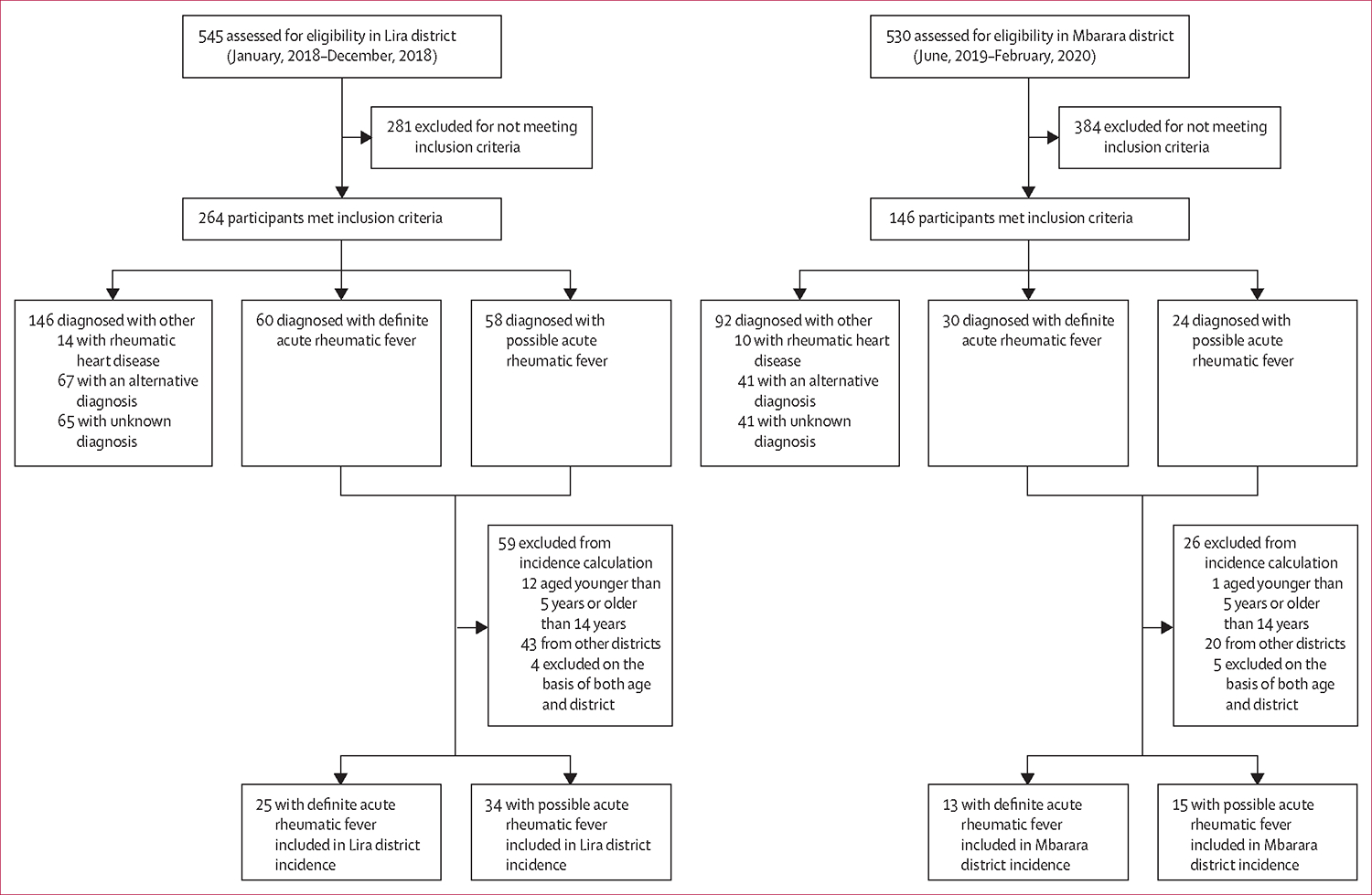
Figure 2: Distribution of primary residence of definite acute rheumatic fever cases by enrolment site.
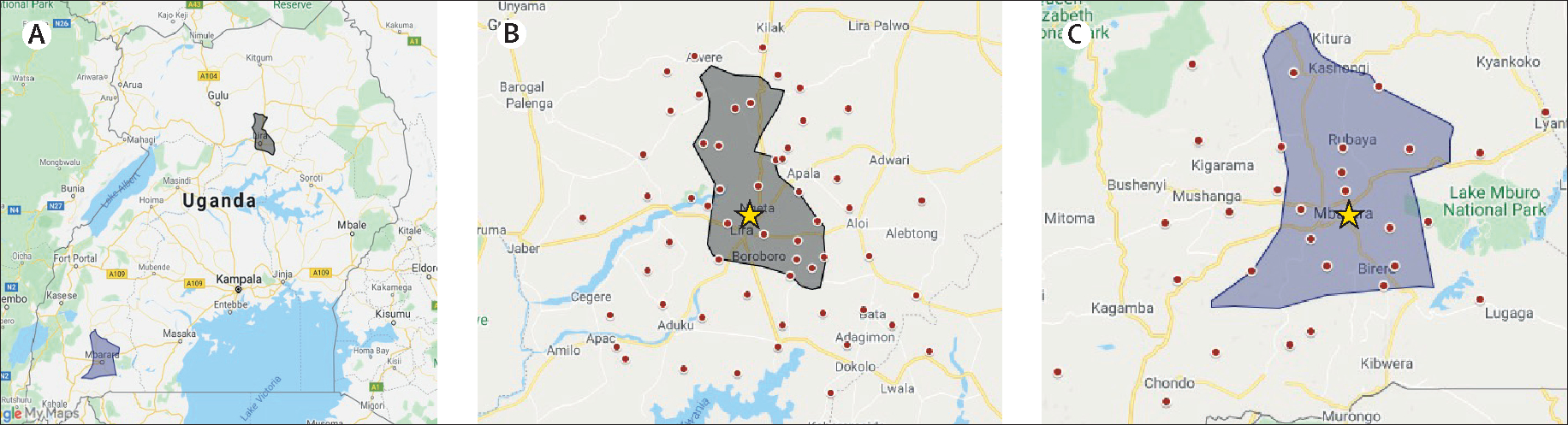
(A) Location of participating Ugandan districts. (B) Distribution of definite acute rheumatic fever cases enrolled at Lira Regional Referral Hospital (yellow star). (C) Distribution of definite acute rheumatic fever cases enrolled at Mbarara Regional Referral Hospital (yellow star). Each red dot represents a case of definite acute rheumatic fever. Dots outside of the grey shaded areas (district boundaries) were excluded from the incidence calculation.
The incidence of definite acute rheumatic fever in Lira district was 25 cases (95% CI 13·7–30·3) per 100 000 person-years at risk aged 5–14 years and the incidence of any acute rheumatic fever (possible or definite) in Lira district was 47·9 cases (37·1–61·8) per 100 000 children aged 5–14 years. This value was higher than the incidence in Mbarara district, which was calculated to be 13 cases (95% CI 7·1–21·0) per 100 000 person-years at risk for definite acute rheumatic fever and 26·2 cases (18·1–37·9) per 100 000 children for any acute rheumatic fever (table 2).
Table 2:
Incidence of acute rheumatic fever by enrolment site for children aged 5–14 years
| Lira district | Mbarara district | |
|---|---|---|
|
| ||
| District population | 408 043 | 472 629 |
| Person-years at risk | 408 043 | 354 472 |
| District population years at risk (5–14 years)* | 123 229 | 107 050 |
| In-district definite acute rheumatic fever cases in age range | 25 | 13 |
| Definite acute rheumatic fever incidence, cases per 100 000 person-years (95% CI) | 20·2 (13·7–30·0) | 12·1 (7·1–21·0) |
| In-district possible acute rheumatic fever cases in age range | 34 | 15 |
| Possible acute rheumatic fever incidence, cases per 100 000 person-years (95% CI) | 27·6 (19·7–38·6) | 14·0 (8·5–23·2) |
| Combined definite and possible acute rheumatic fever incidence, cases per 100 000 person-years (95% CI) | 47·9 (37·1–61·8) | 26·2 (18·1–37·9) |
In the 2014 Uganda Census,16 the percentage of the population estimated to be aged 5–14 years was 30·2%.
The demographic, laboratory features, and clinical features of children diagnosed with definite and possible acute rheumatic fever are summarised in table 3. The median age of definite acute rheumatic fever diagnosis was 9·0 years (IQR 7·0–12·0).
Table 3:
Baseline demographics and clinical characteristics associated with definite and possible acute rheumatic fever
| Definite acute rheumatic fever (n=90) | Possible acute rheumatic fever (n=82) | |
|---|---|---|
|
| ||
| Sex | ||
| Male | 42 (47%) | 39 (48%) |
| Female | 48 (53%) | 43 (52%) |
| Age | ||
| Median (IQR) | 9(7–12) | 10 (8–12) |
| Evidence of recent streptococcal infection | ||
| Positive group A streptococcus throat culture | 3 (3%) | 3 (4%) |
| Antistreptolysin O titres (>80% of normal range for age) | 60 (67%) | 23 (28%) |
| Antideoxyribonuclease B titres (>80% of normal range for age) | 68 (76%) | 45 (55%) |
| Antistreptolysin O titres (>80% of normal range for age) and antideoxyribonuclease B titres (>80% of normal range for age) | 46 (51%) | 11 (13%) |
| Joint manifestations | ||
| Any joint manifestation | 77 (86%) | 78 (95%) |
| Polyarthritis | 26 (29%) | 11 (13%) |
| Polyarthralgia | 45 (50%) | 54 (66%) |
| Monoarthritis | 4 (4%) | 1 (1%) |
| Monoarthralgia | 2 (2%) | 12 (15%) |
| Carditis | ||
| Any type of carditis | 44 (49%) | 1 (1%) |
| Mild | 7 (8%) | 1 (1%) |
| Moderate or severe | 37 (41%) | 0 |
| Normal echocardiogram with prolonged PR interval | 6 (7%) | 3 (4%) |
| Chorea | 7 (8%) | 0 |
| Subcutaneous nodules | 0 | 1 (1%) |
| Erythema marginatum | 0 | 0 |
| Fever | ||
| Any type of fever | 84 (93%) | 65 (79%) |
| Subjective | 74 (82%) | 50 (61%) |
| Objective | 10 (11%) | 15 (18%) |
| Elevated markers of inflammation | ||
| C-reactive protein (≥5 mg/dL) | 58 (64%) | 11 (13%) |
| Erythrocyte sedimentation rate (≥30 mm/h) | 46 (51%) | 8 (10%) |
Data are n (%) unless otherwise indicated.
All children presenting with definite acute rheumatic fever had at least one elevated streptococcal antibody titre, with about half (51%) showing elevation of both antideoxyribonuclease B titres and antistreptolysin O titres. Although three children with definite acute rheumatic fever had a positive pharyngeal culture for group A streptococcus, they also showed elevations of streptococcal antibodies.
Joint manifestations were very common in this cohort. Among those with definite acute rheumatic fever, polyarthralgia was the most common presenting manifestation (45 [50%] of 90), followed by polyarthritis (26 [29%] of 90), with a smaller number of patients presenting with monoarthritis (4 [4%] of 90). Almost half of the children with definite acute rheumatic fever showed echocardiographic evidence of carditis (44 [49%] of 90), with most cases having moderate or severe cardiac involvement (37 [84%] of 44). Prolonged PR interval on ECG, in the absence of echocardiographic carditis, occurred in 7% of cases with definite acute rheumatic fever. Chorea was exceedingly rare in this cohort, with only seven children (8%) with definite acute rheumatic fever presenting with this feature. Subcutaneous nodules and erythema marginatum, both major criteria for diagnosis, were absent in this population.
Fever was very common (present in >90% with definite acute rheumatic fever), but most children reported a tactile or subjective fever, with no objective confirmation. C-reactive protein was elevated more commonly than was erythrocyte sedimentation rate (64% vs 51%) among children with definite acute rheumatic fever. Lack of elevation in inflammatory markers was the most common reason that children were assigned a diagnosis of possible rather than definite acute rheumatic fever (61 [74%] of 82). Many children reported use of anti-inflammatory medication before acute rheumatic fever evaluation (217 [53%] of 410), with most (163 [75%] of 217) receiving a non-steroidal medication, a minority (17 [8%] of 217) receiving a corticosteroid, and 37 (17%) of 217 receiving an unknown medication. 52 (58%) of 90 with definite acute rheumatic fever and 46 (56%) of 82 with possible acute rheumatic fever reported use of these medications.
Discussion
We present, to the best of our knowledge, the only acute rheumatic fever incidence data from sub-Saharan Africa. These data suggest that acute rheumatic fever, as diagnosed by the Jones Criteria for moderate-risk or high-risk settings, is present at high incidence in two distinct communities in Uganda, with a range of 13 cases per 100 000 person-years to 25 cases per 100 000 person-years, aged 5–14 years. These incidence rates increase substantially if possible acute rheumatic fever is included: 26·2 cases per 100 000 children to 47·9 cases per 100 000 children, aged 5–14 years.
The mean incidence of acute rheumatic fever globally is 19 cases per 100 000 school-aged children.5 However, the global mean is driven by extremely high rates in low-resource settings such as Indigenous Australia (193 cases per 100 000 school-aged children)17 compensating for extremely low rates in high-income countries such as the USA (two cases per 100 000).18 There are almost no contemporary data on acute rheumatic fever incidence in Africa. In a 2010 systematic review, only three countries in WHO’s Africa region had any source data on acute rheumatic fever incidence since the 1970s, with none of these countries from sub-Saharan Africa.19
Although the acute rheumatic fever incidence (13 cases per 100 000 person-years to 25 cases per 100 000 person-years) we found in Uganda is very high compared with well-resourced populations globally, it is lower than we might have anticipated given that between 1500 and 2500 of every 100 000 Ugandan youths shows echocardiographic evidence of rheumatic heart disease.2 The rates of acute rheumatic fever detected are probably an underestimate, because the condition is not commonly diagnosed in these communities. Achieving this level of diagnosis required intensive community mobilisation and education, including addressing use of traditional medicine,20 establishing clinics with the diagnostic resources to evaluate cases of suspected acute rheumatic fever, and health-care worker education to increase referrals. Although other settings with similar rheumatic heart disease burdens—Australia, Fiji, and New Zealand as the best examples—have found higher acute rheumatic fever incidence, they have benefited from established clinical care, better record-keeping and diagnostic capabilities, and better-established surveillance systems embedded broadly across their public health system. However, underdiagnosis remains a considerable challenge, with a Fijian study estimating that for every case of acute rheumatic fever diagnosed, there was probably another one or two cases missed.21 In the current study, we cannot determine the number of Ugandan children with acute rheumatic fever who did not present to clinic for evaluation, and these incidence figures should be considered the minimum acute rheumatic fever incidence for these districts.
Gaps between acute rheumatic fever incidence and rheumatic heart disease prevalence also emphasise the weaknesses of current diagnostic criteria for acute rheumatic fever, which rely on fulfilling a series of clinical, laboratory, and imaging features—features that have changed very little since their initial publication in the 1940s.7 We are currently investigating whether modern multi-omics techniques might identify an acute rheumatic fever biological signature to aid diagnosis, which remains a clinical diagnosis.
The clinical presentation of definite acute rheumatic fever in this study was less severe on average, than previously reported. Overall, the percentage of children with carditis and chorea were slightly lower than previously reported rates from Africa: 45% versus 63% for carditis, and 6% versus 9% for chorea.22 However, of children with definite acute rheumatic fever with carditis in this study, more than 80% had moderate or severe valvular involvement. Although moderate and severe valvular damage can occur with the first acute rheumatic fever episode, the condition (along with rheumatic heart disease) is a progressive, cumulative process.6 The high percentage of more severe valvular involvement probably reflects missed previous episodes, as very few children (<2%) had a known history of acute rheumatic fever or rheumatic heart disease, making it challenging to accurately distinguish new from recurrent acute rheumatic fever. Notably, two of the five major criteria for acute rheumatic fever, subcutaneous nodules and erythema marginatum,7 were absent or nearly absent in this population, calling into question whether they should remain key diagnostic factors.
Joint manifestations were also less severe than those reported in previous studies from Africa, with only a quarter of patients presenting with polyarthritis, while two thirds of patients had polyarthralgia.7 Monoarthritis, also now considered a major criterion in moderate-risk and high-risk populations, was less common than reported from Australia, with less than 10% of children presenting with this compared with up to 30% in Australia’s Northern Territory.22 Because in 2015 the criteria changed to include monoarthritis, no previous data from Africa are available. These less severe manifestations probably reflect our active case finding strategy, which identified children with milder acute rheumatic fever symptoms who would have been missed in a tertiary hospital study.
This is the first study to our knowledge, other than our previously published pilot study, to use an active case finding strategy to diagnose acute rheumatic fever in the community. Our study shows that the epidemiological study of acute rheumatic fever in sub-Saharan Africa and other low-resource settings is possible by raising community and health-care worker awareness and by overcoming diagnostic challenges that are pervasive in these settings. Building local capacity for surveillance of acute rheumatic fever will require more research. We consulted a panel of experts including a cardiologist, infectious disease expert, dermatologist, and neurologist, to make a final diagnosis, which was more accurate and specific than could be expected in a general community health setting.
Follow-up of children diagnosed in this study will also provide additional data on outcomes of acute rheumatic fever in this context, and risk of rheumatic heart disease in children with possible acute rheumatic fever and unknown alternative diagnoses. Children diagnosed with definite acute rheumatic fever were placed on secondary antibiotic prophylaxis with intramuscular benzathine penicillin G and were enrolled into the Ugandan National Rheumatic Heart Disease Registry. Children with possible acute rheumatic fever were placed on secondary antibiotic prophylaxis for 12 months, with repeated echocardiographic evaluation scheduled for 1 year after the study end. Children with unknown alternative diagnosis were not prescribed secondary antibiotic prophylaxis but will be re-evaluated at 1 year with echocardiography and clinical review.
There are several limitations to this study that might have introduced some bias into our incidence values. First, we used an approximate denominator (from the 2014 census) to calculate incidence for children aged 5–14 years living in the primary enrolment districts. Second, the location of our clinics at the Regional Referral Hospitals could have introduced some selection bias, on the basis of distance to the clinic, although our case distribution maps do not show obvious clustering. However, we cannot know how many children with acute rheumatic fever were missed through our community enrolment strategy, and the incidence rates should be considered minimal estimates.
Another study limitation and potential confounder is that nearly half of the children presenting at the clinic had already received anti-inflammatory medication in the community. Anti-inflammatory medication can rapidly reduce joint symptoms and fever and lower markers of inflammation, all components of the Jones Criteria used for diagnosis, and could have reduced the number of children diagnosed with definite acute rheumatic fever.7 A specific diagnostic test might overcome some of these challenges, and a multi-omics approach is currently being employed by our team to identify a specific biological signature. Finally, whether children diagnosed with possible acute rheumatic fever, very few of whom had cardiac involvement at presentation, are at risk of developing rheumatic heart disease, the most consequential sequela of acute rheumatic fever, remains to be determined. Children with both definite and possible rheumatic heart disease were prescribed secondary antibiotic prophylaxis and will be followed up at 1 year to assess long-term outcomes.
Overall, we can conclude that acute rheumatic fever exists at an increased prevalence in low-resource settings such as Uganda, even though routine diagnosis remains uncommon. Although these incidence data have probably underestimated the cases of acute rheumatic fever in two districts, they show that opportunity exists to improve community sensitisation and health-care worker training to increase awareness of the condition. Ultimately, this leads to diagnosing more children with acute rheumatic fever before they develop rheumatic heart disease, so that they can be offered secondary prophylaxis. A group A streptococcus vaccine will probably be the most effective strategy to prevent rheumatic heart disease. Until that time, novel strategies are needed to improve acute rheumatic fever diagnosis in low-resource settings.
Research in context.
Evidence before this study
To date, there are no incidence data for acute rheumatic fever in sub-Saharan Africa. Studies from other geographical regions are generally decades old and based on retrospective review of clinical records from tertiary facilities, biasing towards more severe cases. Furthermore, there are no studies that used the 2015 revision of the Jones Criteria, which recognised the need for increased sensitivity in countries in which rheumatic heart disease is endemic and provided a separate set of criteria for moderate-risk and high-risk populations.
Added value of this study
Acute rheumatic fever incidence in western Uganda is 13 cases per 100 000 person-years and in northern Uganda is 25 cases per 100 000 person-years among children aged 5–14 years. When compared with estimates over the past 10 years of rheumatic heart disease prevalence in Uganda (1500–2500 cases per 100 000 children), the incidence is lower than expected.
Implications of all the available evidence
These data dispel the long-held hypothesis that acute rheumatic fever does not exist in sub-Saharan Africa. This study emphasises the challenge of acute rheumatic fever diagnosis and compels vigilance to improve and implement prevention efforts including primary prevention, streptococcal vaccine development, and continued study of early rheumatic heart disease diagnosis using echocardiography.
Acknowledgments
We would like to thank the participants, parents, and clinicians in Lira district and Mbarara district who participated in case identification and supported this study. This work was supported by the American Heart Association Children’s Strategically Focused Research Network Grant (#17SFRN33670607), DEL-15-011 to THRiVE-2, General Electric, and the Cincinnati Children’s Heart Institute Research Core.
Footnotes
Declaration of interests
TP reports grant funding from the National Institute for Health Research (ACF-2016–20-001). All other authors declare no competing interests.
Contributor Information
Emmy Okello, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda; Department of Medicine, Makerere University, Kampala, Uganda.
Emma Ndagire, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Babu Muhamed, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
Rachel Sarnacki, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
Meghna Murali, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
Jafesi Pulle, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Jenifer Atala, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Asha C Bowen, Wesfarmers Centre for Vaccines and Infectious Diseases, Telethon Kids Institute, and Department of Infectious Diseases, Perth Children’s Hospital, Nedlands, WA, Australia.
Marc P DiFazio, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
M G Nakitto, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Nada S Harik, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
Rosemary Kansiime, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Chris T Longenecker, Case Western Reserve University, Health Education Campus, Cleveland, OH, USA.
Peter Lwabi, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Collins Agaba, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Scott A Norton, Department of Dermatology, George Washington University School of Medicine and Health Sciences, Washington DC, USA.
Isaac Otim Omara, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Linda Mary Oyella, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Tom Parks, Wellcome Center for Human Genetics, The London School of Tropical Medicine and Hygiene, London, UK.
Joselyn Rwebembera, The Uganda Heart Institute, Mulago Hospital Complex, Kampala, Uganda.
Christopher F Spurney, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
Elizabeth Stein, University of Washington School of Medicine, Seattle, WA, USA.
Laura Tochen, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
David Watkins, Department of Medicine and Department of Global Health, University of Washington, Seattle, WA, USA.
Meghan Zimmerman, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA.
Jonathan R Carapetis, Wesfarmers Centre for Vaccines and Infectious Diseases, Telethon Kids Institute, and Department of Infectious Diseases, Perth Children’s Hospital, Nedlands, WA, Australia.
Craig A Sable, Makerere School of Health Sciences, Children’s National Hospital, Washington DC, USA.
Andrea Beaton, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; The University of Cincinnati School of Medicine, Cincinnati, OH, USA.
Data sharing
Deidentified participant data will be available through reasonable request to the corresponding author.
References
- 1.WHO. Rheumatic fever and rheumatic heart disease. Geneva: Switzerland, 2018.
- 2.Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation 2012; 125: 3127–32. [DOI] [PubMed] [Google Scholar]
- 3.Ekure EN, Amadi C, Sokunbi O, et al. Echocardiographic screening of 4107 Nigerian school children for rheumatic heart disease. Trop Med Int Health 2019; 24: 757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sims Sanyahumbi A, Sable CA, Beaton A, et al. School and community screening shows Malawi, Africa, to have a high prevalence of latent rheumatic heart disease. Congenit Heart Dis 2016; 11: 615–21. [DOI] [PubMed] [Google Scholar]
- 5.Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart 2008; 94: 1534–40. [DOI] [PubMed] [Google Scholar]
- 6.Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet 2018; 392: 161–74. [DOI] [PubMed] [Google Scholar]
- 7.Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation 2015; 131: 1806–18. [DOI] [PubMed] [Google Scholar]
- 8.Parks T, Kado J, Colquhoun S, Carapetis J, Steer A. Underdiagnosis of acute rheumatic fever in primary care settings in a developing country. Trop Med Int Health 2009; 14: 1407–13. [DOI] [PubMed] [Google Scholar]
- 9.Nkoke C, Luchuo EB, Jingi AM, Makoge C, Hamadou B, Dzudie A. Rheumatic heart disease awareness in the South West region of Cameroon: a hospital based survey in a sub-Saharan African setting. PLoS One 2018; 13: e0203864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman GM, Abdelrahman SM, Ali SK. Evaluation of physicians’ knowledge about prevention of rheumatic fever and rheumatic heart disease before and after a teaching session. Sudan J Paediatr 2015; 15: 37–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson S, Duber HC, Achan J, et al. Capacity for diagnosis and treatment of heart failure in sub-Saharan Africa. Heart 2017; 103: 1874–79. [DOI] [PubMed] [Google Scholar]
- 12.Okello E, Ndagire E, Atala J, et al. Active case finding for rheumatic fever in an endemic country. J Am Heart Assoc 2020; 9: e016053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Uganda: country profiles. Geneva: Switzerland, 2020.
- 14.Okello E, Murali M, Rwebembera J, et al. Cross-sectional study of population-specific streptococcal antibody titres in Uganda. Arch Dis Child 2020; 105: 825–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ugandan Bureau of Statistics. National Census main report. Kampala: Uganda, 2014. [Google Scholar]
- 17.Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation 2013; 128: 492–501. [DOI] [PubMed] [Google Scholar]
- 18.Miyake CY, Gauvreau K, Tani LY, Sundel RP, Newburger JW. Characteristics of children discharged from hospitals in the United States in 2000 with the diagnosis of acute rheumatic fever. Pediatrics 2007; 120: 503–08. [DOI] [PubMed] [Google Scholar]
- 19.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol 2011; 3: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein E, Pulle J, Zimmerman M, et al. Previous traditional medicine use for sore throat among children evaluated for rheumatic fever in northern Uganda. Am J Trop Med Hyg 2020; 104: 842–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steer AC, Kado J, Jenney AW, et al. Acute rheumatic fever and rheumatic heart disease in Fiji: prospective surveillance, 2005–2007. Med J Aust 2009; 190: 133–35. [DOI] [PubMed] [Google Scholar]
- 22.Carapetis JR, Currie BJ. Rheumatic fever in a high incidence population: the importance of monoarthritis and low grade fever. Arch Dis Child 2001; 85: 223–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data will be available through reasonable request to the corresponding author.


