Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin released into the gastrointestinal tract after food ingestion, and stimulates insulin secretion from the beta cells of the pancreatic islets. Incretins have recently been reported to have extrapancreatic actions, and they are anticipated to have potential efficacy for conditions such as male infertility as well as diabetes. However, the effects of incretins on male reproductive function remain unclear. In this study, GLP-1 receptor expression and the effects of GLP-1 on spermatogenesis-associated genes were investigated using mouse testes and testis-derived cultured cell lines. Glp1r mRNA and GLP-1 protein were expressed in mouse testes at levels comparable to or greater than those in positive control adipose tissue, and the liver and intestine, and also in a Sertoli cell line (TM4) and a Leydig cell line (MA-10) as well as the GC-1 spg and GC-2 spd (ts) germ cell lines. TM4 cells treated with the GLP-1 receptor agonist exenatide showed transiently and significantly upregulated Kitl, Pdgfa, and Glp1r mRNA expression. Furthermore, at 1 hr post-exenatide administration to male mice, Kitl and Glp1r mRNA expression levels were significantly increased, and Pdgfa mRNA expression level also showed a tendency toward increase. TM4 cells were treated with various cell-activating agents, and bucladesine elicited significantly increased Glp1r mRNA expression. We suggest that GLP-1 provides acute stimulation of Sertoli cells in the mouse testis and has a stimulatory effect on the expression of spermatogenesis-related genes.
Keywords: bucladesine, exenatide, Glucagon-like peptide-1 receptor, Sertoli cell, spermatogenesis
Obesity is a metabolic disorder that is increasing in many countries around the world, and is the largest risk factor for type 2 diabetes. It can aggravate diabetes-related conditions including cardiovascular disease, nephropathy, and neuropathy, which can be fatal. Obesity and diabetes are linked to a range of other complications, including male infertility [32]. Obesity reportedly leads to reduced numbers of spermatozoa and impaired spermatic function in humans and laboratory animals (specifically, rats) [11, 18, 36]. Diabetes causes lesions such as reduced testicular weight, reduced seminiferous tubule diameter, and degeneration and vacuolation of spermatogonia, spermatocytes, and spermatids. Reduced serum levels of testosterone, luteinizing hormone, and follicle-stimulating hormone are also observed in diabetes [32]. Obesity and diabetes mellitus may also be risk factors for poor outcomes with artificial insemination and spontaneous conception, considering that male obesity is reportedly associated with reduced pregnancy rates with in vitro fertilization in humans [28]. Obesity and diabetes are thus suggested to be significant risk factors for reproductive disorders in animals. Obesity- and diabetes-related infertility and reproductive dysfunction represent a critical problem in terms of decreased quality of life and economic losses from decreased litter size; however, the molecular basis of this phenomenon is not well understood.
Incretins are hormones released into the gastrointestinal tract after food intake in response to stimulation by nutrients, including sugars and lipids, and promote insulin secretion by acting on the beta cells of the pancreas. Incretins include glucose-dependent insulinotropic polypeptide (GIP), produced by K cells in the upper small intestine, and glucagon-like peptide-1 (GLP-1), produced by L cells in the lower small intestine and colon [31]. The insulinotropic action of these incretins is triggered when they bind to G protein-coupled receptors on the plasma membrane of pancreatic beta cells. This ligand binding results in activation of G-protein-coupled adenylate cyclase, which increases intracellular cAMP levels and stimulates insulin secretion via the protein kinase A (PKA) pathway [30]. Incretins have also been reported to promote pancreatic β-cell proliferation through various pathways, including the protein kinase C (PKC) pathway [4, 9]. Recently, incretin-based pharmaceutical agents have been used to treat obesity and type 2 diabetes [24, 33].
Incretin receptors are expressed in a range of extrapancreatic tissues in vivo [24, 33, 41], and reportedly trigger diverse effects when activated by a ligand. For example, activation of the GLP-1 receptor (after binding its incretin ligand or an agonist) has been shown to suppress food intake through an action on the central nervous system [23], to exert protective effects on the myocardium and kidneys [23, 34, 40], to promote lipolysis and inhibit lipogenesis in the liver [38], and to promote bone formation and inhibit bone resorption [17]. According to one recent report, administration of GLP-1 receptor agonists to obese mice suppressed testicular inflammation and improved sperm characteristics [42]. However, the mechanism by which incretins—molecules closely associated with obesity and diabetes—exert an effect on reproductive function has not been well characterized.
Accordingly, in the present study, we investigated the role of incretins in male reproductive function, with the aim of elucidating part of the molecular basis of infertility and reproductive dysfunction associated with obesity and diabetes. In particular, the effects of GLP-1 receptor agonists were investigated by altering gene expression in mouse testis and testis-derived lineage cells. Furthermore, mouse testis-derived cells were used to ascertain regulators of GLP-1 receptor expression.
MATERIALS AND METHODS
Animal experiments
Inbred mice (C57BL/6J, male, 8 weeks old, Japan SLC Inc., Shizuoka, Japan) were housed in plastic cages at the Experimental Animal Center of Kagoshima University (temperature: 23 ± 3°C; humidity: 50 ± 20%; light/dark cycle: 12 hr [7:00–19:00]). They were fed a commercial chow (CE-2; CLEA Japan, Inc., Tokyo, Japan) and allowed to drink water ad libitum.
Exenatide (Toronto Research Chemicals, Toronto, Canada) was used as the GLP-1 receptor agonist in the relevant experiments in this study. Exenatide was dissolved in a dimethyl sulfoxide (DMSO; Nacalai Tesque Inc., Kyoto, Japan) solution, to prepare 1 mM, and the resultant solution was stored at −80°C until use. Immediately before use, the exenatide solution was diluted to 1 µM with saline (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan). Male mice were fasted for 18 hr (6:00 pm–12:00 noon) and then assigned to the control (n=3) or exenatide-treated (n=3) group. Each mouse in the exenatide-treated group received an intraperitoneal dose of the diluted solution (2.5 nmol/kg). The dose was set based on a previous report on exendin administration to mice [8]. Each mouse in the control group received 1,000-fold diluted DMSO saline solution intraperitoneally. Each mouse was euthanized by cervical dislocation at 1 hr post-administration, and its testes were collected. The collected testes were stored at −80°C until evaluation.
This study was approved by the Animal Care and Use Committee of Kagoshima University (Protocol Approval Numbers VM180006, VM180064, VM19053, and VM22043). The program for the care and use of laboratory animals at the Experimental Animal Center has been accredited by AAALAC International.
Cultured cell lines and culture conditions
The following mouse-derived cell lines were used in this study: a spermatogonial cell line (GC-1 spg: CRL-2053), a spermatocyte cell line {GC-2 spd (ts): CRL-2196}, a Sertoli cell line (TM4: CRL-1715), and a Leydig cell line (MA-10: CRL-3050). Each cell line was each obtained from the American Type Culture Collection (Manassas, VA, USA).
GC-1 spg and GC-2 spd (ts) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific K.K, Tokyo, Japan). TM4 cells were cultured in a 1:1 mixture of Ham’s F12 medium (FUJIFILM Wako) supplemented with 5% horse serum (Thermo Fisher) and 2.5% FBS. MA-10 cells were cultured in DMEM supplemented with L-glutamine, phenol red, HEPES, and sodium pyruvate (DMEM/Ham’s F-12) medium (FUJIFILM Wako) supplemented with 15% horse serum. All media used for cell culture were supplemented with 100 units/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B (Nacalai). All cell lines were cultured at 37°C in 5% CO2. Plates and dishes used for culture of MA-10 cells were precoated with 0.1% gelatin (Nacalai) on the inner surface.
Bucladesine (dibutyryl cAMP; Cayman, Ann Arbor, MI, USA), phorbol 12-myristate 13-acetate (PMA, protein kinase C activator; LC Laboratories, Woburn, MA, USA), WY-14643 (PPARα agonist; Cayman), and all-trans-retinoic acid (Vitamin A acid; FUJIFILM Wako) were dissolved in DMSO. The relevant drug solution was applied to the cell culture medium at a rate of 10 µL/mL. The final concentrations of the drug solutions were 1 mM for bucladesine, 100 nM for PMA, 10 µM for WY-14643, and 100 nM for all-trans-retinoic acid. The drug-solution doses were set based on the reports from previous in vitro studies in male mouse reproductive cells [3, 14, 22, 26]. DMSO was applied at 10 µL/mL, as a vehicle control.
Exenatide was dissolved in DMSO. The solution was added to a medium containing TM4 cells at a rate of 1 µL/mL and a final concentration of 10 nM [10]. The mRNA expression levels of Kitl, Pdgfa, and Glp1r were quantified after at 1, 12, and 24-hr treatment with real-time RT-PCR, and evaluated by comparison with levels noted for vehicle control treatment, which involved supplementation of the media with 1 µL/mL DMSO.
Total RNA extraction and reverse transcription reaction
Mouse tissues and mouse-derived cultured cells were homogenized using TriPure total RNA isolation reagent (Roche Diagnostics K.K, Tokyo, Japan). Total RNA extraction was performed in accordance with the manufacturer’s protocol. Reverse transcription reactions were performed using 2 µg total RNA, oligo dT primer, oligo d (N) primer, and reverse transcriptase (ReverTra Ace; TOYOBO Inc., Osaka, Japan). Reverse transcription products were stored at −80°C until use.
Quantification of mRNA levels in each tissue or cell line
Quantitative real-time RT-PCR was performed using KAPA SYBR FAST Master Mix (x 2) (Kapa Biosystems Inc., Wilmington, MA, USA), gene-specific primers, and the StepOne real-time PCR system (Thermo Fisher) or the CFX Connect real-time PCR system (Bio-Rad Laboratories, Inc., Tokyo, Japan). Relative comparative quantification with standard curves was used to quantify gene expression level. Dilutions of cDNA from normal mouse testes were used to generate the standard curves. Primers used for real-time RT-PCR are listed in Table 1.
Table 1. Lists of the primers used in the quantitative real-time PCR.
| Gene | Forward/Reverse | Primer sequence |
|---|---|---|
| Gapdh | Forward | TCCTGCACCACCAACTGCTT |
| Reverse | GTCTTCTGGGTGGCAGTGAT | |
| Glp1r | Forward | GTCATCGCTTCAGCCATCCT |
| Reverse | TACATCCACTTGAGGGCAGC | |
| Kitl | Forward | ATCTGCGGGAATCCTGTGAC |
| Reverse | CATCCCGGCGACATAGTTGA | |
| Pdgfa | Forward | TGGGTCCCATGCCATTAACC |
| Reverse | AATGACCGTCCTGGTCTTGC |
Immunoblotting
Proteins from mouse tissues and mouse-derived cultured cells were solubilized with RIPA buffer (Nacalai), and separated by SDS-PAGE on 10% polyacrylamide gels. The separated proteins were transferred from the gel to a PVDF membrane (FUJIFILM Wako). Rabbit anti-human GLP-1 receptor polyclonal antibody (26196-1-AP; Proteintech Group, Inc., Rosemont, IL, USA) and mouse anti-GAPDH monoclonal antibody (5A12; FUJIFILM Wako) were used as primary antibodies. Goat peroxidase-conjugated anti-mouse IgG (H+L) antibody {5220-0341; Seracare Life Sciences Inc., Milford, MA, USA} and goat peroxidase-conjugated anti-rabbit IgG (H+L) antibody (5220-0458; Seracare) were used as secondary antibodies. Immobilon Forte Western HRP substrate (Merck KGaA, Darmstadt, Germany) and LAS-1000 (FUJIFILM Wako) were used for secondary antibody detection. ImageJ software [29] was used for quantitative analysis of the detected bands.
Statistical analysis
All data are expressed as mean ± standard error. GraphPad Prism 6 software (GraphPad Software, Boston, MA, USA) was used to test for statistical significance. One-way analysis of variance was used to test for differences between three or more groups, and multiple comparisons were made using Tukey’s post-hoc method. Unpaired t-test with equal SD was used to test for differences between two groups. P values below 0.05 were considered statistically significant.
RESULTS
Evaluation of GLP-1 receptor expression in mouse tissues and cultured cell lines derived from mouse testis
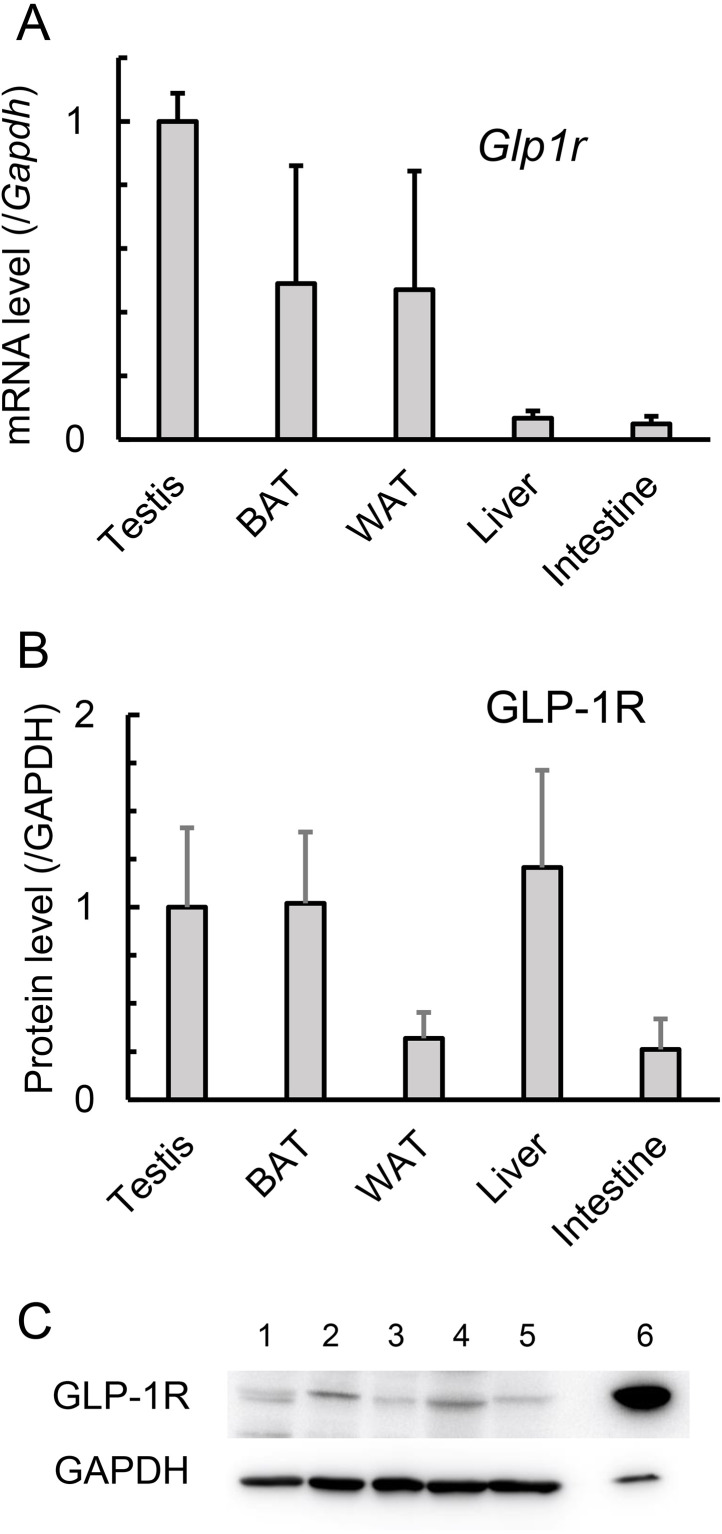
To compare the expression of GLP-1 receptors between mouse testicular and non-testicular tissues, we determined relevant tissue Glp1r mRNA expression levels in a real-time RT-PCR assay. The assay revealed that testicular Glp1r mRNA expression was equal to or greater than that in brown adipose tissue, white adipose tissue, the small intestine, and the liver, tissues for which GLP-1 receptor expression has been previously reported (Fig. 1A). Specifically, the testicular expression level was approximately 20-fold higher than those in the small intestine and liver. For a further comparison, the expression levels of the GLP-1 receptor (GLP-1R) protein were compared between the same tissues using immunoblot analysis, which revealed that GLP-1R protein expression level in the testis was comparable to those in brown adipose tissue and liver, and 3.1 to 3.8-fold higher than those in white adipose tissue and small intestine (Fig. 1B).
Fig. 1.
Expression of Glucagon-like peptide-1 receptor (GLP-1) receptor (GLP-1R) in the testis of mice. A. Glp1r mRNA expression was determined using quantitative RT-PCR. Glp1r mRNA levels were normalized to Gapdh mRNA levels and are expressed relative to that of the testis. Values are mean ± SEM of three samples. B. GLP-1R protein expression was determined using immunoblotting. GLP-1R protein levels were normalized to the GAPDH protein level and are expressed relative to that of the testis. Values are mean ± SEM of three samples. C. Immunoblotting of GLP-1R and GAPDH proteins. 1: Testis, 2: Brown adipose tissue (BAT), 3: White adipose tissue (WAT), 4: Liver, 5: Intestine, 6: Pancreas.
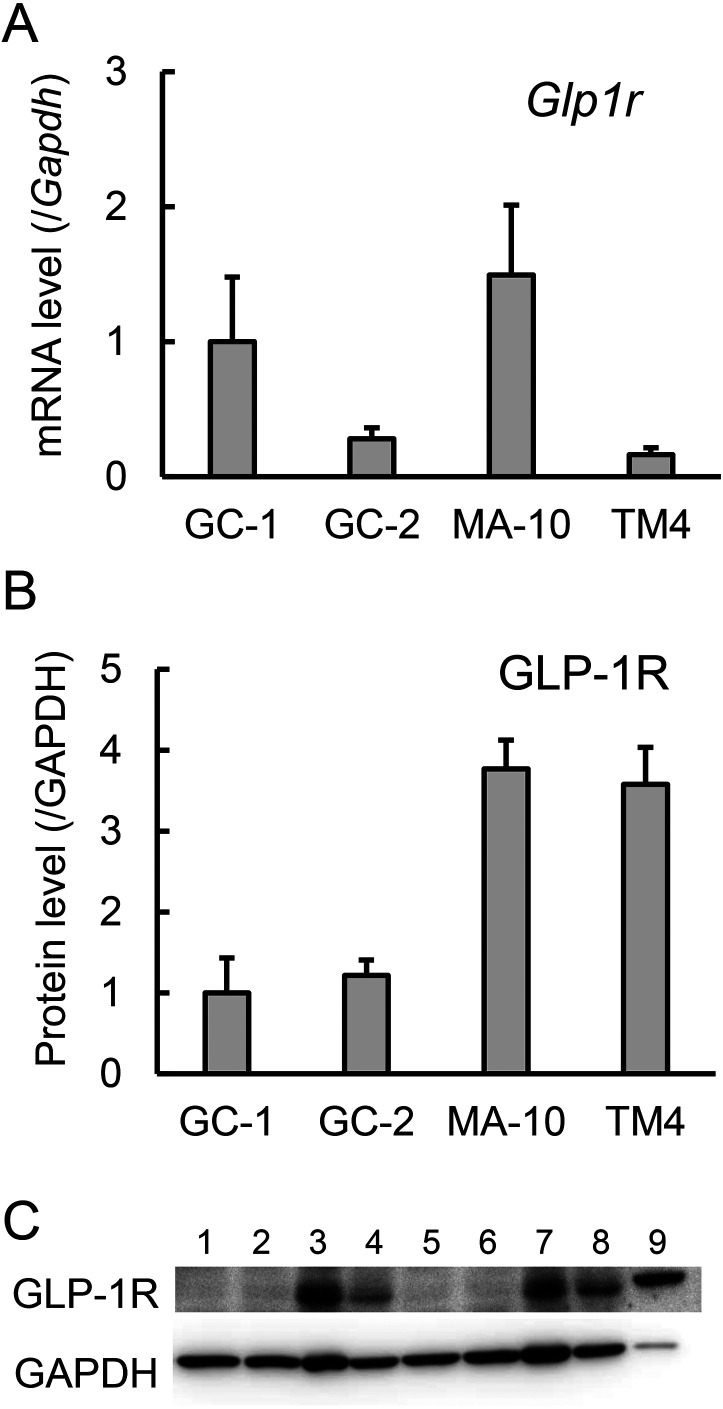
To clarify the distribution of GLP-1 receptors in the testis and to select cell lines for an investigation of GLP-1 action at the cellular level, we then quantified Glp1r mRNA expression in multiple cultured mouse testis cell lines. This expression was highest in the MA-10 Leydig cell line, with the GC-1 spg spermatogonial cell line showing a similar level, but relatively low in the GC-2 spd (ts) spermatocyte cell line (mean value: 27%), and the TM4 Sertoli cell line (mean value: 16%) (Fig. 2A). Further immunoblot analysis for the GLP-1 receptor protein revealed lower expression levels in the GC-1 spg and GC-2 spd (ts) cell lines than in the other cell lines, and an approximately 3-fold higher level in TM4 cells—relative to GC-1 spg and GC-2 spd (ts) cells—that was comparable to the level in MA-10 cells (Fig. 2B, 2C).
Fig. 2.
Glucagon-like peptide-1 receptor (GLP-1) receptor (GLP-1R) expression in the mouse testis-derived cell lines. A. Glp1r mRNA expression was determined using quantitative RT-PCR. Glp1r mRNA levels were normalized to Gapdh mRNA levels and are expressed relative to that of GC-1 spg cells. Values are mean ± SEM of three samples. B. GLP-1R protein expression was determined using immunoblotting. GLP-1R protein levels were normalized to GAPDH protein levels and are expressed relative to that of GC-1 spg cells. Values are mean ± SEM of three samples. C. Immunoblotting of GLP-1R and GAPDH proteins. 1, 5: GC-1 spg cells, 2, 6: GC-2 spd (ts) cells, 3, 7: MA-10 cells, 4, 8: TM4 cells, 9: Pancreas.
Effects of GLP-1 receptor stimulation in mouse TM4 cells
Based on the relatively high GLP-1 receptor protein expression level in TM4 cells described above, we selected the TM4 cell line as the test system for an in vitro investigation into the effects of GLP-1 at the cellular level in testicular tissue.
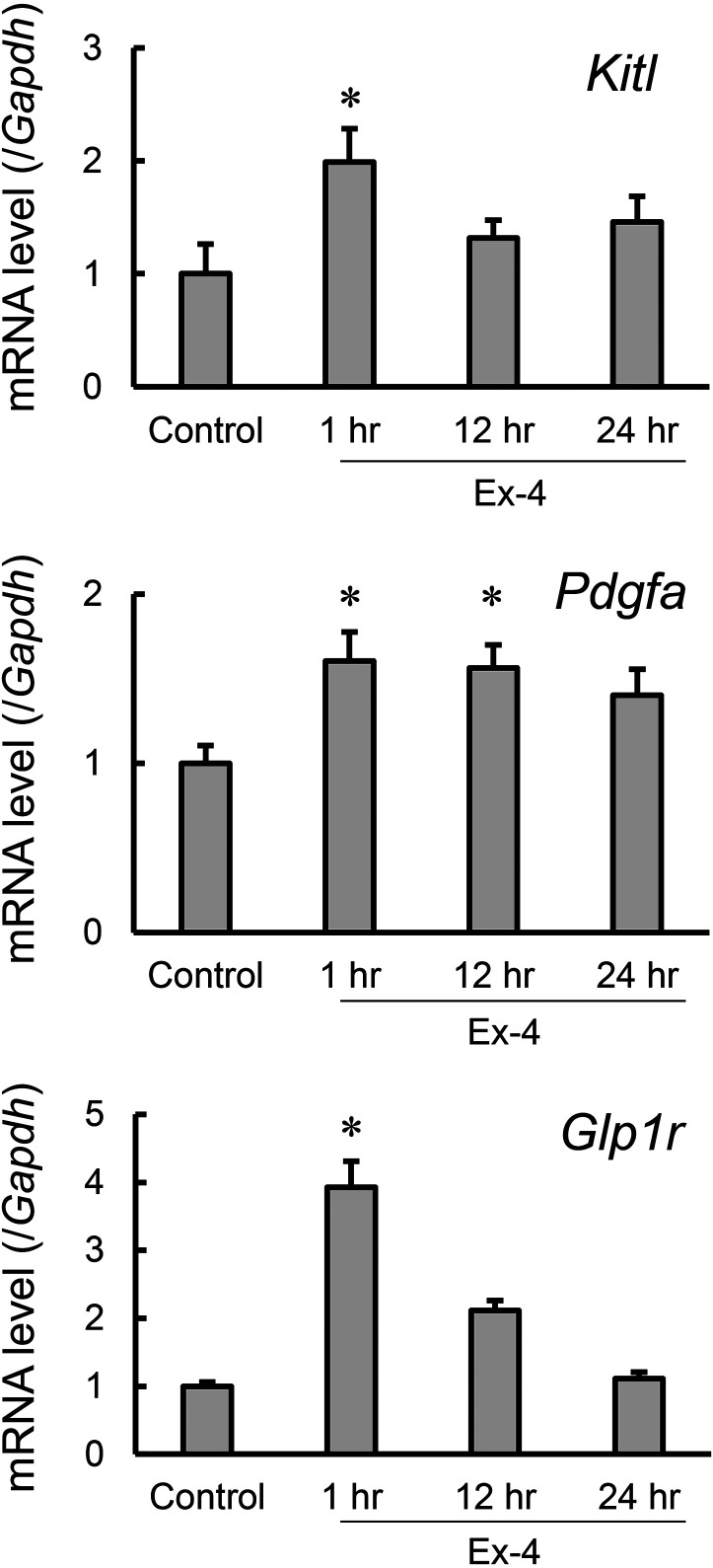
Kitl and Pdgfa encode Kit receptor ligands and platelet-derived growth factor A (PDGF-A), respectively. Kit receptor ligands and PDGF-A are each produced by Sertoli cells and are known to be involved in testicular function [1, 2, 6, 13]. Therefore, to investigate whether GLP-1 receptor activation can modulate the production of the above-stated cytokines by Sertoli cells, we applied exenatide, a stable GLP-1 receptor agonist, to TM4 cells, and compared the expression levels of Kitl, Pdgfa and Glp1r relative to those noted with controls at 1, 12 and 24 hr (Fig. 3). Kitl, Pdgfa and Glp1r mRNA expression levels were all significantly increased in exenatide-treated cells; Kitl and Glp1r showed transient, approximately two-fold and four-fold increases, respectively, after 1-hr treatment, relative to the vehicle control level. In contrast, Pdgfa showed an approximately 1.6-fold increase after 1-hr treatment, relative to the vehicle control level and maintained similar expression levels to the vehicle control level after 12-hr treatments.
Fig. 3.
The effect of exenatide on the expression of genes associated with the spermatogenic function of Sertoli cells in TM4 cells. TM4 cells were treated with exenatide (Ex-4) for 1 to 24 hr. mRNA expression of Kitl, Pdgfa, and Glp1r was determined using quantitative RT-PCR. mRNA levels of each gene were normalized to the Gapdh mRNA level and are expressed relative to that of vehicle control cells (Control). Values are mean ± SEM of six samples. *P<0.05 vs. control by ANOVA with Tukey’s post hoc test.
Effects of GLP-1 receptor agonists on gene expression in mouse testis
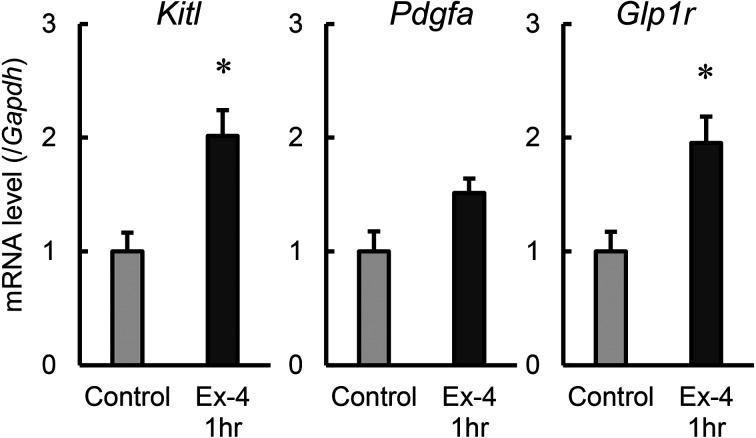
Mouse TM4 cells had shown increased expression levels for spermatogenesis-related genes known to be expressed in Sertoli cells, after the treatment with exenatide in vitro; therefore, we then administered exenatide intraperitoneally to healthy male mice and determined testicular gene expression at 1 hr post-administration (Fig. 4). Exenatide treatment resulted in a significant, two-fold increase in Kitl and Glp1r mRNA expression versus the control. In contrast, Pdgfa mRNA expression showed a tendency toward increase with exenatide treatment, but the difference versus the control was not significant. These results suggest that GLP-1 agonists have a stimulatory effect on mouse testes and transiently increase the expression of spermatogenesis-related genes.
Fig. 4.
The effect of exenatide on the expression of genes associated with spermatogenic function of Sertoli cells in mouse testis. Mice were treated with exenatide (Ex-4) for 1 hr. mRNA expression of Kitl, Pdgfa, and Glp1r was determined using quantitative RT-PCR. mRNA levels of each gene were normalized to Gapdh mRNA level and are expressed relative to that of vehicle control cells (control). Values are mean ± SEM of three samples. *P<0.05 vs. control by unpaired t-test with equal SD.
Variation in GLP-1 receptor mRNA expression levels in TM4 cells
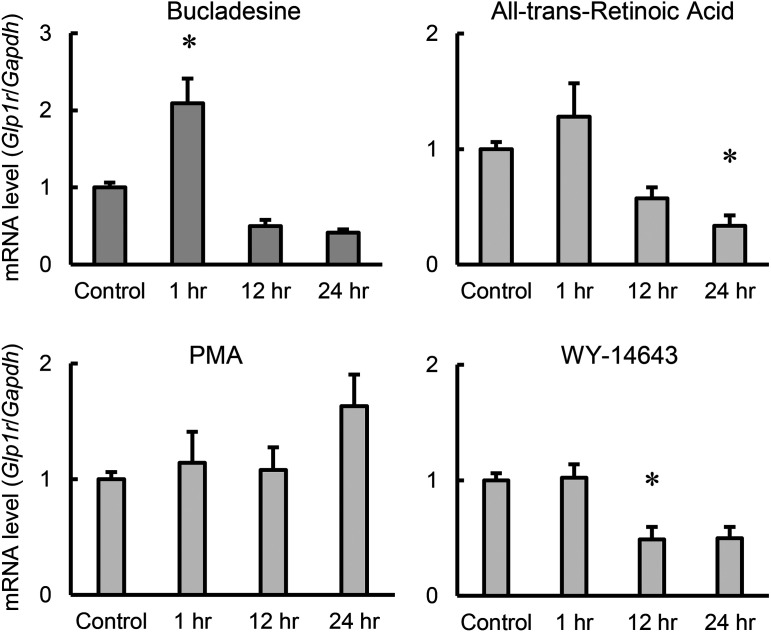
To elucidate the role of GLP-1 in the regulatory mechanism for spermatogenesis, we investigated modulators of GLP-1 receptor expression, using four cell-activating drugs, bucladesine, PMA, WY-14643, and all-trans-retinoic acid. TM4 cells were treated with each stimulant for 1, 12 and 24 hr, and Glp1r mRNA expression was then quantified and compared with that measured for vehicle control treatment (Fig. 5). Glp1r expression was upregulated approximately two-fold after 1-hr treatment with bucladesine, but significantly downregulated (decrease of approximately 50%) by 12-hr treatment with WY-14643. In addition, 24-hr all-trans-retinoic acid treatment resulted in significantly downregulated expression (decrease of approximately 50%).
Fig. 5.
The effect of stimulating drugs on the expression of genes associated with spermatogenic function of Sertoli cells in TM4 cells. TM4 cells were treated with bucladesine, all-trans-retinoic acid, phorbol 12-myristate 13-acetate (PMA) or WY-14643 for 1 to 24 hr. mRNA expression of Glp1r was determined using quantitative RT-PCR. mRNA levels of each gene were normalized to the Gapdh mRNA level and are expressed relative to that of vehicle control cells (Control). Values are mean ± SEM of six samples. *P<0.05 vs. control by ANOVA with Tukey’s post hoc test.
DISCUSSION
In the present study, the first step involved determining the relative mRNA expression levels for genes encoding GLP-1 receptors, in mouse testicular tissues with real-time RT-PCR. We found that testicular Glp1r mRNA was expressed at comparable levels in the testes, and white and brown adipose tissues (Fig. 1A). Immunoblot analysis for the GLP-1 receptor protein then revealed similar levels of expression in the testis and other tissues (Fig. 1B). In adipose tissue, GLP-1 receptor stimulation has been reported to induce lipolysis and brown remodeling [35, 37, 39]. Furthermore, GLP-1 is involved in enhancing lipolysis and inhibiting lipogenesis in the liver [38]. Taken together with these reports, our results indicate that GLP-1 receptors are expressed in the testes, as well as in adipose and other tissues, and suggest that GLP-1 receptor ligands have an effect on testicular function.
Analysis of Glp1r mRNA (Fig. 2A) and GLP-1 receptor protein (Fig. 2B, 2C) expression in cultured cell lines suggest that the GLP-1 receptor is relatively highly expressed in Leydig cells, and is clearly present in germ cells and Sertoli cells, in mice. According to recent reports on the human testis, both Glp1r mRNA and GLP-1 receptor protein are mainly expressed in Leydig cells [5]. GLP-1 reportedly stimulates differentiation of stem Leydig cells, but does not affect their proliferation, in rats [15]. It is thus plausible that GLP-1 can affect the function of Leydig cells in mice. On the other hand, in mouse testes, GLP-1 receptor protein expression is reportedly localized to Sertoli cells [42]. Moreover, human Sertoli cells also reportedly express GLP-1 receptors [19]. GLP-1 has the potential to induce conversion of glucose to lactate, mTOR phosphorylation, and attenuation of mitochondrial membrane potential and oxidative damage in human Sertoli cells [19]. However, there have been few reports on the effects of GLP-1 on spermatogenesis, particularly on the regulation of spermatogenesis via Sertoli cells. Therefore, in the present study, we focused on the effect of GLP-1 on the supportive function of Sertoli cells for spermatogenesis. Somewhat surprisingly, we observed no positive correlation between mRNA and protein expression levels in TM4 cells and the liver, and it is not possible to offer an explanation for these findings currently. Further detailed analyses of the distribution of GLP-1 receptors in the testis using histological or cytological techniques are required.
Considering the previous reports and our findings on cell lines in the first step in this study, we then investigated the effect of a GLP-1 agonist (exenatide) on the spermatogenic function of Sertoli cells using TM4 cells (Fig. 3). Exenatide treatment elicited a significant, transient increase in Kitl, Pdgfa and Glp1r expression versus the vehicle control. Furthermore, administration of exenatide to male mice resulted in significantly upregulated Kitl and Glp1r expression and a tendency toward upregulated Pdgfa expression, results which were similar to our in vitro findings in TM4 cells. Kit ligand is a membrane-bound cytokine that functions as a ligand for the KIT receptor, a tyrosine kinase-type receptor, and is produced by Sertoli cells. Kit ligand are reported to be involved in spermatogonial cell differentiation and proliferation, and in differentiation and steroidogenesis of Leydig cells [6, 13]. PDGF-A is another cytokine expressed in the mouse testis and is produced mainly in Sertoli cells, whereas its receptor, PDGFR-α, is expressed on Leydig cells [1]. PDGF-A-deficient adult mice show loss of Leydig cells, progressively reduced testicular size, and arrested spermatogenesis, indicating that PDGF-A is involved in the differentiation and proliferation of Leydig cells and their steroidogenic functions [1, 2]. Sertoli cells are known to promote self-renewal, proliferation, and differentiation of male germ cells in the seminiferous tubules [7, 27]. The present results suggest that GLP-1 plays a role in promoting spermatogenesis by stimulating the proliferation and differentiation of spermatogonia and Leydig cells, and steroidogenesis in Leydig cells in vivo by acting on Sertoli cells to stimulate the production of Kit ligand and PDGF-A. Although the blood-testis barrier is formed in the seminiferous tubules [16], it has not been established whether this barrier is permeable to incretins and incretin receptor agonists. This has implications for any consideration of the pathways by which endogenous GLP-1 and exogenous GLP-1 receptor agonists may exert an effect on male germ cells. The consideration of potential pathways should encompass both direct pathways to cells in the seminiferous tubules and pathways by which indirect effects are exerted on testicular tissue before the blood-testis barrier. In contrast, the follicle-stimulating hormone (FSH), a peptide hormone, acts as a survival factor for spermatogonia by activating surface receptors located exclusively on Sertoli cells in the adult phase [21, 25]. The reported accessibility of FSH to Sertoli cells underlines that possibility that other peptide hormones such as GLP-1 can reach Sertoli cells in the same way as FSH.
Finally, stimulation experiments with four different drugs were performed on TM4 cells to explore modulators of incretin receptor expression. The results showed that Glp1r mRNA expression levels were significantly increased by bucladesine (Fig. 5), which is consistent with the results showing upregulation following GLP-1 agonist stimulation (Figs. 3 and 4). Long-term exenatide treatment reportedly elicits a tendency to increased GLP-1 receptor protein expression in the testis [42]. The findings in the present study suggest that increased Glp1r mRNA expression also occurs as an acute effect of exenatide. Taking our findings together with previous reports, we suggest that there is a positive feedback mechanism for GLP-1 signaling involving induction of GLP-1 receptor expression, in the testis. Glp1r mRNA expression was significantly reduced by all-trans-retinoic acid and WY-14643 treatment, suggesting it is suppressed by activation of RAR and PPARα. RAR has previously been reported to suppress the expression of matrix metalloproteinase-2 (MMP-2), an enzyme that degrades denatured collagen in dental pulp cells [12]. PPARα suppresses the expression of thrombin activatable fibrinolysis inhibitor (TAFI), a factor that promotes thrombus formation in the liver [20]. Similar mechanisms may be responsible for the suppression of Glp1r expression.
The present study revealed that GLP-1 receptor expression in the testis is comparable to that in other GLP-1 receptor-positive tissues, suggesting that GLP-1 agonists and GLP-1 receptor modulators are involved in stimulation of Sertoli cells, leading to proliferation and differentiation of spermatogonia and Leydig cells. Further detailed studies are needed to determine the effects and mechanisms of action of GLP-1 agonists on testicular function in males with impaired reproductive function due to obesity and diabetes mellitus.
CONFLICT OF INTEREST
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 18K06033.
REFERENCES
- 1.Basciani S, Mariani S, Spera G, Gnessi L. 2010. Role of platelet-derived growth factors in the testis. Endocr Rev 31: 916–939. doi: 10.1210/er.2010-0004 [DOI] [PubMed] [Google Scholar]
- 2.Bergeron F, Bagu ET, Tremblay JJ. 2011. Transcription of platelet-derived growth factor receptor α in Leydig cells involves specificity protein 1 and 3. J Mol Endocrinol 46: 125–138. doi: 10.1530/JME-10-0145 [DOI] [PubMed] [Google Scholar]
- 3.Braun KW, Tribley WA, Griswold MD, Kim KH. 2000. Follicle-stimulating hormone inhibits all-trans-retinoic acid-induced retinoic acid receptor alpha nuclear localization and transcriptional activation in mouse Sertoli cell lines. J Biol Chem 275: 4145–4151. doi: 10.1074/jbc.275.6.4145 [DOI] [PubMed] [Google Scholar]
- 4.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. 2001. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 50: 2237–2243. doi: 10.2337/diabetes.50.10.2237 [DOI] [PubMed] [Google Scholar]
- 5.Caltabiano R, Condorelli D, Panza S, Boitani C, Musso N, Ježek D, Memeo L, Colarossi L, Rago V, Mularoni V, Spadola S, Castiglione R, Santoro M, Aquila S, D’Agata R. 2020. Glucagon-like peptide-1 receptor is expressed in human and rodent testis. Andrology 8: 1935–1945. doi: 10.1111/andr.12871 [DOI] [PubMed] [Google Scholar]
- 6.Chapman KM, Medrano GA, Chaudhary J, Hamra FK. 2015. NRG1 and KITL signal downstream of retinoic acid in the germline to support soma-free syncytial growth of differentiating spermatogonia. Cell Death Discov 1: 15018. doi: 10.1038/cddiscovery.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SR, Liu YX. 2015. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 149: R159–R167. doi: 10.1530/REP-14-0481 [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. 2006. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173–181. doi: 10.1002/hep.21006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, Nielsen JH, Møldrup A. 2006. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol 188: 481–492. doi: 10.1677/joe.1.06160 [DOI] [PubMed] [Google Scholar]
- 10.Iwaya C, Nomiyama T, Komatsu S, Kawanami T, Tsutsumi Y, Hamaguchi Y, Horikawa T, Yoshinaga Y, Yamashita S, Tanaka T, Terawaki Y, Tanabe M, Nabeshima K, Iwasaki A, Yanase T. 2017. Exendin-4, a Glucagonlike Peptide-1 Receptor Agonist, Attenuates Breast Cancer Growth by Inhibiting NF-κB Activation. Endocrinology 158: 4218–4232. doi: 10.1210/en.2017-00461 [DOI] [PubMed] [Google Scholar]
- 11.Katib A. 2015. Mechanisms linking obesity to male infertility. Cent European J Urol 68: 79–85. doi: 10.5173/ceju.2015.01.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JM, Kang SW, Shin SM, Su Kim D, Choi KK, Kim EC, Kim SY. 2014. Inhibition of matrix metalloproteinases expression in human dental pulp cells by all-trans retinoic acid. Int J Oral Sci 6: 150–153. doi: 10.1038/ijos.2013.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P. 2000. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J 19: 1312–1326. doi: 10.1093/emboj/19.6.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Wong CKC. 2008. Effects of dexamethasone and dibutyryl cAMP on stanniocalcin-1 mRNA expression in rat primary Sertoli and Leydig cells. Mol Cell Endocrinol 283: 96–103. doi: 10.1016/j.mce.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Chen L, Wang Y, Li H, Zhu Q, Ge RS. 2022. Glucagon-like peptide-1 promotes Leydig cell regeneration from stem cells in rats. Reproduction 165: 19–30. doi: 10.1530/REP-22-0136 [DOI] [PubMed] [Google Scholar]
- 16.Luaces JP, Toro-Urrego N, Otero-Losada M, Capani F. 2023. What do we know about blood-testis barrier? current understanding of its structure and physiology. Front Cell Dev Biol 11: 1114769. doi: 10.3389/fcell.2023.1114769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y, Hu J, He G, Luo X. 2013. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res 28: 1641–1652. doi: 10.1002/jbmr.1898 [DOI] [PubMed] [Google Scholar]
- 18.Mah PM, Wittert GA. 2010. Obesity and testicular function. Mol Cell Endocrinol 316: 180–186. doi: 10.1016/j.mce.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 19.Martins AD, Monteiro MP, Silva BM, Barros A, Sousa M, Carvalho RA, Oliveira PF, Alves MG. 2019. Metabolic dynamics of human Sertoli cells are differentially modulated by physiological and pharmacological concentrations of GLP-1. Toxicol Appl Pharmacol 362: 1–8. doi: 10.1016/j.taap.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 20.Masuda Y, Saotome D, Takada K, Sugimoto K, Sasaki T, Ishii H. 2012. Peroxisome proliferator-activated receptor-alpha agonists repress expression of thrombin-activatable fibrinolysis inhibitor by decreasing transcript stability. Thromb Haemost 108: 74–85. doi: 10.1160/TH12-02-0101 [DOI] [PubMed] [Google Scholar]
- 21.Meachem SJ, Mclachlan RI, Stanton PG, Robertson DM, Wreford NG. 1999. FSH immunoneutralization acutely impairs spermatogonial development in normal adult rats. J Androl 20: 756–762, discussion 755. doi: 10.1002/j.1939-4640.1999.tb03382.x [DOI] [PubMed] [Google Scholar]
- 22.Menegaz D, Barrientos-Duran A, Kline A, Silva FRMB, Norman AW, Mizwicki MT, Zanello LP. 2010. 1alpha,25(OH)2-Vitamin D3 stimulation of secretion via chloride channel activation in Sertoli cells. J Steroid Biochem Mol Biol 119: 127–134. doi: 10.1016/j.jsbmb.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 23.Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschöp MH. 2019. Glucagon-like peptide 1 (GLP-1). Mol Metab 30: 72–130. doi: 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. 2021. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab 23 Suppl 3: 5–29. doi: 10.1111/dom.14496 [DOI] [PubMed] [Google Scholar]
- 25.Recchia K, Jorge AS, Pessôa LVF, Botigelli RC, Zugaib VC, de Souza AF, Martins DDS, Ambrósio CE, Bressan FF, Pieri NCG. 2021. Actions and roles of FSH in germinative cells. Int J Mol Sci 22: 10110. doi: 10.3390/ijms221810110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regueira M, Riera MF, Galardo MN, Pellizzari EH, Cigorraga SB, Meroni SB. 2014. Activation of PPAR α and PPAR β/δ regulates Sertoli cell metabolism. Mol Cell Endocrinol 382: 271–281. doi: 10.1016/j.mce.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 27.Rossi P, Dolci S. 2013. Paracrine mechanisms involved in the control of early stages of Mammalian spermatogenesis. Front Endocrinol (Lausanne) 4: 181. doi: 10.3389/fendo.2013.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallmén M, Sandler DP, Hoppin JA, Blair A, Baird DD. 2006. Reduced fertility among overweight and obese men. Epidemiology 17: 520–523. doi: 10.1097/01.ede.0000229953.76862.e5 [DOI] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seino S, Shibasaki T. 2005. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85: 1303–1342. doi: 10.1152/physrev.00001.2005 [DOI] [PubMed] [Google Scholar]
- 31.Seino Y, Fukushima M, Yabe D. 2010. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig 1: 8–23. doi: 10.1111/j.2040-1124.2010.00022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi GJ, Li ZM, Zheng J, Chen J, Han XX, Wu J, Li GY, Chang Q, Li YX, Yu JQ. 2017. Diabetes associated with male reproductive system damages: onset of presentation, pathophysiological mechanisms and drug intervention. Biomed Pharmacother 90: 562–574. doi: 10.1016/j.biopha.2017.03.074 [DOI] [PubMed] [Google Scholar]
- 33.Tan Q, Akindehin SE, Orsso CE, Waldner RC, DiMarchi RD, Müller TD, Haqq AM. 2022. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front Endocrinol (Lausanne) 13: 838410. doi: 10.3389/fendo.2022.838410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka T, Higashijima Y, Wada T, Nangaku M. 2014. The potential for renoprotection with incretin-based drugs. Kidney Int 86: 701–711. doi: 10.1038/ki.2014.236 [DOI] [PubMed] [Google Scholar]
- 35.Vendrell J, El Bekay R, Peral B, García-Fuentes E, Megia A, Macias-Gonzalez M, Fernández Real J, Jimenez-Gomez Y, Escoté X, Pachón G, Simó R, Selva DM, Malagón MM, Tinahones FJ. 2011. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 152: 4072–4079. doi: 10.1210/en.2011-1070 [DOI] [PubMed] [Google Scholar]
- 36.Vigueras-Villaseñor RM, Rojas-Castañeda JC, Chávez-Saldaña M, Gutiérrez-Pérez O, García-Cruz ME, Cuevas-Alpuche O, Reyes-Romero MM, Zambrano E. 2011. Alterations in the spermatic function generated by obesity in rats. Acta Histochem 113: 214–220. doi: 10.1016/j.acthis.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 37.Wei Q, Li L, Chen JA, Wang SH, Sun ZL. 2015. Exendin-4 improves thermogenic capacity by regulating fat metabolism on brown adipose tissue in mice with diet-induced obesity. Ann Clin Lab Sci 45: 158–165. [PubMed] [Google Scholar]
- 38.Xiao Y, Han J, Wang Q, Mao Y, Wei M, Jia W, Wei L. 2017. A novel interacting protein SERP1 regulates the N-Linked glycosylation and function of GLP-1 receptor in the liver. J Cell Biochem 118: 3616–3626. doi: 10.1002/jcb.26207 [DOI] [PubMed] [Google Scholar]
- 39.Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H, Liang H, Weng J. 2016. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 59: 1059–1069. doi: 10.1007/s00125-016-3896-5 [DOI] [PubMed] [Google Scholar]
- 40.Yamada S, Tanabe J, Ogura Y, Nagai Y, Sugaya T, Ohata K, Natsuki Y, Ichikawa D, Watanabe S, Inoue K, Hoshino S, Kimura K, Shibagaki Y, Kamijo-Ikemori A. 2021. Renoprotective effect of GLP-1 receptor agonist, liraglutide, in early-phase diabetic kidney disease in spontaneously diabetic Torii fatty rats. Clin Exp Nephrol 25: 365–375. doi: 10.1007/s10157-020-02007-2 [DOI] [PubMed] [Google Scholar]
- 41.Yamada Y, Tsukiyama K, Sato T, Shimizu T, Fujita H, Narita T. 2016. Novel extrapancreatic effects of incretin. J Diabetes Investig 7 Suppl 1: 76–79. doi: 10.1111/jdi.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang E, Xu F, Liang H, Yan J, Xu H, Li Z, Wen X, Weng J. 2015. GLP-1 receptor agonist exenatide attenuates the detrimental effects of obesity on inflammatory profile in testis and sperm quality in mice. Am J Reprod Immunol 74: 457–466. doi: 10.1111/aji.12420 [DOI] [PubMed] [Google Scholar]