Abstract
Neural epidermal growth factor-like 1 protein (NELL1) is a target antigen of membranous nephropathy (MN). NELL1-associated MN (NELL1-MN) was originally described as a primary form but has subsequently been associated with other diseases, including malignancies, pre-exposure to certain drugs, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, and rheumatoid arthritis (RA). We present a case of a 78-year-old woman with long-standing RA who developed persistent proteinuria and was diagnosed with MN. Evaluation of the underlying cause revealed chronic active HCV infection and past HBV infection. The underlying cause was less likely to be drug-related; however, there was no evidence of malignancy. The patient was diagnosed with HCV-associated MN. At 4 years after the diagnosis of MN, the patient died of breast cancer with multiple metastases. Subsequent immunohistological analysis revealed that she had NELL1-MN, and her breast cancer tissue stained positive for NELL1. Our case illustrates the difficulty in establishing the underlying cause of NELL1-MN, even after diagnosis. However, the incidence of malignancies, particularly breast and prostate cancers, is higher in NELL1-MN than in MN with other target antigens. Therefore, malignancies are considered a priority for investigation because of their frequency and prognosis among patients with NELL1-MN.
Keywords: Membranous nephropathy, Rheumatoid arthritis, Chronic hepatitis C, Hepatitis B, Breast cancer, Neural epidermal growth factor-like 1 protein
Introduction
Membranous nephropathy (MN) is the most common cause of adult-onset nephrotic syndrome [1]. Approximately 70–80% of cases of MN are classified as primary; the remainder are classified as secondary MN associated with autoimmune disease, infection, drug exposure, or malignancies [2–4].
Neural epidermal growth factor-like 1 protein (NELL1) is a recently discovered target antigen of MN, which accounts for 5–10% of cases [5]. An initial study reported that NELL1-positive MN (NELL1-MN) is a primary disease without underlying contributing illnesses. Subsequently, NELL1-MN has been found in conjunction with malignant diseases, pre-exposure to certain drugs, infections, autoimmune diseases, hematopoietic stem cell transplants, de novo MN in a kidney transplant, and sarcoidosis [5–10]. Therefore, NELL1 has been recognized as a target antigen of secondary MN with heterogeneous underlying diseases.
Herein, we report a case of a potential secondary MN in a patient with several underlying illnesses who was eventually diagnosed with NELL1-MN.
Case report
A 78-year-old woman was referred to our hospital for persistent proteinuria. At 22 years prior, the patient developed rheumatoid arthritis (RA) and was treated with oral prednisolone. Several years later, methotrexate and sulfasalazine were added, and her joint symptoms were well controlled. At 5 months before referral, the patient was first noted to have proteinuria. Her urinary protein persisted at approximately 3.0 g/g creatinine (Cr).
Other medical history included appendicitis at the age of 17 years. Her family history included uterine cancer (mother) and chronic kidney disease of unknown etiology (brother). The patient smoked 10 cigarettes daily for 57 years but had no history of alcohol consumption, illicit drug use, or blood transfusions.
The patient was admitted to the hospital for further examination. Her blood pressure was 130/70 mmHg, pulse was regular at 90 bpm, and temperature was 37.8°C. Peripheral edema was observed in both legs. The patient had moderately impaired kidney function but exhibited no evidence of nephrotic syndrome. Serum tests indicated chronic active hepatitis C virus (HCV) infection and inactive past hepatitis B virus (HBV) infection. Serum C4 and CH50 levels were low, whereas serum C3 level was within normal range. A test for antinuclear antibodies was positive at a dilution of 1:80. Antibodies to double-stranded DNA were negative. Serum cryoglobulin showed a weakly positive result. Urinary protein excretion was 3.1 g/day with a urinary red blood cell count of 0–1/high power field. Complete laboratory test results are shown in Table 1.
Table 1.
Laboratory data at the time of kidney biopsy
| Reference range | Patient results | |
|---|---|---|
| Total protein(g/dL) | 6.6–8.1 | 6 |
| Albumin (g/dL) | 4.1–5.1 | 2.8 |
| Total bilirubin (mg/dL) | 0.4–1.5 | 0.3 |
| Alanine aminotransferase (U/L) | 13–30 | 8 |
| Aspartate aminotransferase (U/L) | 7–23 | 19 |
| Alkaline phosphatase (U/L) | 106–322 | 207 |
| Creatinine (mg/dL) | 0.46–0.79 | 1.17 |
| C-reactive protein (mg/L) | > 0.14 | 0.37 |
| Serum IgG (mg/dL) | 870–1700 | 1502 |
| Serum IgA (mg/dL) | 110–410 | 245 |
| Serum IgM (mg/dL) | 35–220 | 205 |
| Serum C3 (mg/dL) | 65–135 | 103 |
| Serum C4 (mg/dL) | 13–35 | 9 |
| Serum CH50 (U/mL) | 30–50 | < 4.5 |
| Serum antinuclear antibody | 0–39 | × 80 (nucleolar pattern) |
| Rheumatoid factor | 0–15 | 45 |
| Serum cryoglobulin | Negative | Weakly positive |
| Monoclonal protein | ||
| Serum | Negative | Negative |
| Urine | Negative | Negative |
| Hepatitis B surface antigen | Negative | Negative |
| Hepatitis B surface antibody | Negative | Positive |
| Hepatitis B envelope antibody | Negative | Positive |
| Hepatitis B core antibody | Negative | Positive |
| Hepatitis B virus DNA | Negative | Negative |
| Hepatitis C virus antibody | Negative | Positive |
| Hepatitis C virus RNA (log IU/mL) | Negative | 6.2 |
| Urinalysis | ||
| Proteinuria (g/day) | 3.1 | |
| Urine RBC (/high power field) | 0–1 |
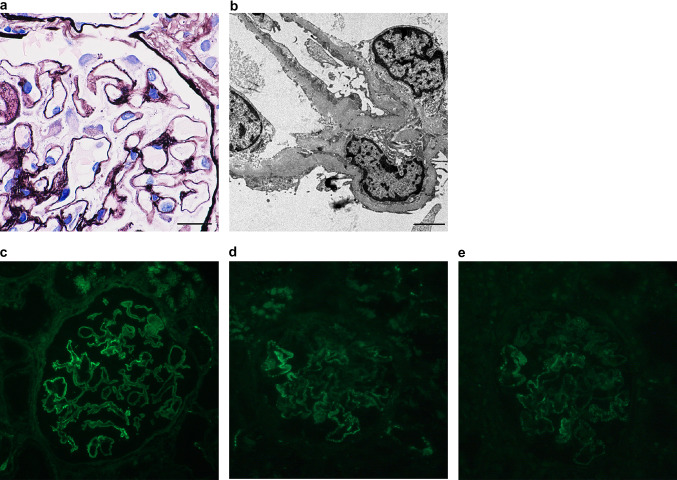
A kidney biopsy was performed to evaluate the etiology of the patient’s proteinuria. Out of the total 44 glomeruli identified, three showed global sclerosis, and one showed segmental sclerosis with neither extracapillary nor endocapillary hypercellularity in the remaining glomeruli. Glomerular capillary walls were segmentally thickened, with spike and crater formation (Fig. 1a). Mesangial hypercellularity was not observed. Mild intimal thickening of the interlobular artery was observed, but not hyaline arteriolosclerosis. Interstitial fibrosis and tubular atrophy involved approximately 10% of the renal cortex. Electron microscopy showed the presence of subepithelial electron-dense deposits of glomeruli but no mesangial deposits (Fig. 1b). There were no microstructures in the deposits. The patient was diagnosed with stage I–II MN. Immunofluorescence studies revealed positive granular staining on the segmental glomerular basement membrane for IgG (3 +) (Fig. 1c), C3 (1 +) (Fig. 1d) C1q (1 +) (Fig. 1e), IgA ( – ), and IgM ( – ) based on a 5-point scale from ( – ) to (3 +). IgG subclass analysis showed positive granular staining for IgG1 (1 +), IgG2 (2 +), and IgG4 (2 +) along the glomerular capillary wall, as well as negative staining for IgG3 ( – ). Histopathological findings, including incomplete segmental deposits and various IgG subclass immunostaining patterns, suggested a secondary form of MN. However, no apparent lesion suspected of being a malignancy was found. Therefore, the patient was considered to have developed secondary MN associated with chronic HCV infection. Considering the patient’s age, supportive therapy was given with salt restriction and imidapril administration. Even after the initiation of supportive therapy, the patient’s urinary protein fluctuated from 0.4 to 1.3 g/g Cr with a stable serum Cr level.
Fig. 1.
Kidney biopsy specimen. a Silver staining shows segmental thickening of the glomerular basement membrane with spike and crater formation (original magnification, × 1000; scale bar, 20 μm). b Electron microscopy reveals the presence of subepithelial electron-dense deposits (original magnification, × 6000; scale bar, 2 μm). c–e Immunofluorescence assay showing positive granular staining on the segmental glomerular basement membrane for IgG (c) and less intense staining for C3 (d) and C1q (e) (original magnification, × 200)
At 4 years after MN diagnosis, the patient noticed a lump in the right breast and was diagnosed with scirrhous pattern-dominant invasive ductal carcinoma of the right breast with brain and lung metastases. The patient requested no further evaluation and treatment. At 1 year later, she died of advanced terminal cancer.
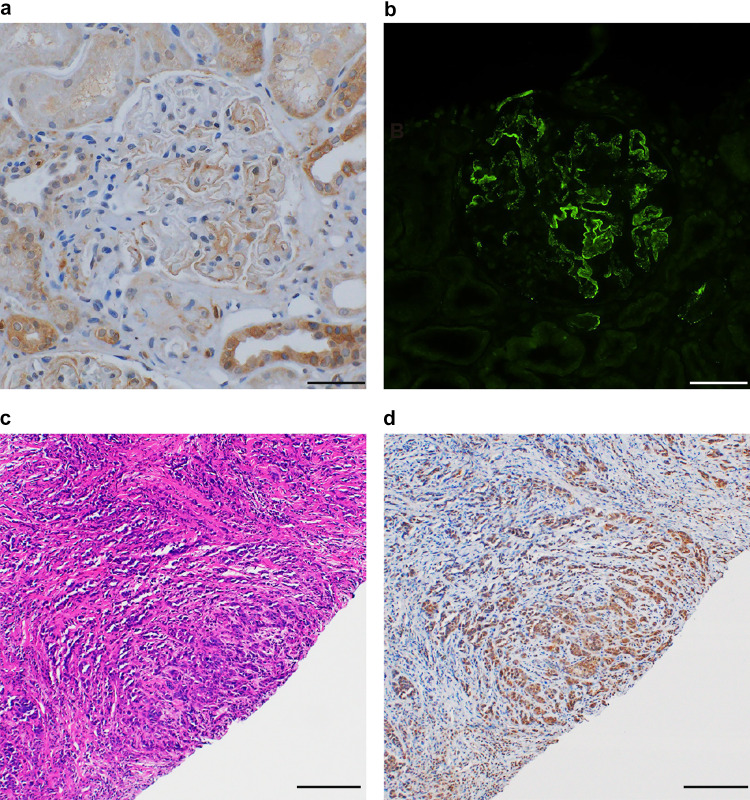
Several years after her death, we performed immunohistological analysis of her previous kidney biopsy specimens using antibodies against phospholipase A2 receptor (PLA2R), thrombospondin type-1 domain-containing 7A (THSD7A), and NELL1. Immunohistochemical analysis revealed segmental NELL1-positive staining along the glomerular capillary wall (Fig. 2a and b), whereas PLA2R and THSD7A were negative. Furthermore, the breast biopsy specimen showed intense staining for anti-NELL1 antibodies on ductal carcinoma cells (Fig. 2c and d) but negative staining for anti-PLA2R antibodies and anti-THSD7A antibodies.
Fig. 2.
Immunohistochemistry with anti-NELL1 antibody on kidney biopsy tissue and breast cancer tissue. a and b Immunohistochemistry with anti-NELL1 antibody on kidney biopsy tissue shows both incomplete and segmental distributions of NELL1 positivity on paraffin-embedded (a) and frozen (b) sections (original magnification, × 200; scale bar, 50 μm). c Hematoxylin–eosin staining of breast biopsy tissue. Ductal carcinoma was diagnosed (original magnification, × 100; scale bar, 200 μm). d Immunohistochemistry with anti-NELL1 antibody on breast biopsy tissue shows strong positive staining on ductal carcinoma (original magnification, × 100; scale bar, 200 μm)
Discussion
Our patient developed MN with several diseases that were potentially associated with secondary MN, such as RA, past HBV infection, chronic HCV infection, and breast cancer. Immunohistological analysis after death revealed that the patient had developed NELL1-MN.
The patient had a long-term history of RA. MN is a glomerular disease frequently associated with RA. The most common cause is pre-exposure to certain drugs, particularly D-penicillamine and bucillamine [11]. MN complicated by RA has been reported; however, its prevalence is lower than that of drug-associated MN [12]. Our patient received methylprednisolone, sulfasalazine, and methotrexate as a treatment for RA prior to the development of proteinuria. These drugs are rarely related to drug-associated MN. Recently, we reported four cases of NELL1-MN complicated by RA [13]. In this study, 60% of Japanese patients with NELL1-MN had a history of RA, and 66% of cases were MN associated with drugs (mostly bucillamine), indicating that NELL1-MN in Japanese people is frequently complicated by RA and bucillamine-associated MN. Our patient did not have a history of bucillamine use. NELL1 has been reported to have osteogenic, pro-chondrogenic, and anti-inflammatory properties in osteoarthritis models. As such, the osteogenic properties of NELL1 in the joint involvement of patients with RA may cause immune antigenicity against MN.
In our case, HBV infection was unlikely to contribute to MN development because of its inactive state; however, the patient’s serum tests indicated chronic active HCV infection. Several small studies suggested that MN might be induced by chronic HCV infection [14]. Additionally, Sethi [5] recently reported that NELL1-MN was detected in 25% of hepatitis B-associated MN cases and has also been detected in a patient with active hepatitis C. Thus, active HCV infection may have affected the development of NELL1-MN in our case.
Our patient was diagnosed with breast cancer 4 years after MN diagnosis. It has previously been reported that the risk of cancer development persists for at least 5 years after MN diagnosis [15]. Furthermore, the median time from MN diagnosis to malignancy diagnosis was 40 months [16]. Considering the multiple metastases at the time of breast cancer diagnosis in our case, the association between breast cancer and NELL1-MN is probable. Immunohistochemistry of her breast biopsy tissue revealed intense NELL1-positive staining on ductal carcinoma cells. This finding is consistent with the data from the Human Protein Atlas [17] and supports the previously reported possibility of breast cancer as a source of NELL1 antigen [6].
Our case depicts the difficulty in interpreting NELL1-MN, reinforcing the findings reported in the recent review by Sethi [5] NELL1-MN is associated with almost all causes of secondary MN. Therefore, we cannot conclude which underlying diseases have affected MN development in our case, even after establishing the diagnosis of NELL1-MN. However, it is important to note that malignancy is the most commonly reported disease in secondary NELL1-MN, albeit with a wide range of incidence rates [6, 9, 18]. Additionally, the incidence of malignancies was much higher in NELL1-MN (33%) than in MN with other target antigens (i.e., PLA2R-positive MN [4.2%] and THSD7A-positive MN [10.8%]) [6]. While NELL1 is expressed in various cancer tissues [17], breast cancer has been reported to express a higher NELL1 level among them and to be the second most common malignancy (14.7%) following prostate cancer (17.6%) among patients with NELL1-MN [6]. Therefore, patients with NELL1-MN, in particular, should be screened for breast cancers.
In conclusion, we report a patient who developed advanced breast cancer at 4 years after being diagnosed with NELL1-MN complicated by long-standing RA and chronic active HCV infection. Our case suggests that NELL1-MN is associated with several diseases related to both primary and secondary MN; nonetheless, malignant disease should be addressed with the highest priority for investigation because of its frequency and prognosis.
Acknowledgements
We would like to thank Ms. Moeno Ishida for her technical support in the immunofluorescence staining of the kidney specimens of the patient.
Abbreviations
- Cr
Creatinine
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- MN
Membranous nephropathy
- NELL1
Neural epidermal growth factor-like 1 protein
- PLA2R
Phospholipase A2 receptor
- RA
Rheumatoid arthritis
- THSD7A
Thrombospondin type-1 domain-containing 7A
Authors’ contributions
RM, HU, MO, and AS collected clinical data. RM and HU drafted the manuscript. KJ provided advice on histopathological interpretation. All authors approved the final version of the manuscript.
Funding
The authors declare that they received no funding for this research.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
Ethics approval was waived as consent for publication was obtained from the patient in this case report.
Informed consent
Informed consent was obtained from the patient’s family because the patient is deceased.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers. 2021;7:69. doi: 10.1038/s41572-021-00303-z. [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014;86:905–914. doi: 10.1038/ki.2014.49. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan J, Perazella MA. Drug-induced glomerular disease: attention required! Clin J Am Soc Nephrol. 2015;10:1287–1290. doi: 10.2215/CJN.01010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plaisier E, Ronco P. Screening for cancer in patients with glomerular diseases. Clin J Am Soc Nephrol. 2020;15:886–888. doi: 10.2215/CJN.09000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S. The many faces of NELL1 MN. Clin Kidney J. 2022;16:442–446. doi: 10.1093/ckj/sfac237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caza TN, Hassen SI, Dvanajscak Z, Kuperman M, Edmondson R, Herzog C, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967–976. doi: 10.1016/j.kint.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caza TN, Larsen CP. Lipoic acid in neural epidermal growth factor-like 1-associated membranous nephropathy: more than a coincidence? Kidney Int. 2022;101:418–419. doi: 10.1016/j.kint.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Kurien AA, Prema KSJ, Walker P, Caza T. Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int. 2022;102:1424–1426. doi: 10.1016/j.kint.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Iwakura T, Ema C, Isobe S, Fujikura T, Ohashi N, Kato A, et al. Prevalence of neural epidermal growth factor-like 1- and exostosin 1/exostosin 2-associated membranous nephropathy: a single-center retrospective study in Japan. Sci Rep. 2022;12:2967. doi: 10.1038/s41598-022-07037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Münch J, Krüger BM, Weimann A, Wiech T, Reinhard L, Hoxha E, et al. Posttransplant nephrotic syndrome resulting from NELL1-positive membranous nephropathy. Am J Transpl. 2021;21:3175–3179. doi: 10.1111/ajt.16610. [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, Ponticelli C. Secondary membranous nephropathy. A narrative review. Front Med (Lausanne). 2020;7:611317. doi: 10.3389/fmed.2020.611317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida A, Wada Y, Hayashi J, Tachibana S, Inaba T, Iyoda M, et al. Membranous nephropathy caused by rheumatoid arthritis. CEN Case Rep. 2019;8:233–238. doi: 10.1007/s13730-019-00399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki R, Ueda H, Hayashi A, Okabe M, Katsuma A, Shimizu A, et al. Neural epidermal growth factor-like 1-positive membranous nephropathy with rheumatoid arthritis. Kidney Int Rep. 2023;8:921–924. doi: 10.1016/j.ekir.2022.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchiyama-Tanaka Y, Mori Y, Kishimoto N, Nose A, Kijima Y, Nagata T, et al. Membranous glomerulonephritis associated with hepatitis C virus infection: case report and literature review. Clin Nephrol. 2004;61:144–150. doi: 10.5414/CNP61144. [DOI] [PubMed] [Google Scholar]
- 15.Bjørneklett R, Vikse BE, Svarstad E, Aasarød K, Bostad L, Langmark F, et al. Long-term risk of cancer in membranous nephropathy patients. Am J Kidney Dis. 2007;50:396–403. doi: 10.1053/j.ajkd.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Alnasrallah B, Collins JF, Zwi LJ. Malignancy in membranous nephropathy: evaluation of incidence. Int J Nephrol. 2017;2017:e8409829. doi: 10.1155/2017/8409829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Sun L, Dong H, Wang Y, Xu X, Zhao Z, et al. Neural epidermal growth factor–like 1 protein–positive membranous nephropathy in Chinese patients. Clin J Am Soc Nephrol. 2021;16:727–735. doi: 10.2215/CJN.11860720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.