Abstract
Aims
Current guidelines advise against the use of lipid-lowering drugs during pregnancy. This is based only on previous observational evidence demonstrating an association between statin use and congenital malformations, which is increasingly controversial. In the absence of clinical trial data, we aimed to use drug-target Mendelian randomization to model the potential impact of fetal LDL-lowering, overall and through PCSK9 drug targets, on congenital malformations.
Methods and results
Instrumental variants influencing LDL levels overall and through PCSK9-inhibitor drug targets were extracted from genome-wide association study (GWAS) summary data for LDL on 1 320 016 individuals. Instrumental variants influencing circulating PCSK9 levels (pQTLs) and liver PCSK9 gene expression levels (eQTLs) were extracted, respectively, from a GWAS on 10 186 individuals and from the genotype-tissue expression project. Gene-outcome association data was extracted from the 7th release of GWAS summary data on the FinnGen cohort (n = 342 499) for eight categories of congenital malformations affecting multiple systems. Genetically proxied LDL-lowering through PCSK9 was associated with higher odds of malformations affecting multiple systems [OR 2.70, 95% confidence interval (CI) 1.30–5.63, P = 0.018], the skin (OR 2.23, 95% CI 1.33–3.75, P = 0.007), and the vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb association (VACTERL) (OR 1.51, 95% CI 1.16–1.96, P = 0.007). An association was also found with obstructive defects of the renal pelvis and ureter, but this association was suggestive of horizontal pleiotropy. Lower PCSK9 pQTLs were associated with the same congenital malformations.
Conclusion
These data provide genetic evidence supporting current manufacturer advice to avoid the use of PCSK9 inhibitors during pregnancy.
Keywords: Low-density lipoprotein, PCSK9-inhibitors, congenital malformations, pregnancy, Mendelian randomization
See the editorial comment for this article ‘The use of PCSK9 inhibitors should be avoided during pregnancy: results from a Mendelian randomization study', by J.T. Efird, https://doi.org/10.1093/eurjpc/zwae018.
Introduction
Elevated low-density lipoprotein (LDL) is a cardinal risk factor for cardiovascular disease.1 Proprotein convertase subtilisin–kexin type 9 (PCSK9)-inhibiting therapies, including monoclonal antibodies (mAb) and silencing RNA therapies (siRNA), can achieve profound, long-lasting reductions in LDL. Based on current guidelines, as many as 4% of the adult population are eligible for PCSK9 mAb therapies,2 and this is set to increase with progressively lower treatment targets. Recently, the Food and Drug Administration expanded their remit by approving their use for familial hypercholesterolaemia down to age 10.3
The use of LDL-lowering therapies during pregnancy is currently avoided except in very severe cases. This is partly due to concerns regarding a previously reported association of statin use with congenital malformations,4 though opinions regarding the true causal nature of this association are conflicted, as subsequent studies have not replicated it.5,6 Overall, however, the concern appears biologically justified: LDL metabolism is central to the development of the fetus, playing key roles in cell proliferation as well as sonic hedgehog signalling, both of which are important during human development. Additionally, PCSK9 is known to play an important role in regulating fetal LDL levels, both through its direct role in regulating fetal LDL metabolism, and through modulation of LDL-receptor expression on the placenta regulating maternal-to-fetal LDL transport.7 Indeed, it is known that oligogenic conditions perturbing LDL synthesis, such as Smith-Lemli-Opitz syndrome (SLOS), are associated with high risk of congenital abnormalities.8 In addition to this, previous evidence has suggested an association of the loss-of-function R46L mutation in the PCSK9 gene with risk of neural tube defects,9 though this association was only nominally statistically significant in the setting of a phenome-wide association study.
Given these biologically important safety concerns, clinical trials to test the safety of PCSK9 inhibitors are not ethically justified. This implies that there is currently no randomized evidence to either support or refute the hypothetical risks of congenital malformations associated with PCSK9 inhibitor use in pregnancy. In clinical practice, despite the lack of clinical data, the medication is advised against by manufacturers.10,11 The ongoing contraindication of these therapies in the setting of limited evidence-based data does disservice to women who rely on these therapies for LDL-lowering. Upon discontinuation of this drug during pregnancy, the exposure to high LDL levels lasting the pregnancy, which will be further aggravated by the physiological increase in LDL occurring secondary to the pregnancy itself, will contribute to an increase in lifetime maternal and offspring atherosclerotic disease risk. Further investigation is needed to provide additional evidence to either corroborate or question the current recommendation against use of these agents in pregnancy.
In the absence of clinical trial data, drug-target Mendelian randomization (MR) can be used to inform potential efficacy and safety12 of medications. Drug-target MR leverages the natural variability in genetic variants encoding drug targets to explore potential effects of their perturbation. Since allocation of genetic variants occurs randomly through the process of mating and allele assortment at conception, this is akin to randomization in a clinical trial. Importantly, when modelling the potential fetal effects of administration of a drug in pregnancy, the framework behind drug-target MR can only hold when modelling direct fetal effects of drug-target perturbation in the fetus directly. In this case, this assumption can be assumed to hold, because it is established that both mAb and siRNA molecules cross the placenta.10,11 However, it must be highlighted that the framework is only strictly applicable to agents that cross the placenta, and that it models the potential effects of LDL metabolism perturbation in the fetus rather than in the mother. In this study, we aim to leverage drug-target MR to model the potential impact of LDL-lowering, overall and through PCSK9-inhibition, on risk of congenital malformations.
Methods
Ethical approval, data availability, and reporting
Data used in this study is publicly available and all relevant sources are cited. Ethical approval and participant consent were obtained in the original studies that generated the data. Statistical analysis was carried out using R version 4.2.2 (2022-10-31).13
Instrumental variable selection
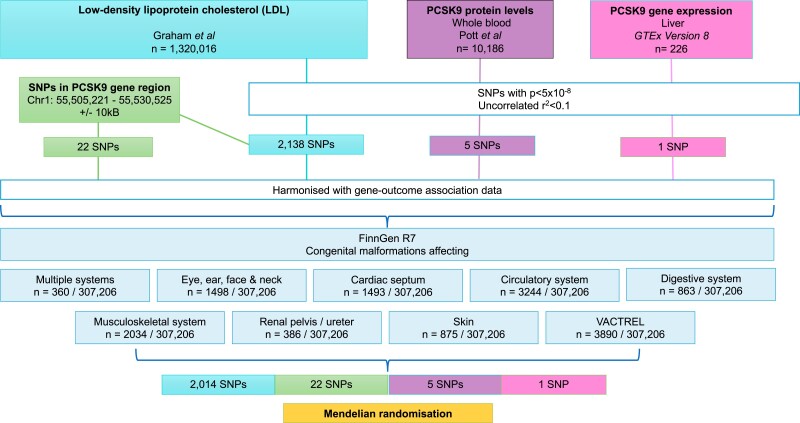
Genetic association estimates for LDL were acquired from the most recent genome-wide association study (GWAS) on 1 320 016 European ancestry individuals included in the global lipids genetic consortium (GLGC).14 Uncorrelated (r2 < 0.1) single-nucleotide polymorphisms associated with LDL (P < 5 × 10−8) overall and in the PCSK9 gene region ±10kB (Table 1, derived from DrugBank15) were selected as instrumental variants. The final instrumental variants utilized in the analysis and respective association estimates with LDL are reported in Supplementary material online, Tables S1 and S2.
Table 1.
Data sources for exposures and outcomes with international statistical classification of diseases and related health problems 10th revision (ICD-10) codes
| Exposures | ||||
|---|---|---|---|---|
| Drug target | Data source | Gene | Chromosome: base pair position (excluding 10 kB window) | n uncorrelated SNPs (r2 < 0.1) |
| LDL | Graham et al | — | — | 2014 |
| LDL via PCSK9 | Graham et al | PCSK9 | Chr1: | 22 |
| 55 505 221–55 530 525 | ||||
| PCSK9 protein levels in whole blood | Graham et al | PCSK9 | Chr1: | 5 |
| 55 505 221–55 530 525 | ||||
| PCSK9 gene expression in liver | GTEx 8 | PCSK9 | Chr1: | 1 |
| 55 505 221–55 530 525 | ||||
| PCSK9 gene expression in whole blood | GTEx 8 | PCSK9 | Chr1: | 2 |
| 55 505 221–55 530 525 | ||||
| Outcomes | ||||
|---|---|---|---|---|
| Phenotype | Data source | ICD-10 codes | Cases | Control |
| Congenital malformations affecting multiple systems | FinnGen R7 | Q87 | 360 | 307 206 |
| Congenital malformations of eye, ear, face, and neck | FinnGen R7 | Q10-Q18 | 1498 | 307 656 |
| Congenital malformations of the cardiac septum | FinnGen R7 | Q21 | 1493 | 305 910 |
| Congenital malformations of the circulatory system | FinnGen R7 | Q20-Q28 | 3244 | 305 910 |
| Congenital malformations of the digestive system | FinnGen R7 | Q38-Q45 | 863 | 308 291 |
| Congenital malformations of the musculoskeletal system | FinnGen R7 | Q65-Q79 | 2034 | 307 120 |
| Congenital malformations of the skin | FinnGen R7 | Q82 | 875 | 307 206 |
| Congenital obstructive defects of renal pelvis and ureter | FinnGen R7 | Q62 | 386 | 307 923 |
| Vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb anomaly | FinnGen R7 | Q8726|Q76| Q675| | 3890 | 305 264 |
| Q42|Q43[5–9]| Q20|Q21|Q22| Q23|Q24|Q25| Q26[1–4]|Q39| Q6[0–4]| Q69[0–1]| Q70[014]|Q71 | ||||
| Sensitivity analysis: Exposures | ||||
|---|---|---|---|---|
| Drug target | Data source | Gene | Chromosome and base pair position (excludes 10 kB window) | #Uncorrelated SNPs, r2 < 0.1 |
| LDL | Neale lab | — | — | 941 |
| LDL via PCSK9 | Neale lab | PCSK9 | Chr1: | 16 |
| 55 505 221–55 530 525 | ||||
SNP, single-nucleotide polymorphism; LDL, low-density lipoprotein; PCSK9, proprotein convertase subtilisin–kexin type 9.
In addition to the drug-target MR, we tested the associations of circulating PCSK9 protein levels and PCSK9 gene expression in the liver with congenital malformations. For these analyses, genome-wide significant (P < 5 × 10−8) uncorrelated (r2 < 0.1) instrumental variants acting in cis (±100kB from PCSK9 gene region) were extracted for PCSK9 protein levels in whole blood [PCSK9 protein quantitative trait loci (pQTLs)] from a GWAS on 10 186 individuals,16 and for PCSK9 gene expression levels in the liver [PCSK9 expression quantitative trait loci (eQTLs)] from the genotype-tissue expression (GTEx) Project summary data (Version 8, n = 266).17,18 Because pQTLs and eQTLs were extracted from association studies with limited sample sizes, the cis region for instrument selection was expanded to ±100 kb to increase power. The final instrumental variants utilized are reported in Supplementary material online, Tables S3 and S4.
A validation analysis for the primary drug-target MR was carried out by repliating the entire workflow utilizing data from Neale Lab’s R2 data including 469 897 UK Biobank participants (http://www.nealelab.is/uk-biobank/). Similar to the main analysis, uncorrelated (r2 < 0.1) single-nucleotide polymorphisms associated with LDL (P < 5 × 10−8) overall, and in the PCSK9 gene region ±10 kB were extracted as instrumental variants for this analysis.
Study outcomes
Genetic association estimates for congenital malformations were extracted from FinnGen Round 7,19 for the outcomes of congenital malformations affecting multiple systems (n cases = 360, n controls = 307 206), of the eye, ear, face and neck (n cases = 1498, n controls = 307 206), of the cardiac septum (n cases = 1493, n controls = 307 206), of the circulatory system (n case = 3244, n controls = 307 206), of the digestive system (n case = 833, n controls = 307 206), of the musculoskeletal system (n case = 2034, n controls = 307 206), of the renal pelvis and ureter (n case = 386, n controls = 307 206), of the skin (n case = 876, n controls = 307 206), and the vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb (VACTERL) association (n case = 3890, n controls = 307 206). Cohort numbers and International Classification of Disease (ICD) codes utilized for outcome definitions are reported in Table 1, and the study design is summarized in Figure 1.
Figure 1.
Study flowchart outlining study design. SNP, single-nucleotide polymorphism, PCSK9, proprotein convertase subtilisin–kexin type 9.
Statistical analysis
Inverse-variance weighted models were used for primary analysis20 using the Mendelianrandomization21 package in R. Bayesian tests for genetic co-localization22 were performed to investigate the posterior probability that exposure-outcome pairs share causal variants for LDL and congenital malformation risk within the PCSK9 gene region.
For the primary analyses, an expected 5% false discovery rate (FDR) was controlled for using Benjamini-Hochberg correction of P-values. Results for the primary analyses are presented as odds ratio (OR) and 95% confidence intervals (95%CI) for every 1-standard deviation (SD) lower genetically predicted LDL, and FDR-adjusted P-values, with FDR-adjusted P < 0.05 considered statistically significant in the primary analysis, and nominal P < 0.05 considered statistically significant in the replication analyses and the further analyses using PCSK9 pQTLs and eQTLs. Results for PCSK9 pQTLs are presented as OR and 95%CI per unit lower normalized PCSK9 protein level, and results for PCSK9 eQTLs per transcript per million (TPM) lower PCSK9 gene expression.
Instrumental variable assumptions
A number of additional sensitivity analyses were carried out to evaluate the instrumental variable assumptions. The instrumental variable assumptions state that for the results of MR analysis to be valid, the genetic variants must satisfy three key conditions:
The variants are able to predict the exposure.
There are no common causes of the genetic variant and the outcome.
The variant only influences the outcome through the exposure, and not directly or through alternative phenotypes.
The first assumption can be formally evaluated through the calculation of combined instrument F-statistics. In this study, these were calculated using the following formula:
where is the variance explained by the SNPs, n is the number of participants in the study, and k is the number of SNPs. The was calculated as the sum of single-nucleotide polymorphism (SNP)-wise of instruments, which is calculated as follows:
where β represents the effect size of the genetic variant per additional effect allele, and SE(β) represents the standard error of β.
The second assumption cannot be formally tested, but was mitigated through the use of data sources for gene-exposure and gene-outcome association data from studies that included only European ancestry populations, to limit the potential for confounding from population stratification.
The third assumption was tested through sensitivity analyses using weighted median MR23 and MR-Egger.24 The weighted median method can provide consistent estimates assuming at least half the weight is derived from valid SNPs.23 The MR-Egger method can be used to identify the presence of directional pleiotropy under a weaker assumption that the instrument strength is independent of direct effects (InSIDE assumption).24
The third assumption was tested further to confirm that the genetic instruments used for MR analysis were valid by performing a phenome-wide scan. Phenome-wide scanning was performed for all traits associated with the SNPs that were used as instrumental variables within this study to proxy the effects of modifying the following:
LDL cholesterol levels via PCSK9
PCSK9 protein levels in whole blood (pQTL)
PCSK9 gene expression in whole blood (eQTL)
PCSK9 gene expression in liver (eQTL)
LDL cholesterol levels overall
Results
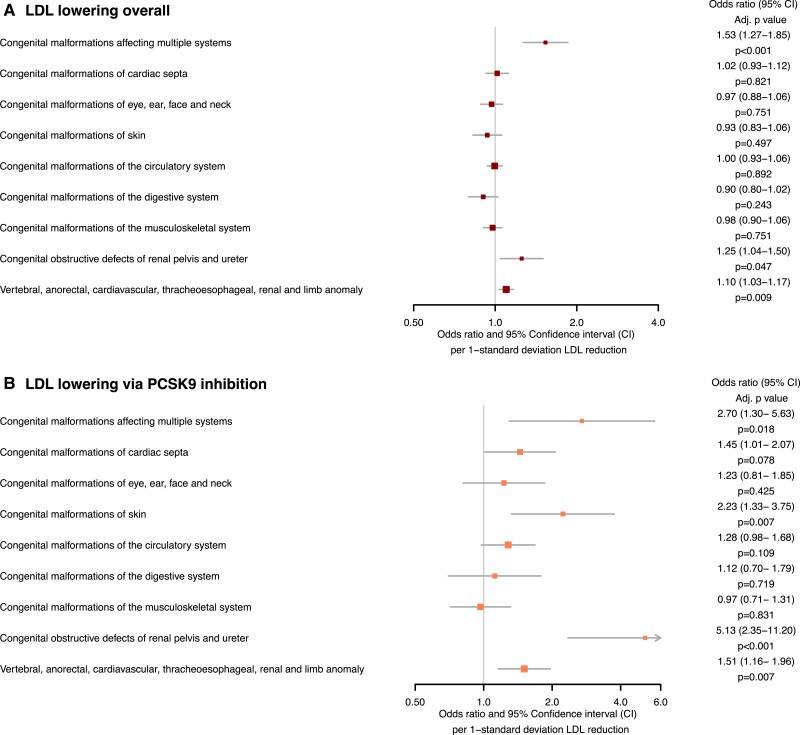
Genetically proxied fetal LDL-lowering overall was associated with higher odds of the VACTREL association [OR 1.10 (1.03–1.17) FDR-adjusted P = 0.009], malformations affecting multiple systems [OR 1.53 (1.27–1.85), FDR-adjusted P = 9.88 × 10−5] and obstructive defects of the renal pelvis and ureter [OR 1.25 (1.04–1.50), FDR-adjusted P = 0.047], as shown in Figure 2A. Sensitivity analyses did not identify potential directional pleiotropy (all MR-Egger intercept P > 0.05; Table 2). The combined F-statistic for LDL instruments was 294.24, as reported in Supplementary material online, Table S5.
Figure 2.
Forest plots displaying the Mendelian randomization estimates for the association between genetically predicted low-density lipoprotein-lowering: (A) by any means (B) via the proprotein convertase subtilisin–kexin type 9 (PCSK9) drug-target, with congenital malformations.
Table 2.
Mendelian randomization sensitivity analyses for the genetically predicted LDL-c lowering overall, through PCSK9 drug targets, and for PCSK9 protein levels in whole blood
| Exposure | Outcome | Method | Beta | Standard error | P-value |
|---|---|---|---|---|---|
| LDL-lowering overall (1-SD) | Congenital malformations affecting multiple systems | Weighted median | 0.582 | 0.177 | 0.001 |
| MR Egger | 0.119 | 0.044 | 0.007 | ||
| intercept | −0.003 | 0.004 | 0.370 | ||
| Congenital malformations of cardiac septa | Weighted median | −0.054 | 0.085 | 0.522 | |
| MR Egger | −0.004 | 0.070 | 0.960 | ||
| intercept | 0.001 | 0.002 | 0.694 | ||
| Congenital malformations of eye, ear, face, and neck | Weighted median | −0.058 | 0.082 | 0.475 | |
| MR Egger | −0.028 | 0.068 | 0.677 | ||
| intercept | 0.000 | 0.002 | 0.960 | ||
| Congenital malformations of skin | Weighted median | 0.069 | 0.115 | 0.548 | |
| MR Egger | 0.520 | 0.142 | 0.000 | ||
| intercept | −0.001 | 0.002 | 0.683 | ||
| Congenital malformations of the circulatory system | Weighted median | −0.048 | 0.060 | 0.422 | |
| MR Egger | 0.006 | 0.048 | 0.900 | ||
| intercept | 0.000 | 0.001 | 0.765 | ||
| Congenital malformations of the digestive system | Weighted median | −0.079 | 0.114 | 0.486 | |
| MR Egger | −0.110 | 0.092 | 0.232 | ||
| intercept | 0.000 | 0.002 | 0.895 | ||
| Congenital malformations of the musculoskeletal system | Weighted median | 0.030 | 0.073 | 0.683 | |
| MR Egger | −0.075 | 0.059 | 0.205 | ||
| intercept | 0.002 | 0.001 | 0.221 | ||
| Congenital obstructive defects of renal pelvis and ureter | Weighted median | 0.038 | 0.159 | 0.810 | |
| MR Egger | 0.409 | 0.136 | 0.003 | ||
| intercept | −0.006 | 0.003 | 0.062 | ||
| Vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb anomaly | Weighted median | 0.058 | 0.053 | 0.275 | |
| MR-Egger | −0.041 | 0.091 | 0.654 | ||
| intercept | −0.001 | 0.001 | 0.432 | ||
| LDL-lowering via PCSK9-inhibition (1-SD) | Congenital malformations affecting multiple systems | Weighted median | 1.017 | 0.450 | 0.024 |
| MR Egger | 0.238 | 0.182 | 0.204 | ||
| intercept | 0.001 | 0.044 | 0.986 | ||
| Congenital malformations of cardiac septa | Weighted median | 0.250 | 0.221 | 0.258 | |
| MR Egger | 0.191 | 0.251 | 0.456 | ||
| intercept | 0.022 | 0.021 | 0.315 | ||
| Congenital malformations of eye, ear, face, and neck | Weighted median | 0.036 | 0.208 | 0.862 | |
| MR Egger | −0.122 | 0.278 | 0.664 | ||
| intercept | 0.040 | 0.024 | 0.102 | ||
| Congenital malformations of skin | Weighted median | 0.772 | 0.254 | 0.002 | |
| MR Egger | 0.988 | 0.518 | 0.070 | ||
| intercept | 0.003 | 0.033 | 0.919 | ||
| Congenital malformations of the circulatory system | Weighted median | 0.219 | 0.142 | 0.123 | |
| MR Egger | 0.215 | 0.195 | 0.284 | ||
| intercept | 0.004 | 0.017 | 0.805 | ||
| Congenital malformations of the digestive system | Weighted median | 0.018 | 0.278 | 0.948 | |
| MR Egger | −0.115 | 0.329 | 0.730 | ||
| intercept | 0.028 | 0.028 | 0.327 | ||
| Congenital malformations of the musculoskeletal system | Weighted median | −0.073 | 0.180 | 0.686 | |
| MR Egger | −0.060 | 0.214 | 0.782 | ||
| intercept | 0.003 | 0.018 | 0.856 | ||
| Congenital obstructive defects of renal pelvis and ureter | Weighted median | 0.912 | 0.444 | 0.040 | |
| MR Egger | 0.490 | 0.495 | 0.333 | ||
| intercept | 0.138 | 0.042 | 0.003 | ||
| Vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb anomaly | Weighted median | 0.285 | 0.137 | 0.037 | |
| MR-Egger | 0.778 | 0.363 | 0.044 | ||
| intercept | 0.021 | 0.015 | 0.183 | ||
| Lower PCSK9 protein levels in whole blood (normalized protein expression units) | Congenital malformations affecting multiple systems | Weighted median | 1.544 | 0.613 | 0.012 |
| MR Egger | 1.359 | 0.846 | 0.207 | ||
| intercept | 0.027 | 0.066 | 0.709 | ||
| Congenital malformations of cardiac septa | Weighted median | 0.368 | 0.312 | 0.238 | |
| MR Egger | 0.359 | 0.487 | 0.515 | ||
| intercept | 0.015 | 0.038 | 0.716 | ||
| Congenital malformations of eye, ear, face, and neck | Weighted median | 0.057 | 0.313 | 0.855 | |
| MR Egger | −0.094 | 0.410 | 0.833 | ||
| intercept | 0.026 | 0.032 | 0.481 | ||
| Congenital malformations of skin | Weighted median | 1.124 | 0.380 | 0.003 | |
| MR Egger | 1.128 | 0.670 | 0.191 | ||
| intercept | 0.005 | 0.057 | 0.939 | ||
| Congenital malformations of the circulatory system | Weighted median | 0.307 | 0.222 | 0.168 | |
| MR Egger | 0.395 | 0.419 | 0.415 | ||
| intercept | 0.000 | 0.033 | 0.993 | ||
| Congenital malformations of the digestive system | Weighted median | 0.029 | 0.407 | 0.944 | |
| MR Egger | −0.111 | 0.537 | 0.849 | ||
| intercept | 0.033 | 0.042 | 0.491 | ||
| Congenital malformations of the musculoskeletal system | Weighted median | −0.101 | 0.260 | 0.698 | |
| MR Egger | −0.157 | 0.350 | 0.684 | ||
| intercept | 0.008 | 0.028 | 0.795 | ||
| Congenital obstructive defects of renal pelvis and ureter | Weighted median | 1.669 | 0.634 | 0.008 | |
| MR Egger | 0.548 | 0.963 | 0.609 | ||
| intercept | 0.165 | 0.074 | 0.113 | ||
| Vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb anomaly | Weighted median | 0.440 | 0.191 | 0.021 | |
| MR-Egger | 0.407 | 0.468 | 0.448 | ||
| intercept | 0.017 | 0.037 | 0.671 |
Sensitivity analyses could not be carried out for analyses with exposures of PCSK9 gene expression (in liver and whole blood) as <3 SNPs were available.
Genetically proxied fetal LDL-lowering via PCSK9 was associated with malformations affecting multiple systems [OR 2.70 (1.30–5.63), FDR-adjusted P = 0.018], malformations of the skin [OR 2.23 (1.33–3.75), FDR-adjusted P = 0.007], obstructive defects of the renal pelvis and ureter [OR 5.13 (2.35–11.20), FDR-adjusted P = 3.64 × 10−4], and the VACTERL association [OR 1.51 (1.16− 1.96), FDR-adjusted P = 0.007], as shown in Figure 2B. Sensitivity analyses identified directional pleiotropy in the association with malformations of the renal pelvis and ureter (MR-Egger intercept P = 0.001). No evidence of directional pleiotropy was identified for the other outcomes (all MR-Egger intercept P > 0.05) as reported in Table 2. The combined F-statistic for LDL via PCSK9 was 750.33, as reported in Supplementary material online, Table S5.
Colocalization analyses revealed weak evidence of shared causal variants for LDL in the PCSK9 region with malformations of the skin (H4 = 55.75%; H3 = 1.32%). The results were inconclusive for malformations affecting multiple systems (H1 = 79.71%, H3 = 2.54%, H4 = 17.74%), obstructive defects of the renal pelvis and ureter (H1 = 84.17%, H3 = 4.60%, H4 = 11.23%) and the VACTERL association (H2 = 79.32%, H3 = 5.97%, H4 = 14.71%). Full results of PCSK9 colocalization analyses are presented in Supplementary material online, Table S6.
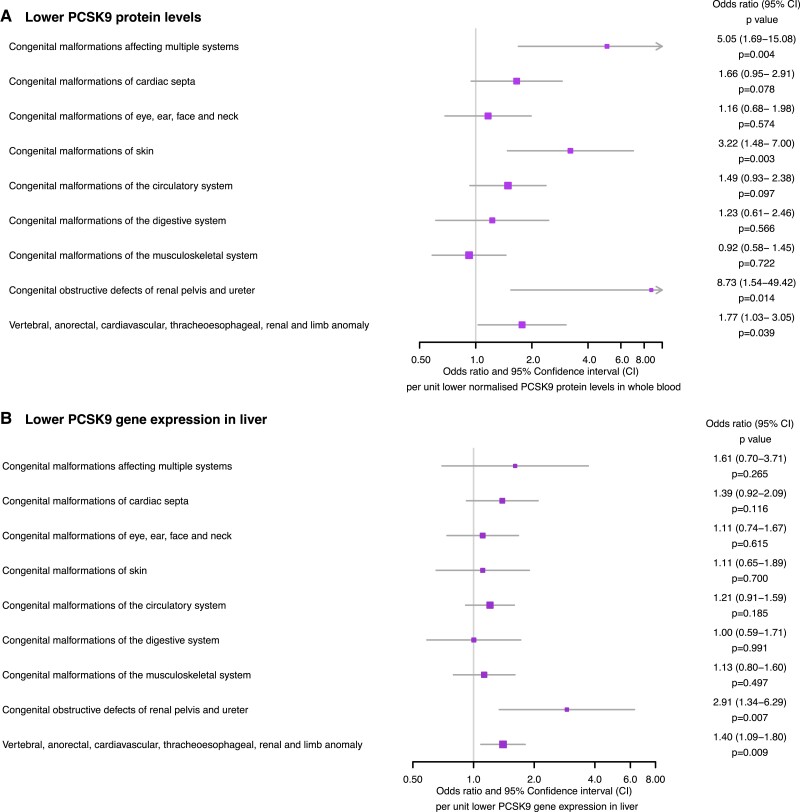
Consistent with the PCSK9 drug-target MR findings, a unit lower normalized genetically predicted PCSK9 protein level was associated with a greater risk of congenital malformations affecting multiple systems [OR 5.05 (1.69–15.08), P = 0.004], the skin [OR 3.22 (1.48–7.00), P = 0.003], the renal pelvis and ureter [OR 8.73 (1.54–49.42), P = 0.014] and the VACTERL association [OR 1.77 (1.03–3.05), P = 0.039], as displayed in Figure 3A. Sensitivity analyses did not identify evidence of directional pleiotropy (all MR-Egger intercept P > 0.05), as reported in Table 2. The combined F-statistic for PCSK9 pQTL instruments was 18.90, as reported in Supplementary material online, Table S5.
Figure 3.
Forest plots displaying the Mendelian randomization estimates for the association of (A) lower genetically predicted circulating proprotein convertase subtilisin–kexin type 9 (PCSK9) levels (B) lower genetically predicted PCSK9 gene expression in the liver.
Lower genetically predicted PCSK9 gene expression in the liver was associated with malformations of the renal pelvis and ureter [OR 2.91 (1.34–6.29), P = 0.007] as well as the VACTREL association [OR 1.40 (1.09–1.80), P = 0.009], as reported in Figure 3B. These results might be biased toward the observational estimate due to weak instruments, as the combined F-statistic for PCSK9 liver eQTL instruments was 4.91, as reported in Supplementary material online, Table S5.
UK Biobank replication analyses yielded findings broadly consistent with the main analyses, as presented in Table 3, though statistical significance was lost for the association between LDL-lowering overall and congenital malformations of the renal pelvis and ureter [OR 1.25 (0.99–1.58), P = 0.059] as well as the VACTREL association [OR 1.08 (1.00–1.16), P = 0.066] despite association estimates consistent in magnitude and direction.
Table 3.
Results of the UK biobank replication analyses: tabulated Mendelian randomization summary estimates for the genetic association between low-density lipoprotein-lowering generally, and via the PCSK9 drug-target
| Exposure | nSNP | Outcome | Odds ratio | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|---|---|
| LDL-lowering overall (1-SD) | 607 | Congenital malformations of cardiac septa | 0.96 | 0.85 | 1.08 | 0.478 |
| 607 | Congenital malformations of the circulatory system | 0.99 | 0.91 | 1.07 | 0.737 | |
| 607 | Congenital malformations of the musculoskeletal system | 1.00 | 0.91 | 1.10 | 0.966 | |
| 607 | Congenital malformations of eye, ear, face, and neck | 0.99 | 0.88 | 1.11 | 0.846 | |
| 607 | Congenital obstructive defects of renal pelvis and ureter | 1.25 | 0.99 | 1.58 | 0.059 | |
| 607 | Congenital malformations of the digestive system | 0.94 | 0.81 | 1.11 | 0.478 | |
| 607 | Congenital malformations of skin | 0.91 | 0.78 | 1.07 | 0.256 | |
| 607 | Congenital malformations affecting multiple systems | 1.58 | 1.25 | 2.01 | 0.000 | |
| 607 | Vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb anomaly | 1.08 | 1.00 | 1.16 | 0.066 | |
| LDL-lowering via PCSK9-inhibition (1-SD) | 11 | Congenital malformations of cardiac septa | 1.44 | 0.89 | 2.32 | 0.133 |
| 11 | Congenital malformations of the circulatory system | 1.23 | 0.89 | 1.69 | 0.217 | |
| 11 | Congenital malformations of the musculoskeletal system | 0.97 | 0.65 | 1.46 | 0.882 | |
| 11 | Congenital malformations of eye, ear, face, and neck | 1.24 | 0.77 | 1.99 | 0.374 | |
| 11 | Congenital obstructive defects of renal pelvis and ureter | 4.52 | 1.78 | 11.51 | 0.002 | |
| 11 | Congenital malformations of the digestive system | 1.11 | 0.59 | 2.06 | 0.747 | |
| 11 | Congenital malformations of skin | 3.00 | 1.49 | 6.03 | 0.002 | |
| 11 | Congenital malformations affecting multiple systems | 4.48 | 1.68 | 11.89 | 0.003 | |
| 11 | Vertebral, anorectal, cardiovascular, tracheo-esophageal, renal, and limb anomaly | 1.46 | 1.08 | 1.96 | 0.012 |
All data is from Neale Lab’s second release within UK Biobank, n = 469 897 (http://www.nealelab.is/uk-biobank/).
Phenome-wide scanning for SNPs instrumenting LDL levels via PCSK9, PCSK9 protein levels in whole blood (pQTL), PCSK9 gene expression in whole blood (eQTL), PCSK9 gene expression in liver (eQTL), and SNPs instrumenting LDL cholesterol levels overall identified 7119 phenotypic traits in total, reported in Supplementary material online, Table S7.
Discussion
This study leverages genetic variants associated with LDL levels in the PCSK9 region, as well as variants associated with actual PCSK9 protein levels and gene expression in the liver, to explore potential fetal effects of administration of a PCSK9 inhibitor capable of crossing the placenta during pregnancy. Within this framework, the results support an association between fetal LDL-lowering via PCSK9-inhibition and multiple types of congenital malformations. The results, therefore, corroborate current manufacturer recommendations against the use of PCSK9-inhibition during pregnancy.
The results of this study extend current knowledge regarding the importance of LDL in pregnancy. Fetal cholesterol metabolism is vital in placentation and early embryogenesis,25 and PCSK9 plays a key role in its regulation.7 In syndromes characterized by extremely low LDL levels, such as SLOS, a panoply of malformations occur.8 Additionally, previous studies have described an association of lower PCSK9 levels with neural tube defects.26 There are many potential mechanisms underlying these associations supported by the results of our study. Of central importance, cholesterol plays a major role in the normal maturation and signalling of hedgehog (Hh) proteins, a family of proteins that are critical for pattern formation during embryonic development.27 Impaired Hh signalling due to low cholesterol levels has been suggested to underlie at least some of the malformations that are typical of SLOS, including holoprosencephaly, agenesis of the corpus callosum, and postaxial polydactyly.28 Supporting this, Cooper et al,28 previously demonstrated significant compromise in Hh signal in cells from mouse models of SLOS and lathosterolosis, but also in normal cells that were pharmacologically depleted of cholesterol. Thus, previous studies investigating the pathophysiology of SLOS support the notion that cholesterol deficiency plays a role in altering membrane properties and promoting congenital malformations, in addition to the increased levels of dehydrocholesterol, a sterol precursor that is elevated in SLOS. While the latter mechanism is expected to be unique to the inborn error of metabolism that characterizes SLOS and bears no relevance to PCSK9 signalling, the cholesterol deficiency mechanism is likely relevant in the associations of low PCSK9 activity, be due to the R46L PCSK9 mutation or administration of a PCSK9 inhibitor that crosses the placenta, with congenital malformations.
An investigational in vivo clustered regularly interspaced short palindromic repeats (CRISPR) base editing therapy, VERVE-101 is currently under development29 with the aim to permanently hinder hepatic PCSK9 production by altering a single DNA base in the PCSK9 gene. If this approach proves effective, this might be a theoretically safer option for women planning to conceive, as long as pregnancy occurs after the ‘active’ delivery phase. Unfortunately, this cannot be inferred with confidence, as this study does not exclude that potential ‘indirect’ effects of lowering maternal LDL might occur on the fetus. However, this therapy would not be expected to interfere with fetal LDL metabolism, given that pre-clinical data in primates has promisingly suggested that PCSK9 gene editing in liver (i.e. non-germline) cells is not heritable, and would, therefore, not be expected to exhibit the associations described in this study.29 Considering the results of this study, we highlight the importance of thorough investigation of the potential reproductive safety of this novel therapy, to avoid its contraindication due to insufficient data which contributes to inequity of care for women during reproductive years.
This study has important clinical implications. First, in practical terms, the results do not suggest that any change should be made to the current recommendations to avoid these drugs in pregnancy. Second, it follows that reproductive wishes should be discussed with women of reproductive age taking PCSK9 inhibitors. Contraception advice and appropriate pre-conception planning are warranted. At present, no guidelines or consensus statements exist to provide advice on the appropriate timing to stop PCSK9-inhibitors relative to attempts to conceive. Despite the recognition that limited data is available, these would be useful to guide advice for the pre-conception stage and to guide conversations with women who conceive accidentally during therapy. From a research perspective, the findings call for curation of a registry to monitor outcomes among individuals with inadvertent PCSK9i exposure in pregnancy to see if any signals are recapitulated. Finally, clinicians must be aware of the importance of meticulous care for women of reproductive age on PCSK9 inhibitors, because these safety concerns risk widening existing sex-based disparities in cardiovascular care.30
Limitations
There are a number of limitations to discuss. First, we unfortunately cannot ascertain in which trimester LDL-lowering was highest risk for malformations, and it is plausible that risk may differ throughout different stages of pregnancy. Second, as mentioned, we cannot exclude additional effects that might relate to indirect influence of maternal LDL-lowering. Third, the instruments for the analysis of PCSK9 gene expression in both the liver were weak (F-statistics <10) and might therefore be biased towards observational estimates. Once larger data sources are available when further releases of GTEx data are available, the analyses should be repeated to ensure the findings for these exposures are not influenced by weak instrument bias. However, it is important to note that weak instrument bias in a two-sample MR setting using nonoverlapping cohorts typically results in estimates that are biased toward the null, it would therefore not be expected to exaggerate inferences made in this study for these instruments. Finally, horizontal pleiotropy can limit MR investigations and if present, may result in violation of the third instrumental variable assumption whereby genetic variants must only influence the outcome through the exposure, and not directly or through alternative phenotypes. In order to test this, we performed sensitivity analyses using robust methods including weighted median and MR Egger approaches which did not identify the presence of directional pleiotropy. To further maintain confidence in our results, we performed phenome-wide scanning to identify phenotypic traits associated with the genetic variants selected as instrumental variables to identify any alternative pathways between the exposure and outcome unrelated to the biomarker of interest. These demonstrated that the SNPs instrumenting LDL via PCSK9 and all PCSK9 pQTL and eQTLs were mostly associated with cholesterol and cholesterol-related traits, further supporting the absence of horizontal pleiotropy in these MR analyses. The range of associations with SNPs instrumenting LDL overall was much broader, so the existence of pleiotropy cannot be fully excluded from these data. However, evidence to refute this possibility is provided by the sensitivity analyses using robust methods failing to detect directional pleiotropy. Taken together, this suggests that if pleiotropy exists, it is balanced and would not be expected to influence the direction or magnitude of the association of genetically predicted LDL with the outcomes.
Conclusion
In conclusion, the results of this study support current manufacturer recommendations to avoid the use of PCSK9-inhibition during pregnancy. By extension, it is prudent for physicians looking after women of reproductive age on PCSK9 inhibitors to counsel patients regarding contraception and to encourage planned pregnancy with appropriate pre-conception care.
Supplementary Material
Acknowledgements
We thank all investigators and participants of the FinnGen study, UK Biobank, and Global Lipids Genetic Consortium (GLGC). The views expressed are those of the authors and not necessarily those of King’s Health Partners, Guy’s and St. Thomas’s Hospital, the NHS, the NIHR, or the Department of Health and Social Care. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 8 June 2023.
Contributor Information
Maddalena Ardissino, National Heart and Lung Institute, Imperial College London, Hammersmith Campus, London, UK; Department of Medicine, School of Clinical Medicine, University of Cambridge, London, UK.
Eric A W Slob, MRC Biostatistics Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK; Department of Applied Economics, Erasmus School of Economics, Erasmus University Rotterdam, Rotterdam, The Netherlands; Erasmus University Rotterdam Institute for Behavior and Biology, Erasmus University Rotterdam, Rotterdam, The Netherlands.
Rohin K Reddy, National Heart and Lung Institute, Imperial College London, Hammersmith Campus, London, UK.
Alec P Morley, Department of Medicine, School of Clinical Medicine, University of Cambridge, London, UK.
Art Schuermans, Program in Medical and Population Genetics and Cardiovascular Disease Initiative, Broad Institute of Harvard and MIT, Cambridge, MA, USA; Cardiovascular Research Center and Center for Genomic Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Phoebe Hill, Royal Oldham Hospital, Northern Care Alliance NHS Foundation Trust, Manchester, UK.
Catherine Williamson, Institute of Reproductive and Developmental Biology, Imperial college London, London, UK.
Michael C Honigberg, Program in Medical and Population Genetics and Cardiovascular Disease Initiative, Broad Institute of Harvard and MIT, Cambridge, MA, USA; Cardiovascular Research Center and Center for Genomic Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Antonio de Marvao, British Heart Foundation Centre of Research Excellence, School of Cardiovascular Medicine and Sciences, King’s College London, London, UK; Medical Research Council, London Institute of Medical Sciences, Imperial College London, London, UK.
Fu Siong Ng, National Heart and Lung Institute, Imperial College London, Hammersmith Campus, London, UK.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology.
Author contribution
M.A. contributed to conceptualization, data curation, formal analysis, investigation, methodology, writing (original draft). E.A.W.S. contributed to conceptualization, data curation, formal analysis, investigation, methodology, writing (review and editing). R.K.R. contributed to conceptualization, investigation, methodology, project administration, writing (original draft). A.P.M. contributed to conceptualization, investigation, project administration, writing (original draft). A.S. contributed to investigation, project administration, writing (review & editing). P.H. contributed to investigation, project administration, writing (review & editing). C.W. contributed to supervision, project administration, writing (review & editing). M.C.H. contributed to supervision, project administration, writing (review & editing). A.d.M. contributed to supervision, project administration, writing (review & editing). F.S.N. contributed to conceptualization, supervision, project administration, writing (review & editing).
Funding
This work was supported by the National Institute for Health Research (MA, RKR, CW, FSN), the Fetal Medicine Foundation (MA, CW, AdM), the United Kingdom Research and Innovation Medical Research Council (grant number MC_UU_00002/7) (EAWS), National Institute for Health Research Cambridge Biomedical Research Centre grant number (BRC-1215-20014) (EAWS), Belgian American Educational Foundation (AS), Diabetes UK (CW), Medical Research Council (CW), U.S. National Heart, Lung, and Blood Institute (grant number K08HL166687) (MCH), American Heart Association (grant numbers 940166, 979465) (MCH), and the British Heart Foundation (grant numbers RG/F/22/110078 and RE/18/4/34215).
Data availability
The data underlying this article were derived from sources in the public domain and these data sources have been appropriately cited within the manuscript.
References
- 1. Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 2. Shen M, Aghajani Nargesi A, Nasir K, Bhatt DL, Khera R. Contemporary national patterns of eligibility and use of novel lipid-lowering therapies in the United States. J Am Heart Assoc 2022;11:e026075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol 2022;80:1366–1418. [DOI] [PubMed] [Google Scholar]
- 4. Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med 2004;350:1579–1582. [DOI] [PubMed] [Google Scholar]
- 5. Chang J-C, Chen Y-J, Chen I-C, Lin W-S, Chen Y-M, Lin C-H. Perinatal outcomes after statin exposure during pregnancy. JAMA Netw Open 2021;4:e2141321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM, et al. Statins and congenital malformations: cohort study. BMJ 2015;350:h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pecks U, Rath W, Maass N, Berger B, Lueg I, Farrokh A, et al. Fetal gender and gestational age differentially affect PCSK9 levels in intrauterine growth restriction. Lipids Health Dis 2016;15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeBarber AE, Eroglu Y, Merkens LS, Pappu AS, Steiner RD. Smith–lemli–opitz syndrome. Expert Rev Mol Med 2011;13:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jerome RN, Pulley JM, Roden DM, Shirey-Rice JK, Bastarache LA, R Bernard G, et al. Using human ‘experiments of nature’ to predict drug safety issues: an example with PCSK9 inhibitors. Drug Saf 2018;41:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Medicines Agency. Leqvio (inclisiran) product information. https://www.ema.europa.eu/en/medicines/human/EPAR/leqvio#ema-inpage-item-product-info (20 May 2023).
- 11. European Medicines Agency. Repatha (evolovumab) product information. https://www.ema.europa.eu/en/medicines/human/EPAR/repatha#ema-inpage-item-product-info (20 May 2023).
- 12. Schmidt AF, Finan C, Gordillo-Marañón M, Asselbergs FW, Freitag DF, Patel RS, et al. Genetic drug target validation using Mendelian randomisation. Nat Commun 2020;11:3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. R Core Team . R: A language and environment for statistical computing. R Found Stat Comput Vienna, Austria URL https://www.R-project.org/. 2022.
- 14. Graham SE, Clarke SL, Wu K-HH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 2021;600:675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res 2008;36:D901–D906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pott J, Gådin JR, Theusch E, Kleber ME, Delgado GE, Kirsten H, et al. Meta-GWAS of PCSK9 levels detects two novel loci at APOB and TM6SF2. Hum Mol Genet 2022;31:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The genotype-tissue expression (GTEx) project. Nat Genet 2013;45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. GTEx Consortium . Genetic effects on gene expression across human tissues. Nature 2017;550:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv. January 2022:2022.03.03.22271360. 10.1101/2022.03.03.22271360 [DOI]
- 20. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res 2020;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017;46:1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet 2014;10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-egger method. Eur J Epidemiol 2017;32:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porter FD. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr Opin Pediatr 2003;15:607–613. [DOI] [PubMed] [Google Scholar]
- 26. Erol SA, Tanacan A, Firat Oguz E, Anuk AT, Goncu Ayhan S, Neselioglu S, et al. A comparison of the maternal levels of serum proprotein convertase subtilisin/kexin type 9 in pregnant women with the complication of fetal open neural tube defects. Congenit Anom (Kyoto) 2021;61:169–176. [DOI] [PubMed] [Google Scholar]
- 27. Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science 1996;274:255–259. . [DOI] [PubMed] [Google Scholar]
- 28. Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, et al. A defective response to hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet 2003;33:508–513. [DOI] [PubMed] [Google Scholar]
- 29. Lee RG, Mazzola AM, Braun MC, Platt C, Vafai SB, Kathiresan S, et al. Efficacy and safety of an investigational single-course CRISPR base editing therapy targeting PCSK9 in non-human primate and mouse models. Circulation 2023;147:242–253. [DOI] [PubMed] [Google Scholar]
- 30. Zhao M, Woodward M, Vaartjes I, Millett ERC, Klipstein-Grobusch K, Hyun K, et al. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were derived from sources in the public domain and these data sources have been appropriately cited within the manuscript.