Abstract
Human T-lymphotropic virus type 1 (HTLV-1) causes adult T-cell leukemia/lymphoma and is associated with a variety of immune-mediated disorders. The role of four open reading frames (ORFs), located between env and the 3′ long terminal repeat of HTLV-1, in mediating disease is not entirely clear. By differential splicing, ORF II encodes two proteins, p13II and p30II, both of which have not been functionally defined. p13II localizes to mitochondria and may alter the configuration of the tubular network of this cellular organelle. p30II localizes to the nucleolus and shares homology with the transcription factors Oct-1 and -2, Pit-1, and POU-M1. Both p13II and p30II are dispensable for infection and immortalization of primary human and rabbit lymphocytes in vitro. To test the role of ORF II gene products in vivo, we inoculated rabbits with lethally irradiated cell lines expressing the wild-type molecular clone of HTLV-1 (ACH.1) or a clone containing selected mutations in ORF II (ACH.30/13.1). ACH.1-inoculated animals maintained higher HTLV-1-specific antibody titers than animals inoculated with ACH.30/13.1. Viral p19 antigen was transiently detected in ex vivo cultures of peripheral blood mononuclear cells (PBMC) from only two ACH.30/13.1-inoculated rabbits, while PBMC cultures from all ACH.1-inoculated rabbits routinely produced p19 antigen. In only three of six animals exposed to the ACH.p30II/p13II clone could provirus be consistently PCR amplified from extracted PBMC DNA and quantitative competitive PCR showed the proviral loads in PBMC from ACH.p30II/p13II-infected rabbits to be dramatically lower than the proviral loads in rabbits exposed to ACH. Our data indicate selected mutations in pX ORF II diminish the ability of HTLV-1 to maintain high viral loads in vivo and suggest an important function for p13II and p30II in viral pathogenesis.
Human T-lymphotropic virus type 1 (HTLV-1) is a complex retrovirus causally linked with adult T-cell leukemia/lymphoma (ATLL), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), and a number of other immune-mediated disorders (32). Along with the typical retroviral genes gag, pol, and env, the genome contains various regulatory and accessory genes encoded by the pX region (31). The pX region, located between env and the 3′ long terminal repeat (LTR), contains four open reading frames (ORFs). ORFs IV and III of HTLV-1 encode the well-characterized Tax and Rex proteins, respectively (15). Tax is a 40-kDa nuclear phosphoprotein which increases viral transcription from the HTLV-1 LTR. The ability of HTLV-1 to cause cell transformation is likely the result of dysregulation of cellular gene expression and cell cycle checkpoints by Tax (13, 17, 26). Rex is a 27-kDa nucleolar phosphoprotein which increases the cytoplasmic accumulation of nonspliced and singly spliced viral RNA and stabilizes interleukin-2 receptor alpha (IL-2Rα) mRNA (15, 19).
In contrast to the extensive knowledge of Tax and Rex structure and function, less is known about the role of pX ORF I- and II-encoded proteins in the replication or pathogenesis of HTLV-1. p12I of ORF I is a 99-amino-acid protein that contains four minimal SH3 binding motifs (PXXP) and when overexpressed associates with the vacuolar H+ ATPase and appears to bind the β and γ chains of the IL-2R complex (28). p12I has similar structural features and cooperates with the E5 protein of bovine papillomavirus type 1 in transformation of mouse C127 cells (14). Using infectious molecular clones of HTLV-1 capable of CD4+ lymphocyte transformation, we have selectively ablated the mRNA for p12I and are the first to identify a functional role of pX ORF I in establishment of infection in an animal model (10).
Separate ORF II mRNA sequences are spliced to the promoter region located in the 5′ LTR to encode the proteins p30II and p13II, which when expressed in HeLa/Tat cells appear to localize to the nucleolus and nucleus, respectively (22). Recently p13II has been demonstrated to localize to mitochondrial membranes (5). The cellular segregation of ORF II gene products suggests specific roles for these proteins in the regulation of the expression of HTLV-1 or as determinants of virus-cell interactions. The p30II protein contains serine- and threonine-rich regions with distant homology to transcription factors Oct-1 and -2, Pit-1, and POU-M1 (6). Interestingly, cells transformed by HTLV-1 molecular clones with mutations in ORF II have differential patterns of phosphorylation of the signal transduction adapter protein Vav, suggesting their role in alteration of T-cell signaling (25).
We constructed the ACH.p30II/p13II viral clone, which destroys the initiator methionine of the mRNA encoding p13II and inserts an artificial termination codon in the mRNA encoding p30II (30). The resultant incomplete translation of both p30II and p13II does not influence the ability of ACH.p30II/p13II to infect and immortalize peripheral blood mononuclear cells (PBMC) in vitro and does not appear to affect the functions of Tax or Rex (30). We now present data for T-cell lines immortalized by either ACH (ACH.1) or ACH.p30II/p13II (ACH.30/13.1) proviral clones to examine the role of ORF II in viral infectivity and replication in vivo.
Upon inoculation of γ-irradiated ACH.1 and ACH.30/13.1 cell lines into rabbits, both viral clones elicited anti-HTLV-1 antibodies, but on average ACH.30/13.1-inoculated animals had lower titers and less reactivity to specific viral epitopes. Viral replication was confirmed by detection of proviral DNA in ACH.1-inoculated animals by PCR and HTLV-1 p19 antigen enzyme-linked immunosorbent assay (ELISA) from ex vivo cultures of rabbit PBMC. However, provirus was detected in only three of six of the ACH.30/13.1-inoculated animals, and only two of six rabbits were transiently positive for p19 antigen from PBMC cultures. Finally, ACH.30/13.1-inoculated rabbits had significantly lower viral loads compared to ACH.1-inoculated rabbits, demonstrated by quantitative competitive PCR (qcPCR). These data provide the first evidence of a functional role for pX ORF II in the maintenance of high viral load in vivo and suggest mechanisms for the interaction of p13II and p30II in the viral life cycle.
MATERIALS AND METHODS
Viral clones and cell lines.
The derivation and infectious properties of the full-length ACH viral clone have been reported elsewhere (11, 21). The ACH.p30II/p13II clone was produced by creating two separate mutations in ACH (30). A 24-bp linker inserted at a SacII site located 291 bp into the pX ORF II encoding p30II results in an artificial termination codon 16 bp downstream from the SacII site. A second mutation destroys the initiator methionine codon of the p13II mRNA by altering the nucleotide sequences from ATG to GAT.
ACH.1 and ACH.30/13.1 cell lines were obtained from the outgrowth of immortalized PBMC previously transfected with the ACH and ACH.p30II/p13II clones, respectively (9, 30). PBMC were isolated from normal human donors by Ficoll-Hypaque (Pharmacia, Piscataway, N.J.) centrifugation as described elsewhere (29). Cell cultures were maintained in RPMI 1640 supplemented with 15% fetal bovine serum, l-glutamine (0.3 mg/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), and recombinant IL-2 (10 U/ml) (complete medium). Surface membrane receptor expression was determined by direct labeling of the cell lines with fluorescein isothiocyanate-conjugated monoclonal antibodies against CD3 (UCHT1; Pharmingen, San Diego, Calif.), HLA class I (W6/32; Sigma, St. Louis, Mo.), or HLA-DR (HK14; Sigma) or with phycoerythrin-conjugated monoclonal antibodies against CD4 (RPA-T4; Pharmingen) and CD8 (PRA-T8; Pharmingen) as described elsewhere (9).
Detection of viral p19 matrix antigen.
To compare virus production between ACH.1 and ACH.30/13.1 cell lines, duplicate samples of 106 cells from each line were washed and seeded in a 24-well plate in 2 ml of complete RPMI 1640. Culture supernatants were collected at 24 and 72 h, serially diluted 10-fold, tested for HTLV-1 p19 matrix antigen by a commercially available ELISA (Cellular Products, Buffalo, N.Y.) with a detection sensitivity of 25 pg of p19 protein per ml. Resultant absorbance values were compared to a standard curve generated in the same assay to estimate p19 protein production.
For detection of HTLV-1 p19 antigen ex vivo, rabbit PBMC were isolated from whole blood by density gradient (Cedarlane, Hornby, Ontario, Canada), stimulated with 3 μg of concanavalin A (Sigma) per ml, and cultured in complete RPMI 1640. Culture supernatants were collected at day 7 and assayed for p19 antigen as described above.
Detection of proviral sequences.
For detection of provirus in cell lines and rabbit PBMC, genomic DNA was harvested by affinity column (Qiagen, Valencia, Calif.) and examined for the presence of HTLV-1 sequences following PCR amplification. Five hundred nanograms of DNA (approximately 5 × 105 cells) was amplified by using a primer pair specific for the HTLV-1 pX ORF II region (7047, 5′-TGCCGATCACGATGCGTTTC-3′; 7492, 5′-AGCCGATAACGCGTCCATCGAT-3′), which yielded a 445-bp product from the wild-type ACH.1 cell line and 469 bp from ACH.30/13.1. The ACH.30/13.1 amplicon included both an XbaI site at nucleotide 7128 and a BglII site at nucleotide 7286 (30). As a positive control and to provide for semiquantitative comparison of HTLV-1 products, simultaneous amplification was performed with a primer pair specific for β-actin, which yielded a 415-bp product from rabbit DNA (10). After an initial 10-min incubation at 94°C to activate the Taq polymerase (AmpliTaq Gold; Perkin-Elmer, Norwalk, Conn.), 37 cycles of PCR were performed with the following cycle parameters: denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 45 s, followed by a final extension at 72°C for 5 min. The amplified products were separated in a 1.5% agarose gel and stained with ethidium bromide.
Titrations of HTLV-1-positive (MT-2) and -negative (Jurkat) cellular DNA were performed to determine the sensitivity of the assay; detection of as little as 0.05 ng of MT-2 DNA (approximately 50 cells) per 500 ng of Jurkat DNA was achieved. We have previously determined that this MT-2 clone contains, on average, 2.1 proviral copies per cell (1). Thus, we estimated the sensitivity of the PCR assay to be at least one proviral copy per 5,000 cells.
HTLV-1-specific PCR products resulting from the 7047-7492 pX primer pair were sequenced to further confirm specificity and ensure the absence of second-site mutations within ORF I or II. PCR products were purified (Qiagen) and sequenced by the automated dye terminator cycle sequencing method (ABI PRISM dye terminator cycle sequencing kit; Applied Biosystems Inc., Foster City, Calif.), using the primer pair as mentioned above.
qcPCR.
Estimates of in vivo viral loads were determined with qcPCR as previously described (1). DNA was extracted from rabbit PBMC at 4, 8, and 9 weeks postinoculation. Primers SG 166 and SG 196 were used to amplify a 284-bp segment of the HTLV-1 gag region. The competitor StyIΔ28, which contains nucleotide sequence identical to that of the 284-bp gag amplicon with the addition of a 28-bp linker, was varied in concentration over 2 orders of magnitude while genomic DNA remained constant. Aliquots of the reactions were separated on 10% polyacrylamide gels, stained with ethidium bromide, and analyzed under UV light, and equivalence points were determined by plotting regression curves. From the equivalence points, the amount of provirus per cell was calculated by a conversion of 5 amol of competitor ≅ 3 × 106 copies.
Infectivity in cultured rabbit lymphocytes and rabbit inoculation.
Virus infectivity for rabbit lymphocytes was tested in vitro by coculturing 106 naive rabbit PBMC with 105 gamma-irradiated (7,500 R) ACH.1 or ACH.30/13.1 cells as described previously (10). After maintenance in complete RPMI 1640 for 3 weeks, cells were washed to ensure measurement of de novo antigen production and cultured in fresh 24-well plates for 7 days, with aliquots of culture supernatant obtained at days 1 and 7 and assayed for HTLV-1 p19 antigen as described above.
To test the in vivo replication capacity of each viral clone, 12-week-old specific-pathogen-free New Zealand White rabbits (Hazelton, Kalamazoo, Mich.) were inoculated via the lateral ear vein. Inocula were equilibrated by two distinct methods, cell number and viral protein production; 107 gamma-irradiated (7,500 R) cells from either the ACH.1 (n = 2) or ACH.30/13.1 (n = 2) line were injected. Relative levels of viral production per ACH.1 and ACH.30/13.1 cell were compared by p19 antigen ELISA as described above. To equilibrate total virus production, a separate group of six animals was injected with 5.0 × 106 ACH.1 (n = 2) or 107 ACH.30/13.1 (n = 4) gamma-irradiated (7,500 R) cells. Rabbits inoculated with uninfected, normal human PBMC (n = 1) or left uninoculated (n = 1) were used as controls. Representative samples from each inoculum type were cultured and monitored for viability daily to confirm the lethality of the gamma irradiation procedure.
Serologic, clinical, and hematologic analysis.
Plasma antibody response to HTLV-1 in inoculated rabbits was determined by use of a commercial ELISA (Organon Teknika, Durham, N.C.) which was adapted for use with rabbit plasma by substitution of alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (1:400 dilution; Sigma). Plasma was diluted 1:12,000 (to obtain values in the linear range of the assay), and data were expressed as absorbance values. Reactivity to specific viral antigenic determinants was detected using a commercial HTLV-1 Western blot assay (Cambridge Biotech, Worcester, Mass.) adapted for rabbit plasma by use of avidin-conjugated goat anti-rabbit immunoglobulin G (1:3,000 dilution; Vector, Burlington, Calif.). Plasma showing reactivity to Gag (p24 or p19) and Env (p21 or gp46) antigens was classified as positive for HTLV-1 seroreactivity. Complete hematologic analysis was performed by automated cell counting (Coulter Immunology Corp., Hialeah, Fla.) and differential enumeration of leukocytes and erythrocyte morphology in blood films. The animals were regularly evaluated for any overt signs of clinical disease. Rabbits were euthanized for necropsy and gross and microscopic examination of major organ systems at postinoculation intervals of 9 or 12 weeks.
RESULTS
Comparative infectivity of viral clones in vitro.
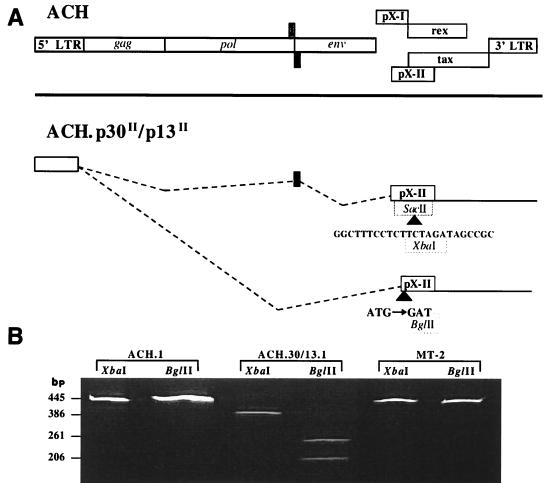
We have reported that ACH clones containing mutations of ORF II designed to selectively eliminated p13II and p30II protein expression do not affect Gag and Env protein compositions of virus particles (evidence of Rex function), are dispensable for in vitro viral infectivity of human PBMC, and have functional Tax activity (30). To further investigate the role of ORF II, we developed immortalized human T-cell lines which continually produce either wild-type HTLV-1 (ACH.1) or HTLV-1 containing mutations in ORF II (ACH.30/13.1). As we have reported, the cell lines were representative of the phenotype of T cells immortalized by HTLV-1 and had typical expression profiles of CD3, CD4, or CD8, as well as similar expression of CD25 (IL-2Rα) and major histocompatibility complex class I and II molecules (9, 30). The ACH.p30II/p13II viral clone was constructed by the insertion of a 24-bp linker into a SacII site of the p30II ORF, which adds an XbaI site, and disruption of the p13II ORF start site, adding a BglII site (Fig. 1A). To ensure that the mutations were present prior to inoculation, a region of ORF II containing the mutation sites was amplified by PCR from the ACH.30/13.1 line. The product was then digested with the appropriate restriction endonucleases. As expected, XbaI digestion of DNA amplified from the ACH.30/13.1 cell line yielded fragments of 386 and 81 bp, and BglII digestion yielded fragments of 261 and 206 bp. ACH.1 and MT-2 cell line DNA was analyzed concurrently, using the same primer pair. As expected for the wild-type provirus, XbaI and BglII failed to cut the amplified DNA (Fig. 1B).
FIG. 1.
Mutations in ORF II of the full-length HTLV-1 molecular clone ACH add two diagnostic restriction endonuclease sites. (A) The top schematic drawing represents the organization of the HTLV-1 provirus, including the four ORFs (ORF I and II, tax, and rex) located in the pX region between env and the 3′ LTR. The lower schematic demonstrates the two mutations created in ORF II of ACH, which are present in the ACH.30/13.1 cell line. A 24-bp linker, including a novel XbaI site, was inserted into a SacII site, producing a premature stop codon in the doubly spliced p30II transcript. The ATG start sequence of the singly spliced p13II transcript was changed to GAT by site-directed PCR mutagenesis; the sequence disruption produced a new BglII site. (B) PCR amplification with the primer pair 7047-7492, specific for ORF II, produced a fragment of 445 bp from wild-type genomic DNA. Lanes 1, 2, 5, and 6 demonstrate the absence of XbaI and BglII sites in sequences amplified from the ACH.1 and MT2 cell lines. XbaI digestion of the amplicon from the ACH.30/13.1 cell line produced the predicted fragments of 386 (lane 3) and 81 (not shown) bp. Lane 4 shows the 261- and 206-bp fragments resulting from digestion of the ACH.30/13.1-derived amplicon with BglII.
As an estimate of relative viral infectivity for ACH.1 and ACH.30/13.1, we serially diluted the cell lines and measured the production of HTLV-1 p19 antigen in vitro by ELISA. All of the detected absorbance values fell within the limits of the standard curve at a dilution of 1:100. By interpolation from the standard, the concentration of p19 produced by ACH.1 was approximately two times the concentration produced by ACH.30/13.1 (data not shown). This variation in p19 antigen production is typical of HTLV-1-infected cell lines (24).
Serologic response of rabbits to viral clones.
To evaluate the function of HTLV-1 ORF II in vivo, we compared the abilities of ACH.1 and ACH.30/13.1 cell lines to establish and maintain infection in our rabbit model. To ensure comparable infection potential, inocula were equilibrated by either cell number or total HTLV-1 p19 antigen production (Table 1).
TABLE 1.
Rabbit inocula
| Rabbit | Inoculum typea | No. of cells | Time (wk) in study |
|---|---|---|---|
| R1 | ACH.1 | 5 × 106 | 9 |
| R2 | ACH.1 | 5 × 106 | 9 |
| R3 | ACH.1 | 107 | 12 |
| R4 | ACH.1 | 107 | 12 |
| R5 | ACH.30/13.1 | 107 | 9 |
| R6 | ACH.30/13.1 | 107 | 9 |
| R7 | ACH.30/13.1 | 107 | 9 |
| R8 | ACH.30/13.1 | 107 | 9 |
| R9 | ACH.30/13.1 | 107 | 12 |
| R10 | ACH.30/13.1 | 107 | 12 |
| R11 | Uninfected PBMC | 107 | 9 |
| R12 | None | 0 | 12 |
Twelve-week-old specific-pathogen-free New Zealand White rabbits were inoculated via the lateral ear vein as described in Materials and Methods. The ACH.1 cell line was obtained by outgrowth of immortalized PBMC previously transfected with the full-length HTLV-1 molecular clone ACH (9). The ACH.30/13.1 cell line was obtained as described above and contains select mutations in ORF II of ACH (30).
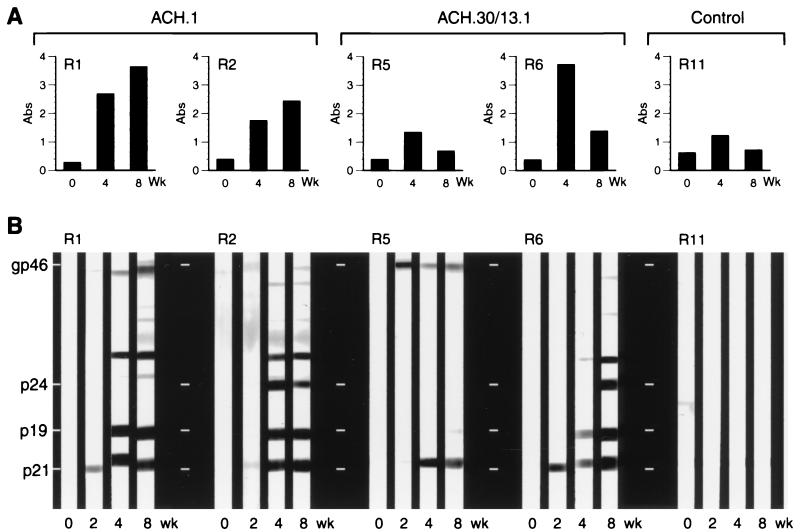
Serologic response of the rabbits to the inocula was determined by measuring titers of antibody directed against inactivated HTLV-1 viral antigens and recombinant envelope protein by ELISA. Antibody levels in control animals (R11 and R12) remained below the positive cutoff point (absorbance ≥ 1.3) for all time points assayed. ACH.1-inoculated rabbit (R1 to R4) titers were significantly higher (week 8 mean absorbance, 3.3 ± 0.6; P = 0.01; analysis of variance) than the variable levels seen for ACH.30/13.1-inoculated animals (week 8 absorbance range, 3.1 to 0.4; mean absorbance, 1.5 ± 1.0). The antibody titers detected for ACH.1-inoculated rabbits continued to rise at all time points evaluated. In contrast, the titers for ACH.30/13.1-inoculated animals diminished after the 4-week time point (Fig. 2A).
FIG. 2.
HTLV-1-specific serologic response of inoculated rabbits. Rabbits R1 and R2 were inoculated with the ACH.1 cell line and represent a group of four animals. Six animals were injected with the ACH.30/13.1 cell line; data for rabbits R5 and R6 are shown from the group. Control animals R11 (shown) and R12 (not shown) were inoculated with uninfected PBMC and left uninoculated, respectively. Data shown are absorbance (Abs) values from plasma samples diluted 1:12,000 and determined by anti-HTLV-1 antibody ELISA (A) or specific reactivity to HTLV-1 epitopes measured by Western blot analysis (B). Note that the reactivity of R11 detected by ELISA is HTLV-1 nonspecific as demonstrated by Western blot analysis and is attributable to cross-reactivity of inoculum cellular antigens.
Reactivity to specific HTLV-1 antigens was subsequently confirmed at 2-week intervals throughout the study by Western blot analysis; band intensity was evaluated visually and correlated with ELISA titers (Fig. 2B). All ACH.1-inoculated rabbits were considered seropositive for HTLV-1 (reactivity to both Gag and Env antigens), while rabbits inoculated with ACH.30/13.1 were either seropositive or indeterminate (weaker reactivity to fewer antigens). Control rabbits failed to seroconvert to any HTLV-1-specific antigens (Fig. 2B).
Detection of p19 antigen and provirus from rabbit PBMC.
To compare the in vitro infectivities of the two viral clones for rabbit primary cells, we lethally irradiated and cocultured both ACH.1 and ACH.30/13.1 cell lines with naive but mitogen-activated rabbit PBMC. De novo viral p19 antigen was produced in similar amounts by both cultures, indicating that the p30.13 mutation did not affect the ability of HTLV-1 to infect rabbit PBMC in vitro (data not shown).
To determine the HTLV-1 infection status of inoculated rabbits, we measured soluble p19 antigen in ex vivo PBMC culture supernatants. Viral antigen was detected in cultures from all ACH.1-inoculated rabbits (R1 to R4) at 4 weeks postinoculation. However, p19 production in PBMC cultures derived from ACH.30/13.1-inoculated rabbits (R5 to R10) occurred in only two rabbits at a single time point (R6 at week 2; R8 at week 8). No p19 production was observed in either of the two control animals (R11 and R12) (Table 2).
TABLE 2.
Viral detection in PBMC of rabbits by p19 antigen ELISA and PCR
| Inoculum | Rabbit | p19a/PCRb at wk:
|
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 12 | ||
| ACH.1 | R1 | −/− | +/+ | +/+ | +/+ | +/+ | Ec |
| R2 | −/− | +/+ | +/+ | +/+ | +/+ | E | |
| R3 | −/− | −/− | +/+ | +/+ | +/+ | +/+ | |
| R4 | −/− | −/+ | +/+ | +/+ | +/+ | −/+ | |
| ACH.30/13.1 | R5 | −/− | −/+ | −/− | −/− | −/− | E |
| R6 | −/− | +/+ | −/+ | −/+ | −/+ | E | |
| R7 | −/− | −/− | −/+ | −/+ | −/+ | E | |
| R8 | −/− | −/+ | −/+ | −/+ | +/+ | E | |
| R9 | −/− | −/+ | −/− | −/− | −/− | −/− | |
| R10 | −/− | −/− | −/− | −/− | −/− | −/− | |
| PBMC | R11 | −/− | −/− | −/− | −/− | −/− | E |
| None | R12 | −/− | −/− | −/− | −/− | −/− | −/− |
Measurement of p19 antigen in 7-day ex vivo PBMC cultures; + indicates concentrations of at least 25 pg/ml.
Amplification of HTLV-1 ORF II-specific proviral sequence (+), sensitivity estimated to be one viral copy per 5,000 cells.
E, euthanized at 9 weeks (termination of study).
As a further means of detecting infection in rabbits, we attempted to amplify HTLV-1-specific pX proviral sequences from rabbit PBMC DNA by PCR using the 7047-7492 primer pair. Provirus was detected in ACH.1-inoculated rabbits by 2 weeks postinoculation; however, the ACH.30/13.1-inoculated rabbits showed a mixed response. Provirus was detectable by PCR from PBMC of three of the six animals (R6 to R8) throughout the study, starting at 2 weeks. The remaining three ACH.30/13.1-inoculated rabbits (R5, R9, and R10) and the control rabbits (R11 and R12) were HTLV-1 negative by PCR following the 2-week time point (Table 2). We sequenced the PCR products derived from the 7047-7492 primer pair to ensure that there were no unexpected mutations or reversions within ORF II. Except for the previously described p30II/p13II mutations, no sequence differences were noted between the two viral clones (data not shown).
qcPCR.
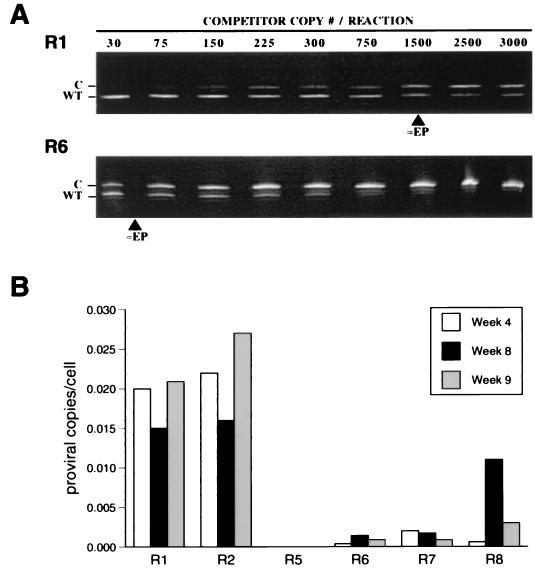
To measure the ability of each HTLV-1 clone to maintain viral loads in vivo, we determined the number of proviral copies per cell (rabbit PBMC) by qcPCR for six animals (R1, R2, and R5 to R8) at 4, 8, and 9 weeks postinoculation and for R3 and R4 at 9 weeks postinoculation. Band intensities were evaluated and regression curves were plotted to determine equivalency points (Fig. 3A). Viral load was calculated from the resultant equivalencies. ACH.30/13.1-inoculated rabbits contained lower PBMC proviral load at all time points tested (Fig. 3B). Typical results were obtained at the 9-week time point, with ACH.1-inoculated rabbit PBMC (R1 and R2) found to contain an average of 0.02 ± 0.004 proviral copies per cell, which was 10-fold greater than the average for ACH.30/13.1-inoculated rabbit PBMC of 0.002 ± 0.001 copies per cell. Nine-week samples of PBMC from R3 and R4, both ACH.1-inoculated rabbits, contained 20-fold more proviral copies per cell compared to ACH.30/13.1-inoculated rabbits (data not shown). The increased viral load seen for R8 at 8 weeks postinoculation is consistent with the production of detectable p19 antigen at the same time point (Fig. 3B). These data taken together with the variability in serologic response and lack of detection of p19 antigen from culture PBMC from ACH.30/13.1-inoculated rabbits indicate that mutations in ORF II profoundly affected the ability of HTLV-1 to maintain high viral loads in vivo.
FIG. 3.
Viral loads of inoculated rabbits determined by qcPCR. (A) HTLV-1-specific sequences (wild type [WT]) were amplified from genomic DNA extracted from the PBMC of rabbits inoculated with ACH.1 or ACH.30/13.1 cells in the presence of increasing competitor (C) concentrations. Samples collected at 9 weeks into the study from R1 and R6 represent ACH.1- and ACH.30/13.1-injected animals, respectively; approximately equivalency points (≈EP) are shown for each. (B) Proviral copy number per cell for representative rabbits was calculated from the equivalency points determined at 4, 8, and 9 weeks postinoculation.
DISCUSSION
To date a function for the proteins encoded by HTLV-1 ORF II, p30II and p13II, remains elusive. Our group has demonstrated that selective mutations of our ACH clone designed to eliminated p30II or p13II expression do not affect in vitro viral infectivity of HTLV-1 in human PBMC, alter Gag and Env composition of virus particles, or influence Tax function in transfected cell lines (30). Others have shown ORF II to be dispensable for in vitro replication and immortalization of primary T lymphocytes, and the expression of p30II or p13II protein has never been conclusively demonstrated in cells derived from ATLL patients (3, 12). However, it would be unique among retroviruses for HTLV-1 to retain highly conserved sequences of DNA which serve no purpose in viral propagation or alteration of the host cell environment. Although in quantities lower than seen for the well-characterized Tax and Rex, mRNA sequences coding for ORF II have been demonstrated in mammalian cells transfected with full-length HTLV-1 molecular clones, HTLV-1-infected cell lines, and uncultured primary cells from ATLL patients (2, 6, 23). Antibody specific for p30II can also be found in serum samples from both ATLL and HAM/TSP patients (4). These data suggest a significant role for ORF II in vivo.
HTLV-1 inoculation of the rabbit has been established as an appropriate model of the persistent asymptomatic infection in humans (27). We and others have used this animal model extensively to investigate the mechanisms of transmission, antiviral immune responses, and the role of ORF I in viral expression in vivo (10, 11, 18, 24). Here we used the rabbit model to test the influence of mutations in ORF II of HTLV-1 on viral replication in vivo.
We confirmed the integrity of the ORF II mutations prior to exposing the rabbits to ACH.1 and ACH.30/13.1 cell lines. The mutations added two diagnostic restriction endonuclease sites, which proved to be intact upon digestion with XbaI and BglII. Analogous to our previous report of the ACH clone containing a ORF I mutation (10), herein we demonstrate that ORF II mutations do not affect the ability of HTLV-1 to infect cultured rabbit lymphocytes. To test the effects of these mutations in vivo, we inoculated rabbits with lethally irradiated cell lines expressing either wild-type ACH or ACH.p30II/p13II. Inocula were equilibrated by cell number and p19 production. As expected, the wild-type ACH clone induced a vigorous and continuous humoral response against major viral antigenic determinants; however, the response to the ACH.p30II/p13II clone varied from rapid and complete to indeterminate seroconversion. Viral transcription is typically low in asymptomatic HTLV-1-infected humans as well as in rabbits (7, 34). However, viral replication can be induced upon mitogenic stimulation of ex vivo cultures of infected PBMC (24, 35). The absence of p19 antigen in most PBMC cultures at 2 weeks into the study suggests that further p19 antigen production resulted from the infection of rabbit PBMC and not residual inoculum. By 4 weeks we were able to detect high levels of HTLV-1 p19 antigen from all PBMC cultures of ACH.1-inoculated rabbits. In contrast, only two of the ACH.30/13.1-inoculated rabbits (R6 at week 2; R8 at week 8) transiently produced detectable p19 antigen from PBMC cultures. These findings suggest that the ACH.p30II/p13II mutant clone exhibits less efficient viral infectivity in vivo compared to the wild-type ACH.
Previously we have shown ACH to be consistently infectious in rabbits (10, 11). Similarly, here we were able to amplify, by PCR, HTLV-1-specific sequences from ACH.1-inoculated rabbits at all weeks postinoculation, even with our typical inoculum of 107 cells reduced to 5 × 106 cells in two rabbits (R1 and R2). In contrast to the ACH.1-inoculated animals, we could amplify viral sequences from only half of the ACH.30/13.1-inoculated rabbits (R6 to R8). To ensure that the PCR products were specific, the original ORF II mutations were intact, and no second site mutations had occurred in the region spanned by the 7047-7492 primer pair, we sequenced the resultant amplicon from four rabbits (R1, R3, R6, and R8). No sequence differences other than the expected mutations in ACH.p30II/p13II were observed. If in vivo mutations of alternate viral genes were accountable for the observed ACH.p30II/p13II phenotype, they would have had to occur independently in all ACH.30/13.1-inoculated rabbits but in none of the ACH.1-inoculated animals. Although we cannot exclude the possibility that second-site mutations occurred, we consider it unlikely.
As a further assessment of the function of ORF II, we quantified the in vivo proviral loads from rabbits infected with each clone (ACH and ACH.p30II/p13II). The PBMC from ACH.1-inoculated rabbits contained high viral loads at all time points evaluated, which is similar to results of our previous study (1). In contrast, 10- to 100-fold less provirus was found in PBMC derived from ACH.30/13.1-inoculated animals. Although we cannot rule out the possibility there is a tissue reservoir for the ACH.p30II/p13II clone, these findings provide strong evidence ACH.p30II/p13II is less replication efficient in vivo and ORF II is critical for maintenance of high viral loads.
Ours is the first study to demonstrate a functional role for p30II and p13II in vivo, resulting from specific mutations in ORF II. Lower viral loads in vivo also resulted when extensive regions, analogous to ORF I and II of HTLV-1, were deleted from bovine leukemia virus and HTLV-2; however, no deleterious effect was seen in vitro (8, 16, 33). Our results are analogous to studies of the simian immunodeficiency virus (SIV) protein Nef, which has been shown to be important for establishment of SIV infection in monkeys (20). However, the predicted protein structure of p30 and p13 does not resemble that of SIV Nef, and the influence of these HTLV-1 proteins in viral replication remains to be determined. Our current data regarding mutations in HTLV-1 ORF II differ from our studies with ACH containing mutations which eliminate ORF I expression. We have recently shown ablation of HTLV-1 p12I mRNA to completely eliminate infectivity in vivo yet show no in vitro effect (10). In contrast, our ORF II mutations reduced cell-associated viremia of HTLV-1 infections but did not eliminate the ability of the virus to establish a persistent albeit milder infection. These results suggest divergent functions for these two pX ORFs in viral replication in vivo.
The exact functions of p30II and p13II remain to be elucidated. The localization of p30II to the nucleolus and homology with cellular transcription factors suggest that it may regulate the expression of important viral or host genes. It is important to note here that the mutation in the region of ORF II encoding p30II is predicted to produce a truncated product upon translation. In the unlikely event this truncated protein retains active sites and is folded in the proper conformation, p30II may contribute to the phenotype that we describe in this study. It will be imperative in the future to identify the crucial functional motifs of p30II. Destruction of the translation start site should prevent production of the p13II protein. While we cannot completely rule out a role for the untranslated p13II mRNA, function for the protein product has been suggested by the recent report of localization to mitochondria (5). Since p13II may alter the normal mitochondrial tubular network, it could be involved in derangement of cellular Ca2+ homeostasis and signaling pathways. An association between constitutive Vav phosphorylation in cells expressing an HTLV-1 clone, with mutations predicted for amino acid position 17 of p13II and position 171 of p30II, has been shown (25). Since Vav is involved in signaling cascades and is phosphorylated upon T-cell activation, control of the state of Vav phosphorylation by p13II or p30II has been hypothesized to determine the onset of ATLL in HTLV-1-infected individuals (25).
ATLL and the immune-mediated disorders apparently initiated by HTLV-1, including HAM/TSP, are a significant health problem worldwide. Our continuing effort to both identify and characterize the regulatory proteins p12I, p13II, and p30II will contribute to the pursuit of a more complete understanding of the infectivity and pathogenesis of HTLV-1. These regulatory proteins may be ideal targets for the development of antiviral agents. Further studies will be required to determine the exact pathway by which pX ORF II expression increases the potential for HTLV-1 to replicate in vivo.
ACKNOWLEDGMENTS
This work was supported by grants CA-55185, RR-14324, CA-16058, and CA-70259 from the National Institutes of Health. Björn Albrecht is a Boehringer Ingelheim Fellow. Michael D. Lairmore is supported by Independent Scientist Career Award AI-01474 from the National Institutes of Health.
We thank Tim Vojt for preparation of figures and Wei Ding, Celine D'Souza, Weiqing Zhang, and John Nisbet for critical reviews of the manuscript.
REFERENCES
- 1.Albrecht B, Collins N D, Newbound G C, Ratner L, Lairmore M D. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. doi: 10.1016/s0166-0934(98)00087-1. [DOI] [PubMed] [Google Scholar]
- 2.Berneman Z N, Gartenhaus R B, Reitz M S, Blattner W A, Manns A, Hanchard B, Ikehara O, Gallo R C, Klotman M E. Expression of alternatively spliced human T-lymphotrophic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci USA. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caputo A, Haseltine W A. Reexamination of the coding potential of the HTLV-1 pX region. Virology. 1992;188:618–627. doi: 10.1016/0042-6822(92)90516-r. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y M A, Chen S H, Fu C Y, Chen J Y, Osame M. Antibody reactivities to tumor-suppressor protein p53 and HTLV-I Tof Rex and Tax in HTLV-I-infected people with differing clinical status. Int J Cancer. 1997;71:196–202. doi: 10.1002/(sici)1097-0215(19970410)71:2<196::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Ciminale V, Zotti L, D'Agostino D M, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotopic virus type 1 (HTLV-1) Oncogene. 1999;18:4505–4514. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- 6.Ciminale V, Pavlakis G N, Derse D, Cunningham C P, Felber B K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockerell G L, Lairmore M D, De B, Rovnak J, Hartley T, Miyoshi I. Persistent infection of rabbits with HTLV-I: patterns of anti-viral reactivity and detection of virus by gene amplification. Int J Cancer. 1990;45:127–130. doi: 10.1002/ijc.2910450123. [DOI] [PubMed] [Google Scholar]
- 8.Cockerell G L, Rovnak J, Green P L, Chen I S Y. A deletion in the proximal untranslated pX region of human T-cell leukemia virus type II decreases viral replication but not infectivity in vivo. Blood. 1996;87:1030–1035. [PubMed] [Google Scholar]
- 9.Collins N D, D'Souza C, Albrecht B, Robek M D, Ratner L, Ding W, Green P L, Lairmore M D. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T cell lymphotropic virus type 1 is independent of open reading frame I expression. J Virol. 1999;73:9642–9649. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins N D, Newbound G C, Albrecht B, Beard J L, Ratner L, Lairmore M D. Selective ablation of human T-cell lymphotropic virus type 1 p12(I) reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 11.Collins N D, Newbound G C, Ratner L, Lairmore M D. In vitro CD4+ lymphocyte transformation and infection in a rabbit model with a molecular clone of human T-cell lymphotropic virus type 1. J Virol. 1996;70:7241–7246. doi: 10.1128/jvi.70.10.7241-7246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 13.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 14.Franchini G, Mulloy J C, Koralnik I J, Lo Monico A, Sparkowski J J, Andresson T, Goldstein D J, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green P L, Chen I S Y. Molecular features of the human T-cell leukemia virus. Mechanisms of transformation and leukemogenicity. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1994. pp. 277–311. [Google Scholar]
- 16.Green P L, Ross T M, Chen I S Y, Pettiford S. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J Virol. 1995;69:387–394. doi: 10.1128/jvi.69.1.387-394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollsberg P. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol Mol Biol Rev. 1999;63:308–333. doi: 10.1128/mmbr.63.2.308-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwahara Y, Takehara N, Kataoka R, Sawada T, Ohtsuki Y, Nakachi H, Maehama T, Okayama T, Miyoshi I. Transmission of HTLV-1 to rabbits via semen and breast milk from seropositive healthy persons. Int J Cancer. 1990;45:980–983. doi: 10.1002/ijc.2910450534. [DOI] [PubMed] [Google Scholar]
- 19.Kanamori H, Suzuki N, Siomi H, Nosaka T, Sato A, Sabe H, Hatanaka M, Honjo T. HTLV-1 p27rex stabilizes human interleukin-2 receptor alpha chain mRNA. EMBO J. 1990;9:4161–4166. doi: 10.1002/j.1460-2075.1990.tb07639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high viral loads and for the development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 21.Kimata J T, Wong F, Wang J, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 22.Koralnik I J, Fullen J, Franchini G. The p12, p13, and p30 proteins encoded by human T-cell leukemia/lymphotropic virus type 1 open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koralnik I J, Gessain A, Klotman M E, Lo Monico A, Berneman Z N, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type 1. Proc Natl Acad Sci USA. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lairmore M D, Roberts B, Frank D, Rovnak J, Weiser M G, Cockerell G L. Comparative biological responses of rabbits infected with human T-lymphotropic virus type I isolates from patients with lymphoproliferative and neurodegenerative disease. Int J Cancer. 1992;50:124–130. doi: 10.1002/ijc.2910500125. [DOI] [PubMed] [Google Scholar]
- 25.Mahana W, Zhao T M, Teller R, Robinson M A, Kindt T J. Genes in the pX region of human T cell leukemia virus I influence Vav phosphorylation in T cells. Proc Natl Acad Sci USA. 1998;95:1782–1787. doi: 10.1073/pnas.95.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesnard J M, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi I, Yoshimoto S, Kubonishi I, Fujishita M, Ohtsuki Y, Yamashita M, Yamato K, Hirose S, Taguchi H, Niiya K. Infectious transmission of human T-cell leukemia virus to rabbits. Int J Cancer. 1985;35:81–85. doi: 10.1002/ijc.2910350113. [DOI] [PubMed] [Google Scholar]
- 28.Mulloy J C, Crowley R W, Fullen J, Leonard W J, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newbound G C, Andrews J M, O'Rourke J, Brady J N, Lairmore M D. Human T-cell lymphotropic virus type 1 Tax mediates enhanced transcription in CD4+ T lymphocytes. J Virol. 1996;70:2101–2106. doi: 10.1128/jvi.70.4.2101-2106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robek M D, Wong F H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Willems L, Kerkhofs P, Dequiedt F, Portetelle D, Mammerickx M, Burny A, Kettmann R. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc Natl Acad Sci USA. 1994;91:11532–11536. doi: 10.1073/pnas.91.24.11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida M. Expression of the HTLV-1 genome and its association with a unique T-cell malignancy. Biochim Biophys Acta. 1987;907:145–161. doi: 10.1016/0304-419x(87)90003-5. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]