Abstract
Aims
Elevated small dense LDL cholesterol (sd-LDL-C) increases atherosclerotic cardiovascular disease (CVD) risk. Although coronary artery calcification (CAC) is widely used for predicting CVD events, few studies have examined the relationship between sd-LDL-C and CAC.
Methods and results
This study included 4672 individuals with directly measured baseline sd-LDL-C and CAC from the Multi-Ethnic Study of Atherosclerosis [mean (standard deviation) age: 61.9 (10.4) years; 52.5% women; 47.3% with baseline CAC (mean score >0)]. We used multi-variable general linear models and restricted cubic splines with the goodness of fit testing to evaluate the association of sd-LDL-C with the presence of CAC. Odds ratios [OR (95% confidence interval)] were adjusted for demographics and cardiovascular risk factors, including estimated total LDL-C. Higher quartiles of sd-LDL-C were associated with the presence of CAC, even after accounting for total LDL-C. Compared with the lowest quartile of sd-LDL-C, participants in Quartiles 2, 3, and 4 had higher odds for the presence of baseline CAC [Quartile 2 OR: 1.24 (1.00, 1.53); Quartile 3 OR: 1.51 (1.19, 1.93); and Quartile 4 OR 1.59 (1.17, 2.16)]. Splines suggested a quadratic curvilinear relationship of continuous sd-LDL-C with CAC after adjustment for demographics and CVD risk factors (quadratic vs. first-order sd-LDL-C terms likelihood ratio test: P = 0.015), but not after accounting for total LDL-C (quadratic vs. first-order terms: P = 0.156).
Conclusion
In a large, multi-ethnic sample without known CVD, higher sd-LDL-C was associated with the presence of CAC, above and beyond total LDL-C. Whether selective direct measurement of sd-LDL-C is indicated to refine cardiovascular risk assessment in primary prevention warrants further investigation.
Keywords: Small dense low-density lipoprotein cholesterol, Coronary artery calcium, Cardiovascular disease
Graphical Abstract
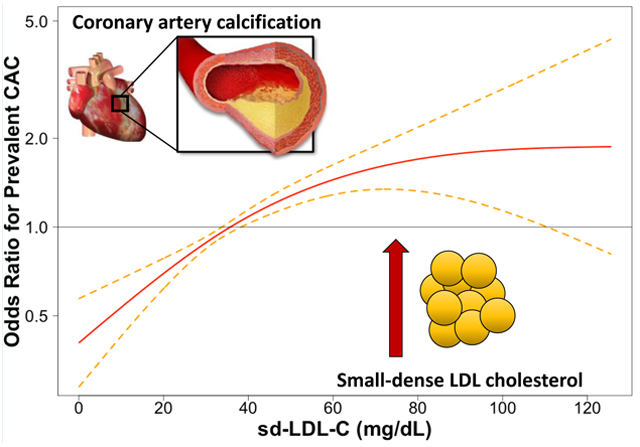
Lay summary
Higher levels of small dense particles of LDL cholesterol, better known as the ‘bad cholesterol’, are associated with a greater risk for the presence of coronary artery calcium, a strong marker for heart disease, even when accounting for estimated total (small dense + large body particles) LDL cholesterol.
This risk is stronger in older individuals.
Peak risk seems to occur between 49 and 71 mg/dL and does not increase further at higher levels.
Introduction
Multiple lines of evidence suggest a causal association between LDL cholesterol (LDL-C) and atherosclerotic cardiovascular disease (CVD).1 The size of LDL particles varies considerably among individuals, yielding two distinct phenotypes (A and B).2 Phenotype A is characterized by predominantly large buoyant LDL (lb-LDL), whereas individuals with predominantly small dense LDL (sd-LDL) comprise phenotype B.2 Numerous epidemiologic studies and randomized controlled trials have shown an independent association of sd-LDL cholesterol (sd-LDL-C) with coronary heart disease.2–9 Additionally, sd-LDL may be the most atherogenic lipoprotein, even compared with other apolipoprotein B (apoB) lipoproteins including total LDL content and lipoprotein(a) [Lp(a)],10 due in part to its longer circulation time and presumed higher likelihood of arterial wall penetration and oxidation.2
The presence of computed tomography (CT)-detected coronary artery calcification (CAC) is a strong marker of subclinical atherosclerosis and a worldwide decision tool used to risk-stratify individuals in primary prevention.11,12 Several studies have shown an association of elevated apoB lipoproteins with CAC;13–18 however, few have evaluated directly measured sd-LDL-C. Recently, a study in a community-based sample of 10 357 men and women found LDL-C/apoB ratio, a proxy for sd-LDL-C, was a superior predictor for CAC compared with total apoB alone.19 In the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing, population-based, longitudinal cohort study investigating the causes and progression of subclinical vascular disease, it was previously shown that CAC score independently predicted time to CVD events across the four most common racial and ethnic groups in the USA.20 To build upon the findings of this large, multi-ethnic study in primary prevention, we used directly measured sd-LDL-C to evaluate its association with prevalent baseline CAC in MESA.
Methods
The study objectives and design of MESA have been previously described.21 Briefly, MESA is a community-based cohort that recruited 6814 White, Chinese-American, Black, and Hispanic men and women across six US field centres to better understand predictors of subclinical CVD. Participants were 45–84 years of age and free from clinically known CVD at enrolment (2000–02). The field centres included the academic medical centres of Columbia University (New York, NY, USA), Johns Hopkins University (Baltimore, MD, USA), Northwestern University (Chicago, IL, USA), University of California at Los Angeles (Los Angeles, CA, USA), University of Minnesota (Minneapolis, MN, USA), and Wake Forest University (Winston-Salem, NC, USA). The present study included 4672 participants with measured baseline sd-LDL-C and CAC. Individuals on lipid-lowering medications or with limited serum sample availability (n = 2142) did not have sd-LDL-C measured and were excluded from the present study.5 Informed consent was collected from all participants prior to enrolment, and all participating field centres obtained Institutional Review Board approval for the study.
Demographic and cardiovascular risk factor data were obtained at the baseline MESA clinical examination (Exam 1) that occurred over a period of 24 months from 15 July 2000 to 14 July 2002.21 Participants self-reported their age, sex, race/ethnicity (White, Chinese-American, Black, or Hispanic), highest level of educational attainment [no schooling, Grades 1–8, Grades 9–11, completed high school (Grade 12), some university but no degree, technical school certificate, associate degree, bachelor degree, or graduate or professional school degree], smoking status (never, former, or current, defined as any cigarette use in the last 30 days), medical history, and medication use. Resting blood pressure was measured in triplicate in a seated position at two-minute intervals using an automated Dinamap Pro 100 oscillometric sphygmomanometer (Critikon, Tampa, FL, USA). The average of the latter two measurements were used to define hypertension status: systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or blood pressure–lowering medication use. Diabetes mellitus was defined by fasting plasma glucose ≥126 mg/dL (6.99 mmol/L), or glucose-lowering medication use or insulin for the treatment of diabetes.
Total cholesterol, HDL cholesterol (HDL-C), and triglycerides were obtained from overnight fasting blood samples stored in EDTA-coated tubes at −70°C at the MESA central laboratory (Minneapolis, MN, USA).21 Total cholesterol and HDL-C were measured using the cholesterol oxidase method (Roche Diagnostics, Indianapolis, IN, USA). Triglycerides were measured using a triglyceride glycerol-blanked reagent (Roche Diagnostic, Indianapolis, IN, USA). The total LDL-C level was estimated according to the Friedewald,22 Martin–Hopkins,23 and Sampson–National Institutes of Health (NIH)24 equations for validity. A fully automated homogenous method with a short assay time was used to measure sd-LDL-C particles, defined as 15–20 nm in size (sd-LDL-EX, Denka Sieken Co., Tokyo, Japan).7,25
Coronary artery calcification was measured using either electron-beam CT (Columbia University, Northwestern University, and University of California at Los Angeles) or four-detector row CT (Johns Hopkins University, University of Minnesota, and Wake Forest University).26 In participants who weighed more than 100 kg, the CT scanner tube current was increased by 25%. All images used cardiac gating: one image for electron beam and four images for four-detector row CT per cardiac cycle. Participants had two consecutive scans completed at baseline that were assessed for quality by trained image analysts. Cardiologists or radiologists interpret CT scans using a specialized in-house program (University of California at Los Angeles) to assess for calcified coronary artery plaque. Images were reconstructed using phantom calibration to adjust for changes in CT image attenuation between different scanners (Image Analysis, Columbia, KY, USA). After phantom adjustment, the mean CAC score from the two scans was calculated. Intra- and inter-observer agreement was high (kappa statistics of 0.93 and 0.90, respectively).
Baseline characteristics across sd-LDL-C quartiles were compared using χ2 tests for proportions and one-way analysis of variance for continuous variables. We then compared the characteristics of participants included and not included in this analysis using t-tests and χ2 tests (see Supplementary material online, Table S1).
Based on differences in included and excluded participants, we used multi-variable logistic regression with inverse probability weighting for exclusion to assess the association of quartiles of sd-LDL-C with the presence of baseline CAC (mean score >0). Stabilized inverse probability weights were generated for participants contributing and not contributing sd-LDL data, conditioned on age, sex, race/ethnicity, CAC status, hypertension, diabetes, and smoking status. A comparison of standardized mean differences in weighted and unweighted variables indicates that better covariate distributional balance was achieved by inverse probability weighting (see Supplementary material online, Table S2). In addition, we imputed missing covariate values using multiple imputations by chained equations. Continuous covariates were imputed via predictive mean matching and categorical covariates were imputed via logistic regression processes. Results from 10 iterations of 20 imputed data sets were combined for validity. Missingness did not exceed 1.1% for any covariate.
We report adjusted odds ratios (ORs) with 95% confidence interval (CI) for quartiles of sd-LDL-C from general linear models with the lowest quartile (Quartile 1) serving as the referent: Model 1 was minimally adjusted for field centre (to account for differences in CAC data acquisition, i.e. electron-beam or four-detector row CT); Model 2 was adjusted for field centre, age, sex, race/ethnicity, and educational attainment; and Model 3 was adjusted for Model 2 covariates and hypertension, diabetes, smoking status, body mass index, triglycerides (log-transformed to correct skewed distribution), and HDL-C. We then assessed a fourth model (Model 4) that was additionally adjusted for Model 3 covariates plus estimated total LDL-C according to the Friedewald equation (Model 4a), Martin–Hopkins equation (Model 4b), and Sampson–NIH equation (Model 4c). To evaluate the consistency of results by sex, race/ethnicity, and age group (45–54, 55–64, 65–74, and 75–84 years), we added appropriate interaction terms to Model 3 and Model 4a, and stratified analyses by significant interactions.
Finally, we evaluated associations of continuous sd-LDL-C using restricted cubic splines and compared the goodness of fit for nested models with first-order, quadratic, and cubic sd-LDL-C terms via likelihood ratio test to determine if associations with prevalent CAC were linear or curvilinear. Hypothesis testing was completed using a two-sided alpha of 0.05 to determine the statistical significance of main effects. Statistical analyses were completed using R version 4.2.3 (2023). Multiple imputation was performed using the mice package (version 3.15) for R.
Results
Baseline characteristics of the 4672 participants included in this analysis stratified by sd-LDL-C quartiles are depicted in Table 1. Participants in higher quartiles of sd-LDL-C tended to be younger, less likely to be female, more likely to be of Chinese-American or Hispanic ethnicity, less likely to have completed high school (12 years of compulsory schooling), and had a higher prevalence of hypertension and diabetes. Additionally, higher quartiles of sd-LDL-C were associated with lower HDL-C levels, higher triglycerides, and higher estimated total LDL-C according to the Friedewald, Martin–Hopkins, and Sampson–NIH equations. In total, CAC was prevalent among 2212 participants (47.3%) and was higher among Quartile 3 (52.7%) and Quartile 4 (51.8%) of sd-LDL-C than among Quartile 1 (40.0%) and Quartile 2 (44.9%) (P < 0.001).
Table 1.
Baseline characteristics by quartile of directly measured small dense LDL cholesterol
| Total | sd-LDL-C 1st quartile | sd-LDL-C 2nd quartile | sd-LDL-C 3rd quartile | sd-LDL-C 4th quartile | P-value | |
|---|---|---|---|---|---|---|
| n = 4672 | n = 1168 | n = 1168 | n = 1168 | n = 1168 | ||
| Range, mg/dL | 0.1, 26.2 | 26.2, 35.6 | 35.6, 48.9 | 49.0, 214.8 | — | |
| Age, years | 61.9 (10.4) | 62.2 (10.9) | 62.2 (10.4) | 62.5 (10.3) | 60.8 (9.8) | <0.001 |
| Women, n (%) | 2453 (52.5) | 648 (55.5) | 680 (58.2) | 570 (48.8) | 555 (47.5) | <0.001 |
| Race/ethnicity, n (%) | <0.001 | |||||
| White | 1707 (36.5) | 413 (35.4) | 444 (38.0) | 420 (36.0) | 430 (36.8) | |
| Chinese-American | 559 (12.0) | 117 (10.0) | 125 (10.7) | 142 (12.2) | 175 (15.0) | |
| Black | 1343 (28.7) | 457 (39.1) | 375 (32.1) | 313 (26.8) | 198 (17.0) | |
| Hispanic | 1063 (22.8) | 181 (15.5) | 224 (19.2) | 293 (25.1) | 365 (31.3) | |
| Educational attainment, ≥high school, n (%) | 3787 (81.3) | 999 (86.2) | 964 (82.8) | 937 (80.4) | 887 (76.2) | <0.001 |
| Hypertension, n (%) | 1950 (41.7) | 455 (39.0) | 477 (40.8) | 528 (45.2) | 490 (42.0) | 0.019 |
| Diabetes, n (%) | 1185 (25.4) | 211 (18.1) | 261 (22.3) | 335 (28.7) | 378 (32.4) | <0.001 |
| Current cigarette use, n (%) | 607 (13.0) | 167 (14.3) | 133 (11.4) | 158 (13.5) | 149 (12.8) | 0.189 |
| Body mass index, kg/m2 | 28.3 (5.5) | 27.5 (5.9) | 28.0 (5.6) | 28.5 (5.3) | 28.8 (5.1) | <0.001 |
| HDL-C, mg/dL | 51.1 (15.1) | 56.6 (15.9) | 53.8 (15.2) | 49.1 (14.6) | 45.1 (11.5) | <0.001 |
| Triglycerides, log(U) | 4.7 (0.5) | 4.3 (0.4) | 4.5 (0.4) | 4.8 (0.4) | 5.2 (0.4) | <0.001 |
| Estimated total LDL-C, mg/dL | ||||||
| Friedewald equation | 119.7 (31.4) | 94.4 (22.8) | 116.0 (21.5) | 126.4 (26.5) | 142.6 (32.5) | <0.001 |
| Martin–Hopkins equation | 121.4 (31.1) | 93.6 (22.2) | 116.2 (19.6) | 128.5 (24.3) | 147.3 (29.8) | <0.001 |
| Sampson–NIH equation | 122.1 (31.7) | 95.4 (23.2) | 118.1 (21.0) | 129.4 (26.2) | 145.3 (32.0) | <0.001 |
| Prevalent CAC, n (%) | 2212 (47.3) | 467 (40.0) | 524 (44.9) | 616 (52.7) | 605 (51.8) | <0.001 |
Values displayed are mean (standard deviation) for normally distributed continuous variables or total number (%) for categorical variables.
CAC, coronary artery calcification; HDL-C, HDL cholesterol; NIH, National Institutes of Health; sd-LDL-C, small dense LDL cholesterol; LDL-C, LDL cholesterol.
Adjusted odds ratios from multi-variable logistic regression of the association of baseline sd-LDL-C quartiles and the presence of baseline weighted CAC are displayed in Table 2. Higher quartiles of sd-LDL-C were significantly associated with baseline CAC in all models. For example, after adjusting for demographics and cardiovascular risk factors (Model 3), participants in sd-LDL-C Quartile 2 (OR: 1.38; 95% CI: 1.13, 1.68), Quartile 3 (OR: 1.80; 95% CI: 1.46, 2.22), and Quartile 4 (OR: 2.08; 95% CI: 1.63, 2.65) had higher odds for the presence of CAC compared with Quartile 1 (Table 2). Further adjustment for estimated total LDL-C (Model 4a–c) attenuated associations, though each higher quartile remained significantly associated with baseline CAC relative to Quartile 1 (Table 2); the equation used to estimate total LDL-C (i.e. Friedewald, Martin–Hopkins, or Sampson–NIH equations) did not affect results. There was no suggestion that associations between Model 3 and Model 4a differed by sex or race/ethnicity (all interactions P > 0.10). However, associations appeared to be driven most strongly by the oldest age group (75–84 years) in Model 3 (interaction P = 0.011) and Model 4a (interaction P = 0.010) results, as shown in Table 3.
Table 2.
Weighted multi-variable associations of baseline small dense LDL cholesterol quartiles and the presence of coronary artery calcium among 4672 middle-aged and older adults
| Odds ratio (95% CI) per quartile of sd-LDL-C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1a | P-valueb | Model 2c | P-valueb | Model 3d | P-valueb | Model 4ae | P-valueb | Model 4be | P-valueb | Model 4ce | P-valueb | |
| Quartile 1 | Ref | <0.001 | Ref | <0.001 | Ref | <0.001 | Ref | 0.002 | Ref | 0.001 | Ref | 0.002 |
| Quartile 2 | 1.24 (1.05, 1.46) | 1.35 (1.12, 1.64) | 1.38 (1.13, 1.68) | 1.24 (1.00, 1.53) | 1.24 (1.00, 1.53) | 1.23 (1.00, 1.52) | ||||||
| Quartile 3 | 1.69 (1.43, 1.99) | 1.77 (1.46, 2.14) | 1.80 (1.46, 2.22) | 1.51 (1.19, 1.93) | 1.53 (1.20, 1.95) | 1.51 (1.19, 1.93) | ||||||
| Quartile 4 | 1.63 (1.38, 1.92) | 1.94 (1.59, 2.35) | 2.08 (1.63, 2.65) | 1.59 (1.17, 2.16) | 1.60 (1.18, 2.18) | 1.59 (1.17, 2.17) | ||||||
CI, confidence interval; HDL-C, HDL cholesterol; sd-LDL-C, small dense LDL cholesterol.
Model 1 adjusted for field centre.
P-value for linear trend.
Model 2 adjusted for field centre, age, sex, race/ethnicity, and educational attainment.
Model 3 adjusted for Model 2 covariates + hypertension, diabetes, smoking status (never, former, or current), body mass index, plasma triglycerides (log-transformed), and HDL-C.
Model 4(a–c) adjusted for Model 1, Model 2, and Model 3 covariates, and estimated total LDL-C by (a) Friedewald equation; (b) Martin–Hopkins equation; and (c) Sampson–NIH equation.
Table 3.
Weighted multi-variable associations of baseline small dense LDL cholesterol quartiles and the presence of coronary artery calcium among 4672 middle-aged and older adults, stratified by age group
| Odds ratio (95% CI) per quartile of sd-LDL-C | |||||
|---|---|---|---|---|---|
| 45–54 years | 55–64 years | 65–74 years | 75–84 years | Interaction P | |
| n = 1400 | n = 1266 | n = 1332 | n = 674 | ||
| Model 3a | 0.011 | ||||
| Quartile 1 | Ref | Ref | Ref | Ref | |
| Quartile 2 | 1.03 (0.66, 1.59) | 1.11 (0.77, 1.61) | 1.56 (1.11, 2.19) | 2.61 (1.46, 4.65) | |
| Quartile 3 | 1.83 (1.18, 2.83) | 1.42 (0.97, 2.08) | 1.89 (1.30, 2.72) | 2.53 (1.38, 4.63) | |
| Quartile 4 | 2.19 (1.33, 3.61) | 1.58 (1.03, 2.41) | 2.13 (1.38, 3.29) | 2.58 (1.19, 5.57) | |
| Model 4ab | 0.010 | ||||
| Quartile 1 | Ref | Ref | Ref | Ref | |
| Quartile 2 | 0.89 (0.55, 1.43) | 1.02 (0.69, 1.50) | 1.37 (0.95, 1.97) | 2.67 (1.42, 5.02) | |
| Quartile 3 | 1.46 (0.87, 2.44) | 1.22 (0.80, 1.86) | 1.52 (0.98, 2.33) | 2.61 (1.26, 5.39) | |
| Quartile 4 | 1.54 (0.80, 2.98) | 1.22 (0.72, 2.07) | 1.56 (0.91, 2.67) | 2.71 (1.02, 7.21) | |
CI, confidence interval; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; sd-LDL-C, small dense LDL cholesterol.
Model 3 adjusted for field centre, sex, race/ethnicity, educational attainment, hypertension, diabetes, smoking status (never, former, or current), body mass index, plasma triglycerides (log-transformed), and HDL-C.
Model 4a adjusted for Model 3 covariates and estimated total LDL-C according to the Friedewald equation.
Figure 1 shows restricted cubic splines of the association of continuous sd-LDL-C with baseline CAC adjusted for Model 3 (Figure 1A) and Model 4 (Figure 1B) covariates. Figure 1A suggests a moderately curvilinear relationship, with the odds of baseline CAC increasing linearly until appearing to reach a ceiling near 71.0 mg/dL (the 95th percentile value), above which the odds of baseline CAC do not further increase. A goodness of fit comparison of nested models with first-order, first-order and quadratic, and first-order, quadratic, and cubic sd-LDL-C terms supported a quadratic curvilinear relationship with baseline CAC (likelihood ratio test for quadratic vs. first-order terms: P = 0.015; cubic vs. quadratic terms: P = 0.879). Consistent with results in Table 2, Figure 1B suggests adjustment for estimated total LDL-C further flattens the curve, though the relationship appears to remain linearly proportional until reaching a ceiling at Quartile 4 of sd-LDL-C (range 49.0–214.8 mg/dL; Table 1). However, the goodness of fit testing among nested models did not support curvilinearity, as higher-order sd-LDL-C terms did not provide a better fit for the data than the reduced (linear) Model 4a (quadratic vs. first-order terms: P = 0.156; cubic vs. first-order terms: P = 0.364).
Figure 1.
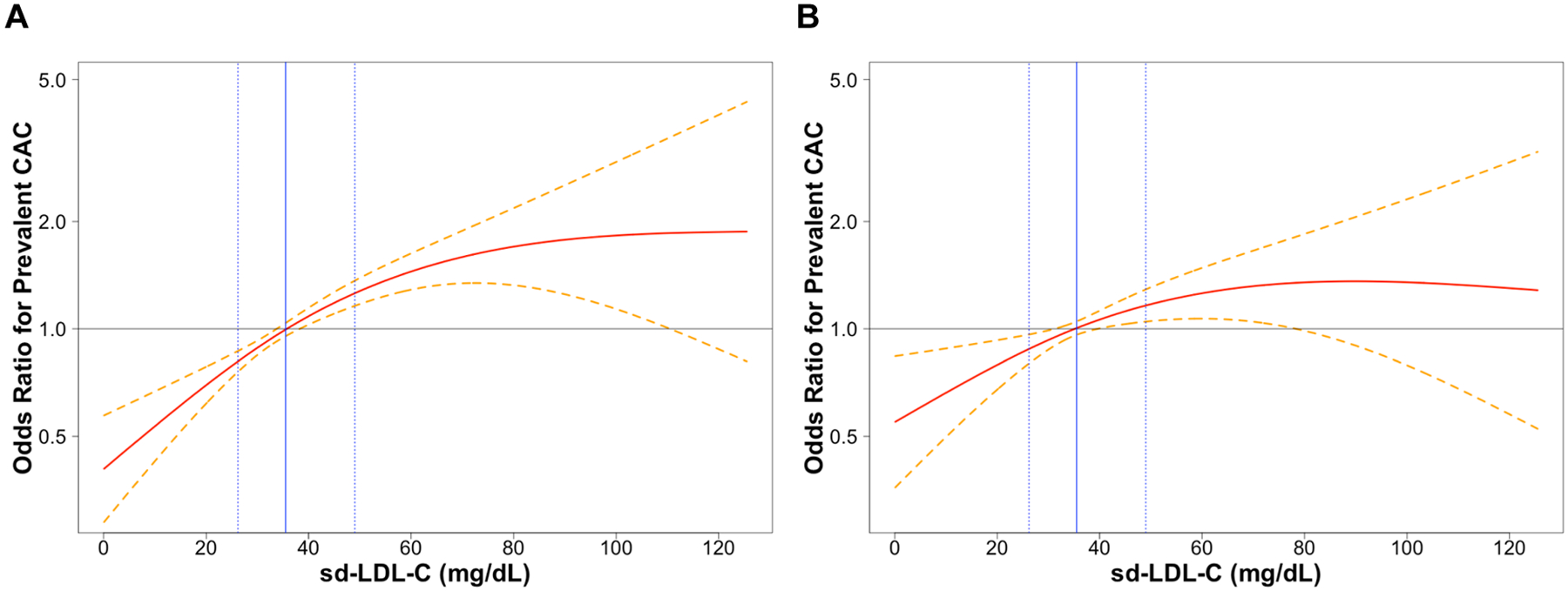
Restricted cubic splines of continuous small dense LDL cholesterol and adjusted odds ratio for the presence of coronary artery calcium at baseline among 4672 middle-aged and older adults. (A) Adjusted for Model 3 covariates (field centre, age, sex, race/ethnicity, educational attainment, hypertension, diabetes, smoking, body mass index, and plasma triglycerides and HDL cholesterol). (B) Adjusted for Model 3 covariates plus estimated total LDL cholesterol according to the Friedewald equation. Dashed lines represent the 95% confidence interval. The solid horizontal line represents the null association value (1.0). The solid vertical line represents the median small dense LDL cholesterol value (35.6 mg/dL; reference value), and the dotted vertical lines represent the 25th percentile (26.2 mg/dL) and 75th percentile (49.0 mg/dL) values, respectively. The Y-axis is on the natural logarithmic scale. sd-LDL-C, small dense LDL cholesterol; CAC, coronary artery calcification.
Discussion
Epidemiologic data support sd-LDL-C as an independent predictor of atherosclerosis and clinical CVD,2–9 and the presence of CAC is a widely adopted surrogate marker of subclinical CVD.11,12 Thus, the purpose of this study was to examine the association of sd-LDL-C with prevalent CAC in a large cohort of middle-aged and older adults free of known clinical CVD at baseline. The primary finding from our analysis of 4672 MESA participants was that higher sd-LDL-C was associated with increased odds for prevalent CAC. These results were independent of traditional cardiovascular risk factors, even after accounting for estimated total LDL-C according to three commonly used equations, and were driven most strongly by the oldest participants (75–84 years of age). Finally, restricted cubic splines and goodness of fit testing suggested that the relationship of sd-LDL-C to prevalent CAC may be curvilinear, with the highest-high values (>95th percentile; 71.0–214.8 mg/dL) appearing not to contribute additional risk.
Few studies have investigated the association between sd-LDL-C and CAC. In contrast to our findings, in a cohort of 160 intermediate CVD-risk individuals, sd-LDL-C was not associated with CAC (OR per 1-unit increment in sd-LDL-C: 1.001; 95% CI: 0.998, 1.003). However, only individuals with a 10-year atherosclerotic CVD risk of 1–5% were referred for CAC assessment in this study.16 For comparison, we note that participants included in our study had a median (interquartile limits) 10-year atherosclerotic CVD risk of 8.8 (3.6, 18.8)%, so this association may be more apparent in individuals with greater than minimal risk. Conversely and consistent with our results, in the Healthy Women Study of 286 postmenopausal women, higher sd-LDL-C was associated with increased risk of CAC [OR per standard deviation increment in sd-LDL-C: 1.36; 95% CI: 1.04, 1.77], as well as with higher triglycerides and LDL-C, and lower HDL-C.17 To our knowledge, our study extends this work to a large, diverse population of older men and women for the first time. Notably, our results did not differ by sex or across the four most common racial/ethnic groups in the USA.
Individuals with an elevated ratio of sd-LDL-C to lb-LDL-C (sd-to-lb-LDL-C) require a greater number of atherogenic lipoprotein particles to convey an equivalent cholesterol load. This mechanistic feature may underlie the observed increased prevalence of CAC in patients with elevated levels of sd-LDL-C.2 However, additional unique properties of sd-LDL-C particles may promote their atherogenicity. For example, sd-LDL has a lower affinity for the LDL receptor, delaying plasma clearance.27 Additionally, studies have found sd-LDL particles to contain lower sialic acid content, which allows for increased affinity to arterial wall proteoglycans, increasing the time sd-LDL remains in the subendothelial space and making it more likely that these particles will undergo modification.27,28 Moreover, sd-LDL is more susceptible to oxidation as it does not contain vitamin E, making it a better substrate for vascular wall macrophages.27
Strengths of this study include its large sample size drawn from a racially and ethnically diverse cohort in primary prevention; an evaluation of the consistency of results across sex, race/ethnicity, and age groups; and the use of statistical techniques to reduce bias beyond covariate adjustment. Furthermore, to our knowledge, we are the first to assess the curvilinearity of the sd-LDL-C to prevalent CAC relationship. However, our results should be interpreted with limitations and other considerations in mind. First, due to the cross-sectional nature of the study, causal relationships cannot be inferred. Second, MESA is a primary prevention population that was free from known clinical CVD at baseline, so results may not be generalizable to higher-risk individuals. Third, since participants using lipid-lowering medications were excluded, we were unable to assess the effect of such medication use on our results. Fourth, although associations of sd-LDL-C with CAC persisted after accounting for estimated total LDL-C, the two measures are not independent of each other, and may not be directly comparable because sd-LDL-C was assayed directly whereas total LDL-C was estimated by the Friedewald, Martin–Hopkins, and Sampson–NIH equations. Our rationale for this approach was to discern whether sd-LDL-C contributes uniquely to the associations we observed, independent of overall LDL-C burden. Nevertheless, we suggest that specific OR estimates from these models be interpreted with caution. Fifth, a single measurement of sd-LDL-C may not reflect the cumulative lifetime exposure to sd-LDL-C, and the lack of longitudinal sd-LDL-C data precludes us from assessing the relationship of sd-LDL-C and CAC over time. Finally, we note that while several methods for measuring sd-LDL-C have been developed, the Denka Sieken (Tokyo, Japan) sd-LDL-EX assay used to quantify sd-LDL-C in MESA offers several advantages such as being fully automated with excellent sensitivity and specificity in detecting sd-LDL particles, its cost-effectiveness, and excellent correlation with density gradient ultracentrifugation methods.29,30 However, limitations of this method to consider include that its results, like many laboratory assays, may be influenced by certain biological variables; that it may not differentiate between various sizes within the spectrum of sd-LDL particles; and that it does not provide information on other lipoprotein fractions that might contribute to cardiovascular risk. Therefore, future studies may benefit from employing complementary techniques to provide a more comprehensive lipid profile analysis.
Conclusions
In a large, diverse primary prevention cohort, sd-LDL-C was associated with increased odds for prevalent baseline CAC, independent of other cardiovascular risk factors including estimated total LDL-C. Further research is warranted to determine whether selective direct measurement of sd-LDL-C is indicated to refine cardiovascular risk assessment in primary prevention, particularly among older men and women with the predominantly sd-LDL phenotype.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. None of the work in this manuscript has been previously presented.
Funding
National Heart, Lung, and Blood Institute (75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169) and National Center for Advancing Translational Sciences (UL1-TR-000040, UL1-TR-001079, UL1-TR-001420).
National Heart, Lung, and Blood Institute (T32HL076132) to R.R.; National Institute on Aging (K01AG073581) to C.L.S.
Footnotes
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology.
Conflict of interest: M.D.S. has participated in scientific advisory boards with the following entities: Amgen, Agepha, Ionis, Novartis, Precision BioScience, Novo Nordisk, and New Amsterdam; and has served as a consultant for Ionis, Novartis, Regeneron, Aidoc, Kaneka, and Shanghai Pharma Biotherapeutics. C.L.S. has received honoraria from the National Institutes of Health for service on grant review panels. All other authors have nothing to disclose.
Disclaimer
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
All data sets analysed in this study can be requested from http://www.mesa-nhlbi.org by individuals trained in human subjects research after approval by the MESA Steering Committee.
References
- 1.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019;139:e1046–e1081. [DOI] [PubMed] [Google Scholar]
- 2.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014;34:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, et al. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb 2008;15:250–260. [DOI] [PubMed] [Google Scholar]
- 4.Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem 2010;56:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb 2013;20:195–203. [DOI] [PubMed] [Google Scholar]
- 6.Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb 2014;21:755–767. [DOI] [PubMed] [Google Scholar]
- 7.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou L, Kaptoge S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: a systematic review and meta-analysis. PLoS One 2020;15:e0241993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaefer EJ, Ikezaki H, Diffenderfer MR, Lim E, Liu C-T, Hoogeveen RC, et al. Atherosclerotic cardiovascular disease risk and small dense low-density lipoprotein cholesterol in men, women, African Americans and non-African Americans: the pooling project. Atherosclerosis 2023;367:15–23. [DOI] [PubMed] [Google Scholar]
- 10.Ikezaki H, Lim E, Cupples LA, Liu CT, Asztalos BF, Schaefer EJ. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham Offspring Study. J Am Heart Assoc 2021;10:e019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golub IS, Termeie OG, Kristo S, Schroeder LP, Lakshmanan S, Shafter AM, et al. Major global coronary artery calcium guidelines. JACC Cardiovasc Imaging 2023;16:98–117. [DOI] [PubMed] [Google Scholar]
- 12.Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ 2021;373:n776. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol 2016;67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung YH, Lee BK, Kwon HM, Min P-K, Choi E-Y, Yoon YW, et al. Coronary calcification is associated with elevated serum lipoprotein (a) levels in asymptomatic men over the age of 45 years: a cross-sectional study of the Korean national health checkup data. Medicine (Baltimore) 2021;100:e24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr 2021;15:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poropat Flerin T, Božič Mijovski M, Jug B. Association between lipoprotein subfractions, hemostatic potentials, and coronary atherosclerosis. Dis Markers 2022;2022:2993309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol 2002;90:71–76. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Nomura SO, Steffen BT, Guan W, Remaley AT, Karger AB, et al. Apolipoprotein B discordance with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in relation to coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Lipidol 2020;14:109–121.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang TY, Chen JD. Low-density lipoprotein cholesterol/apolipoprotein B ratio is superior to apolipoprotein B alone in the diagnosis of coronary artery calcification. Coron Artery Dis 2021;32:561–566. [DOI] [PubMed] [Google Scholar]
- 20.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002; 156:871–881. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Meeusen JW, Lueke AJ, Jaffe AS, Saenger AK. Validation of a proposed novel equation for estimating LDL cholesterol. Clin Chem 2014;60:1519–1523. [DOI] [PubMed] [Google Scholar]
- 24.Sampson M, Ling C, Sun Q, Harb R, Ashmaig M, Warnick R, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol 2020;5:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem 2011;57:57–65. [DOI] [PubMed] [Google Scholar]
- 26.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 27.Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta 2012;414:215–224. [DOI] [PubMed] [Google Scholar]
- 28.Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis 1996;124:261–271. [DOI] [PubMed] [Google Scholar]
- 29.Albers JJ, Kennedy H, Marcovina SM. Evaluation of a new homogenous method for detection of small dense LDL cholesterol: comparison with the LDL cholesterol profile obtained by density gradient ultracentrifugation. Clin Chim Acta 2011;412:556–561. [DOI] [PubMed] [Google Scholar]
- 30.Cooney J, Essa S, Cranson A, Stewart JP, Bedford D, Caslake MJ. Evaluation of an automated method to measure small dense LDL cholesterol. Atherosclerosis 2012;223:532. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sets analysed in this study can be requested from http://www.mesa-nhlbi.org by individuals trained in human subjects research after approval by the MESA Steering Committee.


