Abstract
Plasmodium parasites within mosquitoes are exposed to various physiological processes, such as blood meal digestion activity, the gonotrophic cycle, and host responses preventing the entry of parasites into the midgut wall. However, when in vitro-cultured ookinetes are injected into the hemocoel of mosquitoes, Plasmodium parasites are not affected by the vertebrate host’s blood contents and do not pass through the midgut epithelial cells. This infection method might aid in identifying mosquito-derived factors affecting Plasmodium development within mosquitoes. This study investigated novel mosquito-derived molecules related to parasite development in Anopheles mosquitoes. We injected in vitro-cultured Plasmodium berghei (ANKA strain) ookinetes into female and male Anopheles stephensi (STE2 strain) mosquitoes and found that the oocyst number was significantly higher in males than in females, suggesting that male mosquitoes better support the development of parasites. Next, RNA-seq analysis was performed on the injected female and male mosquitoes to identify genes exhibiting changes in expression. Five genes with different expression patterns between sexes and greatest expression changes were identified as being potentially associated with Plasmodium infection. Two of the five genes also showed expression changes with infection by blood-feeding, indicating that these genes could affect the development of Plasmodium parasites in mosquitoes.
Keywords: Anopheles stephensi, injection, molecules, Plasmodium berghei
Given its involvement in the transmission of the Plasmodium parasites that cause malaria, the genus Anopheles is a key taxon among mosquitoes [19]. Plasmodium parasites enter the midgut lumen when female mosquitoes feed on infected blood [16]. The female and male gametocytes form zygotes approximately 2 hr after entry of the parasites, which then develop into ookinetes. The motile ookinetes invade midgut epithelial cells and differentiate into oocysts under the midgut basal lamina approximately 2 days after blood-feeding. The oocysts gradually enlarge over the next 12 days, during which time a vast number of sporozoites proliferate inside the cysts. The mature oocysts rupture, releasing the sporozoites into the mosquito body cavity (hemocoel). The released sporozoites enter the salivary glands and are transmitted when the mosquitoes feed on blood from mammalian hosts. In parallel with this life cycle, mosquitoes undertake blood meal digestion and the gonotrophic cycle [9]. These reactions alter the development of parasites in mosquitoes. For instance, trypsin produced in the Anopheles mosquito’s midgut allows Plasmodium parasites to pass through the mosquito’s peritrophic matrix [1]. One of the hormones involved in oogenesis, 20-hydroxyecdysone, primes immune responses against Plasmodium parasites [13]. At the same time, the entry of Plasmodium parasites into the midgut wall also triggers activation of the mosquito’s immune responses [3, 16]. Although these phenomena are very important, they are thought to mask the molecules involved in direct interactions between mosquitoes and Plasmodium parasites. A different method for infection than blood-feeding needs to be used to uncover these molecules.
The injection of in vitro-cultured ookinetes into the hemocoel of female mosquitoes as an experimental infection method results in the formation of oocysts in the hemocoel [5, 8, 10]. Injected parasites develop in the mosquito’s body similarly to those introduced through blood-feeding [5, 8, 10]. The injection method has advantages for analyzing the direct interactions between Anopheles mosquitoes and Plasmodium parasites that are not found in blood-feeding infection. In the injection method, mosquitoes do not undergo digestion of the blood meal or the gonotrophic cycle; Plasmodium parasites do not develop via the midgut; the numbers of parasites injected can be controlled; and the prevalence, representing the percentage of mosquitoes that form oocysts among the infected mosquitoes, is high (i.e., injection of 5,000 ookinetes results in 100% prevalence) [5]. The injection method is thought to be suitable for the search for new Plasmodium development-related molecules involved in the direct interaction between mosquitoes and Plasmodium parasites.
Differences in susceptibility to parasitic pathogens between female and male hosts have been reported in various animal species. For instance, male mice are more susceptible to Babesia microti and Plasmodium berghei than females [6, 14]. Differences in sex hormones between male and female mice lead to differences in the immune response against the invaders, resulting in such sex-related differences in susceptibility. In the dipteran tsetse fly (Glossina morsitans), which is a blood-feeding pathogen vector similar to the mosquito, both females and males feed on blood and transmit Trypanosoma brucei [12]. Susceptibility to this parasite differs between females and males, with male salivary glands being more conducive to T. brucei development than female salivary glands [12]. It has been suggested that a sex-linked recessive gene is involved in this difference [12]. Thus, differences in susceptibility between the sexes have revealed factors rooted in the biological characteristics of the vector that are involved in parasite development. Male mosquitoes do not feed on blood, and there are no reports of studies that have examined male mosquitoes’ ability to support Plasmodium parasite development. Our previous study showed that oocysts and sporozoites are formed in female and male mosquitoes injected with in vitro-cultured ookinetes [5]. The injection method enables us to compare Plasmodium development in female and male mosquitoes.
This study was established to investigate novel molecules related to Plasmodium development in mosquitoes. First, we compared the number and size of P. berghei oocysts in female and male Anopheles stephensi mosquitoes to clarify the differences in the ability of the hosts to support the transition of ookinetes to oocysts and the development of oocysts. Next, we used RNA-seq, a simple and powerful tool to evaluate gene expression [13, 17, 18], focusing on early oocyst formation in female and male injected mosquitoes.
MATERIALS AND METHODS
Ethics statement
We performed experiments including animal subjects in accordance with the Guidelines of Animal Experiments of Kitasato University, and these experiments were approved by Kitasato University Institutional Animal Care and Use Committee (Approval number: 18-029 and 21-031).
Mosquitoes and parasites
An. stephensi adult mosquitoes of the STE2 strain were reared in an insectary at 19°C under a 14 hr:10 hr light:dark cycle with 10% (w/v) sucrose solution. Mosquitoes were fed mouse blood and laid eggs. These eggs were collected in distilled water and hatched larvae were reared at 27°C.
Green fluorescent protein (GFP)-expressing P. berghei (ANKA strain) awakened from frozen stock was maintained by serial passage with Institute of Cancer Research (ICR) mice (Charles River Laboratories Japan Inc., Yokohama, Japan).
In vitro culture and purification of P. berghei ookinetes
The culture and purification of P. berghei ookinetes were performed as previously described [5]. In brief, blood collected from infected mice was mixed with medium containing RPMI-1640 medium (Thermo Fisher Scientific Inc., Waltham, MA, USA), 25 mM HEPES (Dojindo Laboratories, Kumamoto, Japan), 0.4 mM hypoxanthine (Sigma-Aldrich, St. Louis, MO, USA), 24 mM NaHCO3 (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and 12.5 mg L−1 gentamicin reagent solution (Thermo Fisher Scientific Inc.) and incubated at 19°C for 20 to 24 hr. A MidiMACS separator system (LS Columns; Miltenyi Biotec, Bergisch Gladbach, Germany) was used to purify ookinetes from the cultured blood.
Examination of mosquitoes injected with P. berghei
A total of 69 nL of 2,000 ookinetes was injected into the hemocoel of <10-day-old female and male mosquitoes using a Nanoject II automatic nanoliter injector (Drummond Scientific Co., Broomall, PA, USA) [5]. Five to sixteen injected female and male mosquitoes per group were dissected at 8, 12, 14, and 16 days post-injection (dPI) to measure the oocyst number (the number of oocysts per mosquito body), oocyst diameter, and prevalence (percentage of mosquitoes that possess oocysts). The oocyst number and prevalence were examined using an Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan). The oocyst diameter was measured using ImageJ software. All experiments were conducted with at least three replicates.
RNA-seq analysis
Female and male mosquitoes were collected 24 and 72 hr post-injection (hPI) with 5,000 P. berghei ookinetes or with only medium (negative control, culture medium for ookinetes) for RNA-seq analysis. Our previous study reported that mosquitoes injected with 5,000 ookinetes had a higher prevalence than those injected with 2,000 ookinetes [5]. We used mosquitoes injected with 5,000 ookinetes in this analysis to make it easier to identify differences in the variation of RNA expression. Three mosquitoes per sample were collected at each time point and stored at −80°C until use. Total RNA was extracted using Nucleo Spin RNA Blood (Macherey-Nagel, Düren, Germany), in accordance with the manufacturer’s protocol, delivered to Macrogen Japan Corp. (Tokyo, Japan) for library construction using the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, USA), and sequenced using the NovaSeq 6000 system (Illumina). FastQCv0.11.7 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was used to analyze the raw read quality, and statistical analysis was performed using fold change per comparison pair of mosquitoes injected with P. berghei ookinetes and with the medium. Gene Ontology (GO) and orthology analysis were performed using VectorBase (https://www.vectorbase.org/).
Real-time polymerase chain reaction (PCR)
A total of 100 to 150 mosquitoes that hatched on the same day per group were allowed to feed blood of P. berghei-infected or naïve mice. Mice were anesthetized and the female mosquitoes were allowed to feed on them for 30 min. Three mosquitoes were collected and pooled every 24 hr until 96 hr after blood-feeding and stored at −80°C. These mosquitoes were homogenized using Micro Smash MS-100R (Tomy Seiko Co., Ltd., Tokyo, Japan) and then total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific) and Phasemaker tubes (Thermo Fisher Scientific), in accordance with the manufacturer’s protocol. The extracted RNA was converted to cDNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd., Osaka, Japan) and then used for the template DNA. Expression levels of target genes were determined by relative quantification by real-time PCR using KOD SYBR qPCR Mix (Toyobo Co., Ltd.). Analysis was conducted using Applied Biosystems StepOnePlus (Thermo Fisher Scientific) with the following cycling conditions: 1 cycle at 98°C for 2 min, followed by 40 cycles of 98°C for 10 sec, 60°C for 10 sec, and 68°C for 30 sec, and 1 cycle of 95°C for 15 sec, 60°C for 1 min, and 99°C for 15 sec. An. stephensi ribosomal protein S7 gene was used as an internal control and the primers used are shown in Table 1. Three technical and two biological replicates were performed.
Table 1. Primer list.
| Target gene | Forward (5'→3') | Reverse (5'→3') |
|---|---|---|
| ASTEI08011 | ATCTCCGACGGATTCACACTTG | CAGAATCACTATCGGCCACTC |
| ASTEI02008 | ATGATGCGTGCGTTCCTTGCG | TCGTTACCGGTGTAAATGTACG |
| ASTEI04832 | CAATGTGGTCACGTCCCAGG | GGATACGATTGGCAGCATTCG |
| An. stephensi ribosomal protein S7 | CCTGGATAAGAACCAGCAGA | TACAAGAAGCTGACTGGCC |
Statistical analysis
All statistical analyses were performed using GraphPad Prism version 8.4.3 software (GraphPad Software Inc., San Diego, CA, USA). We determined the significance of differences in the oocyst number using Mann−Whitney test (P<0.05) and the significance of differences in the prevalence using Fisher’s exact test (P<0.05). Significant differences in oocyst size were determined using the unpaired t-test with Welch’s correction (P<0.05). One-way ANOVA and Tukey’s multiple comparisons test were used to compare relative expressions determined by real-time PCR (P<0.05).
RESULTS
P. berghei development in female and male mosquitoes injected with ookinetes
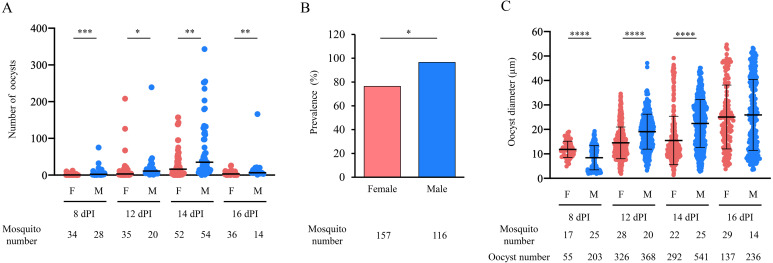
Female and male mosquitoes were injected with 2,000 P. berghei ookinetes to compare oocyst numbers, prevalence, and oocyst diameter. A total of 157 female and 116 male mosquitoes were injected. At 8 dPI, 34 females and 28 males were dissected, and at 12 dPI, 35 females and 20 males were dissected. At 14 dPI, 52 females and 54 males were dissected, and at 16 dPI, 36 females and 14 males were dissected to observe oocysts. The median oocyst numbers were 0.5 and 2.0 at 8 dPI, 3.0 and 11.0 at 12 dPI, 16.0 and 35.0 at 14 dPI, and 3.0 and 6.5 at 16 dPI in females and males, respectively (Fig. 1A). The oocyst number was significantly higher in males than in females in all mosquito groups (P<0.001–0.05; Fig. 1A). The prevalence was 76.4% and 96.6% in females and males, respectively, which was significantly different (P<0.05; Fig. 1B). The mean oocyst diameters were 11.8 ± 3.3 µm and 8.4 ± 5.0 µm at 8 dPI, 14.5 ± 6.5 µm and 19.1 ± 7.2 µm at 12 dPI, 15.5 ± 9.9 µm and 22.4 ± 9.8 µm at 14 dPI, and 25.1 ± 13.1 µm and 26.0 ± 14.5 µm at 16 dPI in females and males, respectively (P<0.0001; Fig. 1C). The mean oocyst diameter was significantly smaller in males than in females at 8 dPI, larger in males than in females at 12–14 dPI, and there was no difference between the sexes at 16 dPI (Fig. 1C). A total of 57 oocysts in males and 26 oocysts in females were larger than 40 µm in diameter at 16 dPI, representing 24% of the oocysts in males and 19% of those in females.
Fig. 1.
Oocyst development in female and male mosquitoes injected with Plasmodium berghei ookinetes. A total of 157 female and 116 male mosquitoes were injected with 2,000 P. berghei ookinetes expressing green fluorescent protein (GFP). (A) The oocyst numbers in female and male mosquitoes were counted with a fluorescence microscope at 8–16 dPI. The number of mosquitoes and oocysts used for each day’s measurements is shown below the figure. Horizontal bars indicate median values. Mann–Whitney test was used for comparison (*P<0.05, **P<0.01, ***P<0.001). F: female, M: male, dPI: days post-injection. (B) The prevalence (the percentage of mosquitoes that possessed oocysts) in female and male mosquitoes was calculated. Fisher’s exact test was used for comparison (*P<0.05). (C) The oocyst diameters in female and male mosquitoes were measured with ImageJ software at 8–16 dPI. Measurements were taken from 96 females and 84 males of the injected mosquitoes. The number of mosquitoes and oocysts used for each day’s measurements is shown below the figure. Boldfaced horizontal bars indicate mean values. Error bars indicate standard deviations. Unpaired t-test with Welch’s correction was used for comparison (****P<0.0001). F: female, M: male, dPI: days post-injection.
RNA-seq analysis of female and male mosquitoes injected with P. berghei ookinetes
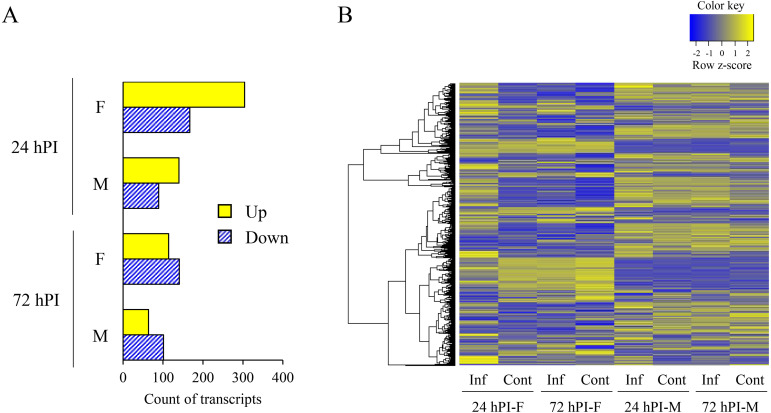
RNA-seq analysis was performed on female and male mosquitoes injected with 5,000 P. berghei ookinetes at 24 and 72 hPI. In a previous report, both ookinetes and oocysts were observed at 24 hPI when ookinetes were injected into Anopheles mosquitoes [10]. At 48 hPI, oocysts were detected in 93% of the injected mosquitoes [10]. This indicates that the differentiation of ookinetes into oocysts progresses at 24 hPI and is complete by 72 hPI, resulting in the formation of early oocysts. The time points examined in this experiment were deemed appropriate for evaluating gene expression related to early oocyst development. In total, 304 genes were upregulated and 167 were downregulated in females, whereas 140 were upregulated and 89 were downregulated in males at 24 hPI (fold change ≥|2|; Fig. 2A). Furthermore, 114 genes were upregulated and 141 were downregulated in females, whereas 64 were upregulated and 101 were downregulated in males at 72 hPI (fold change ≥|2|; Fig. 2A). The expression pattern was different between females and males, especially in females at 24 hPI (Fig. 2B). Expression levels of genes associated with immune-related GO terms and orthologs of known major immune-related genes showed small fold changes and did not differ greatly between females and males (Table 2). We found 458 genes that had differences in the pattern of gene expression (increased or decreased) between females and males. Of these, only five genes had large fold changes in their expression (fold change ≥|10|, Table 3). Functional and transcriptional analysis has not been performed on any five genes, and no reports have found any associations with infection by Plasmodium parasites.
Fig. 2.
RNA-seq analysis of mosquitoes injected with Plasmodium berghei ookinetes. (A) The numbers of upregulated and downregulated genes in mosquitoes at 24 and 72 hPI of 5,000 P. berghei ookinetes (fold change ≥|2|). hPI: hours post-injection, F: female, M: male, Up: number of upregulated genes, Down: number of downregulated genes. (B) Heat map of one-way hierarchical clustering using Z-score for normalized value (log2-based). A total of 861 transcripts satisfied the criterion of fold change ≥|2|. Inf: mosquitoes injected with P. berghei ookinetes, Cont: mosquitoes injected with medium, hPI: hours post-injection, F: female, M: male.
Table 2. Expression changes of immune-related genes.
| Gene ID | Fold change |
Protein function | Function of the ortholog | |||
|---|---|---|---|---|---|---|
| 24 hPI-F | 24 hPI-M | 72 hPI-F | 72 hPI-M | (An. gambiae PEST) | ||
| ASTEI01170 | −1.46 | −1.56 | 1.07 | −1.26 | U. P. | Cecropin2 |
| ASTEI01171 | −2.2 | −1.99 | −1.18 | −1.15 | U. P. | Cecropin-B |
| ASTEI01776 | 1.54 | 1.26 | 2.15 | −1.28 | U. P. | U. P. |
| ASTEI02104 | −3.14 | −1.14 | −1.58 | 3.22 | Leucine-rich immune protein (Short) | LRIM12 |
| ASTEI02158 | −1.67 | −1.21 | −3.42 | −1.11 | U. P. | PGRP-LD |
| ASTEI02214 | 1.14 | −1.16 | −1.5 | −1.28 | U. P. | Attacin C domain-containing protein |
| ASTEI02462 | −1.03 | 2.1 | 1.27 | 1.24 | Rhodanese domain-containing protein | U. P. |
| ASTEI02570 | −1.04 | 2.9 | −1.11 | 1.49 | U. P. | LRIM11 |
| ASTEI02870 | 1.02 | −2.07 | 1.13 | 1.39 | U. P. | TOLL9 |
| ASTEI03016 | 1.19 | 1.06 | −1.11 | 1.23 | NOS | NOS |
| ASTEI03826 | 1.03 | 1.09 | 1 | 1.05 | U. P. | Rel1 |
| ASTEI03923 | 1.17 | 1.84 | −2.74 | 1.37 | Beta-hexosaminidase | Hexosaminidase |
| ASTEI04200 | −1.21 | 1.2 | −1.15 | 1.89 | U. P. | Defensin1 |
| ASTEI04437 | 2.13 | 1.98 | 1.44 | 1.19 | U. P. | Neuronal cell adhesion molecule |
| ASTEI05476 | −2.09 | −1.01 | −1.56 | 1.36 | SH2 domain-containing protein | U. P. |
| ASTEI05828 | 1.39 | −1.22 | 1.33 | −1.07 | U. P. | SOCS |
| ASTEI06240 | −1.08 | 1.12 | −1 | −1.04 | Signal transducer and activator of transcription | STAT2 |
| ASTEI06430 | −2.09 | 1.38 | −1.89 | −1.34 | U. P. | EF-hand_13 domain-containing protein |
| ASTEI06676 | 2.19 | 1.1 | −1.25 | 1.09 | U. P. | U. P. |
| ASTEI06950 | 1.48 | 2.04 | 1.08 | 1.48 | U. P. | U. P. |
| ASTEI07187 | 1.16 | 2.8 | −1.33 | −1.11 | U. P. | Protein-glutamine γ-glutamyltransferase E |
| ASTEI07221 | −1.01 | 1.03 | −1.02 | 1.06 | U. P. | Caspar |
| ASTEI07457 | 1.84 | 2.6 | −1.69 | −1.19 | U. P. | U. P. |
| ASTEI07671 | −1.27 | 1.25 | −1.37 | 1.3 | ANK_REP_REGION domain-containing protein | Cactus |
| ASTEI08051 | 2.76 | −1.14 | −1.01 | −1.01 | Peptidoglycan-recognition protein | PGRP-LB |
| ASTEI08168 | −5.13 | −1.51 | 1.6 | −1.99 | U. P. | U. P. |
| ASTEI08432 | 1.14 | 1.28 | 1.21 | 1.5 | U. P. | TEP1 |
| ASTEI08473 | −1.38 | −1.08 | −1.05 | 1.02 | U. P. | E3 SUMO-protein ligase PIAS2 |
| ASTEI08532 | −2.63 | −1.11 | −1.28 | 1.11 | U. P. | Microtubule-associated protein (RP/EB family) |
| ASTEI08630 | 1.47 | 1.68 | 1.04 | 2.52 | U. P. | 3-Glucan binding protein |
| ASTEI08642 | 1.12 | 1.24 | 1.14 | 1.16 | U. P. | Rel2 |
| ASTEI08645 | −1.84 | −1.79 | −2.99 | 1.01 | Fibrinogen C-terminal domain-containing protein | Fibrinogen C-terminal domain-containing protein |
| ASTEI09465 | −1.18 | −1.25 | 1.19 | 1.22 | Signal transducer and activator of transcription | STAT |
| ASTEI09744 | −1.13 | −2.12 | −1.73 | −1.6 | U. P. | U. P. |
| ASTEI10499 | −1.84 | −3.07 | −1.43 | −1.79 | U. P. | PPO2 |
| ASTEI10663 | −1.58 | −2.47 | −1.14 | −1.12 | U. P. | PPO3 |
| ASTEI11189 | 2.28 | 1 | 1.15 | −1.09 | U. P. | GNBPB5 |
hPI: hours post-injection, F: female, M: male. U.P.: unspecified product, LRIM: leucine-rich repeat immune protein, PGRP: peptidoglycan recognition protein, TOLL: TOLL-like receptor, NOS: nitric oxide synthase, SOCS: suppressor of cytokine signaling, STAT: signal transducers and activators of transcription, TEP1: thioester-containing protein 1, PPOP2: prophenoloxidase 2, PPOP3: prophenoloxidase 3, GNBP: Gram-negative bacteria binding protein.
Table 3. List of genes with the different expression between female and male mosquitoes and large expression change (fold change >|10|).
| Gene ID | Fold change |
Protein function | Function of the ortholog (An. gambiae PEST) |
GO terms |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 24 hPI-F |
24 hPI-M |
72 hPI-F |
72 hPI-M |
Biological Process | Cellular Component | Molecular Function | |||
| ASTEI02744 | 28.09 | 2.36 | −1.51 | 2.59 | U. P. | Cuticular protein unclassified | N. D. | N. D. | N. D. |
| ASTEI04832 | −4.43 | 3.72 | −17.26 | −1.02 | U. P. | Cathepsin B precursor | Proteolysis | N. D. | Cysteine-type peptidase activity |
| ASTEI05475 | −1.28 | 15.86 | −1.03 | 7.03 | U. P. | U. P. | N. D. | N. D. | N. D. |
| ASTEI05892 | −1.1 | 4.48 | 1.01 | 27.59 | Fibrinogen C-terminal domain-containing protein | Fibrinogen and fibronectin, ficolin A, and fibrinogen-related protein 7 | N. D. | N. D. | N. D. |
| ASTEI08011 | −1.08 | 840.05 | −141.4 | −1,465.98 | U. P. | Nuclear pore complex protein Nup88 | G protein-coupled receptor signaling pathway | Membrane | Structural constituent of nuclear pore |
| Ribosomal large subunit export from nucleus | Integral component of membrane | ||||||||
| Nucleocytoplasmic transport | |||||||||
| Phototransduction | |||||||||
hPI: hours post-injection, F: female, M: male. U.P.: unspecified product, N.D.: no data.
Expression patterns of selected genes in mosquitoes fed infected blood or naïve blood
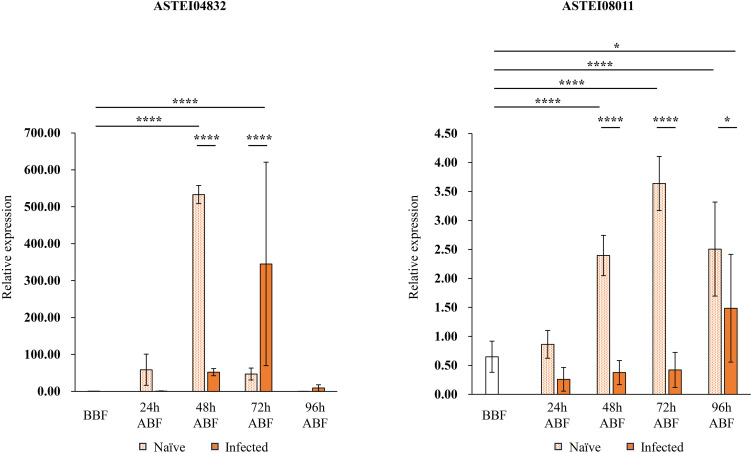
We selected two genes (ASTEI04832 and ASTEI08011) from the five genes based on their GO and ortholog analyses, and quantified their expression levels by real-time PCR. GO analysis showed that three of the five genes were not associated with any GO terms, while two were associated with GO terms (Table 3). The ortholog of ASTEI04832 is cathepsin B precursor, which is a lysosomal cysteine protease [15]. Cathepsin B has been reported to be involved in apoptosis and to be associated with dengue virus infection in Aedes mosquitoes [15]. Nuclear pore complex protein Nup88, which is the ortholog of ASTEI08011, has not been functionally analyzed in mosquitoes. However, ASTEI08011 is annotated with the GO term of the G protein-coupled receptor signaling pathway. Mosquitoes have been found to have G protein-coupled receptors, such as the prostaglandin E2 receptor of An. gambiae [7]. This receptor regulates the mosquito immune system through the prostaglandin E2 signaling and affects the Plasmodium survival [7]. The G protein-coupled receptor signaling pathway may impact the parasite development. Additionally, ASTEI08011 showed the greatest fold change in the RNA-seq results. Therefore, we hypothesized that these two genes may play roles associated with Plasmodium development. However, injection is an experimental method of infection, and the genetic responses may differ from those during infection by blood-feeding. Therefore, the gene expression analysis was done to assess the genes’ relevance to infection by blood-feeding in addition to injection. The expression levels of these genes in female mosquitoes were compared between those fed infected blood and those fed naïve blood: ASTEI04832 was less strongly expressed in the infected group than in the naïve group 48 hr after blood-feeding, whereas this pattern was reversed 72 hr after blood-feeding (Fig. 3). Moreover, ASTEI08011 was less strongly expressed in the infected group than in the naïve group 48 hr to 96 hr after blood-feeding (Fig. 3). Comparing expression levels in mosquitoes before and after blood-feeding, ASTEI04832 showed a continuous increase in expression from 24 to 72 hr after infected blood-feeding; at 96 hr after infected blood-feeding, levels returned to those similar to before blood-feeding (Fig. 3). ASTEI08011 was expressed at a low level at 24 to 72 hr after infected blood-feeding similar to that before blood-feeding (Fig. 3). However, at 96 hr after infected blood-feeding, its expression level surpassed that of before blood-feeding (Fig. 3).
Fig. 3.
Gene expression in female mosquitoes fed blood from Plasmodium berghei-infected or naïve mice. Real-time PCR was performed on two genes (ASTEI04832 and ASTEI08011) selected from the ortholog analysis and the expression pattern to quantify their expression levels. Anopheles stephensi ribosomal protein S7 gene was used as an internal control. Error bars indicate standard deviations. One-way ANOVA and Tukey’s multiple comparisons test was used for comparison (*P<0.05, ****P<0.0001). BBF: before blood-feeding, ABF: after blood-feeding.
DISCUSSION
Our previous study showed that P. berghei developed in female and male mosquitoes injected with ookinetes [5]. The current study investigated the difference in Plasmodium infection between the sexes. Differences in Plasmodium development were observed between male and female mosquitoes injected with ookinetes. The number of oocysts and prevalence were consistently higher in male than in female mosquitoes. These results indicate that the sex of the mosquitoes affects the transition of ookinetes to oocysts and the development of oocysts. The sexes with the largest oocyst size switched with time post-injection. The reason for this change is unknown, but it may include the following factors. Sporoblast formation occurs in 5- to 10-day oocysts and is followed by the formation of sporozoites [2]. Females may have a greater ability to support oocyst growth during sporoblast formation, while males may support oocyst growth more during sporozoite formation. Oocysts rupture when they reach a diameter of 40–80 µm [2]. In this study, the percentage of oocysts larger than 40 µm in diameter was higher in males than females at 16 dPI. This suggests that male mosquitoes had a greater number of oocysts that were likely to rupture sooner than females. It is possible that the larger oocysts ruptured, eliminating the difference in the mean oocyst diameter between female and male mosquitoes at 16 dPI. Male mosquitoes do not feed on blood, whereas female mosquitoes require blood for egg production. As a result, female mosquitoes may have developed immune responses to Plasmodium parasites and blood meals via evolutionary processes over time. Considering this process, it may be natural for males to be more suitable for oocyst formation and development than females.
Next, to explore the genes exhibiting changes in expression, we performed RNA-seq analysis on female and male mosquitoes at 24 and 72 hPI. This is the first study to examine gene expression in female and male mosquitoes injected with Plasmodium parasites. Previous studies have primarily focused on bypassing midgut transit during infection by injection, and there have been no reports analyzing the molecular changes in the injected mosquitoes [4, 8, 10, 11, 20]. The RNA-seq data obtained from this study will provide valuable information on the interaction between Plasmodium parasites and mosquitoes. We found that more genes exhibited changes in expression within females than within males injected with P. berghei ookinetes and that the expression patterns were different between the sexes. Interestingly, in mosquitoes injected with Plasmodium parasites and those injected with medium, female and male gene expression patterns were generally opposite, except for a slightly reactive pattern in infected females at 24 hPI. We have also clarified that genes known to be involved in the immune response during blood-feeding infection showed only small changes in expression. Expression changes of genes known to be involved in melanization, such as TEP1, were small. This is consistent with previous reports that melanization does not occur in An. gambiae mosquitoes infected by injection [10]. In addition, we have identified five genes with different expression patterns between the sexes and large expression changes. All of these genes had unknown functions, and their expression was altered by Plasmodium parasites in mosquitoes. Furthermore, there were distinct expression patterns between the mosquito sexes. Each of these five genes is considered a potential variable associated with infection.
The genetic response to infection by injection may differ from that to infection by blood-feeding. Finally, we performed a gene expression analysis to evaluate the relevance of genes to infection by blood-feeding. We selected two genes (ASTEI04832 and ASTEI08011) from the five genes and quantified their expression levels in female mosquitoes fed infected blood or naïve blood. We found significant differences in expression levels between the infected group and the naïve group, indicating that these genes may respond to infection even in infection by blood-feeding. The expression level of ASTEI04832 was highest 72 hr after infected blood-feeding and returned to baseline 96 hr after infected blood-feeding. This may affect the formation of early oocysts because it coincides with their completion. However, the effect appears to be temporary as the expression returns to normal thereafter. The expression level of ASTEI08011 remained lower than baseline until 72 hr after infected blood-feeding but was increased 96 hr after infected blood-feeding. This suggests that ASTEI08011 may be involved in oocysts that have completed formation. Alternatively, the maintenance of low expression levels of this gene may be involved in early oocyst development.
The gene expression patterns differed between females infected by blood-feeding and those through injection. In the injection, Plasmodium parasites develop throughout the hemocoel, whereas in blood-feeding, they are restricted to the midgut. The difference in the site of infection may be the cause of the differences in gene expression patterns. The RNA-seq data presented here are preliminary results from a single experiment. Confirming expression levels through repeated experiments and quantitative PCR in the future is necessary. Additionally, further analyses, such as gene knockdown or overexpression, are also needed to elucidate the function of these genes.
In conclusion, this study showed the gene expression in female and male mosquitoes injected with Plasmodium parasites. We identified new candidate genes considered to be associated with Plasmodium infection of female and male mosquitoes through parasite injection. Further functional analysis of these genes is expected to reveal new factors associated with Plasmodium parasite infection in mosquitoes.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
The green fluorescent protein-expressing Plasmodium berghei ANKA strain was provided by Dr. Masao Yuda, Mie University, Japan. Anopheles stephensi (STE2 strain, MRA-128) was provided by BEI Resources. This study would not have been possible without the help of our laboratory support staff member Mai Tanaka. This study was supported by KAKENHI of the Japan Society for the Promotion of Science [grant numbers 17H04073 (to Hiromi Ikadai) and 23KJ1873 (to Asako Haraguchi)]; and Cooperative Research Grants of the National Research Center for Protozoan Diseases of Obihiro University of Agriculture and Veterinary Medicine [grant number 2020 joint-11, 2021 joint-15 and 2022 joint-8 (to Hiromi Ikadai)].
REFERENCES
- 1.Adedeji EO, Ogunlana OO, Fatumo S, Beder T, Ajamma Y, Koenig R, Adebiyi E. 2020. Anopheles metabolic proteins in malaria transmission, prevention and control: a review. Parasit Vectors 13: 465. doi: 10.1186/s13071-020-04342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baton LA, Ranford-Cartwright LC. 2005. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol 21: 573–580. doi: 10.1016/j.pt.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 3.Clayton AM, Dong Y, Dimopoulos G. 2014. The Anopheles innate immune system in the defense against malaria infection. J Innate Immun 6: 169–181. doi: 10.1159/000353602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deligianni E, Silmon de Monerri NC, McMillan PJ, Bertuccini L, Superti F, Manola M, Spanos L, Louis C, Blackman MJ, Tilley L, Siden-Kiamos I. 2018. Essential role of Plasmodium perforin-like protein 4 in ookinete midgut passage. PLoS One 13: e0201651. doi: 10.1371/journal.pone.0201651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haraguchi A, Takano M, Hakozaki J, Nakayama K, Nakamura S, Yoshikawa Y, Fukumoto S, Kusakisako K, Ikadai H. 2023. Formation of free oocysts in Anopheles mosquitoes injected with Plasmodium ookinetes. J Vet Med Sci 85: 921–928. doi: 10.1292/jvms.23-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamis AB, Ibrahim JB. 1989. Effects of testosterone on blood leukocytes in plasmodium berghei-infected mice. Parasitol Res 75: 611–613. doi: 10.1007/BF00930957 [DOI] [PubMed] [Google Scholar]
- 7.Kwon H, Hall DR, Smith RC. 2021. Prostaglandin E2 signaling mediates oenocytoid immune cell function and lysis, limiting bacteria and Plasmodium oocyst survival in Anopheles gambiae. Front Immunol 12: 680020. doi: 10.3389/fimmu.2021.680020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nacer A, Underhill A, Hurd H. 2008. The microneme proteins CTRP and SOAP are not essential for Plasmodium berghei ookinete to oocyst transformation in vitro in a cell free system. Malar J 7: 82. doi: 10.1186/1475-2875-7-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oke CE, Ingham VA, Walling CA, Reece SE. 2022. Vector control: agents of selection on malaria parasites? Trends Parasitol 38: 890–903. doi: 10.1016/j.pt.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Paskewitz SM, Shi L. 2005. Bypassing the midgut results in development of Plasmodium berghei oocysts in a refractory strain of Anopheles gambiae (Diptera: Culicidae). J Med Entomol 42: 712–715. doi: 10.1093/jmedent/42.4.712 [DOI] [PubMed] [Google Scholar]
- 11.Patzewitz EM, Guttery DS, Poulin B, Ramakrishnan C, Ferguson DJ, Wall RJ, Brady D, Holder AA, Szöőr B, Tewari R. 2013. An ancient protein phosphatase, SHLP1, is critical to microneme development in Plasmodium ookinetes and parasite transmission. Cell Rep 3: 622–629. doi: 10.1016/j.celrep.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock L, Ferris V, Bailey M, Gibson W. 2012. The influence of sex and fly species on the development of trypanosomes in tsetse flies. PLoS Negl Trop Dis 6: e1515. doi: 10.1371/journal.pntd.0001515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds RA, Kwon H, Smith RC. 2020. 20-Hydroxyecdysone primes innate immune responses that limit bacterial and malarial parasite survival in Anopheles gambiae. MSphere 5: e00983–e19. doi: 10.1128/mSphere.00983-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki M, Fujii Y, Iwamoto M, Ikadai H. 2013. Effect of sex steroids on Babesia microti infection in mice. Am J Trop Med Hyg 88: 367–375. doi: 10.4269/ajtmh.2012.12-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim S, Ramirez JL, Dimopoulos G. 2012. Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog 8: e1002631. doi: 10.1371/journal.ppat.1002631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh M, Suryanshu K, Kanika, Singh G, Dubey A, Chaitanya RK. 2021. Plasmodium’s journey through the Anopheles mosquito: A comprehensive review. Biochimie 181: 176–190. doi: 10.1016/j.biochi.2020.12.009 [DOI] [PubMed] [Google Scholar]
- 17.Wang M, An Y, Gao L, Dong S, Zhou X, Feng Y, Wang P, Dimopoulos G, Tang H, Wang J. 2021. Glucose-mediated proliferation of a gut commensal bacterium promotes Plasmodium infection by increasing mosquito midgut pH. Cell Rep 35: 108992. doi: 10.1016/j.celrep.2021.108992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Wang J. 2020. Glucose transporter GLUT1 influences Plasmodium berghei infection in Anopheles stephensi. Parasit Vectors 13: 285. doi: 10.1186/s13071-020-04155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. 2022. World Malar Rep 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 [accessed on April 24, 2023].
- 20.Yang Z, Shi Y, Cui H, Yang S, Gao H, Yuan J. 2021. A malaria parasite phospholipid flippase safeguards midgut traversal of ookinetes for mosquito transmission. Sci Adv 7: eabf6015. doi: 10.1126/sciadv.abf6015 [DOI] [PMC free article] [PubMed] [Google Scholar]