Abstract
Key Clinical Message
Swift and precise identification of heterotopic cesarean scar pregnancy, coupled with standardized treatment approaches for handling possible serious complications, form an essential component in reaching favorable outcomes for patients experiencing this rare type of pregnancy.
Abstract
Heterotopic pregnancy (HP) denotes a form of multiple gestation where intrauterine and ectopic pregnancies coexist. Cesarean scar ectopic pregnancy, on the other hand, involves the implantation of a fetus over the previous cesarean scar. This condition poses a significant risk of uterine rupture, which may lead to serious health complications, and even death. We report a case of a fit 37‐year‐old woman with two previous cesarean deliveries who was diagnosed with a heterotopic cesarean scar pregnancy at 8 weeks gestation following symptoms of lower abdominal pain and delayed menstruation. Both pregnancies demonstrated cardiac activity and the portion of the myometrium located between the bladder wall and the gestational sac was noted to exhibit considerable thinness. The patient underwent an exploratory laparotomy coupled with dilation and curettage and recovered uneventfully. The proper management of a HCSP requires timely diagnosis through ultrasonography. Early diagnosis allows for immediate intervention to prevent complications such as uterine rupture or potentially lethal bleeding.
Keywords: dilation and curettage, heterotopic cesarean scar pregnancy, laparotomy, ultrasonography
1. INTRODUCTION
Heterotopic pregnancy (HP) is characterized by the existence of intrauterine and ectopic pregnancies occurring simultaneously. 1 This is a rare yet serious condition that can be spontaneous or resultant from assisted reproductive technology (ART). 2 While spontaneous HP has a reported frequency of 1 in 50,000–1 in 10,000, 3 , 4 ART‐related cases of HP have been estimated to occur in 0.2%–1% of patients. 5 Heterotopic cesarean scar pregnancy (HCSP) involves the occurrence of a cesarean scar pregnancy (CSP) accompanied by intrauterine pregnancy (IUP), which poses a high risk of catastrophic complications such as uterine rupture and massive hemorrhage. 6 , 7 , 8 An extremely low incidence of HCSP has been reported during spontaneous cycles. Nevertheless, due to the rising occurrence of cesarean section delivery and the expanding recourse to ARTs, the prevalence of HCSP is anticipated to increase. 9 , 10 , 11 Due to the considerable risk for fetal and maternal morbidity and mortality, timely, and precise diagnosis of HCSP is vital. 12 Medical imaging technology, a baseline serum human chorionic gonadotropin (HCG) concentration, and the history of a cesarean section form the basis of the clinical diagnosis. 13 Patients' reproductive needs, hemorrhage risk, and imaging classification determine the therapeutic strategy. To diagnose, the most common imaging methods are traditional 2D and 3D color/power Doppler ultrasound, contrast‐enhanced ultrasound, and magnetic resonance imaging (MRI). 14 As the first‐line imaging method for CSP, ultrasound is considered the preferred diagnostic method. Ultrasound can detect CSP with a sensitivity of 84.6%. 15 When transvaginal ultrasound (TVS) is performed, the probe is close to the cervix, preventing intestinal gas interference, and displays the GS's location without requiring bladder filling. By performing this procedure, one can find out the size, and shape of the gestational sac, the thickness of the myometrium around the scar, and embryo, as well as the yolk sac and the heartbeat in the gestational sac. Additionally, transabdominal ultrasound (TAS) can demonstrate the relationship between the gestational sac and the bladder when properly filled. 16 In color and power Doppler ultrasounds, abundant low‐impedance flow signals can be obtained to determine the blood flow to the mass. 10 In terms of diagnosing CSP, determining treatment options, and determining prognoses, TVS, TAS, and Doppler ultrasound appear to be of significant value together. 17
In the management of HCSP, preserving the coexistent IUP presents a significant challenge. Guidelines for managing HCSP while preserving the IUP are not universally standardized due to the rarity of this condition. 18 The typical method is to terminate the implantation located within the scarred area, if deemed necessary, at the potential expense of terminating the IUP. 7 The available literature reveals a range of techniques for managing this condition, with medical, and surgical approaches being two distinct options. The surgical interventions, which may involve laparoscopic or hysteroscopic excision of the masses, have been linked with potential complications like the loss of pregnancy and preterm delivery. In our research, we detail a case of HCSP, which was addressed through a combination of exploratory laparotomy and dilation and curettage (D&C) procedures.
2. CASE DESCRIPTION
A healthy 37‐year‐old woman, with a history of two previous cesarean sections due to breech presentation and repeated cesarean delivery 12 and 5 years earlier, without any significant complication, presented to our facility with sudden lower abdominal pain and delay in menstruation. The patient was hemodynamically stable and the abdominal examination was not positive for tenderness, guarding, or rebound tenderness. No blood was detected in the vaginal examination and the cervix was closed. The woman's lab results, including a beta‐human chorionic gonadotropin (β‐hCG) measurement of 174,025 milli‐international units/mL, were all within the accepted range. Transabdominal and Transvaginal ultrasonography revealed a dichorionic diamniotic twin gestation, with cardiac activity, and crown‐rump length appropriate for both fetuses for 7 weeks and 5 days of gestation. There was no apparent difference in size between the embryos (the crown‐rump length of the fetuses was 1.38 cm). Almost the same sac sizes were observed for heterotopic and intrauterine pregnancies, the gestational sac of the IUP, which was identified in the endometrial cavity was 26 mm in diameter and the gestational sac of the HP which was found in the anterior uterine isthmus, specifically at the site of the earlier cesarean scar was 28 mm in diameter. The thickness of the myometrium at the implantation site of the heterotopic sac was measured at 2.5 mm (Figures 1 and 2), and rich blood flow (in color Doppler ultrasound examination) amidst the gestational sac and the wall of the bladder (Figure 3).
FIGURE 1.
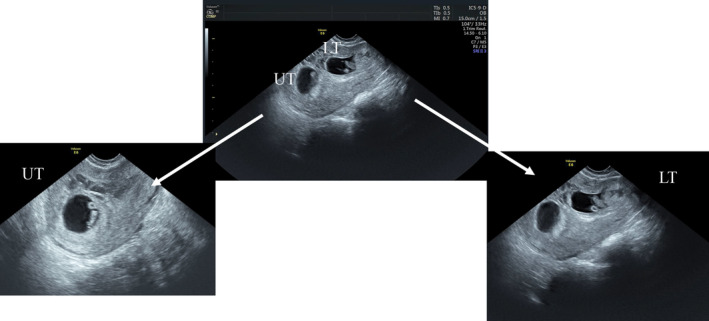
Dichorionic diamniotic pregnancy, with one sac implanted at the cesarean scar. Transvaginal ultrasonography; sagittal view showing two separate intrauterine gestational sacs with two yolk sacs and alive embryos; the upper twin (UT): normally implanted in the endometrial cavity; the lower twin (LT): abnormally implanted at the site of the previous cesarean section scar.
FIGURE 2.
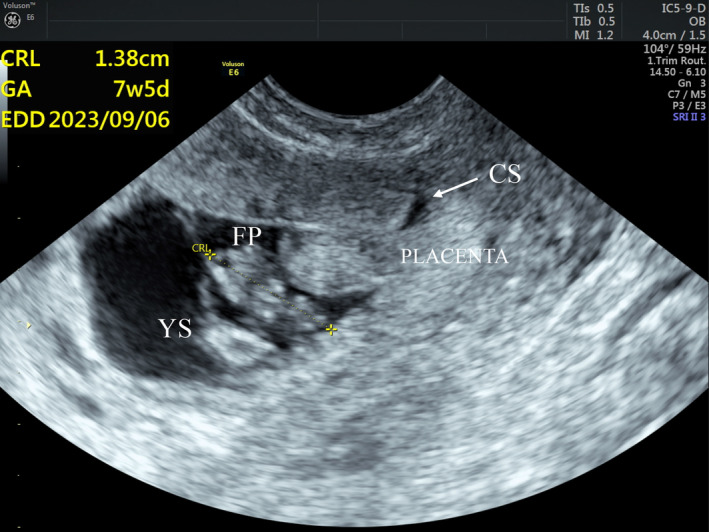
Magnified TVS sagittal view shows the ectopic gestational sac at the lower uterine segment at the site of the cesarean scar (CS) with a yolk sac (YS) and a fetal pole (FP) with cardiac activity and a crown‐rump length (CRL) of 1.38 cm with estimated gestational age (GA) of 7 weeks and 5 days. The ectopic gestational sac extends into the cesarean scar (CS), occupying more than one‐half thickness of the lower uterine segment. The overlying myometrium is thinned out (between cursors).
FIGURE 3.
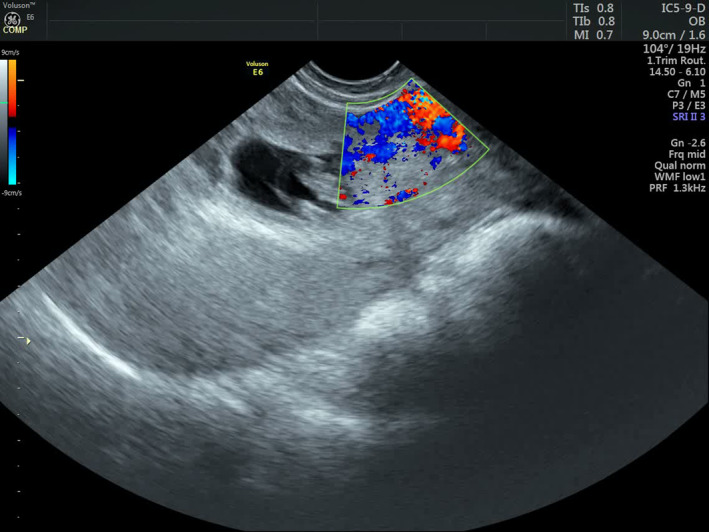
Transvaginal color Doppler ultrasonography shows rich vascularity at the implantation site of the lower twin within the previous cesarean scar.
3. METHODS
The above findings were suggestive of a HCSP. The patient and her husband received guidance regarding the management options and potential maternal and fetal complications associated with the continuation of the pregnancy, such as excessive bleeding, abortion, and the need for subsequent hysterectomy. After thorough counseling, the couple did not want to preserve the intrauterine gestation. The obstetrician arranged for the patient to undergo an exploratory laparotomy coupled with a D & C procedure. An exploratory laparotomy was performed to observe the Isthmus uteri and if necessary, undergo a partial, or total hysterectomy. However, the curettage was uneventful, no perforations were caused, and no incisions were made. The bleeding during surgery was about 500 mL. US was performed the following day which demonstrated complete evacuation of the uterus and β‐hCG was dropped to 68,344 milli‐international units/mL 48 h after the operation.
4. CONCLUSION AND RESULTS
The patient was discharged on postoperative Day 3 and her next follow‐ups were unremarkable. The proper management of a HCSP requires timely diagnosis through ultrasonography. Early diagnosis allows for immediate intervention to prevent complications such as uterine rupture or potentially lethal bleeding.
5. DISCUSSION
HCSP is recognized as one of the least common forms of heterotopic pregnancies, requiring careful observation of a potential IUP. 8 , 19 It has been documented that approximately 1 in 30,000 deliveries conventionally encompasses HP. However, with the advent and continued use of ART, there has been an observable increase in the occurrence of HP, which is currently approximated at 1%. 20 , 21 Several theories have been put forward in an attempt to elucidate the origin of this condition. The most plausible hypothesis posits that the blastocyst makes its way through the uterine wall through a small, non‐continuous pathway. This could potentially be a consequence of damage incurred during a cesarean section, other forms of uterine surgery, or following manual placenta removal. Even without a history of uterine surgery, in vitro fertilization could occasionally lead to this outcome. CSP may demonstrate a symptom‐free clinical trajectory or present with specific clinical signs like unusual vaginal bleeding and/or abdominal discomfort, or sudden abdominal pain due to uterine rupture. 1 Given the potentially fatal complications, such as severe bleeding and rupture, it is vital to diagnose, and manage this condition early. In the early phases of pregnancy, to initially detect a CSP, the primary imaging method recommended is ultrasound scan. 22 Typically, sonographic imaging can identify an increase in the size of the cesarean scar in the lower segment, as well as either a disparate mass or a distinct gestational sac linked to it. There are situations where a vulnerable myometrium, positioned between the bladder wall and the gestational scar, can be seen before rupture. 23 The main sonographic features indicative of a scar pregnancy diagnosis include (i) A vacant uterus, (ii) an unoccupied cervical canal, and (iii) the positioning of the gestational sac at the foremost region of the isthmic portion of the uterus, accompanied by a slender layer of myometrium located between the bladder and the sac. 24 , 25 , 26 , 27 Moreover, a break in the front wall of the uterus can be detected via a sagittal view of the uterus. This is achieved when the ultrasound beam's direction crosses through the amniotic sac. 28 Doppler flow is also important for determining implantation location. 2 Doppler flow evaluations can differentiate a viable pregnancy located in the scar region from a non‐viable intrauterine pregnancy. This distinction affects treatment strategies. If an intrauterine pregnancy proves non‐viable, the gestational sac appears devoid of vasculature, indicating its separation from the implantation site. Conversely, if a CSP maintains viability, the gestational sac displays a well‐vascularized appearance in Doppler examinations. The gestational mass within the scar region exhibits a low‐impedance flow rate (pulsatility index <1) and a high speed (peak velocity >20 cm/s). 29 Various investigators have also noted that the blood flow resistance index is below 0.5, and the peak value ratio of systolic‐to‐diastolic flow is less than 3. 23 TVS, when used in conjunction with color Doppler analysis, exhibits 85% diagnostic sensitivity for CSP detection. 30 Recently, some clinicians have begun utilizing three‐dimensional (3D) ultrasonography and 3D Power Doppler imaging. 31 Based on their findings, employing multiplanar views with 3D‐rendered images can enhance diagnostic precision in such circumstances. In cases where diagnosis becomes intricate or challenging, Magnetic Resonance Imaging may also prove advantageous 32 HCSP handling is usually complex, especially if the woman wants to preserve her current intrauterine embryo. 33 The treatment protocol specific to HP involving CSP is not universally recognized and established. Various strategies are available for managing HCSP, including watchful waiting, medical intervention, and surgical termination. Successful cases of expectant management have been documented in the medical literature. However, given the unfavorable prognosis for an uncomplicated full‐term pregnancy, this approach is generally not advised. 12 There are several approaches to CSP, including conservative approaches (feticide using potassium chloride (KCl) or hyperosmolar glucose, methotrexate (MTX) locally or systemically, and embryo aspiration), surgical approaches (dilatation and curettage, hysteroscopy, laparoscopy, or laparotomy), or combination approaches. 33 In numerous case studies, MTX is an effective treatment for ectopic pregnancies. However, there are concerns that its use alongside an intrauterine pregnancy might cause teratogenic effects. 6 Because of methotrexate's potential teratogenicity and embryotoxicity, its use is not recommended in HCSP. There are a few reports of HCSP with viable pregnancies treated with KCl injections, which are traditionally used for fetal reduction in multiple pregnancies. It is important to note, however, that KCl is associated with certain inherent risks, including abdominal discomfort, miscarriage, excessive vaginal bleeding, preterm birth, a need for further surgery, and spontaneous rupture of the amniotic membranes, which can result in chorioamnionitis. 6 Moreover, embryo aspiration may only be successful in the early stages of pregnancy when the embryo has not yet developed. 34 The foremost operative treatment option for ectopic CSP usually entails the explicit extraction of the ectopic mass situated at the site of the earlier cesarean scar. 35 Some scholars have suggested a surgical approach as the primary management strategy for an HCSP. 18 In a few cases, hysteroscopic removal of the CSP was found to be successful and safe as an alternative treatment for preserving viable intrauterine gestation. 36 Methotrexate and hysteroscopic resection have also been reported to manage term pregnancy following CSP. 37 It has also been reported that hysteroscopic termination of a heterotopic cervical ectopic pregnancy can preserve a concurrent intrauterine pregnancy. 38 It has been reported that laparoscopic removal of heterotopic CSP ectopic mass can lead to good pregnancy outcomes. 39 Nevertheless, laparoscopy should be performed simultaneously with anatomic dissection, trimming of unhealthy tissues, and repair of the uterine defect following the principles of laparotomy. 40 Open laparotomies are popular because of their superior surgical control and the expanded operational field they offer. As a result, excessive bleeding can be effectively controlled more effectively with an open laparotomy. 35 HIFU is a recently developed technique in which acoustic waves are converted to thermal energy when temperature reaches 65°C. 41 Studies have investigated HIFU treatment for CSP in the past few years, an approach that is considered to be effective and noninvasive. 42 , 43 , 44 A further benefit of HIFU is that it can reduce the risk of intraoperative bleeding. 41 In general, HIFU has been successfully applied to studies without complications. As a treatment option for CSP, endovascular surgery, and uterine artery embolization are also proven to be effective. 45 , 46 The patient presented to our facility with mild symptoms, and an ultrasound examination identified heterotopic CSP with one viable intrauterine embryo aged 7 weeks and 5 days. A detailed explanation of HCSP's potential hazards and complexities, as well as management strategies, was given to the couple. Since the couple did not intend to maintain the normally implanted viable intrauterine pregnancy, as well as the thin myometrium between the gestational sac and the bladder wall posing a significant risk of heavy bleeding, it was decided to perform an exploratory laparotomy with D & C. It was a successful surgery, the uterus was preserved without severe blood loss, and the patient recovered smoothly.
AUTHOR CONTRIBUTIONS
Mahsa Karbasi: Conceptualization; data curation; project administration; supervision; writing – original draft. Reza Aletaha: Conceptualization; investigation. Ramin Ahangar‐Sirous: Conceptualization; investigation. Amir Honarmand Alamdari: Conceptualization; investigation. Esmaeil Gharepapagh: Supervision; writing – review and editing. Sahar Rezaei: Conceptualization; supervision; validation; visualization; writing – review and editing.
FUNDING INFORMATION
None.
ETHICS STATEMENT
Not applicable.
CONSENT
The patient provided his written informed consent to publish and allowed for the publication of any accompanying images. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We are thankful for the help of the entire Al‐Zahra Gynecology and Obstetrics Center staff for immediate management and follow‐up of this patient.
Karbasi M, Aletaha R, Ahangar‐Sirous R, Alamdari AH, Gharepapagh E, Rezaei S. A rare case report of heterotopic cesarean scar pregnancy in the 8th week of gestation that was managed successfully by exploratory laparotomy with dilation and curettage. Clin Case Rep. 2024;12:e9025. doi: 10.1002/ccr3.9025
Contributor Information
Esmaeil Gharepapagh, Email: esmaeil.gharepapagh@gmail.com.
Sahar Rezaei, Email: saharrezaei.tums@gmail.com.
DATA AVAILABILITY STATEMENT
All available data are presented in this article and if more explanations are needed, contact the corresponding author.
REFERENCES
- 1. Yu H, Luo H, Zhao F, Liu X, Wang X. Successful selective reduction of a heterotopic cesarean scar pregnancy in the second trimester: a case report and review of the literature. BMC Pregnancy Childbirth. 2016;16(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ouyang Y, Chen H, Lin G, et al. Heterotopic cesarean scar pregnancy: an analysis of 20 cases following in vitro fertilization‐embryo transfer. J Ultrasound Med. 2021;40(10):2239‐2249. [DOI] [PubMed] [Google Scholar]
- 3. Barrenetxea G, Barinaga‐Rementeria L, Lopez de Larruzea A, Agirregoikoa JA, Mandiola M, Carbonero K. Heterotopic pregnancy: two cases and a comparative review. Fertil Steril. 2007;87(2):417.e9‐417.e15. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg JM, Bedaiwy MA. Transvaginal local injection of hyperosmolar glucose for the treatment of heterotopic pregnancies. Obstet Gynecl. 2006;107(2 Part 2):509‐510. [DOI] [PubMed] [Google Scholar]
- 5. Dor J, Seidman DS, Levran D, Ben‐Rafael Z, Ben‐Shlomo I, Mashiach S. The incidence of combined intrauterine and extrauterine pregnancy after in vitro fertilization and embryo transfer. Fertil Steril. 1991;55(4):833‐834. [DOI] [PubMed] [Google Scholar]
- 6. Vikhareva O, Nedopekina E, Herbst A. Normal vaginal delivery at term after expectant management of heterotopic caesarean scar pregnancy: a case report. J Med Case Rep. 2018;12(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. OuYang Z, Yin Q, Xu Y, Ma Y, Zhang Q, Yu Y. Heterotopic cesarean scar pregnancy: diagnosis, treatment, and prognosis. J Ultrasound Med. 2014;33(9):1533‐1537. [DOI] [PubMed] [Google Scholar]
- 8. Kim ML, Jun HS, Kim JY, Seong SJ, Cha DH. Successful full‐term twin deliveries in heterotopic cesarean scar pregnancy in a spontaneous cycle with expectant management. J Obstet Gynaecol Res. 2014;40(5):1415‐1419. [DOI] [PubMed] [Google Scholar]
- 9. Salomon LJ, Fernandez H, Chauveaud A, Doumerc S, Frydman R. Successful management of a heterotopic caesarean scar pregnancy: potassium chloride injection with preservation of the intrauterine gestation: case report. Hum Reprod. 2003;18(1):189‐191. [DOI] [PubMed] [Google Scholar]
- 10. Timor‐Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14‐29. [DOI] [PubMed] [Google Scholar]
- 11. Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maymon R, Halperin R, Mendlovic S, Schneider D, Herman A. Ectopic pregnancies in a caesarean scar: review of the medical approach to an iatrogenic complication. Hum Reprod Update. 2004;10(6):515‐523. [DOI] [PubMed] [Google Scholar]
- 13. Maheux‐Lacroix S, Li F, Bujold E, Nesbitt‐Hawes E, Deans R, Abbott J. Cesarean scar pregnancies: a systematic review of treatment options. J Minim Invasive Gynecol. 2017;24(6):915‐925. [DOI] [PubMed] [Google Scholar]
- 14. Liu D, Yang M, Wu Q. Application of ultrasonography in the diagnosis and treatment of cesarean scar pregnancy. Clin Chim Acta. 2018;486:291‐297. [DOI] [PubMed] [Google Scholar]
- 15. Timor‐Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy: patient counseling and management. Obstet Gynecol Clin North Am. 2019;46(4):813‐828. [DOI] [PubMed] [Google Scholar]
- 16. Nijjar S, Jauniaux E, Jurkovic D. Definition and diagnosis of cesarean scar ectopic pregnancies. Best Pract Res Clin Obstet Gynaecol. 2023;89:102360. [DOI] [PubMed] [Google Scholar]
- 17. Marchiole P, Gorlero F, de Caro G, Podestà M, Valenzano M. Intramural pregnancy embedded in a previous cesarean section scar treated conservatively. Ultrasound Obstet Gynecol. 2004;23(3):307‐309. [DOI] [PubMed] [Google Scholar]
- 18. Chen ZY, Zhou Y, Qian Y, Luo JM, Huang XF, Zhang XM. Management of heterotopic cesarean scar pregnancy with preservation of intrauterine pregnancy: a case report. World J Clin Cases. 2021;9(22):6428‐6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ugurlucan FG, Bastu E, Dogan M, Kalelioglu I, Alanya S, Has R. Management of cesarean heterotopic pregnancy with transvaginal ultrasound‐guided potassium chloride injection and gestational sac aspiration, and review of the literature. J Minim Invasive Gynecol. 2012;19(5):671‐673. [DOI] [PubMed] [Google Scholar]
- 20. Yoder N, Tal R, Martin JR. Abdominal ectopic pregnancy after in vitro fertilization and single embryo transfer: a case report and systematic review. Reprod Biol Endocrinol. 2016;14(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lincenberg KR, Behrman ER, Bembry JS, Kovac CM. Uterine rupture with cesarean scar heterotopic pregnancy with survival of the intrauterine twin. Case Rep Obstet Gynecol. 2016;2016:6832094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Timor‐Tritsch IE, Monteagudo A, Cali G, el Refaey H, Kaelin Agten A, Arslan AA. Easy sonographic differential diagnosis between intrauterine pregnancy and cesarean delivery scar pregnancy in the early first trimester. Am J Obstet Gynecol. 2016;215(2):225.e1‐225.e7. [DOI] [PubMed] [Google Scholar]
- 23. Weimin W, Wenqing L. Effect of early pregnancy on a previous lower segment cesarean section scar. Int J Gynaecol Obstet. 2002;77(3):201‐207. [DOI] [PubMed] [Google Scholar]
- 24. Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997;67(2):398‐400. [DOI] [PubMed] [Google Scholar]
- 25. Seow KM, Cheng WC, Chuang J, Lee C, Tsai YL, Hwang JL. Methotrexate for cesarean scar pregnancy after in vitro fertilization and embryo transfer. A case report. J Reprod Med. 2000;45(9):754‐757. [PubMed] [Google Scholar]
- 26. Seow KM, Huang LW, Lin YH, Yan‐Sheng Lin M, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247‐253. [DOI] [PubMed] [Google Scholar]
- 27. Fylstra DL. Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv. 2002;57(8):537‐543. [DOI] [PubMed] [Google Scholar]
- 28. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6):592‐593. [DOI] [PubMed] [Google Scholar]
- 29. Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First‐trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol. 2003;21(3):220‐227. [DOI] [PubMed] [Google Scholar]
- 30. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373‐1381. [DOI] [PubMed] [Google Scholar]
- 31. Shih JC. Cesarean scar pregnancy: diagnosis with three‐dimensional (3D) ultrasound and 3D power Doppler. Ultrasound Obstet Gynecol. 2004;23(3):306‐307. [DOI] [PubMed] [Google Scholar]
- 32. Dueñas‐Garcia OF, Young C. Heterotopic cesarean scar pregnancy associated with a levonorgestrel‐releasing intrauterine device. Int J Gynaecol Obstet. 2011;114(2):153‐154. [DOI] [PubMed] [Google Scholar]
- 33. Demirel LC, Bodur H, Selam B, Lembet A, Ergin T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: case report. Fertil Steril. 2009;91(4):1293.e5‐1293.e7. [DOI] [PubMed] [Google Scholar]
- 34. Taskn S, Taskn EA, Ciftci TT. Heterotopic cesarean scar pregnancy: how should it be managed? Obstet Gynecol Surv. 2009;64(10):690‐695. [DOI] [PubMed] [Google Scholar]
- 35. Kim H, Koh JH, Lee J, et al. Successful full‐term delivery via selective ectopic embryo reduction accompanied by uterine cerclage in a heterotopic cesarean scar pregnancy: a case report and literature review. Diagnostics (Basel). 2022;12(3):762. 10.3390/diagnostics12030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang C‐J, Tsai F, Chen C, Chao A. Hysteroscopic management of heterotopic cesarean scar pregnancy. Fertil Steril. 2010;94(4):1529.e15‐1529.e18. [DOI] [PubMed] [Google Scholar]
- 37. Korkontzelos I, Tsirkas P, Antoniou N, Souliotis D, Kosmas I. Successful term pregnancy after treatment of a cesarean scar ectopic gestation by endoscopic technique and conservative therapy. Fertil Steril. 2008;90(5):2010.e13‐2010.e15. [DOI] [PubMed] [Google Scholar]
- 38. Jozwiak EA, Ulug U, Akman MA, Bahceci M. Successful resection of a heterotopic cervical pregnancy resulting from intracytoplasmic sperm injection. Fertil Steril. 2003;79(2):428‐430. [DOI] [PubMed] [Google Scholar]
- 39. Demirel LC, Bodur H, Selam B, Lembet A, Ergin T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: case report. Fertil Steril. 2009;91(4):1293.e5‐1293‐1293.e7. [DOI] [PubMed] [Google Scholar]
- 40. Wang C‐N, Chen CK, Wang HS, Chiueh HY, Soong YK. Successful management of heterotopic cesarean scar pregnancy combined with intrauterine pregnancy after in vitro fertilization–embryo transfer. Fertil Steril. 2007;88(3):706.e13‐706.e16. [DOI] [PubMed] [Google Scholar]
- 41. Ouyang Y, Li X, Yi Y, Gong F, Lin G, Lu G. First‐trimester diagnosis and management of cesarean scar pregnancies after in vitro fertilization‐embryo transfer: a retrospective clinical analysis of 12 cases. Reprod Biol Endocrinol. 2015;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu X, Deng X, Wan Y, et al. High‐intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine. 2015;94(18):e854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiao J, Zhang S, Wang F, et al. Cesarean scar pregnancy: noninvasive and effective treatment with high‐intensity focused ultrasound. Am J Obstet Gynecol. 2014;211(4):356.e1‐356.e7. [DOI] [PubMed] [Google Scholar]
- 44. Huang L, Du Y, Zhao C. High‐intensity focused ultrasound combined with dilatation and curettage for cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2014;43(1):98‐101. [DOI] [PubMed] [Google Scholar]
- 45. Failla G, Libra F, Giurazza F, et al. Endovascular treatment of cesarean scar pregnancy: a retrospective multicentric study. Radiol Med. 2022;127(12):1313‐1321. [DOI] [PubMed] [Google Scholar]
- 46. Fornazari VA, Szejnfeld D, Elito Júnior J, Goldman SM. Interventional radiology and endovascular surgery in the treatment of ectopic pregnancies. Einstein (São Paulo). 2015;13(1):167‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data are presented in this article and if more explanations are needed, contact the corresponding author.


