Abstract
Background:
Adult survivors of childhood cancer are at risk for cardiovascular events.
Objective:
To determine the risk for mortality after a major cardiovascular event among survivors compared with non-cancer populations.
Methods:
All-cause and cardiovascular cause-specific mortality risks after heart failure (HF), coronary artery disease (CAD), or stroke were compared among survivors and siblings in the Childhood Cancer Survivor Study (CCSS) and participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Cox proportional hazards regression models estimated hazard ratios (HRs) and 95% confidence intervals (CIs) between groups, adjusted for demographic and clinical factors.
Results:
Among 25,658 childhood cancer survivors (median age at diagnosis 7 years, median age at follow-up/death 38 years), and 5,051 siblings, 1,780 survivors and 91 siblings had a cardiovascular event. Following HF, CAD, or stroke, 10-year all-cause mortality was 30% (CI: 26-33%); 36% (CI: 31-40%); and 29% (CI: 24-33%) respectively among survivors versus 14% (CI: 0-25%); 14% (CI: 2-25%); and 4% (CI: 0-11%) among siblings. All-cause mortality risks among survivors were increased after HF (HR=7.32, CI: 2.56-20.89), CAD (HR=5.54, CI: 2.37-12.93), and stroke (HR=3.57, CI: 1.12-11.37). CAD-specific mortality risk was increased (HR=3.70, CI: 1.05-13.02). Among 5,114 CARDIA participants, 345 had a major event. While CARDIA participants were on average decades older at events (median age 57 years versus 31 years), mortality risks were similar, except all-cause mortality after CAD was significantly increased among survivors (HR=1.85, CI: 1.16-2.95).
Conclusions:
Survivors of childhood cancer represent a population at high risk for mortality after major cardiovascular events.
Keywords: Childhood cancer, cardio-oncology, survivorship, heart failure, coronary artery disease, stroke, anthracyclines, radiation
Condensed abstract:
Adult survivors of childhood cancer are at increased risk for cardiovascular events compared with non-cancer populations. Our study demonstrates an increased risk for mortality after major cardiovascular events. Compared with siblings, childhood cancer survivors’ risk for all-cause mortality after heart failure (HF), coronary artery disease (CAD), or stroke, and risk for cause-specific mortality after CAD were increased. Childhood cancer survivors had risk accelerated by over 2 decades for all-cause mortality after stroke, HF, and particularly CAD compared to a racially diverse, population-based cohort of Black and White participants. Enhanced interventions to reduce risk for mortality in this population are needed.
INTRODUCTION
In the modern era, 85% of children diagnosed with a malignancy will become five-year survivors of cancer.1 However, intensive therapies including chest-directed radiotherapy and anthracycline chemotherapy are associated with increased risk for long term cardiovascular toxicity. While cardiovascular disease is well-established as a leading cause of morbidity and mortality among long-term survivors of childhood cancer,2-5 the impact of a major cardiovascular event including heart failure (HF), coronary artery disease (CAD) or stroke on subsequent mortality risk is not clearly defined. It is possible that survivors of childhood cancer may experience increased mortality risk after major cardiovascular events relative to other populations.
The limited existing evidence suggests that after a major cardiovascular event, cancer survivors are at increased risk for mortality. However, many of these studies do not differentiate cancer survivors based on age at cancer diagnosis, or exclusively examine survivors of adult-onset cancer. Felker et al., observed a three times greater risk for mortality among individuals with HF due to doxorubicin exposure compared with idiopathic HF.6 Increased rates of mortality after heart transplant have also been observed among patients with radiation-induced HF.7,8 Similarly, an increased risk of cardiovascular morbidity and death has been observed among survivors of adult-onset cancer undergoing percutaneous coronary intervention, in comparison with cancer naïve individuals.9 Previously, the Childhood Cancer Survivor Study (CCSS) demonstrated an increased risk for all-cause mortality after stroke among childhood cancer survivors diagnosed between 1970 and 1986.10 However, mortality risk after a major cardiovascular event among survivors of childhood cancer has not been fully quantified.
To address these knowledge gaps, we evaluated survivors from CCSS, expanding the timeframe to include those diagnosed between 1970-1999. We estimated the cumulative incidence of all-cause and cardiovascular cause-specific mortality among survivors of childhood cancer who experienced a major cardiovascular event and a comparison group of siblings. Given known disparities in cardiovascular mortality among different populations,11,12 we also compared outcomes among CCSS cancer survivors with a population-based cohort of racially diverse adults from the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
METHODS
Population
CCSS is a multicenter cohort study including 31 participating centers in the US and Canada. The study is designed to investigate the late effects of cancer and cancer therapies among long-term (five years or longer) survivors of common pediatric cancers including leukemia, brain tumors, Hodgkin lymphoma, non-Hodgkin lymphoma, bone tumors, soft tissue sarcomas, kidney tumors, and neuroblastoma; cohort eligibility required minimum five-year survival following the initial cancer diagnosis.13,14 The study retrospectively ascertained participants diagnosed between January 1, 1970, and December 31, 1999 who were younger than 21 years of age at time of cancer diagnosis (n=25,658). Closest age siblings of a random sample of survivors were recruited to serve as a comparison group (n=5,051). Participating survivors and siblings provide longitudinal follow-up every few years through follow-up surveys (further described below), with data up through the 2014 survey included in this analysis. For this analysis, survivors also had to be free of any major cardiovascular events for the first five years after cancer diagnosis.
CARDIA (n=5,115) is a population-based prospective cohort study designed to investigate risk factors in young adulthood that contribute to cardiovascular disease later in life.15 Population-based sampling was used, with 50% of those invited examined at study baseline. Eligible participants were free of long-term disease or disability that would interfere with participation, and between the ages of 18 and 30 years in 1985-1986. The study recruited participants from four US cities (Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland CA). At each site, geographic areas were selected in order to obtain approximately the same number of individuals in subgroups of race, gender, education (high school or less and more than high school) and age (18-24 and 25-30 years), resulting in a cohort comprised of 48% White participants, 52% Black participants, 54% women and 46% men. An initial in-person clinical examination was completed with additional in-person clinic examinations approximately every 2 to 5 years, most recently in 2020-2023. One person withdrew from CARDIA, leaving 5114 at 33-year follow-up.
CCSS and CARDIA are approved by institutional review boards at participating centers and all participants provided informed consent.
Data Collection
For CCSS cancer survivors, cancer diagnosis and treatment history were obtained through medical record abstraction. Cancer survivors and siblings (or their proxy) periodically completed comprehensive questionnaires including information on demographics, social history, and history of health conditions including new malignant neoplasms (verified by pathology reports) and major cardiovascular events including HF, CAD resulting in myocardial infarction or coronary intervention, and stroke. The severity of these cardiovascular events was graded based on the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE, version 4.03) and categorized based on the CCSS’ adaptation of the CTCAE definitions (Supplemental Table 1).4,16 We considered three cardiovascular conditions in the analysis, HF grade ≥2, CAD grade ≥3, and stroke grade ≥4. If the information needed to distinguish between grades was insufficient, the lower grade was used. At baseline 69% of eligible survivors and 62% of eligible siblings completed the survey.17 The CCSS cohort has been periodically linked with the National Death Index to determine vital status, date of death, and cause of death, most recently in December 2017.
CARDIA participants completed standardized questionnaires at baseline and at each follow-up visit to assess sociodemographic factors, health behaviors (e.g., smoking), and interval medical history. Hospitalizations and vital status were queried annually and medical records were requested for all suspected cardiovascular events; death certificates and autopsy reports (if applicable) were requested for all deaths. Vital status searches were supplemented with National Death Index searches every five years, most recently 2017. CARDIA investigators then applied criteria to adjudicate morbidity and mortality endpoints with further classification as non-fatal events, fatal events, and a combination of fatal or non-fatal events.
Questionnaires and forms used by CCSS and CARDIA can be found on their respective websites: ccss.stjude.org and cardia.dopm.uab.edu.
Statistical Analysis
For cancer survivors, the first major cardiovascular event occurring 5 or more years post-cancer diagnosis was used as the index event. For CCSS siblings and CARDIA participants, the first occurrence of any major cardiovascular event was the index event. If any individual experienced more than one asynchronous cardiovascular event, only the index event was considered in the analysis. Individuals were included in multiple cardiovascular cause-specific analyses in the case of synchronous events (n=168 survivors, n=5 siblings, n=22 CARDIA).
The Kaplan-Meier method was used to estimate the cumulative incidence of all-cause mortality, with differences between survivors and siblings or CARDIA participants assessed using two-sided log-rank tests. The cumulative incidence of cardiovascular cause-specific mortality (i.e., cause of death due to the cardiovascular event of interest) was estimated using a non-parametric estimator, accounting for competing risks posed by other causes of death.18 Time at risk began at the time of the major cardiovascular event of interest, ending with death or censoring at the last follow-up date/NDI request date.
We used Cox proportional hazards models to compare hazard rates of all-cause and cardiovascular-specific mortality between CCSS survivors and siblings or CARDIA participants after the occurrence of HF, CAD, and stroke, estimating hazard ratios (HR) and 95% confidence intervals (CI) using age as the time scale to minimize the potential of biased risk estimates where confounding by age is a concern.19,20, Models were additionally adjusted for sex, cardiovascular risk factors (smoking, pre-existing hypertension, dyslipidemia, and diabetes), and subsequent malignant neoplasm history (in CCSS only). Smoking prior to index event was categorized as never smoker vs. former smoker and current smoker. Pre-existing hypertension, dyslipidemia, and diabetes were limited to those requiring medication treatment. For CCSS-only analyses, subsequent malignant neoplasm history was treated as a time-dependent covariate. For analyses restricted to survivors of childhood cancer, Cox regression models also considered additional covariates, specifically age at cancer diagnosis and treatment exposures (cumulative anthracycline dose [per 100 mg/m2 expressed in terms of doxorubicin equivalent dose]21 and heart/brain/neck radiation dose [per 10 Gy]). Sensitivity analyses were performed to investigate an alternative Cox regression model using follow-up time as the time scale and adjusting for age at cardiovascular event as cubic splines, the influence of more severe grades of HF (≥3) on outcomes, as well as the influence of insurance status at baseline.
All analyses were completed using R v.3.4.4 (R survival package) and STATA/SE v17.0.22
RESULTS
The CARDIA cohort by design had a much greater proportion of Black participants (51.6%) compared with CCSS (6.5% of survivors; 3.0% of siblings; Table 1). Among the 25,658 childhood cancer survivors evaluated, the median age of cancer diagnosis was 7 years (IQR 3-13) and the most common cancer diagnoses were leukemia (40.1%), lymphoma (17.6%), and other solid tumors (20.1%). Approximately half of survivors were treated with anthracyclines (53.1%) or radiation of a field involving the heart (49.8%) and 28.3% received brain radiation. There was a higher prevalence of major cardiovascular events (n=1,780; 6.4%) among cancer survivors compared with siblings overall (n=91; 1.8%), and for each individual cardiovascular outcome: HF 3.0% (n=847 survivors) versus 0.6% (n=31 siblings); CAD 1.8% (n=510 survivors) versus 0.8% (n=39 siblings); and stroke 2.1% (n=568 survivors) versus 0.5% (n=27 siblings). When compared with cancer survivors, CARDIA participants had a 6.7% (n=345) prevalence of cardiovascular events, which included a lower HF prevalence (1.8%; n=90), a higher CAD prevalence (3.3%; n=170), and a similar stroke prevalence (2.2%, n=112). The median age of the first cardiovascular event among cancer survivors (median 31 years, interquartile range (IQR) 22-39 years), siblings (median 35 years, IQR 27-47 years) and CARDIA participants (median 57 years, IQR 25-66 years) also varied. The distribution of potentially modifiable cardiovascular risk factors also varied across the three groups, with fewer current smokers among cancer survivors compared with siblings and especially compared with CARDIA participants, but similar rates of hypertension, and higher rates of dyslipidemia and diabetes. Distributions for age at death are provided in Supplemental Table 2.
Table 1.
Demographic and clinical characteristics of CCSS and CARDIA participants who experienced cardiovascular events
| Overall | Heart failure (HF) | Coronary artery disease (CAD) | Stroke | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Childhood cancer survivors (N=25658) |
Siblings (N=5051) |
CARDIA (N=5114) |
Childhood cancer survivors (N=847) |
Siblings (N=31) |
CARDIA (N=90) |
Childhood cancer survivors (N=510) |
Siblings (N=39) |
CARDIA (N=170) |
Childhood cancer survivors (N=568) |
Siblings (N=27) |
CARDIA (N=112) |
|
| N (%) or median (interquartile range) | ||||||||||||
| Sex | ||||||||||||
| Female | 11937 (46.5%) | 2643 (52.3%) | 2787 (54.5%) | 455 (53.7%) | 17 (54.8%) | 38 (42.2%) | 212 (42.2%) | 14 (35.9%) | 52 (30.6%) | 229 (40.2%) | 13 (48.2%) | 67 (59.8%) |
| Male | 13721 (53.6%) | 2408 (47.7%) | 2327 (45.5%) | 392 (46.3%) | 14 (45.2%) | 52 (57.8%) | 298 (57.8%) | 25 (64.1%) | 118 69.4%) | 339 (59.9%) | 14 (51.9%) | 45 (40.2%) |
| Race/Ethnicitya | ||||||||||||
| Black | 1640 (6.5%) | 151 (3.0%) | 2637 (51.6%) | 68 (8.0%) | 1 (3.2%) | 75 (82.3%) | 32 (6.6%) | 0 (0%) | 76 (44.7%) | 39 (6.7%) | 0 (0%) | 87 (77.7%) |
| Hispanic/Latino | 2032 (8.7%) | 215 (4.3%) | 43 (6.0%) | 0 (0%) | - | 31 (5.9%) | 0 (0%) | - | 36 (5.8%) | 1 (3.7%) | - | |
| White | 20911 (80.5%) | 4377 (86.7%) | 2477 (48.4%) | 703 (82.3%) | 30 (96.8%) | 15 (16.7%) | 433 (84.8%) | 36 (92.3%) | 94 (55.3%) | 475 (84.5%) | 26 (96.3%) | 25 (22.3%) |
| Other | 1075 (4.4%) | 308 (6.1%) | 33 (3.7%) | 0 (0%) | - | 14 (2.7%) | 3 (7.7%) | - | 18 (2.9%) | 0 (0%) | - | |
| Age at cancer diagnosis (median [IQR]) | 7 (3-13) | - | - | 11 (5-15) | - | - | 14 (9-17) | - | - | 8 (4-13) | - | - |
| Cancer diagnosis | ||||||||||||
| Leukemia | 7872 (40.1%) | - | - | 175 (24.8%) | - | - | 85 (18.6%) | - | - | 136 (30.0%) | - | - |
| Lymphomas | 5221 (17.6%) | - | - | 303 (33.9%) | - | - | 304 (58.2%) | - | - | 75 (12.2%) | - | - |
| CNS tumors | 4489 (15.1%) | - | - | 26 (2.9%) | - | - | 26 (5.0%) | - | - | 290 (47.0%) | - | - |
| Bone tumors | 2098 (7.1%) | - | - | 164 (18.3%) | - | - | 38 (7.3%) | - | - | 17 (2.8%) | - | - |
| Other solid tumors | 5977 (20.1%) | - | - | 179 (20.0%) | - | - | 57 (10.9%) | - | - | 50 (8.1%) | - | - |
| Any heart RT | 12375 (49.8%) | - | - | 512 (67.0%) | - | - | 377 (83.6%) | - | - | 395 (80.0%) | - | - |
| Any brain RT | 6721 (28.3%) | - | - | 142 (20.3%) | - | - | 84 (19.3%) | - | - | 333 (67.8%) | - | - |
| Any anthracyclines | 11353 (53.1%) | - | - | 569 (75.1%) | - | - | 159 (36.0%) | - | - | 132 (30.6%) | - | - |
| Age at cardiovascular event (median [IQR]) | - | - | - | 29 (21-38) | 32 (22-37) | 48 (42-52) | 38 (31-44) | 42 (32-53) | 50 (47-54) | 29 (20-38) | 35 (26-45) | 50 (45-55) |
| Attained age (median [IQR]) | 38 (30-45) | 43 (36-51) | 58 (54-61) | 41 (33-49) | 48 (42-55) | 55 (51-61) | 48 (39-54) | 57 (49-64) | 58 (54-61) | 40 (32-48) | 53 (39-61) | 58 (54-62) |
| Smoking statusb,c | ||||||||||||
| Never smoked | 13775 (56.3) | 2469 (49.4) | 2870 (56.1%) | 476 (61.1%) | 17 (56.7%) | 41 (45.6%) | 284 (59.1%) | 15 (38.5%) | 60 (35.3%) | 339 (65.7%) | 11 (40.7%) | 43 (38.4%) |
| Previous smoker | 7654 (30.7) | 1615 (32.3) | 671 (13.1%) | 207 (25.9%) | 5 (16.7%) | 9 (10.0%) | 104 (21.8%) | 12 (30.8%) | 25 (14.7%) | 113 (20.3%) | 10 (37.0%) | 17 (15.2%) |
| Current smoker | 3174 (12.9) | 916 (18.3) | 1572 (30.7%) | 104 (13.0%) | 8 (26.7%) | 40 (44.4%) | 95 (19.2%) | 12 (30.8%) | 85 (50.0%) | 72 (14.0%) | 6 (22.2%) | 52 (46.4%) |
| Pre-existing hypertensionc | 3049 (11.0) | 488 (9.7) | 577 (11.3%) | 359 (41.5%) | 14 (45.2%) | 53 (58.9%) | 262 (50.9%) | 23 (59.0%) | 63 (37.1%) | 173 (29.0%) | 16 (59.3%) | 61 (54.5%) |
| Pre-existing dyslipidemiac | 2140 (7.5) | 315 (6.2) | 223 (4.4%) | 214 (24.4%) | 9 (29.0%) | 22 (24.4%) | 247 (47.3%) | 23 (59.0%) | 65 (38.2%) | 176 (29.5%) | 9 (33.3%) | 29 (25.9%) |
| Pre-existing diabetes mellitusc | 1134 (4.2) | 124 (2.5) | 114 (2.2%) | 97 (12.3%) | 4 (12.9%) | 13 (14.4%) | 88 (17.4%) | 7 (18.0%) | 35 (20.6%) | 52 (8.5%) | 6 (22.2%) | 32 (28.6%) |
| Had health insurancec | 20167 (85.3) | 4474 (90.0) | - | 656 (88.5) | 24 (80.0%) | - | 411 (89.2) | 33 (84.6%) | - | 431 (87.2) | 23 (85.2%) | - |
Abbreviations: CAD, coronary artery disease; CARDIA, Coronary Artery Risk Development in Young Adults; CCSS, Childhood Cancer Survivor Study; CNS, central nervous system; CV, cardiovascular; HF, heart failure; RT, radiation therapy. Percentages reflect primary cancer diagnosis sampling weights in CCSS.
In CCSS, Black and White participants are Non-Hispanic Black and Non-Hispanic White, respectively. In CARDIA, participants were categorized as Black versus White. CARDIA recruited for equal representation of black and white participants.
Missing smoking data for childhood cancer survivors (N=60, HF subgroup; N=27, CAD subgroup; N=44 stroke subgroup) and siblings (N=1, HF subgroup).
In the overall CCSS and CARDIA samples: data was taken from baseline questionnaires. For condition-specific samples: status was evaluated from questionnaires immediately prior to the index event.
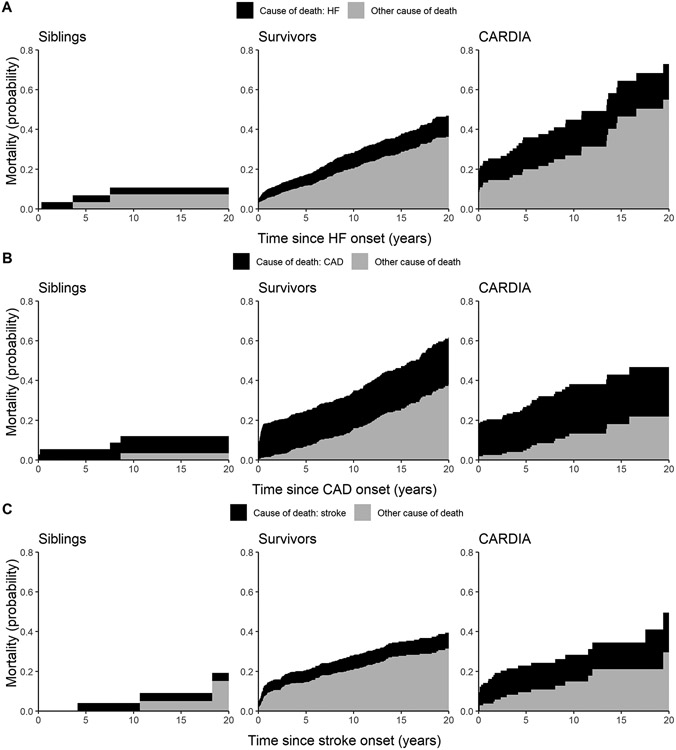
After a major cardiovascular event, mortality was higher among cancer survivors compared with siblings: 37.7% versus 16.1% after HF; 46.3% versus 18.0% after CAD; and 34.0% versus 11.1% after stroke, although numbers of affected siblings were small (Table 2). Compared with cancer survivors, mortality rates for CARDIA participants were higher after HF (55.6%), lower after CAD (37.1%), and similar after stroke (32.1%). Using the Kaplan-Meier method, the probability of all-cause mortality at 10 years since the index cardiovascular event was significantly higher among cancer survivors compared with siblings: 30% (95% CI: 26-33%) versus 14% (95% CI: 0-25%) after HF; 36% (95% CI: 31-40%) versus 14% (95% CI: 2-25%) after CAD; 29% (95% CI: 24-33%) versus 4% (95% CI: 0-11%) after stroke (p<0.001 for all). Compared with CARDIA participants who were on average decades older at the time of their cardiovascular event, the probability of all-cause mortality among cancer survivors was similar after CAD and stroke but lower after HF (Central Illustration, Supplemental Table 3). Accounting for deaths due to other causes as a competing risk, at 10 years there was an increased probability of cardiovascular cause-specific mortality after each major cardiovascular event among cancer survivors compared with siblings (Figure 1, Supplemental Table 4). Compared with CARDIA participants, there was a decreased probability of cardiovascular cause-specific mortality but increased probability of other cause mortality after CAD among cancer survivors. Cancer recurrence and subsequent neoplasms were leading contributors to death among cancer survivors (Table 2), highlighting the importance of non-cardiovascular causes of death after a major cardiovascular event in this population.
Table 2:
Causes of death for CCSS and CARDIA participants who experienced cardiovascular events
| Heart failure (HF) | Coronary artery disease (CAD) | Stroke | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Childhood cancer survivors (N=847) |
Siblings (N=31) |
CARDIA (N=90) |
Childhood cancer survivors (N=510) |
Siblings (N=39) |
CARDIA (N=170) |
Childhood cancer survivors (N=568) |
Siblings (N=27) |
CARDIA (N=112) |
||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Vital status | ||||||||||||||||||
| Deceased | 321 | 37.7 | 5 | 16.1 | 50 | 55.6 | 239 | 46.3 | 7 | 18.0 | 63 | 37.1 | 193 | 34.0 | 3 | 11.1 | 36 | 32.1 |
| Alive | 526 | 62.3 | 26 | 83.9 | 40 | 44.4 | 271 | 53.7 | 32 | 82.1 | 107 | 62.9 | 375 | 66.0 | 24 | 88.9 | 76 | 67.9 |
| Deceased, by causea | ||||||||||||||||||
| Specific cardiovascular condition (HF/CAD/stroke) | 78 | 24.5 | 1 | 25.0 | 14 | 32.6 | 104 | 45.8 | 3 | 60.0 | 37 | 67.3 | 46 | 23.0 | 1 | 33.3 | 15 | 50.0 |
| Other cardiovascular | 32 | 10.9 | 2 | 50.0 | 15 | 34.9 | 27 | 11.6 | 0 | 0 | 2 | 3.6 | 11 | 5.5 | 0 | 0 | 4 | 13.3 |
| Non-cardiovascularb | 192 | 64.6 | 1 | 25.0 | 14 | 32.6 | 99 | 42.6 | 2 | 40.0 | 16 | 29.1 | 126 | 71.5 | 2 | 66.7 | 11 | 36.7 |
| Pulmonary | 16 | 5.0 | 0 | 0 | 13 | 5.6 | 0 | 0 | 7 | 3.5 | 0 | 0 | ||||||
| Recurrence | 50 | 19.1 | 0 | 0 | 10 | 4.3 | 0 | 0 | 56 | 33.4 | 0 | 0 | ||||||
| Subsequent neoplasm | 58 | 18.3 | 0 | 0 | 34 | 14.6 | 0 | 0 | 37 | 20.3 | 1 | 33.3 | ||||||
| External | 15 | 4.7 | 0 | 0 | 10 | 4.3 | 0 | 0 | 6 | 3.0 | 1 | 33.3 | ||||||
| Other | 53 | 17.5 | 1 | 25.0 | 32 | 13.8 | 2 | 40.0 | 20 | 11.3 | 0 | 0 | ||||||
Abbreviations: CAD, coronary artery disease; CARDIA, Coronary Artery Risk Development in Young Adults; CCSS, Childhood Cancer Survivor Study; HF, heart failure. Percentages reflect primary cancer diagnosis sampling weights in CCSS.
Missing cause of death for childhood cancer survivors (N=19, HF subgroup; N=9, CAD subgroup; N=10, stroke subgroup) and siblings (N=1, HF subgroup; N=2, CAD subgroup) and CARDIA (N=7, HF subgroup; N=8 CAD subgroup; N=6 stroke subgroup). Cause-specific mortality represents HF, CAD, or stroke individually and is not a combined CVD endpoint.
Central Figure: All-cause mortality after a major cardiovascular event.
Cumulative incidence of all-cause mortality after heart failure (HF; panel A), coronary artery disease (CAD; panel B), and stroke (panel C) estimated using the Kaplan-Meier method are shown for childhood cancer survivors (red), siblings (grey), and CARDIA participants (blue) with 95% confidence intervals displayed.
Figure 1: Cause-specific mortality after a major cardiovascular event.
Partitioned cumulative mortality probabilities due to heart failure (HF; panel A), coronary artery disease (CAD; panel B), and stroke (panel C) relative to other causes of death for childhood cancer survivors, siblings, and CARDIA participants. Cardiovascular condition-specific and other cause mortality probabilities are represented in black and grey, respectively.
When cancer survivors were compared with siblings in multivariable analyses adjusted for race and pre-existing cardiovascular risk factors, the all-cause mortality rate among survivors was higher after each major cardiovascular event: HF HR 7.32, (95% CI: 2.56-20.89); CAD HR 5.54 (95% CI: 2.37-12.93); and stroke HR 3.57 (95% CI: 1.12-11.37) (Table 3). Cardiovascular cause-specific mortality after a CAD event was also significantly elevated for survivors versus siblings, HR 3.70 (95% CI: 1.05-13.02), while estimates for cause-specific mortality after HF and stroke were increased but not statistically significant. Non-White race was associated with increased risk for all-cause mortality and cause-specific mortality after a HF event. When cancer survivors were compared with CARDIA participants, estimates were generally attenuated, although survivors continued to experience an increased rate of all-cause mortality after CAD. Results from models using time since the major cardiovascular event as the time scale adjusted for age at event (Supplemental Table 5) were consistent with our main results modeling age as the scale.
Table 3:
Multivariable all-cause and cardiac cause-specific mortality models among CCSS and CARDIA participants
| All-cause mortality | Cardiovascular cause-specific mortalitya | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer survivors vs. siblings |
Cancer survivors vs. CARDIA |
Cancer survivors vs. siblings |
Cancer survivors vs. CARDIA |
|||||
| HRc | 95% CIc | HRc | 95% CIc | HRc | 95% CIc | HRc | 95% CIc | |
| After heart failure a | 7.32 | 2.56-20.89 | 1.03 | 0.60-1.77 | 3.62 | 0.48-27.29 | 0.77 | 0.29-2.08 |
| Non-White vs. White, NH (ref.) | 1.80 | 1.32-2.46 | 1.37 | 0.96-1.95 | 2.45 | 1.41-4.25 | 2.06 | 1.11-3.84 |
| Pre-existing hypertensionb | 1.45 | 1.00-2.10 | 0.68 | 0.53-0.87 | 0.98 | 0.40-2.39 | 0.34 | 0.20-0.58 |
| Pre-existing dyslipidemiab | 0.68 | 0.39-1.19 | 0.51 | 0.38-0.70 | 0.49 | 0.11-2.11 | 0.46 | 0.23-0.92 |
| Pre-existing diabetesb | 1.89 | 1.15-3.10 | 1.78 | 1.23-2.57 | 0.91 | 0.21-3.91 | 1.08 | 0.44-2.67 |
| After coronary artery disease a | 5.54 | 2.37-12.93 | 1.85 | 1.16-2.95 | 3.70 | 1.05-13.02 | 1.16 | 0.55-2.45 |
| Non-White vs. White, NH (ref.) | 1.03 | 0.70-1.53 | 1.08 | 0.76-1.55 | 0.87 | 0.48-1.58 | 0.96 | 0.58-1.61 |
| Pre-existing hypertensionb | 1.12 | 0.80-1.57 | 0.77 | 0.59-1.02 | 0.80 | 0.46-1.37 | 0.46 | 0.29-0.72 |
| Pre-existing dyslipidemiab | 0.43 | 0.28-0.68 | 0.39 | 0.29-0.51 | 0.41 | 0.19-0.88 | 0.19 | 0.11-0.33 |
| Pre-existing diabetesb | 0.98 | 0.58-1.67 | 1.12 | 0.80-1.56 | 0.86 | 0.38-1.96 | 0.89 | 0.47-1.67 |
| After stroke a | 3.57 | 1.12-11.37 | 1.36 | 0.70-2.63 | 3.12 | 0.40-24.32 | 0.87 | 0.24-3.17 |
| Non-White vs. White, NH (ref.) | 1.48 | 0.96-2.28 | 1.46 | 0.95-2.24 | 1.13 | 0.49-2.63 | 1.33 | 0.60-2.94 |
| Pre-existing hypertensionb | 1.37 | 0.81-2.32 | 0.74 | 0.53-1.04 | 1.27 | 0.53-3.05 | 0.40 | 0.20-0.80 |
| Pre-existing dyslipidemiab | 1.09 | 0.60-1.99 | 0.55 | 0.37-0.80 | 0.54 | 0.18-1.64 | 0.35 | 0.16-0.72 |
| Pre-existing diabetesb | 0.86 | 0.34-2.20 | 1.00 | 0.62-1.62 | 0.42 | 0.05-3.38 | 1.18 | 0.53-2.36 |
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CCSS, Childhood Cancer Survivor Study; CI, confidence interval; HR, hazard ratio; P, p-value.
Cause-specific mortality represents HF-, CAD-, or stroke-mortality individually and is not a combined CVD mortality.
CCSS definition of hypertension, dyslipidemia, and diabetes limited to respondents requiring medication for the condition.
In addition to the listed exposures, models were adjusted for sex, attained age (as the time scale of Cox regression), cardiovascular index event year, smoking history, and subsequent malignant neoplasms (survivors vs. siblings analysis only).
In sensitivity analyses where CCSS’ HF outcomes were limited to those classified as grade ≥3 (i.e., requiring medication documentation), we found that cancer survivors continued to experience a significantly increased rate of all-cause mortality compared with siblings (HR 5.10 [95% CI: 1.50-17.33]; Supplemental Table 6). The relative hazards of experiencing all-cause or cardiovascular cause-specific mortality after a cardiovascular event among survivors also remained higher when compared with siblings after accounting for insurance status at baseline (Supplemental Table 7).
Among cancer survivors, multivariable analyses that examined treatment-related and cardiovascular risk factors found that higher mean heart doses from radiotherapy were associated with an increased rate for both all-cause and cardiovascular cause-specific mortality after HF and CAD (Table 4). Similarly, increased mean heart dose from radiotherapy was also associated with an increase in the stroke-specific mortality risk, while brain radiotherapy was associated with an increased all-cause risk of death after stroke. In contrast, higher anthracycline dose was associated with a decreased rate of all-cause mortality after HF (HR 0.84 per 100 mg/m2; 95% CI: 0.77-0.92), but not with HF-specific mortality (HR 1.08; 95% CI: 0.93-1.27).
Table 4:
Treatment and cardiovascular risk factor associations with mortality rates among childhood cancer survivors
| All-cause mortality | Cardiovascular cause-specific mortalitya | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Deaths | HRd | 95% CId | N | Deaths | HRd | 95% CId | |
| After heart failure a | 645 | 194 | 638 | 47 | ||||
| Heart RT (per 10 Gy) | 1.27 | 1.12-1.44 | 1.43 | 1.10-1.85 | ||||
| Anthracyclines (per 100 mg/m2) | 0.84 | 0.77-0.92 | 1.08 | 0.93-1.27 | ||||
| Pre-existing hypertensionb | 1.71 | 1.10-2.66 | 1.65 | 0.61-4.49 | ||||
| Pre-existing dyslipidemiab | 0.49 | 0.25-0.97 | 0.23 | 0.03-1.76 | ||||
| Pre-existing diabetes | 1.67 | 0.93-3.01 | 0.57 | 0.07-4.39 | ||||
| Previous smoking | 0.72 | 0.51-1.02 | 0.82 | 0.39-1.70 | ||||
| Current smoking | 1.11 | 0.71-1.71 | 2.47 | 1.18-5.15 | ||||
| After coronary artery disease a | 404 | 172 | 396 | 71 | ||||
| Heart RT (per 10 Gy) | 1.22 | 1.06-1.41 | 1.35 | 1.08-1.68 | ||||
| Anthracyclines (per 100 mg/m2) | 0.98 | 0.87-1.10 | 0.85 | 0.68-1.06 | ||||
| Pre-existing hypertensionb | 1.24 | 0.86-1.80 | 1.04 | 0.58-1.89 | ||||
| Pre-existing dyslipidemiab | 0.43 | 0.26-0.71 | 0.36 | 0.15-0.88 | ||||
| Pre-existing diabetes | 1.11 | 0.62-1.97 | 0.92 | 0.37-2.27 | ||||
| Previous smoking | 0.90 | 0.61-1.32 | 0.63 | 0.32-1.21 | ||||
| Current smoking | 0.93 | 0.60-1.45 | 1.02 | 0.53-1.96 | ||||
| After stroke a | 428 | 113 | 422 | 31 | ||||
| Brain RT (per 10 Gy) | 1.18 | 1.09-1.28 | 1.02 | 0.87-1.20 | ||||
| Heart RT (per 10 Gy) | 1.09 | 0.90-1.32 | 1.43 | 1.01-2.04 | ||||
| Pre-existing hypertensionb | 1.73 | 0.91-3.27 | 1.12 | 0.36-3.45 | ||||
| Pre-existing dyslipidemiab | 0.87 | 0.42-1.83 | 0.45 | 0.10-2.16 | ||||
| Pre-existing diabetesc | 0.34 | 0.08-1.53 | <0.01 | 0.00-1.32 | ||||
| Previous smoking | 0.90 | 0.53-1.54 | 0.76 | 0.26-2.19 | ||||
| Current smoking | 1.12 | 0.62-2.03 | 1.44 | 0.53-3.93 | ||||
Abbreviations: HR, hazard ratio; CI, confidence interval; P, p-value; RT, radiation therapy; Gy, Gray; mg, milligrams; m, meter.
Cause-specific mortality represents HF-, CAD-, or stroke-specific mortality individually and is not a combined CVD mortality. Sample size is slightly smaller due to missing cause of death information in a few cancer survivors.
CCSS definition of hypertension and dyslipidemia limited to respondents requiring medication for the condition.
No stroke-specific deaths were observed among individuals with pre-existing diabetes; 95% CI and p-value were estimated by inverting the likelihood ratio test.
In addition to the listed exposures, models were adjusted for sex, attained age (as the time scale of Cox regression) and year of the cardiovascular index event, race/ethnicity, age at childhood cancer diagnosis, and subsequent malignant neoplasms.
In examining the influence of cardiovascular risk factors on outcomes following a major cardiovascular event among survivors (Table 4), pre-existing hypertension was significantly associated with increased all-cause mortality risk after HF (HR 1.71; 95% CI: 1.10-2.66) and current smoking was associated with an increased cardiovascular cause-specific mortality risk after HF (HR 2.47; 95% CI: 1.18-5.15). HR estimates for hypertension and current smoking tended to be increased for most other outcomes but were not statistically significant. In contrast, pre-existing dyslipidemia was associated with a lower risk of all-cause mortality after both HF (HR 0.49; 95% CI: 0.25-0.97) and CAD (HR 0.43; 95% CI: 0.26-0.71). Dyslipidemia was also associated with lower cause-specific mortality after all three cardiovascular events, although not all estimates were statistically significant. Finally, pre-existing diabetes status was generally not influential, but was relatively rare compared with hypertension and dyslipidemia resulting in estimates with wide confidence intervals.
DISCUSSION
The current study leveraged a large population of childhood cancer survivors with up to five decades of follow up to examine the association of major cardiovascular events and risk for mortality among survivors relative to siblings and a more racially diverse population-based cohort who experienced events appreciably later in life. In comparison with diverse non-cancer populations, among cancer survivors; 1) cardiovascular events occurred at a younger age, 2) cardiovascular cause-specific mortality after CAD events was increased and 3) all-cause mortality after a major cardiovascular event was increased.
These findings provide an opportunity to reassess and potentially optimize cardiovascular care for survivors of childhood cancer. In the setting of a major cardiovascular event, there is evidence that clinical management strategies may differ between cancer survivors and cancer naïve patients. Patients with cancer are often excluded from clinical trials investigating percutaneous coronary intervention and perhaps as a consequence, survivors are more likely to be treated with traditional therapies such as bare metal stents, despite the superior clinical performance of drug eluting stents.9 Additionally there is evidence that survivors may be less likely to receive cardioprotective medications after an acute myocardial infarction.23
Preventative strategies for survivors may also be enhanced. The adversely synergistic relationship between traditional cardiovascular risk factors and cancer therapies is well demonstrated. Relative to cancer survivors without hypertension or exposure to cardiotoxic therapy, cancer survivors with a history of chest radiation who develop hypertension or dyslipidemia are many times more likely to develop cardiomyopathy. Similarly, survivors with a history of anthracycline exposure who develop hypertension or dyslipidemia are significantly more likely to experience serious cardiovascular outcomes.24 Our results support these previous findings by Armstrong et al. and demonstrate that pre-existing hypertension is associated with an increased mortality rate among survivors after a major cardiovascular event. Current ACC/AHA guidelines for hypertension and blood cholesterol do not take cancer diagnosis or treatment history into account when determining individual risk for cardiovascular disease.25,26 Given the increased burden of mortality among survivors who develop cardiovascular disease, more aggressive risk factor modification may be appropriate for this population.
The burden of chronic health conditions, particularly cancer recurrence and subsequent neoplasms among survivors of childhood cancer contributes to our finding of an increased risk for all-cause mortality. Mueller et al. have shown an increased risk for all-cause mortality after stroke among childhood cancer survivors diagnosed between 1970 and 1986.10 In a landmark study, Oeffinger et al. found a more than three times greater rate for chronic health conditions among survivors when compared with siblings.4 In particular, the relative risk for new cancer diagnoses, among survivors versus siblings was more than 14. The severity of these chronic conditions was also disparate with an 8 times greater risk for a severe or life-threatening chronic health condition among survivors. Thus, our data suggest that this population of survivors living with a high burden of chronic health conditions appear less resilient to major cardiovascular events when they occur, and more likely to succumb either to the cardiovascular condition itself or other chronic health conditions, such as cancer. This finding may support consideration for statins as a strategy to lower cancer mortality risk among survivors.27,28 Despite the high burden of chronic health conditions, the majority of childhood, adolescent, and young adult cancer survivors report that they do not receive follow-up care focused on late effects of cancer treatment.29
Our findings also demonstrate the impact of treatment-related risk factors. Higher mean heart doses from radiotherapy were associated with an increased rate for both all-cause and cause-specific mortality after HF and CAD as well as stroke-specific mortality. Brain radiotherapy was associated with an increased all-cause rate of death after stroke. These findings likely reflect the challenges of treating the progressive pathologic changes mediated by radiation therapy30 as well as potential for out-of-field toxicity including an increased risk for hypertension, vascular endothelial dysfunction, and diffuse fibrotic changes.31,32 Studies among individuals with advanced heart failure requiring transplant support our results and demonstrate higher rates of early post-operative mortality in the setting of radiation mediated heart failure as compared with other etiologies of heart failure.7,8 Higher all-cause mortality among individuals with radiation-associated coronary artery disease has also been observed.33 In this study, higher anthracycline dose was associated with a decreased all-cause mortality rate after HF. It is plausible that although higher anthracycline dose mediates risk for HF, once a survivor has developed HF, anthracycline dose does not further augment mortality risk. Additionally, in the past two decades, the recognition of the potential responsiveness of anthracycline mediated cardiomyopathy to cardioactive medications has changed the treatment paradigm for this condition and likely altered the risk of mortality.34
In contrast with Armstrong et al.24 pre-existing dyslipidemia was a protective factor in our study. A recent study by Dixon et al. similarly found dyslipidemia was not associated with increased health related mortality risk.35 These findings may be related to the differences in approach with the study by Armstrong et al. examining factors contributing to cardiovascular events, while the study by Dixon et al. and our study examining factors contributing to mortality. Additionally, in the US, statin use over the past decade has increased and this should be considered.36 It is known that statin therapy decreases mortality among individuals with atherosclerotic cardiovascular disease37 and there is some evidence to suggest statins preserve cardiac function in the setting of anthracycline based chemotherapy, although these results have not been confirmed consistently in all studies.38-41 These findings will require further investigation, but may suggest that statin therapy could play a significant role in future strategies to mitigate cardiovascular events and subsequent mortality among cancer survivors.
The attenuation of mortality risks among survivors compared with CARDIA participants may be explained by two factors. CARDIA is a more racially diverse population, with 50% of participants identifying as Black. Higher mortality rates after cardiovascular events have been well demonstrated among individuals of Black or African American race. 11,12 Age is known to influence mortality risk after a cardiovascular event.42 Compared with survivors, CARDIA participants were considerably older at the time of a major cardiovascular event. The attenuated mortality risk between survivors and CARDIA participants suggests that mortality risk among survivors is more similar to individuals in the general population who are over two 2 decades older. This observation is supported by previous studies demonstrating an accelerated aging phenotype among survivors of childhood cancer.43
Limitations
While our study utilizes a large cohort with well-adjudicated data regarding cancer history, treatment, and mortality, there are some limitations. Our findings may be limited by the small number of cardiovascular events in the sibling population. CCSS, because of the size of the cohort, also had to rely primarily on self-reported outcomes data, introducing a greater likelihood of outcome misclassification. However, any misclassification should be similar between survivors and siblings, and CCSS attempted to minimize the impact of any misclassification via the conditions grading system. Additionally, the influence of cancer treatment related factors reflects total doses of anthracycline and radiation therapy during initial cancer treatment. Lifetime doses may have been greater among individuals who experienced recurrence or a subsequent cancer. Finally, the CCSS population, particularly siblings, was overwhelmingly non-Hispanic White. To address these potential limitations, we utilized data from CARDIA, which featured a roughly contemporaneous adult study population, whose cardiovascular events were more rigorously ascertained and adjudicated. However, CARDIA featured approximately equal percentages Black and White participants and participants experienced events at considerably older ages compared with CCSS cancer survivors. Notably, this allowed a unique comparison between CCSS cancer survivors and a population with higher non-cancer-related mortality risks after major cardiovascular events.
Conclusions
After a cardiovascular event, mortality is higher among survivors of childhood cancer when compared with siblings and is more comparable to a racially diverse adult population who experienced events at considerably older ages. Optimized clinical care in the setting of a cardiovascular event, greater inclusion of survivors in clinical trials, enhanced risk factor modification, and improved access to care that directly considers late effects of cancer therapy, are potential pathways to address disparities in mortality among survivors compared with non-cancer cohorts who experience a major cardiovascular event.
Supplementary Material
CLINICAL PERSPECTIVES.
Competencies in Medical Knowledge:
The risk of developing cardiovascular disease and associated mortality are increased among adult survivors of childhood cancer, particularly among those with hypertension.
Translational Outlook:
Enhanced interventions are needed to reduce the risk of cardiovascular related mortality in this population.
Sources of Funding:
This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator; CA204378, E.J. Chow, Principal Investigator). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC). W. Bottinor is supported by To-morrow’s Research Fund St. Baldrick’s Scholar Award (636214), American Heart Association (19CDA34760181), CTSA award KL2TR002648 from the National Center for Advancing Translational Sciences. C. Im is supported by the National Cancer Institute (R21 CA261833, C. Im/Y. Yuan, Principal Investigators), Children’s Cancer Research Fund, and University of Minnesota Pediatric Foundation. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or sponsors.
Abbreviations:
- HF
heart failure
- CAD
coronary artery disease
- CCSS
Childhood Cancer Survivor Study
- CARDIA
Coronary Artery Risk Development in Young Adults
- HR
hazard ratios
- CI
95% confidence intervals
- CTCAE
Common Terminology Criteria for Adverse Events
- Gy
Gray
- IQR
interquartile range
Footnotes
Disclosures: The authors declare no relationships with industry or financial disclosures relevant to this research.
Data Sharing:
The Childhood Cancer Survivor Study is a US National Cancer Institute funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. CCSS data are publicly available on dbGaP at https://www.ncbi.nlm.nih.gov/gap/through its accession number phs001327.v2.p1. and on the St. Jude Survivorship Portal within the St. Jude Cloud at https://survivorship.stjude.cloud/. In addition, utilization of the CCSS data that leverages the expertise of CCSS Statistical and Survivorship research and resources will be considered on a case-by case basis. For this utilization, a research Application Of Intent followed by an Analysis Concept Proposal must be submitted for evaluation by the CCSS Publications Committee. Users interested in utilizing this resource are encouraged to visit http://ccss.stjude.org. Full analytical data sets associated with CCSS publications since January of 2023 are also available on the St. Jude Survivorship Portal at https://viz.stjude.cloud/community/cancer-survivorship-community~4/publications.
References
- 1.National Cancer Institute Childhood Cancer Data Initiative, [Google Scholar]
- 2.Mertens AC, Liu Q, Neglia JP, et al. : Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 100:1368–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, et al. : Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol 27:2328–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572–82, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mulrooney DA, Armstrong GT, Huang S, et al. : Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann Intern Med 164:93–101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felker GM, Thompson RE, Hare JM, et al. : Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342:1077–84, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Al-Kindi SG, Oliveira GH: Heart Transplantation Outcomes in Radiation-Induced Restrictive Cardiomyopathy. J Card Fail 22:475–8, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Uriel N, Vainrib A, Jorde UP, et al. : Mediastinal radiation and adverse outcomes after heart transplantation. J Heart Lung Transplant 29:378–81, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Landes U, Kornowski R, Bental T, et al. : Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis 28:5–10, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Mueller S, Kline CN, Buerki RA, et al. : Stroke impact on mortality and psychologic morbidity within the Childhood Cancer Survivor Study. Cancer 126:1051–1059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disease Heart and African Americans US Department of Health and Human Services Office of Minority Health, US Department of Health and Human Services Office of Minority Health, 2023 [Google Scholar]
- 12.Zhu C, Shi T, Jiang C, et al. : Racial and Ethnic Disparities in All-Cause and Cardiovascular Mortality Among Cancer Patients in the U.S. JACC CardioOncol 5:55–66, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison LL, Armstrong GT, Boice JD, et al. : The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 27:2308–18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leisenring WM, Mertens AC, Armstrong GT, et al. : Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol 27:2319–27, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. : CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41:1105–16, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events (CTCAE), U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute, 2009 [Google Scholar]
- 17.Robison LL, Mertens AC, Boice JD, et al. : Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol 38:229–39, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Aalen OO, Johansen S: An Empirical Transition Matrix for Non-Homogeneous Markov Chains Based on Censored Observations. Scandinavian Journal of Statistics 5:141–150, 1978 [Google Scholar]
- 19.Thiébaut AC, Bénichou J: Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med 23:3803–20, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Cologne J, Hsu WL, Abbott RD, et al. : Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology 23:565–73, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Feijen EAM, Leisenring WM, Stratton KL, et al. : Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncology 5:864–871, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therneau TM: A Package for Survival Analysis in R, 2021 [Google Scholar]
- 23.Gong IY, Yan AT, Ko DT, et al. : Temporal changes in treatments and outcomes after acute myocardial infarction among cancer survivors and patients without cancer, 1995 to 2013. Cancer 124:1269–1278, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong GT, Oeffinger KC, Chen Y, et al. : Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 31:3673–80, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelton PK, Carey RM, Aronow WS, et al. : 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71:e13–e115, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Stone NJ, Bailey AL, et al. : 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139:e1082–e1143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang W-T, Lin H-W, Lin S-H, et al. : Association of Statin Use With Cancer- and Noncancer-Associated Survival Among Patients With Breast Cancer in Asia. JAMA Network Open 6:e239515–e239515, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig EL, Stopsack KH, Evergren E, et al. : Statins and prostate cancer—hype or hope? The epidemiological perspective. Prostate Cancer and Prostatic Diseases 25:641–649, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellblom AV, Kiserud CE, Rueegg CS, et al. : Self-reported late effects and long-term follow-up care among 1889 long-term Norwegian Childhood, Adolescent, and Young Adult Cancer Survivors (the NOR-CAYACS study). Supportive Care in Cancer 29:2947–2957, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharya S, Asaithamby A: Ionizing radiation and heart risks. Semin Cell Dev Biol 58:14–25, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Farrugia MK, Mattes MD: Radiation-Association Hypertension in Patients Undergoing Treatment for Prostate Cancer. J Radiother Pract 19:112–115, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatesulu BP, Mahadevan LS, Aliru ML, et al. : Radiation-Induced Endothelial Vascular Injury: A Review of Possible Mechanisms. JACC Basic Transl Sci 3:563–572, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed GW, Masri A, Griffin BP, et al. : Long-Term Mortality in Patients With Radiation-Associated Coronary Artery Disease Treated With Percutaneous Coronary Intervention. Circ Cardiovasc Interv 9, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Cardinale D, Colombo A, Lamantia G, et al. : Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 55:213–20, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Dixon SB, Liu Q, Chow EJ, et al. : Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. The Lancet 401:1447–1457, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salami JA, Warraich H, Valero-Elizondo J, et al. : National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiology 2:56–65, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez F, Maron DJ, Knowles JW, et al. : Association of Statin Adherence With Mortality in Patients With Atherosclerotic Cardiovascular Disease. JAMA Cardiology 4:206–213, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neilan TG, Quinaglia T, Onoue T, et al. : Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 330:528–536, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thavendiranathan P, Houbois C, Marwick TH, et al. : Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. European Heart Journal - Cardiovascular Pharmacotherapy 9:515–525, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hundley WG, D'Agostino R Jr., Crotts T, et al. : Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid 1, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel-Qadir H, Bobrowski D, Zhou L, et al. : Statin Exposure and Risk of Heart Failure After Anthracycline- or Trastuzumab-Based Chemotherapy for Early Breast Cancer: A Propensity Score–Matched Cohort Study. J Am Heart Assoc 10:e018393, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damluji AA, Forman DE, Wang TY, et al. : Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation 147:e32–e62, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Q, Song N, Qin N, et al. : Genome-wide association studies identify novel genetic loci for epigenetic age acceleration among survivors of childhood cancer. Genome Med 14:32, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Childhood Cancer Survivor Study is a US National Cancer Institute funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. CCSS data are publicly available on dbGaP at https://www.ncbi.nlm.nih.gov/gap/through its accession number phs001327.v2.p1. and on the St. Jude Survivorship Portal within the St. Jude Cloud at https://survivorship.stjude.cloud/. In addition, utilization of the CCSS data that leverages the expertise of CCSS Statistical and Survivorship research and resources will be considered on a case-by case basis. For this utilization, a research Application Of Intent followed by an Analysis Concept Proposal must be submitted for evaluation by the CCSS Publications Committee. Users interested in utilizing this resource are encouraged to visit http://ccss.stjude.org. Full analytical data sets associated with CCSS publications since January of 2023 are also available on the St. Jude Survivorship Portal at https://viz.stjude.cloud/community/cancer-survivorship-community~4/publications.